Effect of Argon Flow on Oxygen and Carbon Coupled Transport in an Industrial Directional Solidification Furnace for Crystalline Silicon Ingots
Abstract
1. Introduction
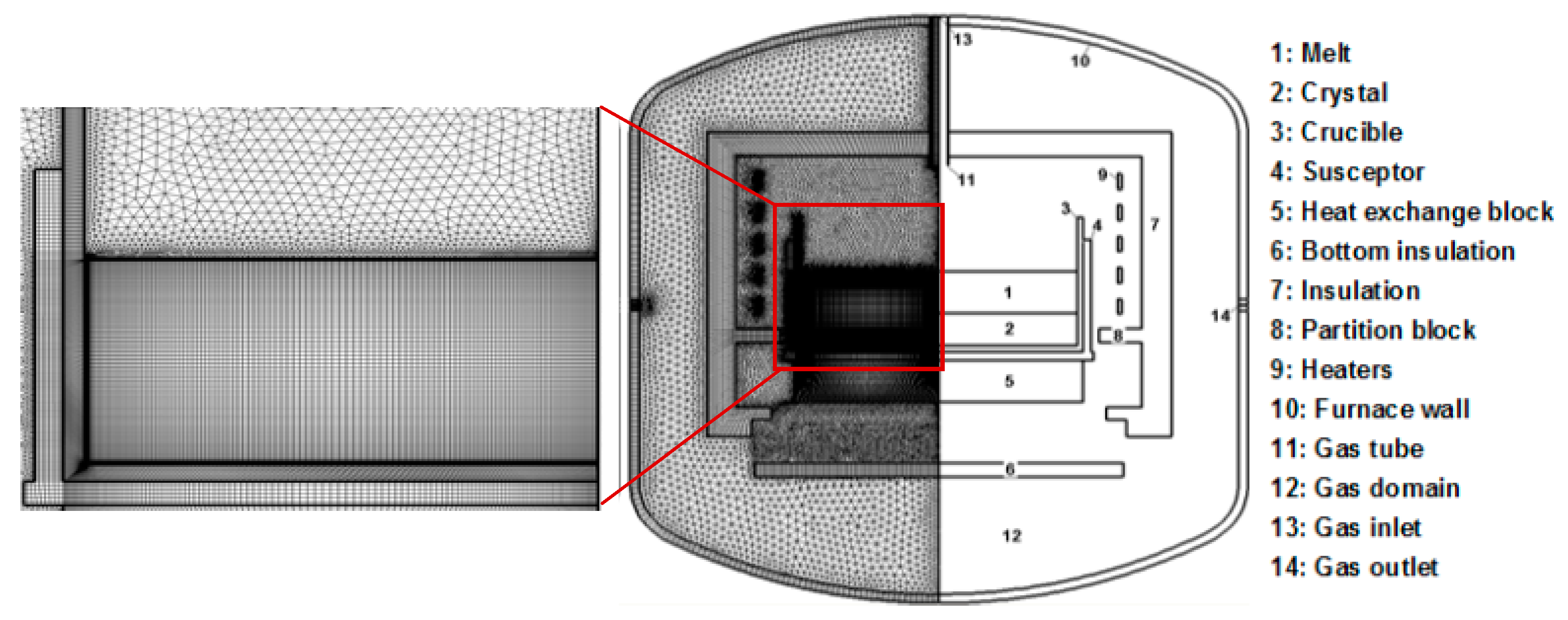
2. Model Description
3. Results and Discussion
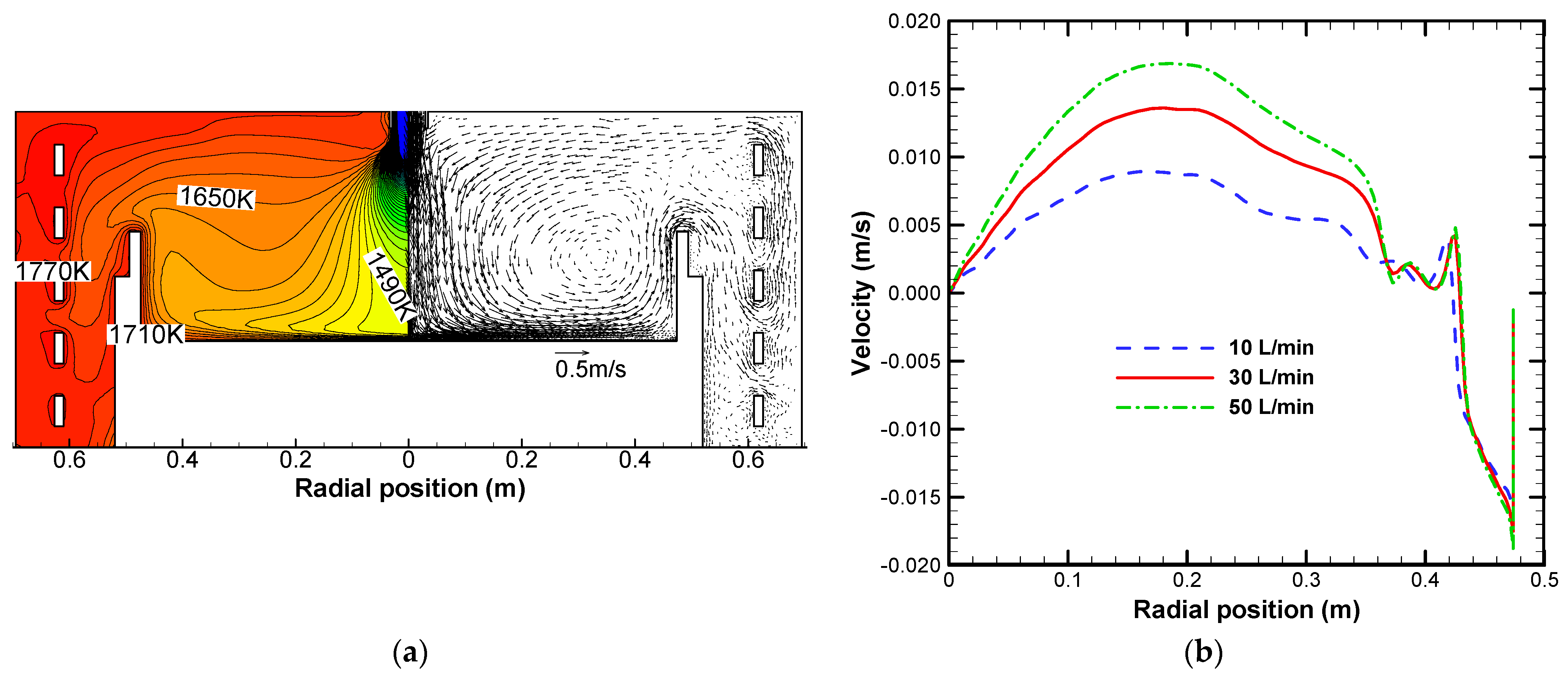
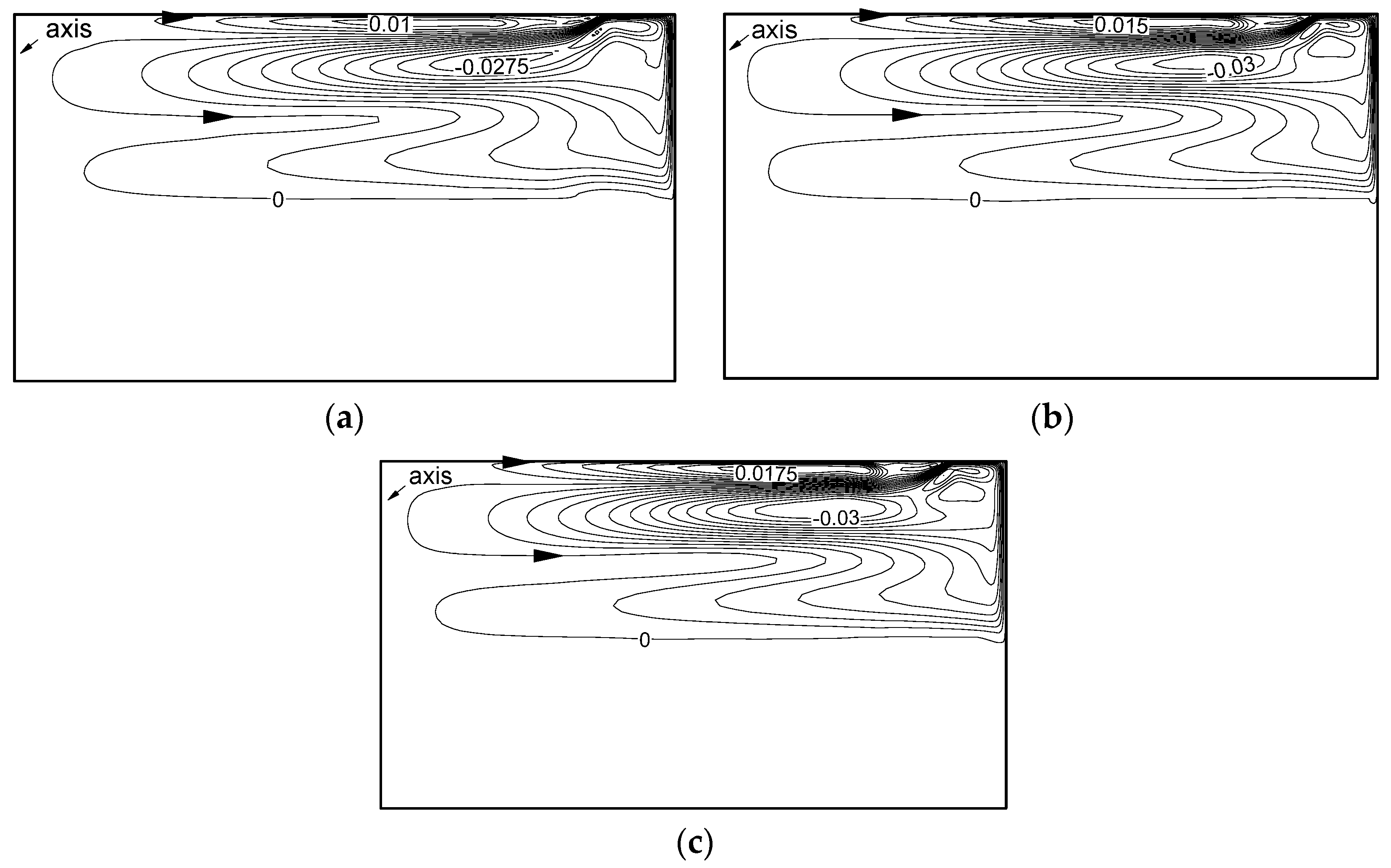
3.1. Effect on Heat Transfer
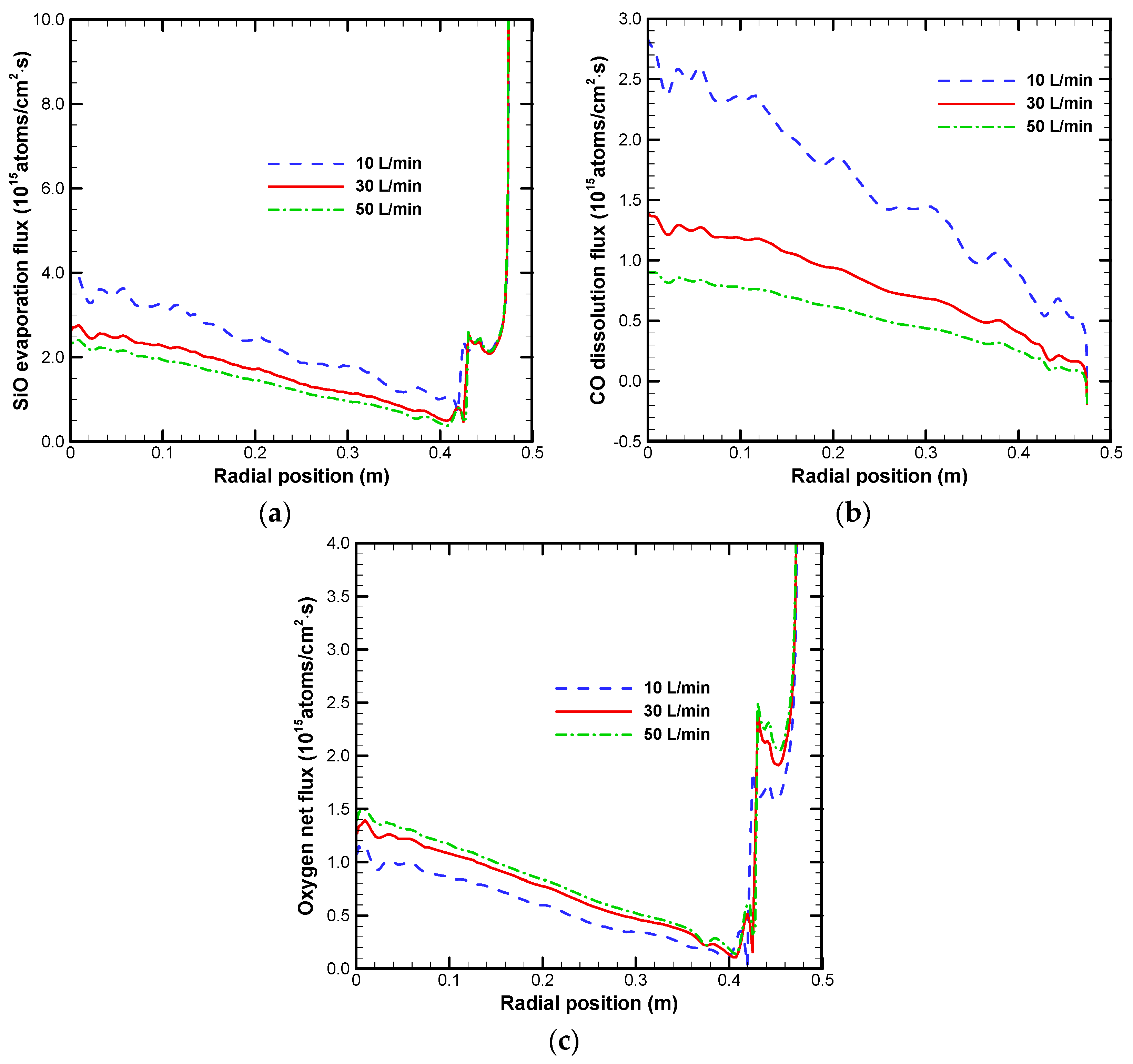
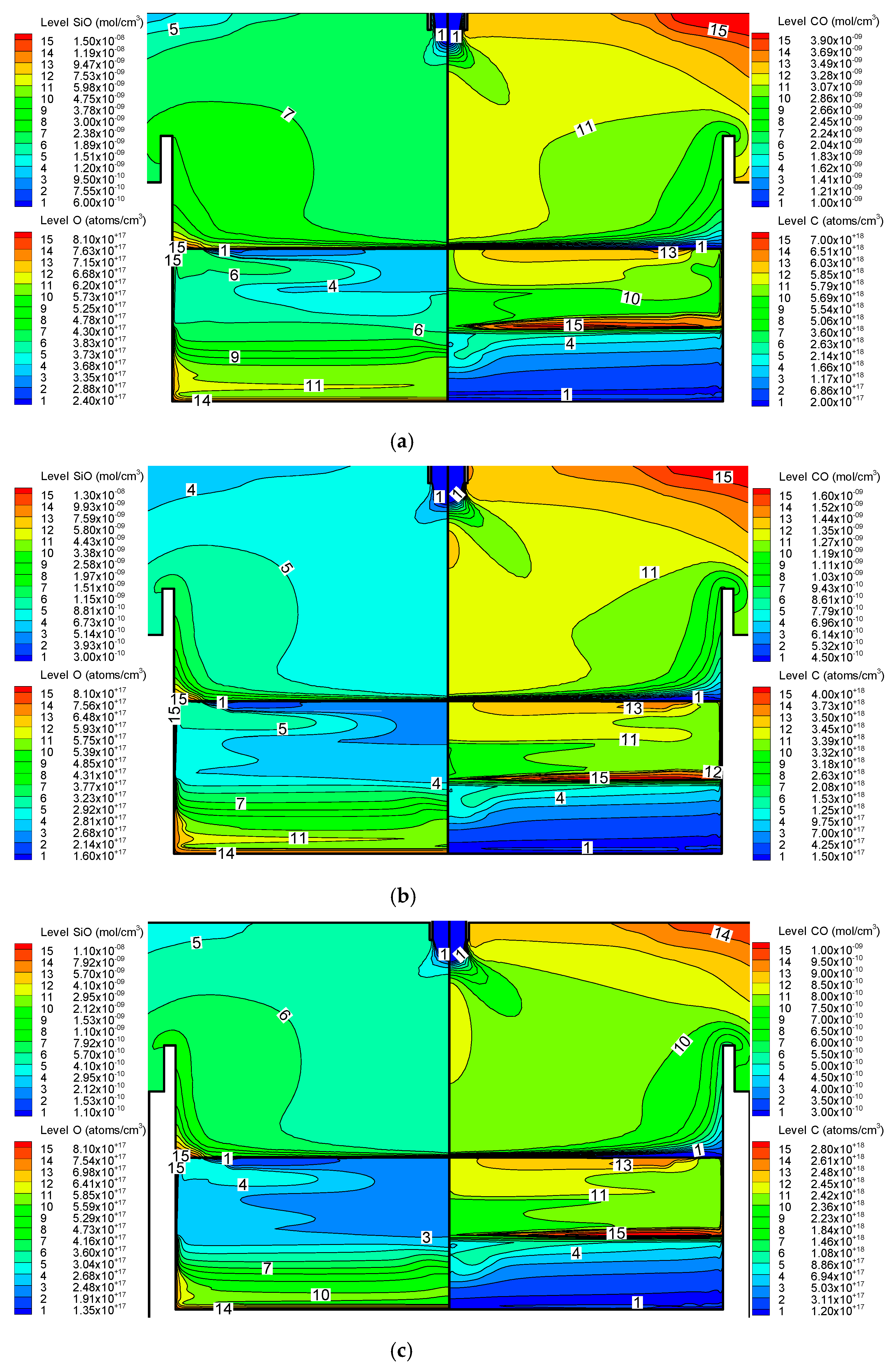
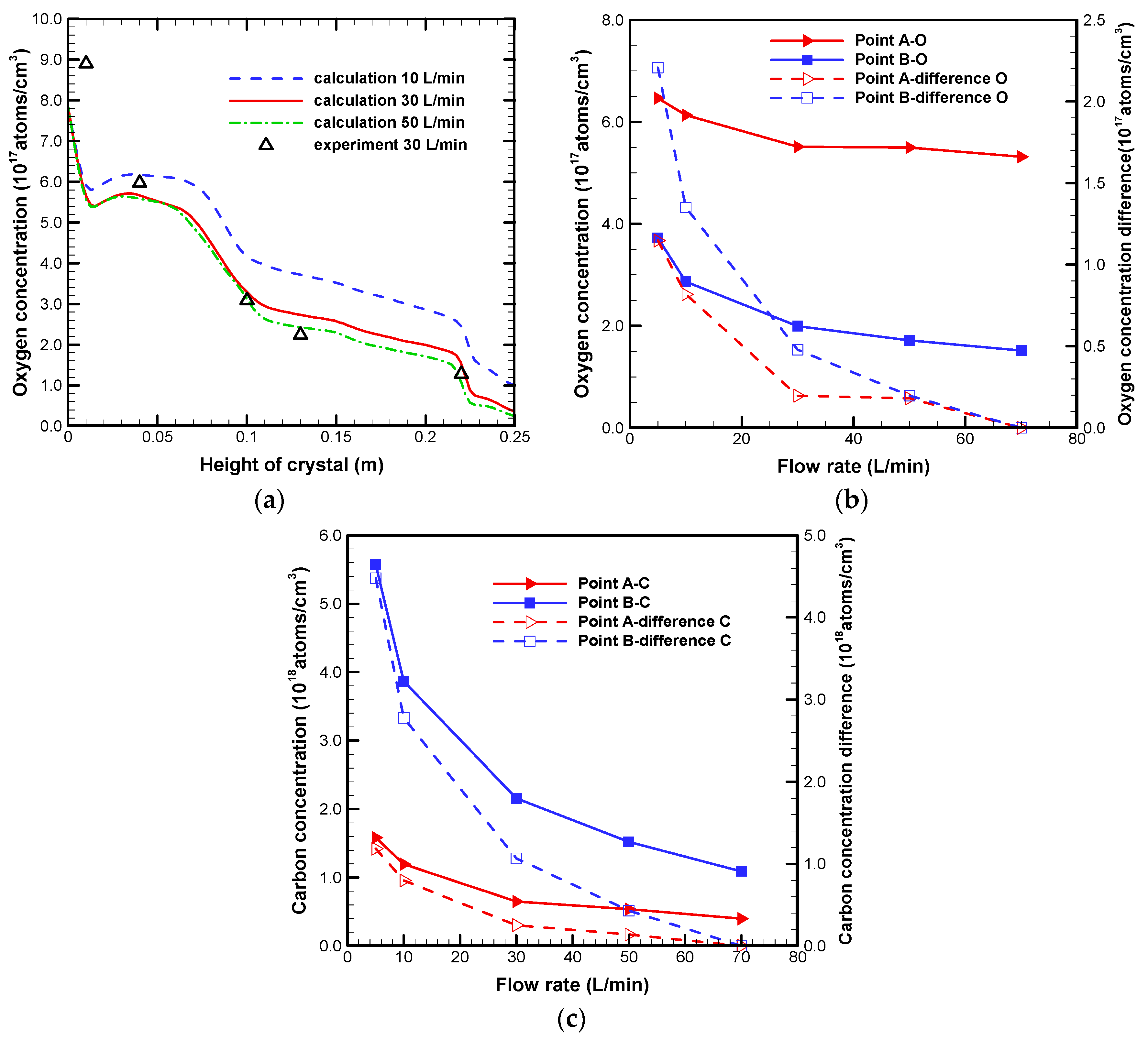
3.2. Effect on Impurities Transport
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stelian, C. Numerical modeling of carbon distribution and precipitation during directional solidification of photovoltaic silicon. Int. J. Heat Mass Tran. 2019, 145, 118775. [Google Scholar] [CrossRef]
- Bellmann, M.P.; Panjwani, B.; Syvertsen, M.; Meese, E.A. Dynamic simulation of impurity transport and chemical reactions in a Bridgman furnace for directional solidification of multi-crystalline silicon. J. Cryst. Growth 2013, 369, 47–54. [Google Scholar] [CrossRef]
- Saitoh, T.; Wang, X.; Hashigami, H.; Abe, T.; Igarashi, T.; Glunz, S.; Rein, S.; Wettling, W.; Yamasaki, I.; Sawai, H.; et al. Suppression of light degradation of carrier lifetimes in low-resistivity CZ-Si solar cells. Sol. Energy Mat. Sol. Cells 2001, 65, 277–285. [Google Scholar] [CrossRef]
- Richter, S.; Bauer, J.; Breitenstein, O. Growth of carbon and nitrogen containing precipitates in crystalline solar silicon and their influence on solar cells. Phys. Status Solidi RRL 2017, 11, 1600354. [Google Scholar] [CrossRef]
- Teng, Y.Y.; Chen, J.C.; Lu, C.W.; Chen, C.Y. Numerical investigation of oxygen impurity distribution during multicrystalline silicon crystal growth using a gas flow guidance device. J. Cryst. Growth 2012, 360, 12–17. [Google Scholar] [CrossRef]
- Bellmann, M.P.; Lindholm, D.; M’Hamdi, M. A novel method for gas flow and impurity control in directional solidification of multi-crystalline silicon. J. Cryst. Growth 2014, 399, 33–38. [Google Scholar] [CrossRef]
- Teng, Y.Y.; Chen, J.C.; Huang, B.S.; Chang, C.H. Numerical simulation of impurity transport under the effect of a gas flow guidance device during the growth of multicrystalline silicon ingots by the directional solidification process. J. Cryst. Growth 2014, 385, 1–8. [Google Scholar] [CrossRef]
- Liu, L.J.; Qi, X.F.; Ma, W.C.; Li, Z.Y.; Zhang, Y.F. Control of the gas flow in an industrial directional solidification furnace for production of high purity multicrystalline silicon ingots. Int. J. Photoenergy 2015, 2015, 513639. [Google Scholar] [CrossRef]
- Su, W.J.; Li, C.; Qi, X.F.; Yang, W.; Wang, J.F. Numerical analysis and optimization of gas flow and impurity control in directional solidification multi-crystalline Si. J. Cryst. Growth 2019, 527, 125244. [Google Scholar] [CrossRef]
- Kesavan, V.; Srinivasan, M.; Ramasamy, P. Optimizing oxygen impurities using different heater design in the directional solidification of multi-crystalline silicon. Mater. Res. Express 2019, 6, 106323. [Google Scholar] [CrossRef]
- Kesavan, V.; Srinivasan, M.; Ramasamy, P. The influence of multiple-heaters on the reduction of impurities in mc-Si for directional solidification. Silicon 2019, 11, 1335–1344. [Google Scholar] [CrossRef]
- Gao, B.; Nakano, S.; Kakimoto, K. Effect of crucible cover material on impurities of multicrystalline silicon in a unidirectional solidification furnace. J. Cryst. Growth 2011, 318, 255–258. [Google Scholar] [CrossRef]
- Liu, L.J.; Ma, W.C.; Qi, X.F.; Li, Z.Y.; Zhang, Y.F. Global simulation of coupled oxygen and carbon transport in an industrial directional solidification furnace for crystalline silicon ingots: Effect of crucible cover coating. Int. J. Heat Mass Tran. 2017, 108, 2355–2364. [Google Scholar] [CrossRef]
- Kumar, M.A.; Srinivasan, M.; Ramasamy, P. Reduction of carbon and oxygen impurities in mc-silicon ingot using molybdenum gas shield in directional solidification process. Silicon 2020. [Google Scholar] [CrossRef]
- Vishnuwaran, M.; Srinivasan, M.; Kesavan, V.; Ramasamy, P. Effect of titanium carbide heat exchanger block and retort on oxygen impurities in Mc-silicon: Numerical modelling. Silicon 2019, 12, 799–803. [Google Scholar] [CrossRef]
- Gao, B.; Nakano, S.; Kakimoto, K. Global simulation of coupled carbon and oxygen transport in a unidirectional solidification furnace for solar cells. J. Electrochem. Soc. 2010, 157, H153–H159. [Google Scholar] [CrossRef]
- Teng, Y.Y.; Chen, J.C.; Lu, C.W.; Chen, H.I.; Chuck, H.; Chen, C.Y. Effects of the furnace pressure on oxygen and silicon oxide distributions during the growth of multicrystalline silicon ingots by the directional. J. Cryst. Growth 2011, 318, 224–229. [Google Scholar] [CrossRef]
- Qi, X.F.; Liu, L.J.; Ma, W.C. Effects of furnace pressure on oxygen and carbon coupled transport in an industrial directional solidification furnace for crystalline silicon ingots. J. Cryst. Growth 2017, 468, 933–938. [Google Scholar] [CrossRef]
- Li, Z.Y.; Liu, L.J.; Ma, W.C.; Kakimoto, K. Effects of argon flow on impurities transport in a directional solidification furnace for silicon solar cells. J. Cryst. Growth 2011, 318, 304–312. [Google Scholar] [CrossRef]
- Trempa, M.; Reimann, C.; Friedrich, J.; Müller, G. The influence of growth rate on the formation and avoidance of C and N related precipitates during directional solidification of multi crystalline silicon. J. Cryst. Growth 2010, 312, 1517–1524. [Google Scholar] [CrossRef]
- Voller, V.R.; Prakash, C. A fixed grid numerical modelling methodology for convection-diffusion mushy region phase-change problems. Int. J. Heat Mass Tran. 1987, 30, 1709–1719. [Google Scholar] [CrossRef]
- Liu, L.J.; Nakano, S.; Kakimoto, K. Investigation of oxygen distribution in electromagnetic CZ–Si melts with a transverse magnetic field using 3D global modeling. J. Cryst. Growth 2007, 299, 48–58. [Google Scholar] [CrossRef]
- Ma, X.; Zheng, L.L.; Zhang, H.; Zhao, B.; Wang, C.; Xu, F.H. Thermal system design and optimization of an industrial silicon directional solidification system. J. Cryst. Growth 2011, 318, 288–292. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qi, X.; Xue, Y.; Su, W.; Ma, W.; Liu, L. Effect of Argon Flow on Oxygen and Carbon Coupled Transport in an Industrial Directional Solidification Furnace for Crystalline Silicon Ingots. Crystals 2021, 11, 421. https://doi.org/10.3390/cryst11040421
Qi X, Xue Y, Su W, Ma W, Liu L. Effect of Argon Flow on Oxygen and Carbon Coupled Transport in an Industrial Directional Solidification Furnace for Crystalline Silicon Ingots. Crystals. 2021; 11(4):421. https://doi.org/10.3390/cryst11040421
Chicago/Turabian StyleQi, Xiaofang, Yiwen Xue, Wenjia Su, Wencheng Ma, and Lijun Liu. 2021. "Effect of Argon Flow on Oxygen and Carbon Coupled Transport in an Industrial Directional Solidification Furnace for Crystalline Silicon Ingots" Crystals 11, no. 4: 421. https://doi.org/10.3390/cryst11040421
APA StyleQi, X., Xue, Y., Su, W., Ma, W., & Liu, L. (2021). Effect of Argon Flow on Oxygen and Carbon Coupled Transport in an Industrial Directional Solidification Furnace for Crystalline Silicon Ingots. Crystals, 11(4), 421. https://doi.org/10.3390/cryst11040421







