Expected and Unexpected Products in Half Curcuminoid Synthesis: Crystal Structures of But-3-en-2-ones and 3-Methylcyclohex-2-enones
Abstract
1. Introduction
2. Materials and Methods
Synthesis and Crystallization
3. Results
4. Discussion
Crystal Packing
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mapoung, S.; Mapoung, S.; Suzuki, S.; Fuji, S.; Naiki-Ito, A.; Kato, H.; Yodkeeree, S.; Yodkeeree, S.; Sakorn, N.; Sakorn, N.; et al. Dehydrozingerone, a Curcumin Analog, as a Potential Anti-Prostate Cancer Inhibitor in Vitro and in Vivo. Molecules 2020, 25, 2737. [Google Scholar] [CrossRef] [PubMed]
- Kondamudi, P.K.; Kovelamudi, H.; Nayak, P.G.; Rao, M.C.; Shenoy, R.R. Curcumin Half Analog Modulates Interleukin-6 and Tumor Necrosis Factor-Alpha in Inflammatory Bowel Disease. Pharmacogn. Mag. 2015, 11, S296–S302. [Google Scholar]
- Yogosawa, S.; Yamada, Y.; Yasuda, S.; Sun, Q.; Takizawa, K.; Sakai, T. Dehydrozingerone, a Structural Analogue of Curcumin, Induces Cell-Cycle Arrest at the G2/M Phase and Accumulates Intracellular ROS in HT-29 Human Colon Cancer Cells. J. Nat. Prod. 2012, 75, 2088–2093. [Google Scholar] [CrossRef] [PubMed]
- Suwito, H.; Jumina; Mustofa; Kristanti, A.N.; Puspaningsih, N.N.T. Chalcones: Synthesis, Structure Diversity and Pharmacological Aspects. J. Chem. Pharm. Res. 2014, 6, 1076–1088. [Google Scholar]
- Mousavi, M.R.; Maghsoodlou, M.T.; Gharari, H. Sodium Carbonate-Catalyzed Claisen-Schmidt Condensation: One-Pot Synthesis of Highly Functionalized Cyclohexenones under Environmental Conditions. Res. Chem. Intermed. 2016, 42, 2233–2246. [Google Scholar] [CrossRef]
- Rahman, A.F.M.M.; Ali, R.; Jahng, Y.; Kadi, A.A.A. Facile Solvent Free Claisen-Schmidt Reaction: Synthesis of α,α’-bis-(Substituted-Benzylidene)cycloalkanones and α,α’-bis-(Substituted-Alkylidene)cycloalkanones. Molecules 2012, 17, 571–583. [Google Scholar] [CrossRef]
- Sreevidya, T.V.; Narayana, B.; Yathirajan, H.S. Synthesis and Characterization of Some Chalcones and Their Cyclohexenone Derivatives. Cent. Eur. J. Chem. 2010, 8, 174–181. [Google Scholar] [CrossRef]
- Gallier, F.; Martel, A.; Dujardin, G. Enantioselective Access to Robinson Annulation Products and Michael Adducts as Precursors. Angew. Chemie Int. Ed. 2017, 56, 12424–12458. [Google Scholar] [CrossRef]
- Ghorai, M.K.; Samanta, S.; Das, S. Synthesis of 3,5-Disubstituted Cyclohex-2-en-1-one via a Five-Step Domino Reaction Catalyzed by Secondary Amines: Formation of (e)-α,β-Unsaturated Methyl Ketones. Asian J. Org. Chem. 2013, 2, 1026–1030. [Google Scholar] [CrossRef]
- Xiang, Z.; Liang, Y.; Chen, X.; Wu, Q.; Lin, X. D-Aminoacylase-Initiated Cascade Aldol Condensation/Robinson Annulation for Synthesis of Substituted Cyclohex-2-enones from Simple Aldehydes and Acetone. Amino Acids 2014, 46, 1929–1937. [Google Scholar] [CrossRef]
- Jasinski, J.P.; Golen, J.A.; Samshuddin, S.; Narayana, B.; Yathirajan, H.S. Ethyl 2-amino-4,6-bis-(4-fluorophenyl)cyclohexa-1,3-diene-1-carboxylate. Acta Crystallogr. Sect. E Struct. Reports Online 2012, 68, o585. [Google Scholar] [CrossRef] [PubMed]
- Rai, S.; Patel, P.N.; Chadha, A. Preparation, Characterisation, and Crystal Structure Analysis of (2E,2′E)-3,3′-(1,4-phenylene)bis(1-(2-aminophenyl)prop-2-en-1-one. Crystallogr. Reports 2016, 61, 1086–1089. [Google Scholar] [CrossRef]
- Singh, V.D.; Salian, V.V.; Narayana, B.; Sarojini, B.K.; Kamni; Anthal, S.; Kant, R. Synthesis and Crystal Structure of a Chalcone Derivative. Crystallogr. Reports 2017, 62, 1157–1159. [Google Scholar] [CrossRef]
- Wang, F.; Liu, Y.; Qi, Z.; Dai, W.; Li, X. Rhodium-Catalyzed Tandem Aldol Condensation-Robinson Annulation Between Aldehydes and Acetone: Synthesis of 3-methylcyclohexenones. Tetrahedron Lett. 2014, 55, 6399–6402. [Google Scholar] [CrossRef]
- MNova Software. Available online: https://mestrelab.com/download/mnova/ (accessed on 10 April 2021).
- Sheldrick, G.M. A Short History of SHELX. Acta Crystallogr. Sect. A Found. Crystallogr. 2008, 64, 112–122. [Google Scholar] [CrossRef]
- Macrae, C.F.; Bruno, I.J.; Chisholm, J.A.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Rodriguez-Monge, L.; Taylor, R.; Van De Streek, J.; Wood, P.A. Mercury CSD 2.0-New features for the visualization and investigation of crystal structures. J. Appl. Crystallogr. 2008, 41, 466–470. [Google Scholar] [CrossRef]
- Collins, A.; Barr, G.; Dong, W.; Gilmore, C.J.; Middlemiss, D.S.; Parkin, A.; Wilson, C.C. The Application of Cluster Analysis to Identify Conformational Preferences in Enones and Enimines from Crystal Structural Data. Acta Crystallogr. Sect. B Struct. Sci. 2007, 63, 469–476. [Google Scholar] [CrossRef]
- Wang, Y.H.; Zou, J.W.; Zhang, B.; Lu, Y.X.; Jin, H.X.; Yu, Q. Sen Enone-Dienol Tautomerism of But-2-enal and Substituent Effect: A Theoretical Study. J. Mol. Struct. THEOCHEM 2005, 755, 31–37. [Google Scholar] [CrossRef]
- Zhang, H.; Li, S.; Shi, X. (E)-2-Methoxy-4-(3-oxobut-1-enyl)phenyl Acetate. Acta Crystallogr. Sect. E Struct. Rep. Online 2008, 64, o1507. [Google Scholar] [CrossRef] [PubMed]
- Patai, S.; Rappopor, Z. The Chemistry of Enones Part 1; Wiley: Hoboken, NJ, USA, 1989; pp. 1–1261. [Google Scholar]
- Lin, F.F.S.; Servis, K.L. Nuclear Magnetic Resonance Spectroscopy. Rotational Isomerism in α,β-Unsaturated Acyl Fluorides. J. Am. Chem. Soc. 1972, 94, 5794–5801. [Google Scholar] [CrossRef]
- Loncharich, R.J.; Schwartz, T.R.; Houk, K.N. Theoretical studies of conformations of acrolein, acrylic acid, methyl acrylate, and their Lewis acid complexes. J. Am. Chem. Soc. 1987, 109, 14–23. [Google Scholar] [CrossRef]
- Cremer, D.; Pople, J.A. A General Definition of Ring Puckering Coordinates. J. Am. Chem. Soc. 1975, 97, 1354–1358. [Google Scholar] [CrossRef]
- Chion, B.; Lajzerowicz, J.; Bordeaux, D.; Collet, A.; Jacques, J. Structural aspects of solid solutions of enantiomers. The 3-hydroxymethyl- and 3-carboxy-2,2,5,5-tetramethylpyrrolidinyl 1-oxyl systems as examples. J. Phys. Chem. 1978, 82, 2682–2688. [Google Scholar] [CrossRef]
- Rekis, T.; Bērziņš, A. On the structural aspects of solid solutions of enantiomers: An intriguing case study of enantiomer recognition in the solid state. CrystEngComm 2018, 20, 6909–6918. [Google Scholar] [CrossRef]
- Brandel, C.; Petit, S.; Coquerel, Y.C. Structural Aspects of Solid Solutions of Enantiomers. Curr. Pharm. Des. 2016, 22, 4929–4941. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Gupta, M. A simple and efficient method for selective single aldol condensation between arylaldehydes and acetone. Synth. Commun. 2005, 35, 213–222. [Google Scholar] [CrossRef]
- Bounora, P.T.; Rosauer, K.G.; Dai, L. Control of the Aqueus Aldol Addition Under Claisen-Schmidt Condition. Tetrahedron Lett. 1995, 36, 4009–4012. [Google Scholar] [CrossRef]
- Bethi, V.; Fernandes, R.A. Traceless OH-Directed Wacker Oxidation-Elimination, an Alternative to Wittig Olefination/Aldol Condensation: One-Pot Synthesis of α,β-Unsaturated and Nonconjugated Ketones from Homoallyl Alcohols. J. Org. Chem. 2016, 81, 8577–8584. [Google Scholar] [CrossRef]
- Walker, G.N. Triton B in Synthesis of 3-Phenylcyclohexenones. J. Am. Chem. Soc. 1955, 77, 3664–3667. [Google Scholar] [CrossRef]
- Surya Prakash Rao, H.; Jothilingam, S. Solvent-free microwave-mediated Michael addition reactions. J. Chem. Sci. 2005, 117, 323–328. [Google Scholar]
- Zhuang, C.; Zhang, W.; Sheng, C.; Zhang, W.; Xing, C.; Miao, Z. Chalcone: A Privileged Structure in Medicinal Chemistry. Chem. Rev. 2017, 117, 7762–7810. [Google Scholar] [CrossRef] [PubMed]

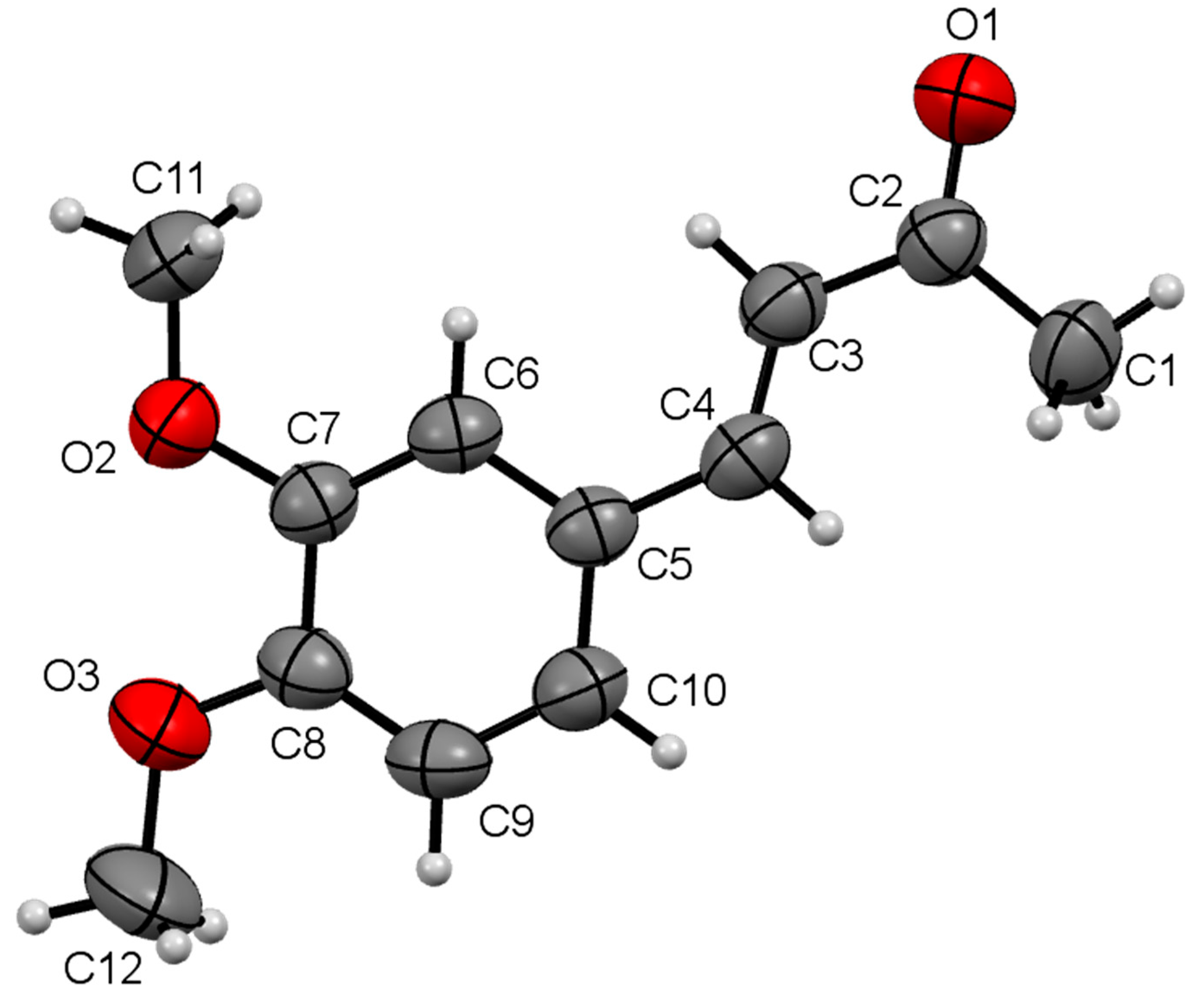
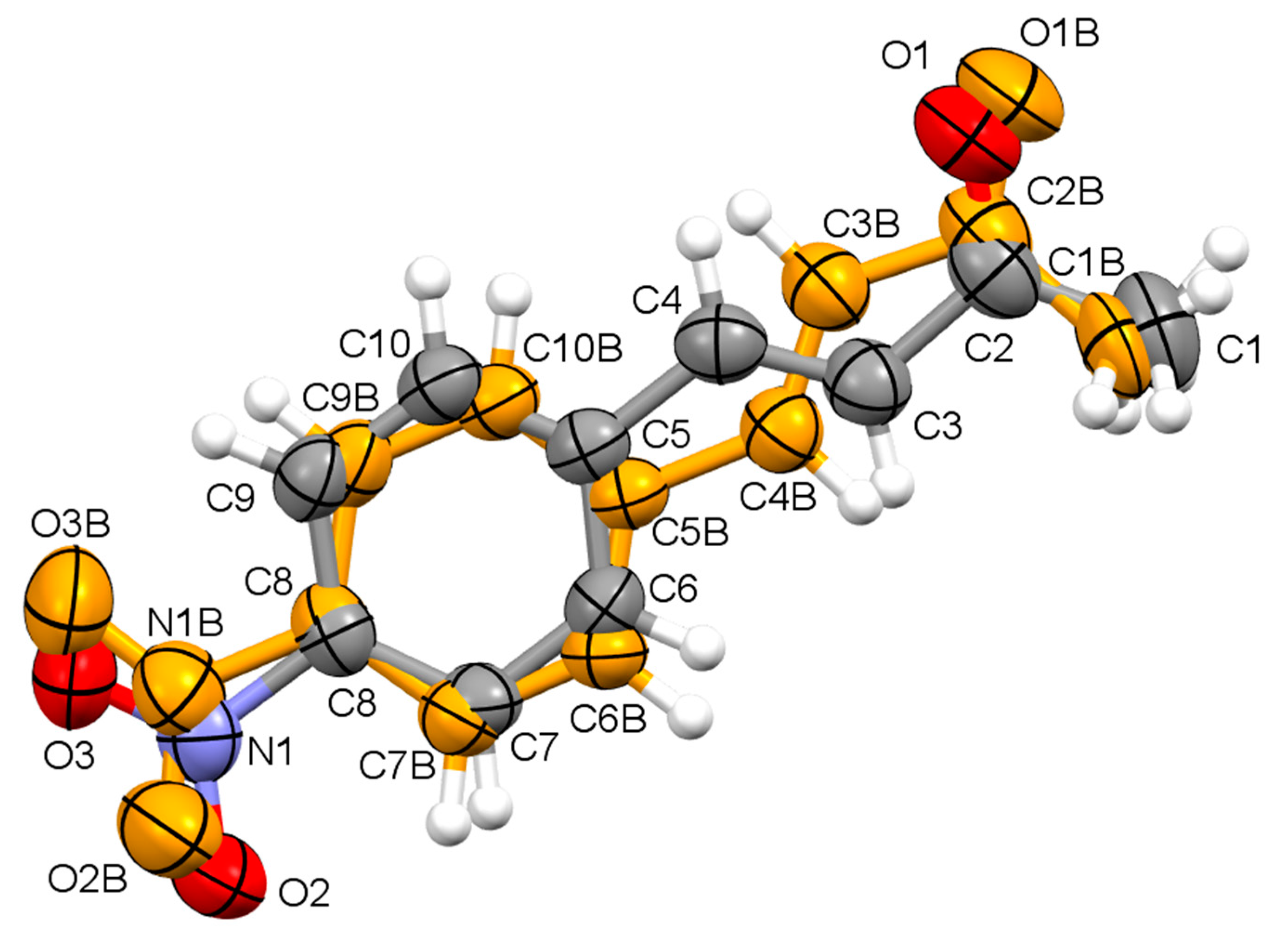
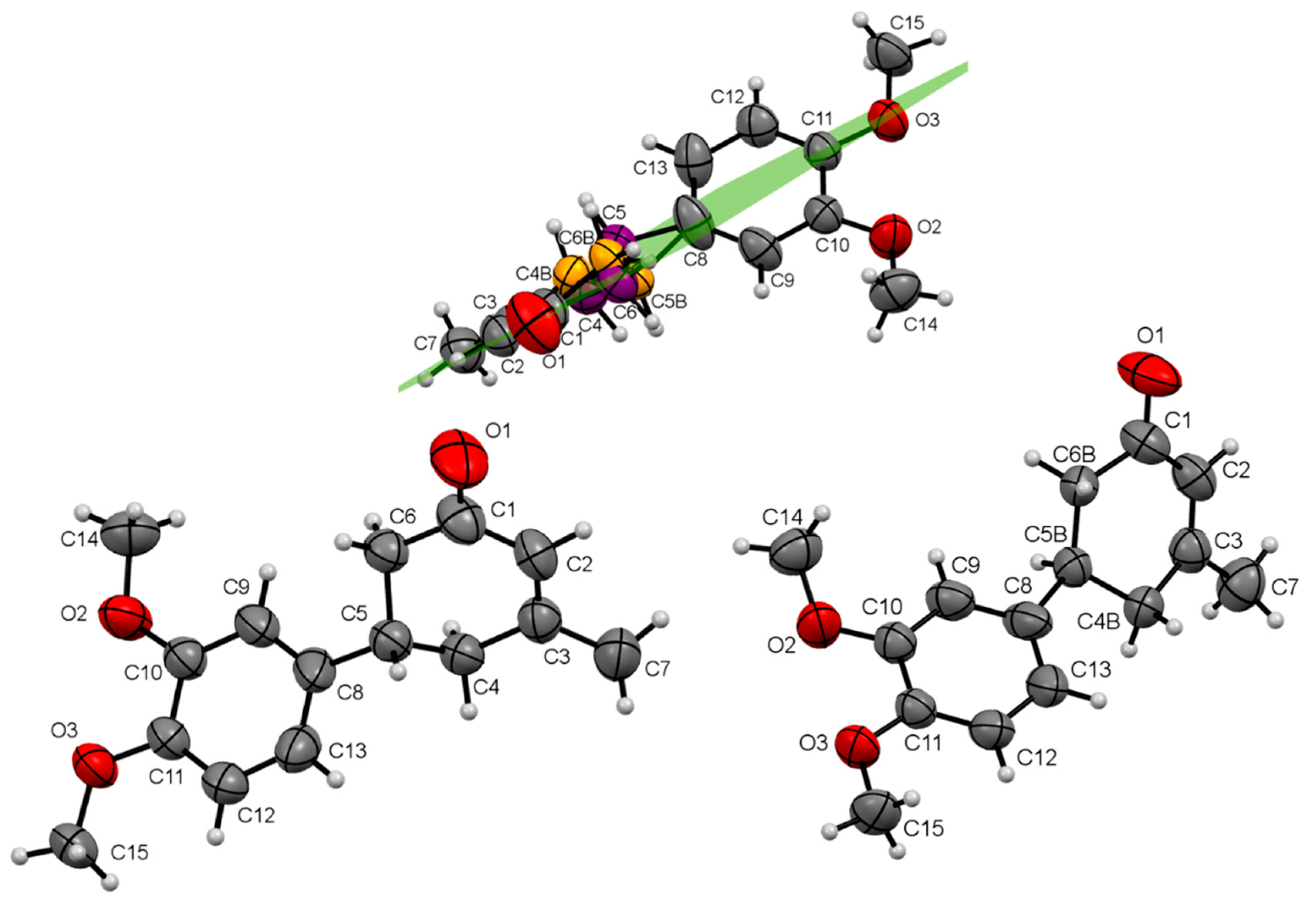
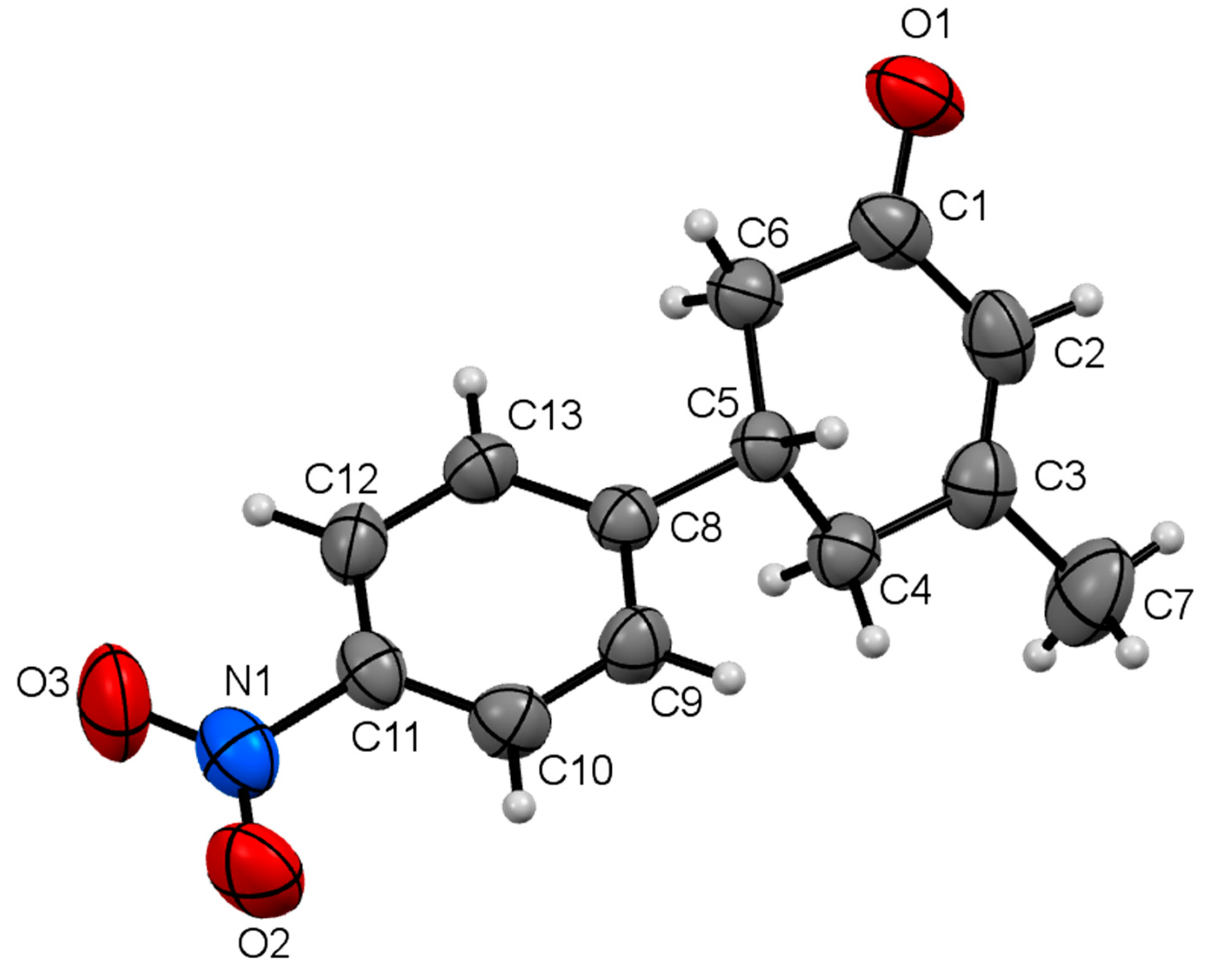
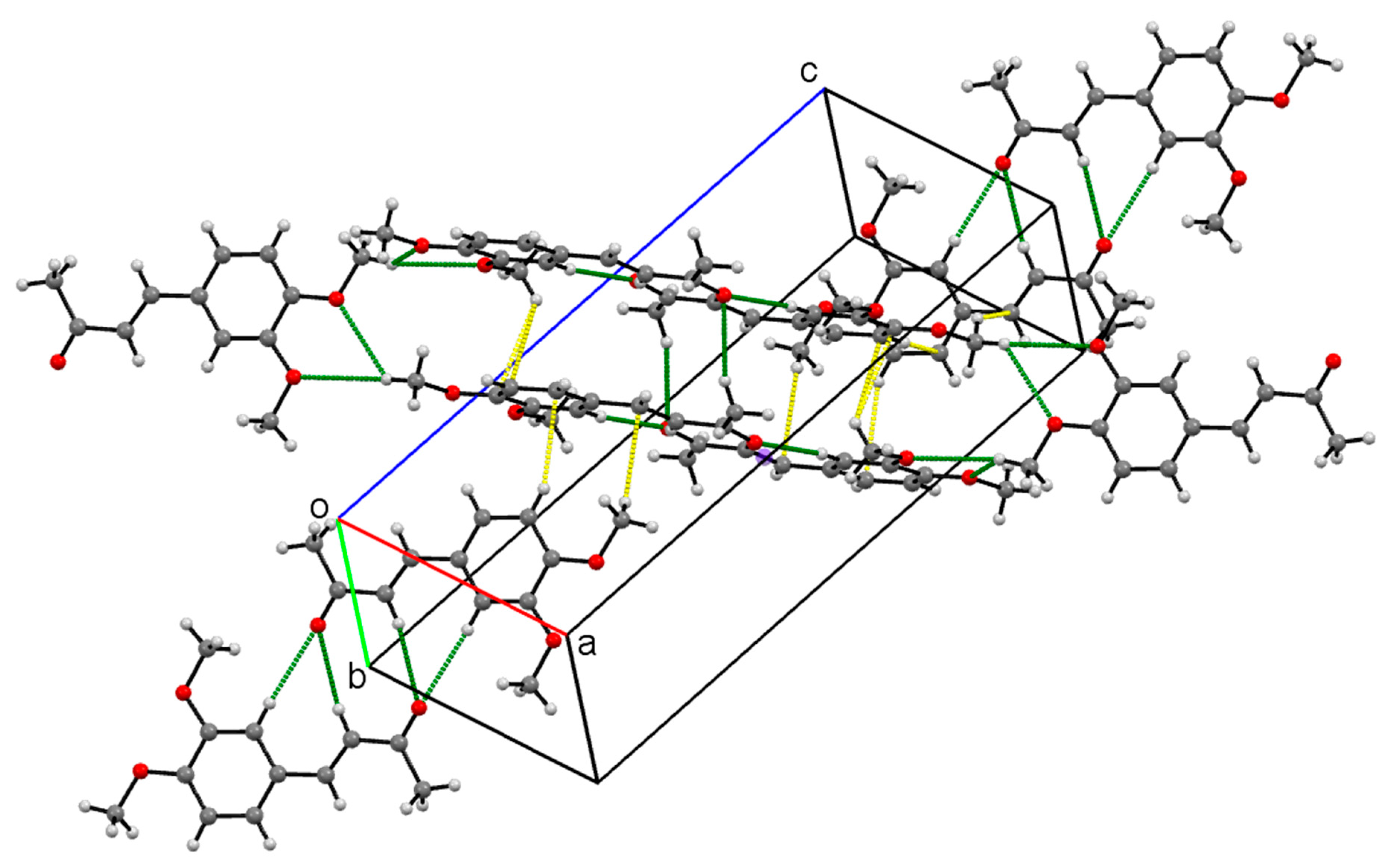
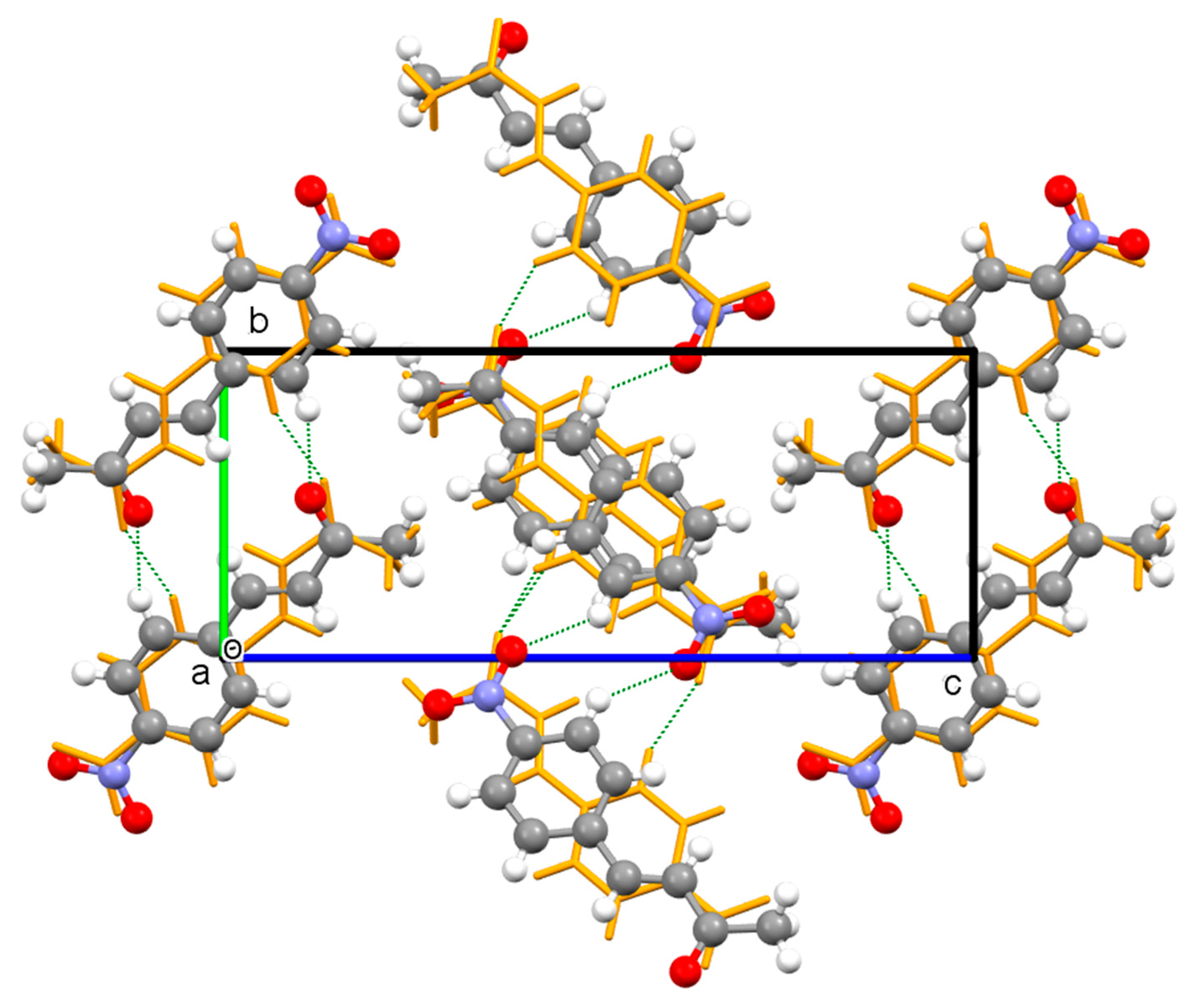
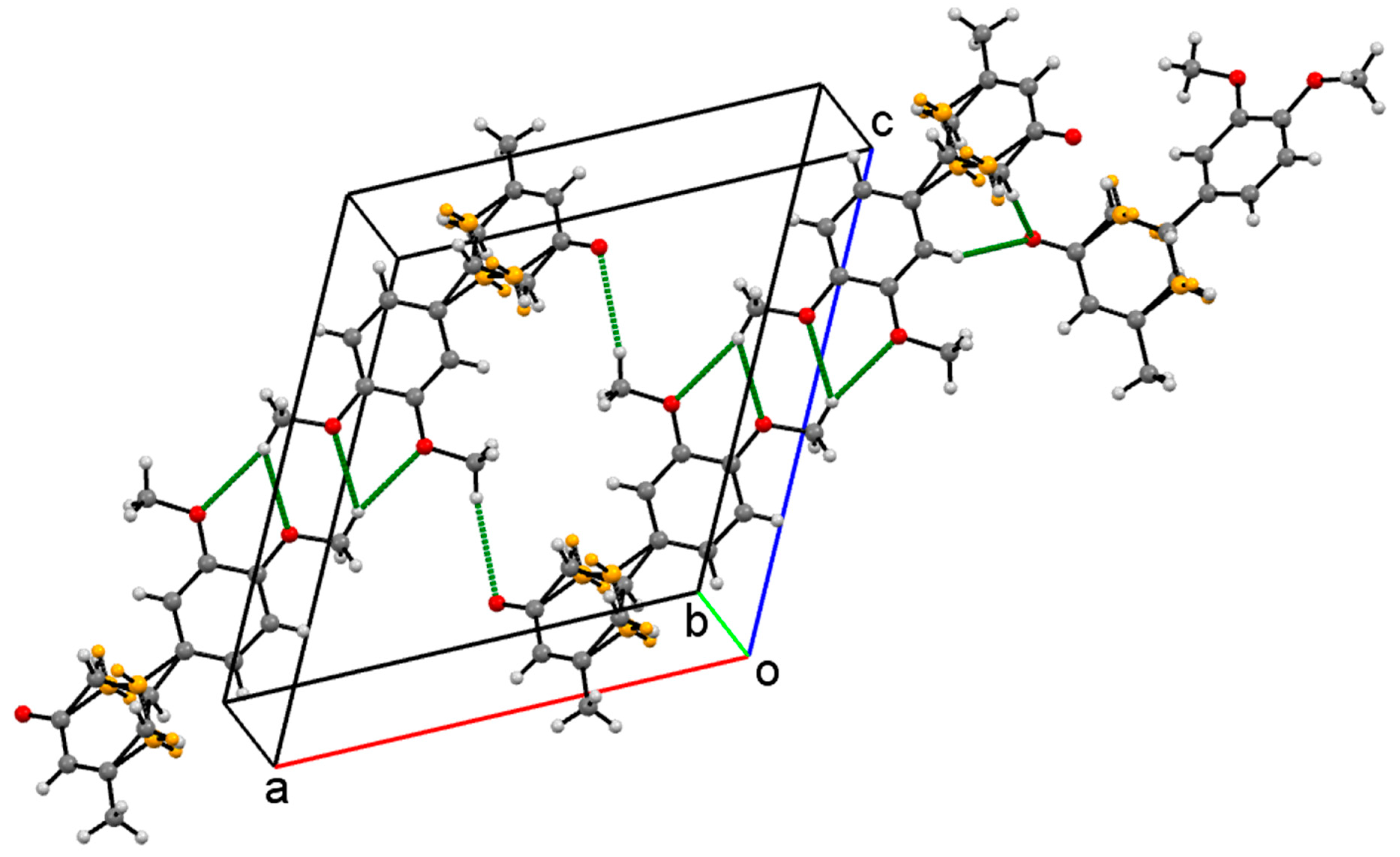
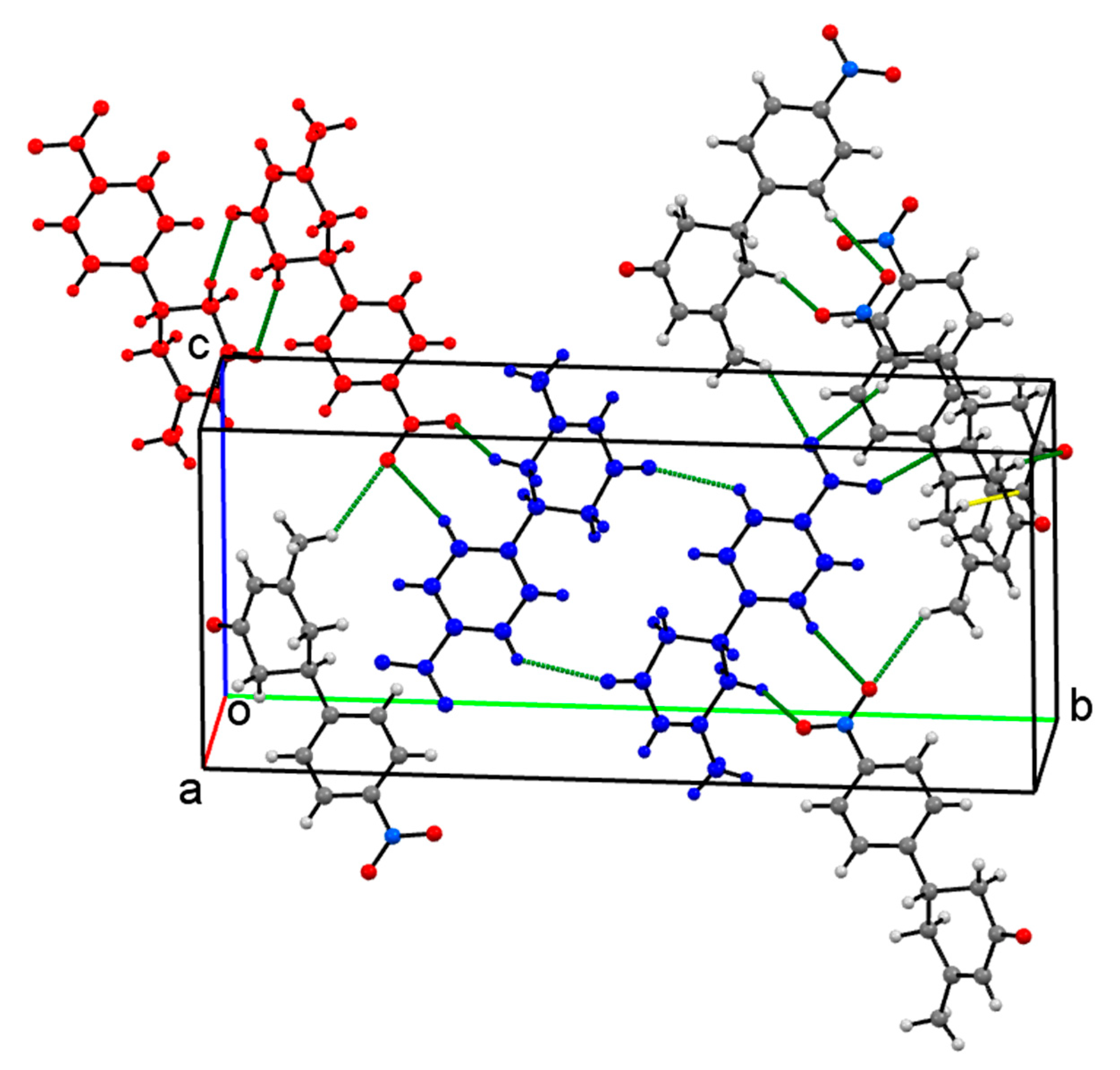
| Compound | I | II | III | IV |
|---|---|---|---|---|
| Crystal system | Monoclinic | Monoclinic | Monoclinic | Monoclinic |
| Unit cell parameters [Å,°] | a = 9.6000(7) b = 5.3426(4) c = 22.353(2) β = 97.307(2) | a = 7.4561(4) b = 7.2591(4) c = 17.7744(10) β = 94.505(2) | a = 13.0205(10) b = 8.0494(6) c = 14.1040(11) β = 114.852(2) | a = 4.9301(18) b = 23.652(9) c = 10.014(4) β = 97.692(12) |
| Volume [Å3] | 1137.17(14) | 959.06(9) | 1341.32(18) | 1157.2(8) |
| Z/Calculated density [mg/m3] | 4/1.205 | 4/1.324 | 4/1.220 | 4/1.327 |
| Z′ | 1 | 1 | 1 | 1 |
| Absorption coefficient [mm−1] | 0.086 | 0.099 | 0.084 | 0.095 |
| Space group, F (000) | P21/n, 440 | P21/n, 400 | P21/c, 528 | P21/c, 488 |
| Index ranges | −11 ≤ h ≤ 11 −6 ≤ k ≤ 6 −25 ≤ l ≤ 26 | −8 ≤ h ≤ 9 −9 ≤ k ≤ 9 −23 ≤ l ≤ 21 | −15 ≤ h ≤ 15 −8 ≤ k ≤ 9 −11 ≤ l ≤ 16 | −6 ≤ h ≤ 6 −30 ≤ k ≤ 30 −13 ≤ l ≤ 13 |
| Reflections collected | 6066 | 16,684 | 5906 | 31,441 |
| Independent reflections | 2071 [R(int) = 0.0722] | 2446 [R(int) = 0.0940] | 2442 [R(int) = 0.1320] | 2663 [R(int) = 0.1187] |
| Observed reflections (I ≥ 2σ(I)) | 1100 | 935 | 1383 | 1290 |
| Completeness to θ = 25.10 | 99.6% | 99.8% | 99.6% | 99.7% |
| Data/restraints/parameters | 2071/0/139 | 2194/247/441 | 2442/ 72/195 | 2663/0/155 |
| Final R indices (I ≥ 2σ(I)) | R1 = 0.0482 wR2 = 0.1026 | R1 = 0.0532 wR2 = 0.1047 | R1 = 0.0631 wR2 = 0.1370 | R1 = 0.0516 wR2 = 0.0907 |
| R indices (all data) | R1 = 0.1005 wR2 = 0.1304 | R1 = 0.1416 wR2 = 0.1670 | R1 = 0.1204 wR2 = 0.1712 | R1 = 0.1469 wR2 = 0.1203 |
| Goodness-of-fit on F2 | 0.940 | 0.995 | 1.025 | 1.005 |
| Largest diff. peak/hole [e·Å−3] | 0.197/−0.172 | 0.114/−0.131 | 0.198/−0.268 | 0.174/−0.139 |
| CCDC deposit number | 2075964 | 2075965 | 2075966 | 2075970 |
| Compound | D-H···A | D-H | H···A | D···A | D-H···A | Symmetry Code |
|---|---|---|---|---|---|---|
| I | C1–H1A···O1 C3–H3···O1 C6–H6···O1 C11–H11B···Cg1 C9–H9···Cg1 C12–H12C···C3,4 | 0.960 0.930 0.930 0.960 0.930 0.960 | 2.61 2.60 2.62 2.84 3.05 2.84 | 3.529(3) 3.503(2) 3.545(3) 3.724(2) 3.733(2) 3.751(2) | 160.3 162.4 173.8 154.0 131.9 143.3 | x, −1+y, z 1−x, 1−y, 1−z 1−x, 1−y, 1−z x, 1+y, z 1/2−x, −1/2+y, 1/2−z 1/2−x, −1/2+y, 1/2−z |
| II | C7–H7···O2 C9–H9···O3 C4-H4···O1 C10–H10···O1 | 0.930 0.930 0.930 0.930 | 2.57 2.57 2.72 2.72 | 3.327(9) 3.301(9) 3.559(7) 3.539(6) | 138.9 135.8 150.0 147.9 | −x, −y, 1−z −x+1/2, y+1/2, −z+3/2 1−x, 2−y, 1−z 1−x, 2−y, 1−z |
| III | C6–H6A···O1 C9–H9···O1 C15–H15A··· O2 C15–H15A···O3 C14–H14A···O1 C4B–H4C···O3 | 0.970 0.930 0.960 0.960 0.960 0.970 | 2.59 2.65 2.62 2.80 2.86 2.66 | 3.539(10) 3.467(3) 3.519(3) 3.446(3) 3.806(3) 3.585(10) | 164.5 146.3 155.5 126.0 170.0 159.0 | −x, −y+1, −z+1 1−x, y+1/2, −z+1/2 −x, 1−y, 1−z −x, 1−y, 1−z 1−x, 1−y, 1−z x, 1/2−y, −1/2+z |
| IV | C6–H6A···O1 C6–H6B···O1 C7–H7B···O3 C12–H12···O1 C4–H4A···O2 C9–H9···O3 C5–H5···Cg1 C4–H4B···C2,3 | 0.970 0.970 0.960 0.930 0.970 0.930 0.980 0.970 | 2.64 2.88 2.68 2.66 2.72 2.73 2.98 2.84 | 3.581(3) 3.829(3) 3.467(3) 3.343(3) 3.327(3) 3.630(3) 3.824(3) 3.788(3) | 162.7 166.0 139.0 131.0 121.0 162.0 145.0 165.0 | −1+x, y, z −x, 1−y, 1−z −1+x, y,1+z 1−x,1−y, 1−z −1+x, 1/2−y, 1/2+z −1+x, 1/2−y, 1/2+z −1+x, y, z −1+x, y, z |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Obregón-Mendoza, M.A.; Arias-Olguín, I.I.; Meza-Morales, W.; Alvarez-Ricardo, Y.; Chávez, M.I.; Toscano, R.A.; Cassani, J.; Enríquez, R.G. Expected and Unexpected Products in Half Curcuminoid Synthesis: Crystal Structures of But-3-en-2-ones and 3-Methylcyclohex-2-enones. Crystals 2021, 11, 404. https://doi.org/10.3390/cryst11040404
Obregón-Mendoza MA, Arias-Olguín II, Meza-Morales W, Alvarez-Ricardo Y, Chávez MI, Toscano RA, Cassani J, Enríquez RG. Expected and Unexpected Products in Half Curcuminoid Synthesis: Crystal Structures of But-3-en-2-ones and 3-Methylcyclohex-2-enones. Crystals. 2021; 11(4):404. https://doi.org/10.3390/cryst11040404
Chicago/Turabian StyleObregón-Mendoza, Marco A., Imilla I. Arias-Olguín, William Meza-Morales, Yair Alvarez-Ricardo, María Isabel Chávez, Rubén A. Toscano, Julia Cassani, and Raúl G. Enríquez. 2021. "Expected and Unexpected Products in Half Curcuminoid Synthesis: Crystal Structures of But-3-en-2-ones and 3-Methylcyclohex-2-enones" Crystals 11, no. 4: 404. https://doi.org/10.3390/cryst11040404
APA StyleObregón-Mendoza, M. A., Arias-Olguín, I. I., Meza-Morales, W., Alvarez-Ricardo, Y., Chávez, M. I., Toscano, R. A., Cassani, J., & Enríquez, R. G. (2021). Expected and Unexpected Products in Half Curcuminoid Synthesis: Crystal Structures of But-3-en-2-ones and 3-Methylcyclohex-2-enones. Crystals, 11(4), 404. https://doi.org/10.3390/cryst11040404







