Abstract
The present study aimed to develop the synthesis of zinc oxide nanoparticles (ZnO-NPs) using the green method, with Aloe barbadensis leaf extract as a stabilizing and capping agent. In vitro antitumor cytotoxic activity, as well as the surface-functionalization of ZnO-NPs and their drug loading capacity against doxorubicin (DOX) and gemcitabine (GEM) drugs, were also studied. Morphological and structural properties of the produced ZnO-NPs were characterized by scanning electron microscopy (SEM), transmission electron microscopy (TEM), energy dispersion X-ray diffraction (EDX), UV-Vis spectrophotometry, Fourier-transform infrared analysis (FTIR), and X-ray diffraction (XRD). The prepared ZnO-NPs had a hexagonal shape and average particle size of 20–40 nm, with an absorption peak at 325 nm. The weight and atomic percentages of zinc (50.58% and 28.13%) and oxygen (26.71% and 60.71%) were also determined by EDAX (energy dispersive x-ray analysis) compositional analysis. The appearance of the FTIR peak at 3420 m–1 confirmed the synthesis of ZnO-NPs. The drug loading efficiency (LE) and loading capacity (LC) of unstabilized and PEGylated ZnO-NPs were determined by doxorubicin (DOX) and gemcitabine (GEM) drugs. DOX had superior LE 65% (650 mg/g) and higher LC 32% (320 mg/g) than GEM LE 30.5% (30 mg/g) and LC 16.25% (162 mg/g) on ZnO-NPs. Similar observation was observed in the case of PEG-ZnO-NPs, where DOX had enhanced LE 68% (680 mg/g) and LC 35% (350) mg/g in contrast to GEM, which had LE and LC values of 35% (350 mg/g) and 19% (190 mg/g), respectively. Therefore, DOX was chosen to encapsulate nanoparticles, along with the untreated nanoparticles, to check their in vitro antiproliferative potential against the triple-negative breast cancer (TNBC) cell line (MDA-MB-231) through the MTT (3-(4,5-Dimethylthiazol-2-Yl)-2,5-Diphenyltetrazolium Bromide) assay. This drug delivery strategy implies that the PEGylated biogenically synthesized ZnO-NPs occupy an important position in chemotherapeutic drug loading efficiency and can improve the therapeutic techniques of triple breast cancer.
1. Introduction
A drug delivery system (DDS) refers to the engineered techniques for approaching, transporting, and formulating therapeutic agents for targeted release. Metal oxide nanoparticles are the residue of conventional drug delivery systems [1]. The major limitations in drug formulation system are poor drug loading capacity, low loading efficiency, and lowered ability to control the size distribution [2]. The defective drug loading efficiency is either due to the amount of the drug not approaching an equate pharmacological concentration in the targeted area, or the carrier substance amount exceeding optimal range associated with toxicity or uncertain side effects. Moreover, frequent release of the encapsulated drug results in a major fraction of the drug released before reaching its target site in the body, which causes inferior efficacy.
Consequently, there is a desperate need for better and more compatible materials for drug delivery systems, which can impart efficient loading and the controlled release of drugs [3]. The prospective application of MO-NPs (Metal oxide nano particles) in drug formulations holds the potential to overcome multidrug resistance (MDR) with improved biodistribution and the slow release of drugs in targeted body sites [4]. The DDS based on nanotechnology provides an approach to reduce MDR. An efficient drug release mechanism based on pH-triggered intracellular acidic environment offers efficient intracellular uptake, therapeutic effect, and cell reflux reduction in target areas of the body.
After completion of the history-making Human Genome Project in 2001, robust advances have been made in the field of cancer diagnostics and therapeutics through an elaborate understanding of novel signal transduction pathways orchestrating neoplastic diseases. A snowballing number of personalized treatment options are now available to manage various types of tumors, but gold-standard chemotherapy (cytotoxic agents that halt the cell cycle of rapidly dividing cells, preventing their division and initiating apoptosis) has always been a frontline strategy to treat various types of advance stage solid tumors [5]. On the other hand, long-term sequelae and side effects of cytostatic agents cannot be belittled and undervalued. Another key challenge is the emergence of multidrug-resistant tumors arising through increased efflux of the drug and decreased cellular uptake, altering epigenetic regulation and drug targets, drug metabolism, and enhanced DNA repair [6,7,8]. This is the reason why considerable attempts have been made for the efficient tumor-targeted delivery of cytotoxic drugs to mitigate toxic effects [9,10]. According to GLOBOCAN 2018 estimates, breast cancer is the second most commonly diagnosed cancer in the world (11.6% of the total cases) and the leading cause of cancer death among females [11]. The heterogeneous nature of this disease proves the bottleneck method to be effective in tumor management, taking into account clinical history (patient symptoms and past treatments), molecular characterization of tumor biology (estrogen receptor-ER positive, progesterone receptor-PR positive, and human epidermal growth factor receptor2-Her2 positive), and quality of life to narrow down therapeutic options [12,13]. Cytostatic chemotherapy (especially anthracyclines) is the solitary option in the case of advanced-stage metastatic breast cancer (MBC), triple-negative condition, or relapse after hormonal therapy [14,15]. Doxorubicin (DOX) is a cytotoxic antibiotic derived from Streptomyces peucetius belonging to an anthracycline class of chemotherapeutic drugs used to treat solid tumors such as breast, bladder and ovary cancer tumors. DOX impedes topoisomerase II, DNA, and RNA synthesis inhibition, causing breakage of DNA strands by intercalating within DNA base pairs [16,17]. Clinical evaluation of DOX has revealed that its therapeutic use is limited in the clinics due to severe side effects, including intrinsic myocardiotoxicity, emesis, alopecia, myelosuppression, and mucositis. Despite these implications, DOX is still under clinical use in combination with frontline chemotherapies [18,19,20].
Gemcitabine (GEM) is a pyrimidine nucleoside analogue antimetabolite chemotherapeutic agent that inhibits DNA synthesis and initiates apoptosis, being employed as a first-line treatment option against pancreatic cancer [21,22]. The drug vows enhanced efficacy in various types of tumors when used in different combinations. For example, anthracycline-resistant breast cancer patients signified susceptibility against gemcitabine/paclitaxel combination therapy [23,24]. However, such combination regimens also have the drawbacks of increased side effects, including diffused alveolar damage, nonspecific interstitial pneumonia, peripheral edema, and adverse hematological events such as thrombocytopenia [25,26]. As a result, efficient pharmaceutical formulations and drug delivery strategies need to be developed to alleviate toxicities of chemotherapeutic drugs and amplify their success to improve metastatic and triple-negative breast cancer management along with patient survival. Among various tumor-targeted drug delivery approaches, nano-size systems have been extensively investigated in a range of solid tumors due to their augmented efficacy and safety profile [27,28]. Some of the drug-encapsulated nano-formulations have been approved by the Food and Drug Administration (FDA-USA), best exemplified by Doxil (liposomal doxorubicin) in 1995 and Marqibo (liposomal Vincristine) in 2012, with reduced myocardiotoxicity and neuropathy, respectively [29]. Nanoparticles improve the pharmacokinetic properties of the anticancer drug and its half-life in plasma. Such drugs not only ameliorate adverse effects but also boost tumor localization through several mechanisms, including nanoparticles’ passive tumor targeting ability to penetrate defective endothelial junctions due to the leakiness of tumor vasculature—a phenomenon known as th enhanced permeability and retention effect (EPR) [30,31]. Emerging drug delivery strategies hold promise to treat triple-negative breast cancer with reduced toxicity and enhanced efficacy. The green synthesis-based metal oxide nano-formulation drug delivery system modifies biodistribution and pharmacokinetics of encapsulated chemotherapeutic drugs, exposing the neoplastic tissue against the enhanced concentration of the drug released and reduced exposure to normal tissue. Active tumor targeting can be achieved by attaching ligands, aptamers, and antibodies at the surface of nanocarriers to bind their appropriate tumor-specific receptors [32,33].
For fabrication of nanocarriers, a broad range of organic (i.e., polyethylene glycol, chitosan, chondroitin sulphate, poly (lactic-co-glycolic acid), and hyaluronic acid) and inorganic (i.e., Zn, Mg, Mn, Cu, Ag, and Fe) matrices have been used to improve tumor targeting ability [34,35,36]. Among inorganic substances, zinc supplementation has been known as an effective therapy to treat age-related macular degeneration, depressive disorder, common cold, and sunburn [37]. Zinc oxide (source of zinc) has been classified as “GRAS” (generally recognized as safe) by Food and Drug Administration (FDA-USA) and reflects unique piezoelectric, catalytic, and optical properties [38]. ZnO-NPs readily dissolve in solution at low pH. Inflammatory and tumor tissues have significantly lower pH than their normal counterparts because of the comparatively high rate of glycolysis. Tumor-targeted increased intracellular drug concentration is facilitated through the fusion of endocytosed drug-loaded nanocarriers with lysosomes and the subsequent low pH, which triggers the dissolution of ZnO nanoparticles in the acidic environment [39]. Another unique anticancerous activity of ZnO nanoparticles is the generation of reactive oxygen species (ROS) responsible for cell death if ROS exceeds the cancerous cell antioxidative capacity [40,41]. In the current investigation, DOX with high loading capacity, loading efficiency, and drug efficiency was encapsulated on ZnO-NPs formulation to study its antitumor effect. In this study, the stealth drug delivery system was an established coating polymer corona, polyethylene glycol (PEG), on the surface of drug-loaded ZnO-NPs, which allows them to evade the immune system. Therefore, such stealth technology reduces protein adsorption and NPs uptake by the reticular endothelial system to prolong drugs’ plasma circulation half-life. However, the presence of PEG may impede the release of a cytotoxic agent acting as a stumbling block between drug and the tumor cells, challenging future improvements to address this aspect [42]. Different synthetic models can be employed for the preparation of inorganic NPs, including physical (thermal ablation, laser ablation, evaporation-condensation) and chemical (reduction, coprecipitation, flow injection, electrochemical) ones. NPs produced by these methods reflect reproducible characters and uniformity in size distribution. These methods are relatively expensive and labor-intensive and generate toxic substances [43]. The emerging and promising green synthesis approach has been reported to be superior to other processes in which a variety of biological systems act as a biolaboratory for the synthesis of effective and safe metal oxide particles at the nanometer scale [44]. Several polyphenolic compounds have been reported in Aloe vera leaf extract that scavenges free radicals to prevent the onset and progression of metabolic diseases (tumors) [45].
Biosynthesis is a green and environmentally friendly technology of synthesizing nanoparticles from natural resources instead of toxic chemicals. Moreover, biogenic techniques offer safe processes for easy handling. Aloe Barbadensis Miller (Aloe Vera) belong to the Aloeaceae family and the genus Aloe vera, in which Barbadensis Miller, Aborescens, and Chinensis are familiar [46]. Additionally, Aloe vera plant leaf extract contains a viscous substance that carries vitamins A, C, beta-carotene antioxidants, choline, and folic acid, as well as contain calcium, copper, magnesium, potassium, and zinc, which are required for the functioning of enzymes in many metabolic pathways. These compounds are also antioxidants [47]. Aloe vera gel is used in food flavoring, cosmetic purposes, food supplements, and herbal remedies, which are enriched in cholesterol, campestral, steroids, sitosterol, and lupeol, having anti-inflammatory properties. Aloe vera gel is also comprised of salicylic acid, an anti-inflammatory agent, as well as the Carboxyl methyl and Sulphonyl groups [48]. These natural chemicals in plant-like salicylic acid and anthraquinones (aloin, aloetic acid, anthranol, cinnamic acid, anthracene) are responsible for the one-step reduction of metals in biogenic synthesis methods [49]. Polyphenols (aloin) in Aloe vera leaf extract can act as chelating agents, as well as capping and reducing agents for the biogenic formulation of metal nanoparticles. The leaf extract also has some chemicals, including polyphenols, acid, vitamins, and catechins (ECG), which are responsible for the reduction of metal [50].
In this study, a green process for the synthesis of ZnO-NPs using Aloe barbadensis (aloe vera) leaf extract was employed. The ZnO-NPs were investigated by UV (Ultra violet), SEM (Scanning electron microscope, TEM (transmission electron microscopy), FT-IR (Fourier transform) and XRD techniques. The obtained ZnO-NPs were used for the preparation of doxorubicin-encapsulated ZnO-nanoparticles (DOX-ZnO-NPs) and doxorubicin-encapsulated PEGylated nanoparticles (DOX-GEM-ZnO-PEGNPs). The anticancer activities of the prepared nanoparticles were investigated. In vitro antiproliferative potential against triple-negative breast cancer cell line (MDA-MB-231) through MTT (3-(4,5-Dimethylthiazol-2-Yl)-2,5-Diphenyltetrazolium Bromide) assay was also employed.
2. Materials and Methods
2.1. Materials
An Aloe barbadensis plant was purchased from a herbal clinic in Lahore, Pakistan, and authenticated by the Department of Botany, G.C. University Lahore, Pakistan. Doxorubicin HCl (DOX) was provided from Pfizer Laboratories (USA), whereas gemcitabine (GEM) was purchased from Novartis Pharma (Switzerland). Zinc nitrate was provided by the Department of Chemistry, University of Punjab (Pakistan). Phosphate buffered saline (PBS), fetal bovine serum (FBS), Dulbecco’s modified Eagle’s medium (DMEM), streptomycin, and penicillin were purchased from Gibco Life Technologies, Inc. (Grand Island, NY, USA). The MTT assay kit was provided from Sigma Aldrich (St-Lou, MO, USA).
2.2. Green Synthesis of ZnO-NPs
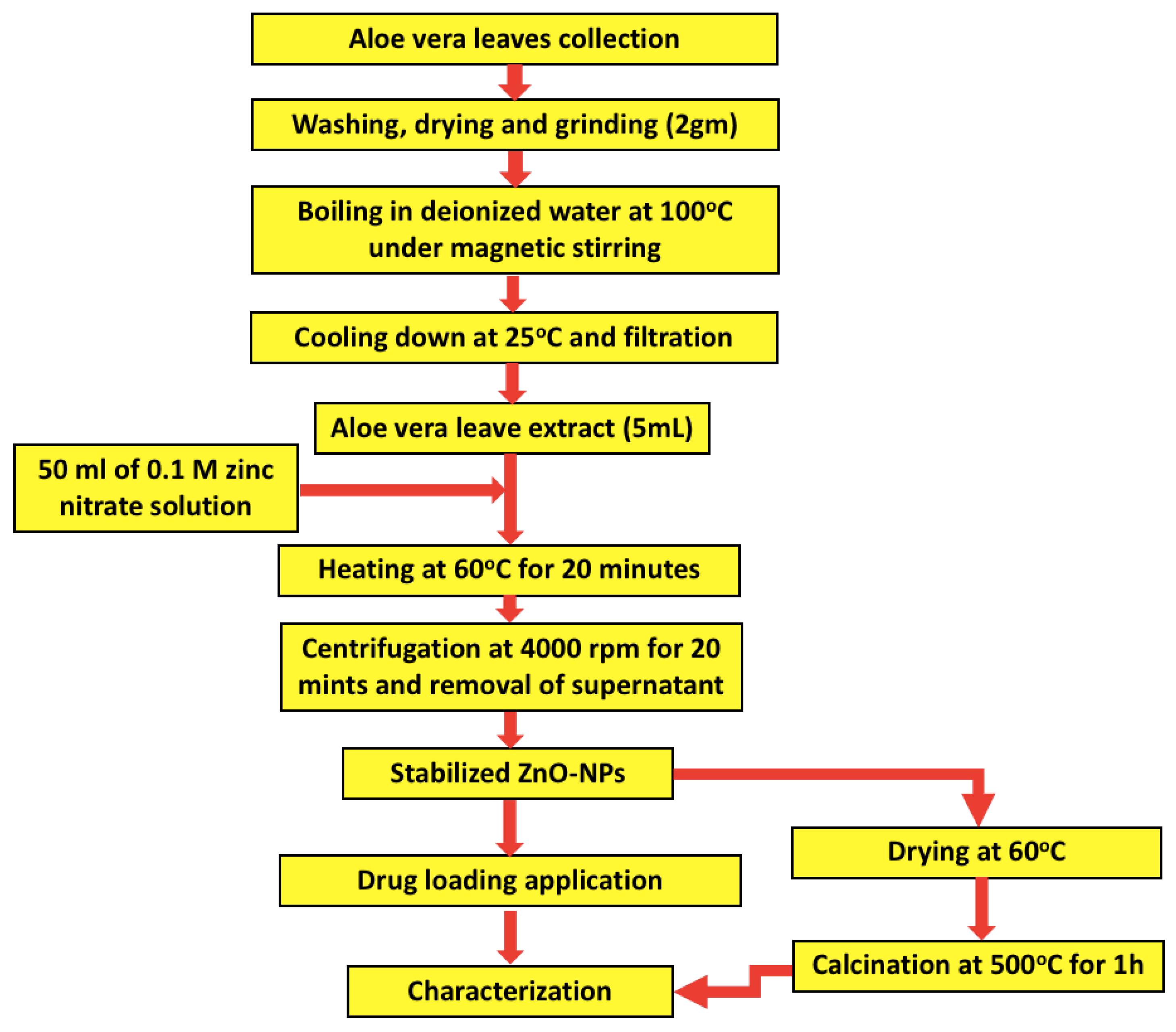
The Aloe barbadensis plant was rinsed 5 times with distilled water to remove any impurities. Then, 2 g of the cleaned leaves were collected, dried, and grinded into a fine powder. The obtained powder was dispersed in 100 mL of deionized distilled water with magnetic stirring and then boiled for 10 min at 100 °C. After allowing the extract to cool down at room temperature, it was filtered through a muslin cloth to collect the clear extract. An Erlenmeyer flask with 50 mL volume of 0.1 M zinc nitrate solution was prepared to react with 5 mL of aloe vera leaves extract through continuous magnetic stirring at 60 °C for 20 min. The color of the obtained solution changed from green to yellow, which confirmed the formation of ZnO-NPs stabilized by the aloe vera leaf extract [47]. The reaction mixture was centrifuged at 4000 rpm for 20 min followed by removal of the supernatant. ZnO nanoparticles were washed 3 times with distilled water and dried in an oven at 60 °C. Finally, the obtained powder was calcined at 500 °C for 1 h, and the formed black particles were collected for downstream processing. The obtained stabilized ZnO-NPs were allowed to interact with polyethylene glycol for 24 h with subsequent purification by dialysis against water, as depicted in Figure 1.
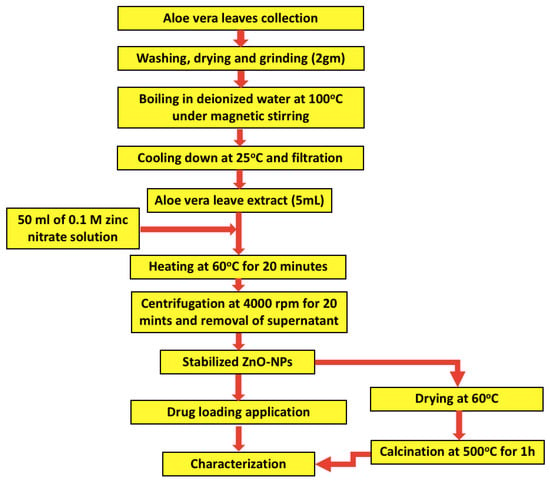
Figure 1.
Schematic flowchart of the synthesis process of zinc oxide nanoparticles (ZnO-NPs) using Aloe barbadensis leaf extract.
2.3. Preparation of Doxorubicin and Gemcitabine Non-PEGylated and PEGylated ZnO-NPs
Doxorubicin (5 mg) was dissolved in 50 mL deionized distilled water to allow subsequent reaction with dispersed ZnO-NPs (10 mg/50 mL) in distilled water of 1:1 volume ratio. The solution was then incubated for 90 min at room temperature. After centrifugation, the supernatant was discarded, washed with ethanol, and dried in a vacuum oven overnight [48]. The same procedure was carried out for the preparation of GEM-ZnO NPs with ZnO-NPs.
The produced ZnO-NPs were modified using polymer polyethene glycol (PEG). Polyethene glycol (Sigma Aldrich) 400 g/mol were dissolved in anhydrous chloroform. The concentration of the hydrophobic chain was adjusted to be at least 0.04 M and was mixed with drug-loaded ZnO-NPs to make 1%weight/volume of the polymer [50]. The ZnO-NPs suspension and polymer solution were then merged in a round flask and heated at 40 °C under N2 gas for 20 min. Finally, the obtained solution was stirred in the evaporator for 5 min and sonicated at 19 w and 40 kHz for 20 min.
2.4. Drug Loading Analysis
The investigated drugs were analyzed by UV-Vis spectrometry. Then, the drug solution was mixed with the lead extract, and the readings were recorded after 30 min, 60 min, 120 min, and 240 min intervals. The drug loading efficiency and loading capacity (LC) of ZnO-NPs can be calculated according to the following equations:
%LC = [entrapped drug/NP weight] × 100
= Drug initial quantity (mg), Free drug (mg) in the supernatant
Carrier (ZnO-NPs drug cargo)
2.5. Characterization of Nanoparticles
Quantitative determination of ZnO-NPs was carried out by UV-Vis spectrophotometry (Lamda 25-Perkin Elmer, Waltham, MA, USA) within the 200–800 nm wavelength range. FT-IR spectrum was generated using (PerkinElmer Inc. Buckinghamshire, UK) within a range of 400–4000 cm−1. X-ray diffraction analysis of the obtained nanoparticles was carried out using (XRD-6000, Shimadzu Corporation, Kyoto, Japan) at 40 kV with a copper source in a range of diffraction angles (10° to 80°). In the case of ZnO-NPs, the Joint Committee on Powder Diffraction Standards Database (JCPDS) was considered for analyzing the XRD reference patterns. The particle size and distribution, shape, and morphology of the prepared nanoparticles were analyzed by transmission electron microscope with the model (JOEL, JEM-2100) and scanning electron microscope with the model (JSM-6480LM, JEOL Ltd, Tokyo, Japan).
2.6. In Vitro Anticancer Activity and MTT Assay on MDA-MB-231 Cell Line
The breast cancer cell line (MDA-MB-231) was obtained from the National Centre of Excellence in Molecular Biology (University of The Punjab, Lahore, Pakistan). Cells were cultured in DMEM medium supplemented with 10% fetal bovine serum and 1% antibiotic/antimycotic. The cell was subcultured at 5000 cells/m2 and extensive washing was performed with 1% FBS before the loading of the drug.
In vitro antiproliferative assessment was carried out when MDA-MB-231 cultured cells accomplished 70–80% confluency. Cultured cells were seeded onto a 96-well plate at a density of 1 × 105 cell into each well and incubated for 24 h at 37 °C. The cancer cells were then treated with various concentrations (3 µg/mL to 200 µg/mL) of DOX-PEG-ZnO-NPs and DOX-ZnO-NPs dispersions and incubated for 72 h at 37 °C under 5% CO₂. After removing the dissolved medium, 25 µL of the 5.5 mg/mL MTT solution (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide) was used to treat cells in each well and was incubated in the aluminum-foiled plate for a further 4 h in the dark. After immediate aspiration of the MTT solution, 100 µL DMSO was added in each well to dissolve the formazan compounds. In each well, absorbance was detected at 570 nm using Spectra Max Plus 384 UV-Vis plate reader. Cell viability was derived by comparison with the untreated group, which was referred to as a control.
For the assessment of cytotoxicity of ZnO-NPs and the modified doxorubicin-ZnO-NPs drug in the cell culture, an MTT assay was performed. MDA-MB 231 cells (10,000) were suspended in 100 µL of RPMI-1640 medium, which was added to each well of the 96-well plates. After 24 h, media was removed, and the wells were washed with 1 × PBS. Then, the treatment of 7 doses (3.12 ug/mL to 200 ug/mL) of the test compound were given to the cells in triplicate, taking each untreated cell as Blank and DMSO as solvent control (0.2%). Media was removed after 24 h, and the wells were washed with 1 × PBS. Then, 20 µL of MTT (5 mg/mL in 1 × PBS) was added to each well. Plates were covered with aluminum foil and incubated at 37 °C with 5% CO2 for 4 h. After incubation for 4 h, MTT was removed carefully and replaced with 100 µL DMSO in each well to dissolve the formazan products formed in the wells. Purple-colored formazan compounds were formed in response to the MTT reaction with live cells. DMSO was used to dissolve the formazan compounds. The appearance of less-intense purple color indicated high cell viability. The plates were kept at room temperature for 20 min. Then, the measured OD (Optical density) at 570 nm and 650 nm was determined, and the cell viability was calculated according to the following equation:
Cell viability (%) = (Test OD570 − OD650 /Blank OD570 − OD650) × 100%
Also, the IC50 (concentration inhibited 50% cell growth) was calculated by the formula:
Inhibition growth = [(OD control − OD test) × 100]/OD control
The results expressed as the ± SD mean of the experiment in triplicate representation experiments.
2.7. Statistical Analysis
The MTT assay was performed in triplicate. The results were expressed as the mean standard error. Statistical analysis of all the data was performed using Origin 2019 software. Observed probability values less than 0.05 were considered to be statistically significant.
3. Results and Discussion
3.1. Characterization of the ZnO-NPs
The green synthesis of zinc oxide NPs could be demonstrated on the basis of the plant’s ability to bioaccumulate metal ions involving the active phytoconstituents as stabilizers and bioreductants. The change in color correlates with the reduction of zinc nitrate to zinc oxide nanoparticles and may be attributed to the surface plasmon resonance phenomenon of nanoparticles [51].
3.1.1. SEM/EDAX Investigations
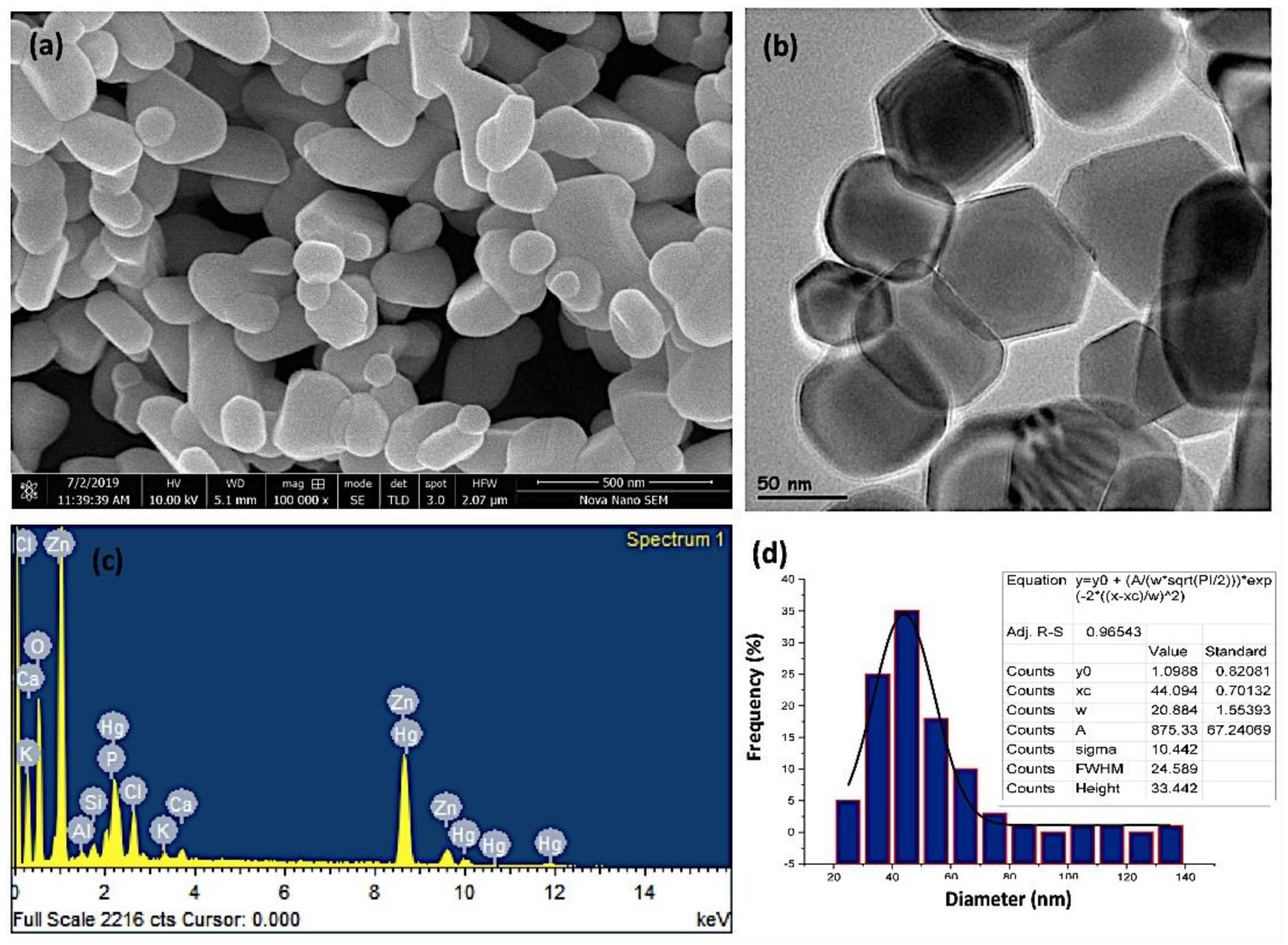
The average particle size of zinc oxide nanoparticles was analyzed by scanning and transmission electron microscopy. The SEM and the high-resolution TEM investigations of the ZnO-NPs, as show in Figure 2a,b, revealed that the ZnO-NPs were discrete, polydispersed, and hexagonal in shape. This structure depicts more iconicity and, consequently, enhanced catalytic activity of ZnO nanoparticles among their three 2D structures. The results are in agreement with the previously reported data in the literature. However, in some studies, oval- and spherical-shaped ZnO nanoparticles have also been reported using different plants [52,53,54]. The presence of dispersion and a few clusters of ZnO-NPs demonstrate the stability of the nanoparticles in aloe vera leaf extract due to the interaction with the flavonoids and phenols. Such compounds play a pivotal role in chelating nanoparticles to ligands [55,56]. The formation of ZnO-NPs was further confirmed by EDAX (energy dispersive x-ray analysis) compositional analysis. As shown in Figure 2c, significant peaks of zinc and oxygen appeared, confirming the formation of ZnO nanoparticles. The weight and atomic percentage of zinc (50.58% and 28.13%) and oxygen (26.71% and 60.71%) were determined and validated the formation of ZnO-NPs. The appearance of foreign peaks other than zinc and oxygen suggests the presence of various elements, including chlorine, calcium, potassium, phosphorous, aluminum, silicon, and mercury, which composed the capping agent that stabilized the ZnO-NPs. The source of these elements is the Aloe vera leaf extract, which acted as a reducing, capping, and stabilizing agent. The appearance of the peak of mercury suggests the accumulation of heavy metals inside the Aloe vera plant leaf tissues, which is related to the composition of the soil [57,58]. Figure 2d shows the particle size distribution of the prepared ZnO-NPs. It was observed from the results that the particle size ranged between 40–60 nm, with a mean particle size of 50 nm. It is well documented that larger surface-to-volume ratio makes the nanoparticles potent anticancer agents, which is subsequently linked with their decrease in size [59].
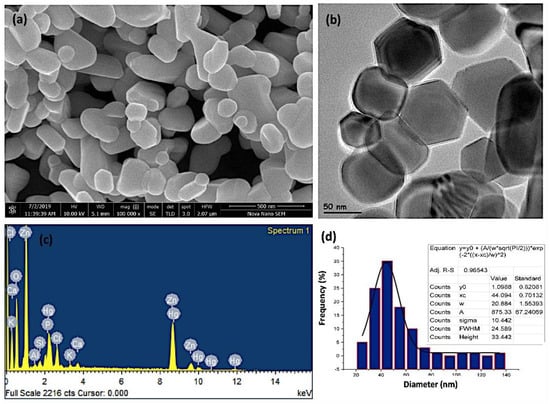
Figure 2.
(a) Scanning electron microscopy (SEM) images, (b) high-resolution transmission electron microscopy (TEM) image, (c) EDAX (energy dispersive X-ray analysis) semi-quantitate compositional analusis, and (d) the particle size distribution of the investigated ZnO-NPs.
3.1.2. Phase Analysis and X-ray Diffraction
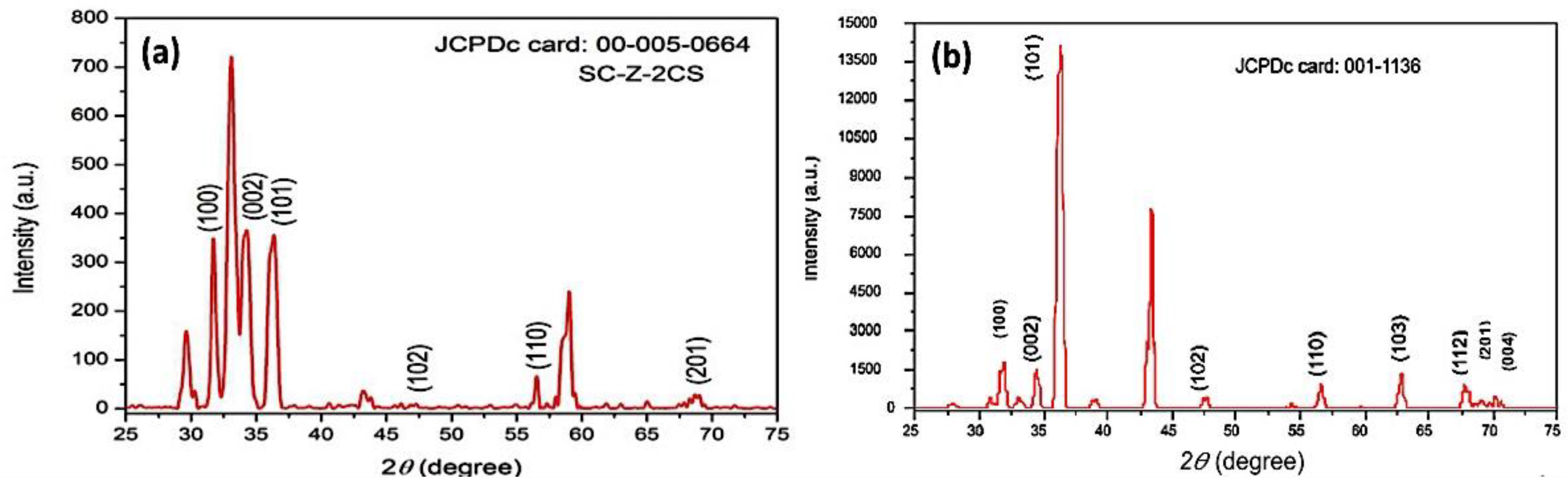
Figure 3a, b shows the X-ray diffraction (XRD) patterns of the as-prepared and calcined ZnO-NPs at 500 °C for 1 h, respectively. The results confirm that the peaks of 2θ correspond to the patterns of the JCPD-00-005-0664 card from high-score software of the as-prepared ZnO-NPs, with miller indices lattices values of a (3.23 Å), b (3.243 Å), c (5.17 Å), and the JCPD-001-1136 of the calcined ZnO-NPs, respectively. The X-ray diffraction pattern further confirms that the ZnO-NPs are highly crystalline, with hexagonal-shaped crystal structure. The XRD patterns further confirm that the ZnO-NPs are of space group (P), with a value of 186, with a calculated density of 5.68 g/cm3 at a temperature of 26 °C. The calculated values of the alpha, beta and gamma angles were 90.0, 90.0, and 120.0, with a Z value of 2. The graphical analysis confirmed the presence of (100), (002), (101), (102), (110), and (201) lattice plans at 2θ angles 34.3, 36.4, 35.4, 47.5, 55.46, and 61.3, respectively (particle size = Kλ/βcosθ) [56]. K reflected the wavelength of X-ray source used (1.541 Å), and β was the full-width-at-half-maximum of the diffraction peak. It was observed from the results that the particle size was in the range of 20–40 nm [60].
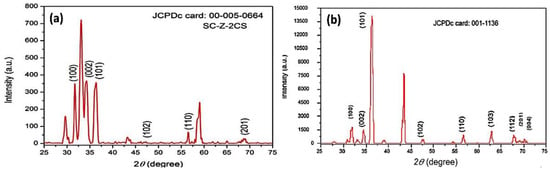
Figure 3.
X-ray diffraction patterns of the investigated nanoparticles, where (a) the as prepared ZnO-NPs and (b) the ZnO-NPs after calcination at 500 °C.
The average crystalline size was calculated by the Scherrer equation (crystallite size = Kλ/βcosθ, where K is a constant, λ is the wavelength of the used X-ray source of 1.541 Å, θ is the angle of diffraction, and β is the peak broadening at full width of max intensity FWHM (β) value of 0.75). A simple estimation of crystallite size from the breadths of a diffraction peak was obtained. It was observed from the results that the average crystallite size was about 37.86 nm [60].
3.1.3. Fourier-Transform Infrared (FTIR) and UV-Visible Spectrophotometry
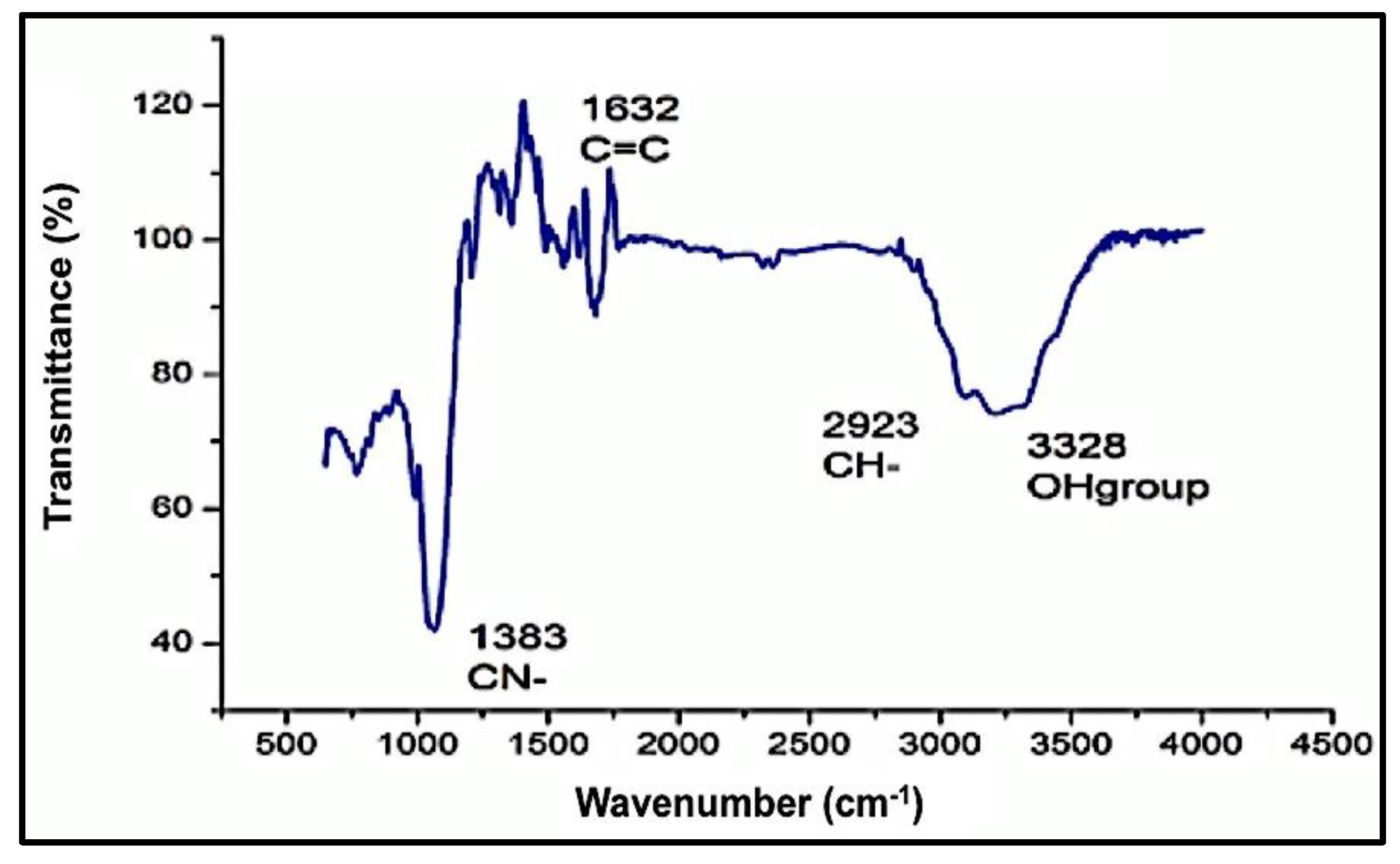
The functional groups of the obtained stabilized ZnO-NPs by the Aloe vera leaf extract were detected by the FTIR spectral band identifications. The obtained data of the spectrum are listed in Table 1, with different bands stretching at 457 cm−1 and 545 cm−1 of the ZnO-NPs predicting the presence of the divalent metal oxide bond. Several other bands were observed at 3215.08 cm–1, 2964.17 cm–1, 1636.68 cm–1, and 1541.82 cm–1, which represent the presence of the –OH (hydroxyl), aromatic ring (C=C), amine (NH), and phenyl (C–H) groups, respectively. Such spectral data justify the presence of phenol, polyphenol, flavanol, and primary amine compounds in Aloe vera leaf extract and may be responsible for the stabilizing and capping of the ZnO-NPs. The presence of weak and broad bands at around 3200 cm–1 was expected due to the formation of the hydroxyl group on the surface of ZnO-NPs (Figure 4). A decrease in the intensity of the hydroxyl bands after the reduction of Zn2+ ions suggests the involvement of phenolic and flavanol sites in the binding of ZnO-NPs [61]. It is assumed that the possible mechanism of interaction between plant phytochemicals and Zn2+ is the repetitive redox reaction, orchestrating the conversion of carbohydrates to energy during glycolysis process, coupled with the opulent hydrogen ions and ATP production. Thus, repetition of this redox reaction corroborates the conversion of zinc ions to Zn0. Our findings are in agreement with previously reported studies addressing characterization of green-synthesized ZnO-NPs [52,62].

Table 1.
Values of the FTIR spectrum bands of the investigated ZnO-NPs.
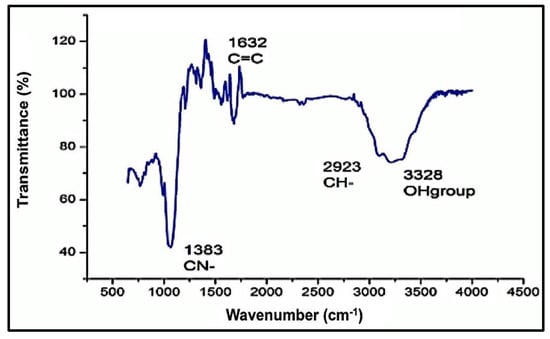
Figure 4.
Fourier-transform infrared spectrum (FTIR) of the investigated ZnO-NPs.
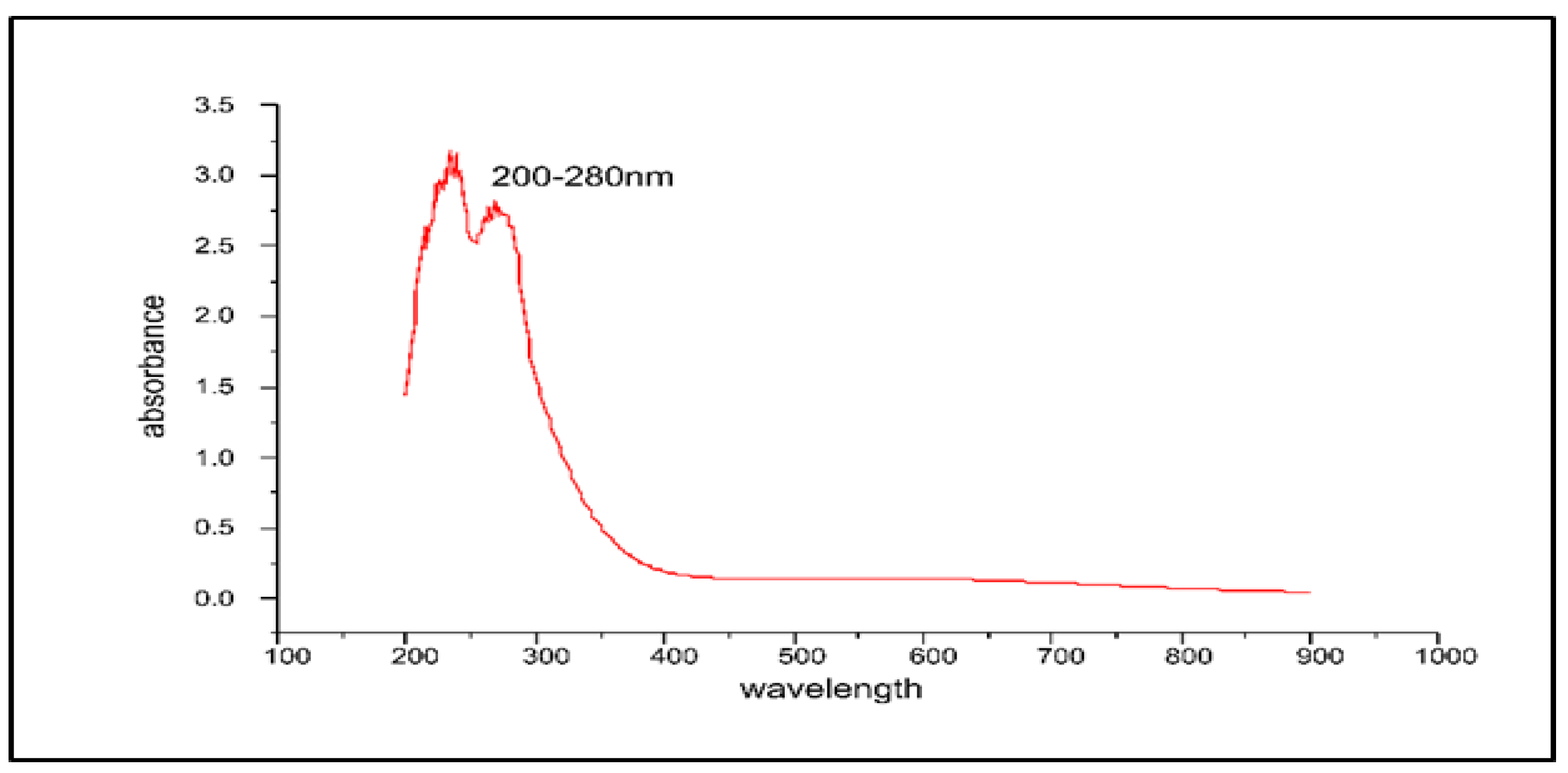
UV-Visible absorption spectrum of the obtained stabilized ZnO-NPs showed a strong peak at ~325 nm, detecting the formation of the stabilized ZnO-NPs which validated the formation of the ZnO-NPs with a characteristic of broad and continuous absorption spectrum. Electron transitions resulting from the valence band to the conduction band (O-2p to Zn-3d) may be attributed to the native band-gap absorption of ZnO-NPs. The catalytic activity and band gap of metal oxide NPs play a pivotal role in their cytotoxic response against biological systems [62,63,64] (Figure 5).
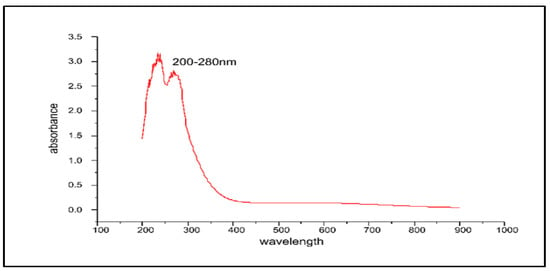
Figure 5.
The UV-Visible spectrum of the ZnO-NPs synthesized by Aloe barbadensis leaf extract at a maximum wavelength of 200–280 nm.
3.2. Anticancer Drug Loading Capacity and Efficiency
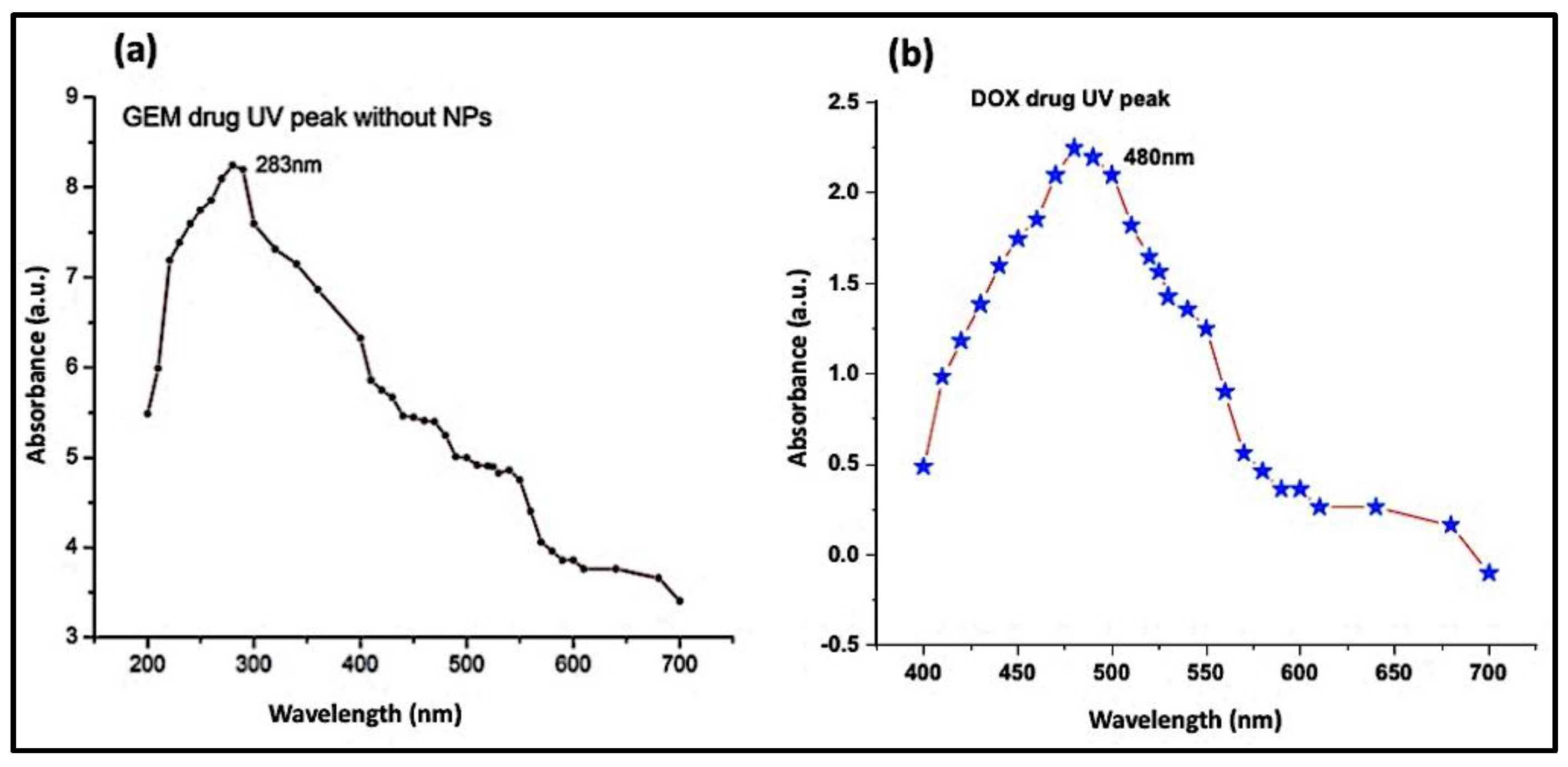
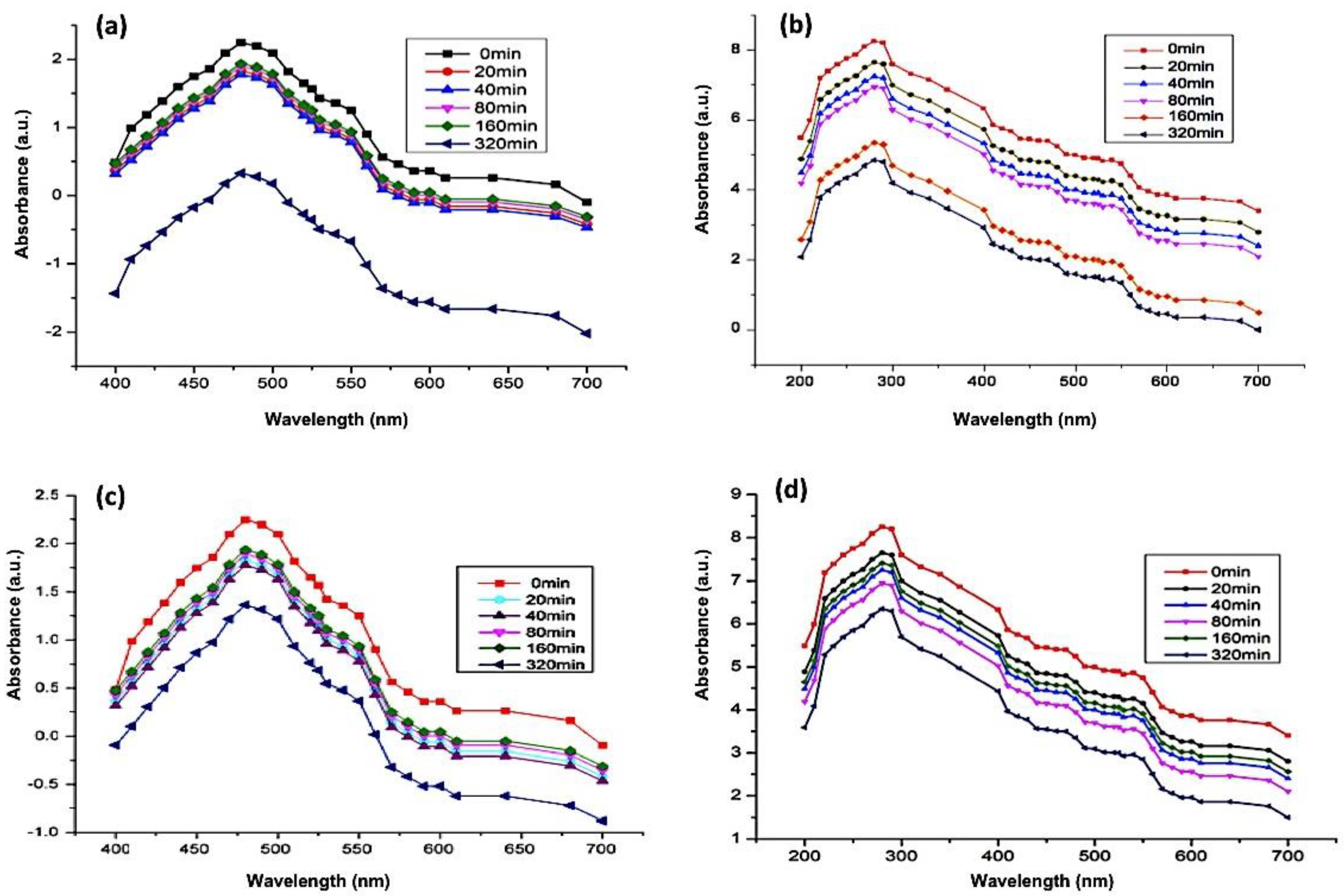
UV-Vis spectrophotometry of DOX (Figure 6a) and GEM (Figure 6b) showed absorbance peaks at 480 nm and 283 nm, respectively. As mentioned in the previous section, the ZnO-NPs can be detected at a wavelength of 325 nm. Subsequent absorbance readings of solution which contained Aloe vera DOX and ZnO-NPs were recorded at discrete time intervals ranging from 0 min to 320 min. The lowering of the absorbance peak of DOX over time indicates the loading of the drug on the ZnO-NPs (Figure 7a). Likewise, the UV-Vis absorption spectrum of the solution containing GEM and ZnO-NPs was also recorded as shown in Figure 7b. It was observed from the results that GEM was successfully loaded on the surface of ZnO-NPs, as indicated by a lowering in the intensity of the absorbance peak (Figure 7b). Similarly, UV-Vis spectrophotometric measurements were observed during the PEGylation of drugs along with the ZnO-NPs. A decrease in the intensity of the absorbance peaks of DOX and GEM guaranteed the generation of DOX-PEG-ZnO-NPs (Figure 7c) and GEM-PEG-ZnO-NPs (Figure 7d), respectively. In the case of DOX-ZnO-NPs, the ZnO-NPs transferred their energy to DOX, which resulted in the reduction of the the corresponding absorption peak due to the π–π interaction phenomenon among the DOX molecules. It was also assumed that a similar phenomenon can take place in the case of GEM [39].
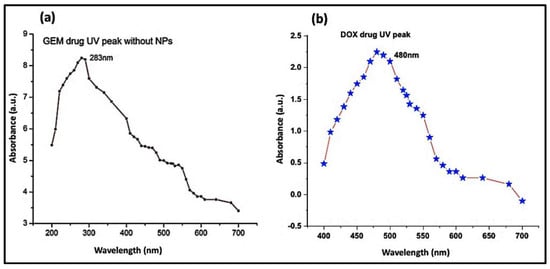
Figure 6.
Absorption spectrum of (a) doxorubicin (DOX) and (b) gemcitabine (GEM).
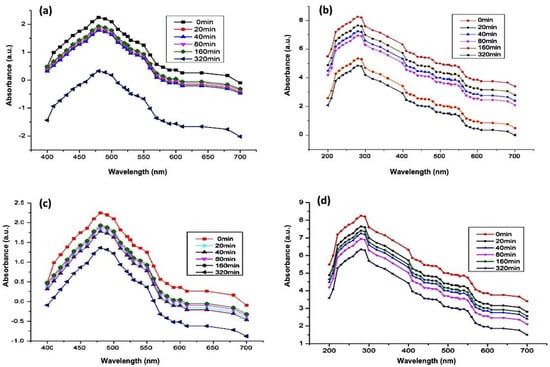
Figure 7.
Absorption spectrum of (a) DOX-encapsulated ZnO-nanoparticles (DOX-ZnO-NPs), (b) GEM-encapsulated ZnO-nanoparticles (GEM-ZnO-NPs), (c) doxorubicin-encapsulated PEGylated nanoparticles (DOX-PEG-ZnO-NPs), and (d) GEM-encapsulated PEGylated nanoparticles (DOX-GEM-ZnO-PEGNPs (GEM-PEG-ZnO-NPs).
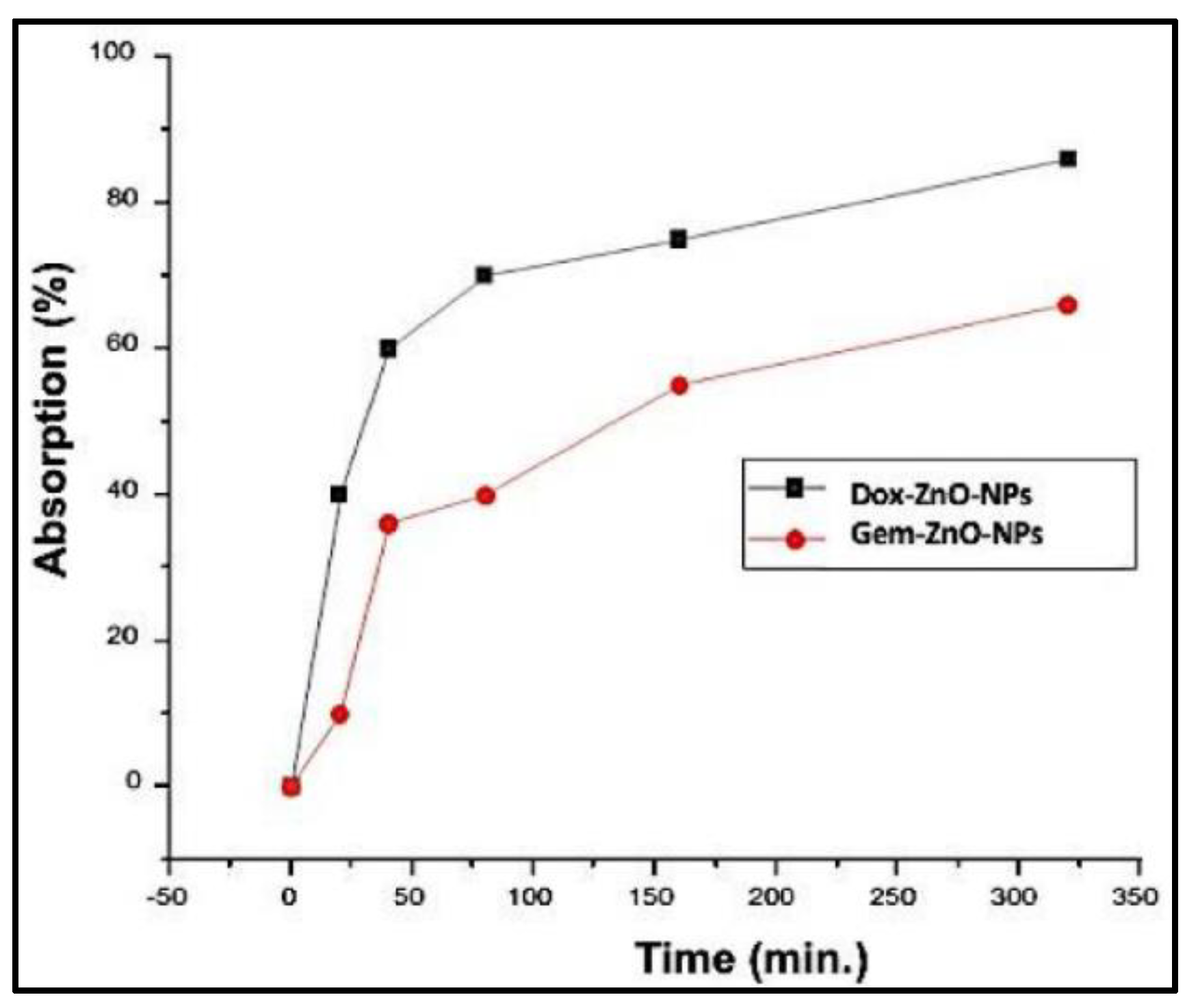
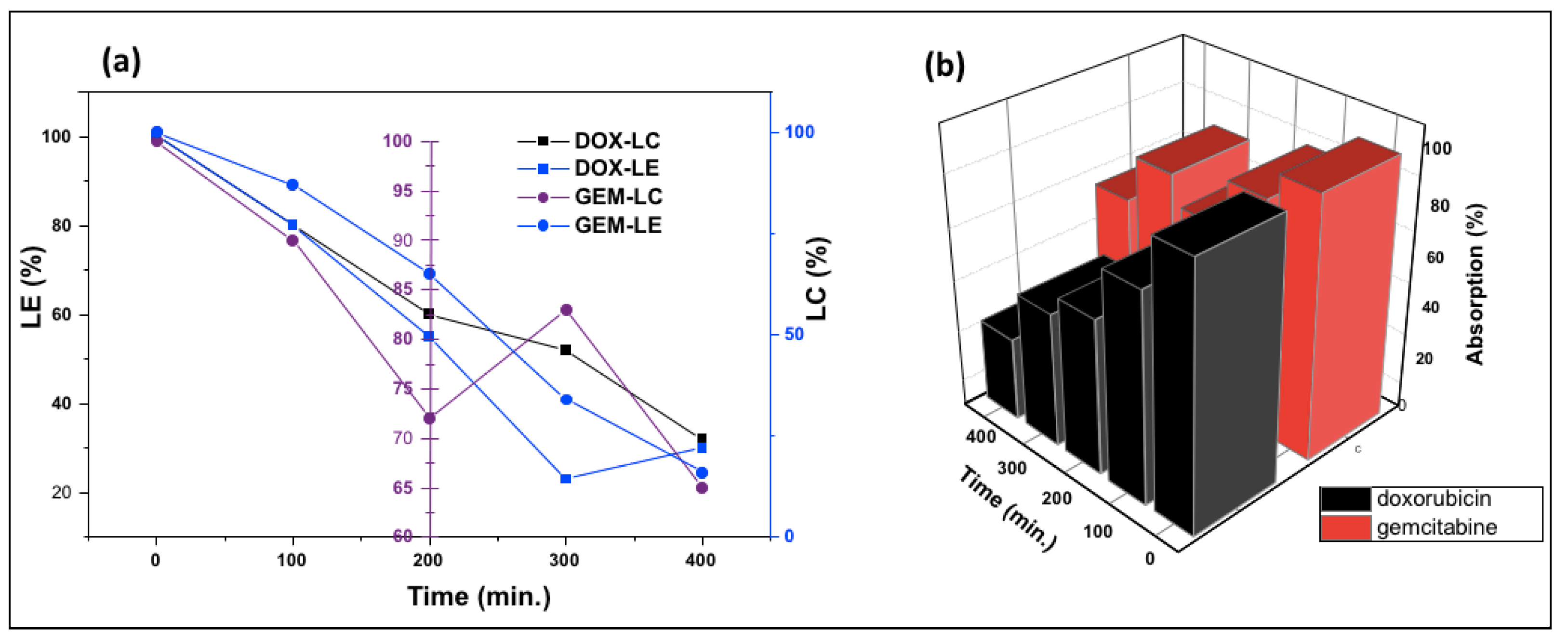
Figure 8 shows the change in the absorption of DOX-PEG-ZnO-NPs and GEM-PEG-ZnO-NPs by time. It was observed from the results that the DOX-loaded ZnO-NPs had a low absorption of nearly 35%. In contrast, GEM had an absorption of about 68% after a time interval of 320 min in the solution. This trend was replicated, with intensified behavior in the case of PEG-ZnO-NPs, which had 30% and 63% absorption, respectively. DOX revealed a high loading efficiency (LE) of 65% (650 mg/g) and a loading capacity (LC) of 32% (320 mg/g). Meanwhile, GEM-loaded ZnO-NPs had an LE of 30.5% (30 mg/g) and LC of 16.25% (162 mg/g). In the case of PEG-ZnO-NPs, DOX exhibited a high LE of 68% (680 mg/g) and LC of 35% (350 mg/g) in contrast to GEM, which exhibited a LE of 35% (350 mg/g) and LC of 19% (190 mg/g) (Figure 9a,b). The main purpose behind this part of analysis is to determine the LC and LE of both investigated drugs on the PEGylated and the non-PEGylated-ZnO-NPs separately, as thse values depend on various factors such as electrostatic stabilization, Van der Waals forces, hydrogen bonding, static repulsions, size of the molecular core, specific surface area-to-volume ratio, molecular weight, and pH of the solution. The superior LE and concentration of DOX in comparison to GEM on ZnO-NPs as confirmed by UV spectrophotometry can be explained by the noncovalent electrostatic interactions between the positively charged DOX and the negatively charged ZnO-NPs. On the other hand, the PEG-coated ZnO-NPs seemed to upload a higher concentration of both drugs. Polyethene glycol is a polymer that can provide a larger molecular core, which makes it possible to upload a higher amount of drug, therefore enhancing the drug LE [65,66,67,68]. It was observed from the results that the DOX loaded more drug as compared to GEM due to its high stability. PEGylated ZnO-NPs seemed to load a higher concentration of both of the investigated anticancer drugs. Different functionalization factors account for drug loading capacity. The main analysis in this study was the LE of the different anticancer drugs on the coated and noncoated ZnO-NPs. The highly efficient loading may be due to different factors such as the electrostatic stabilization, Van der Waals forces, hydrogen bonding, and static repulsions. Drug loading and releasing can also be affected by experimental conditions such as pH.
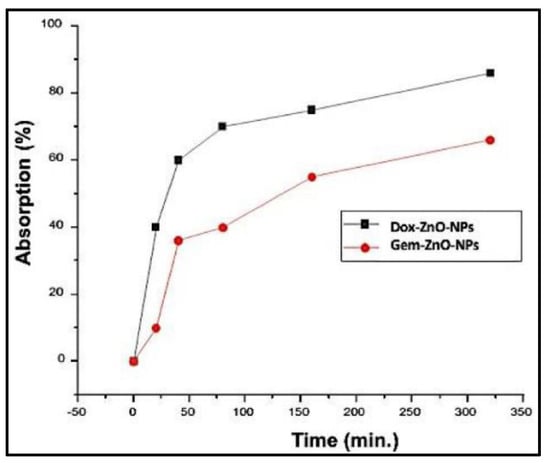
Figure 8.
UV absorption spectra of DOX-ZnO-NPs and GEM-ZnO-NPs after 320 min.
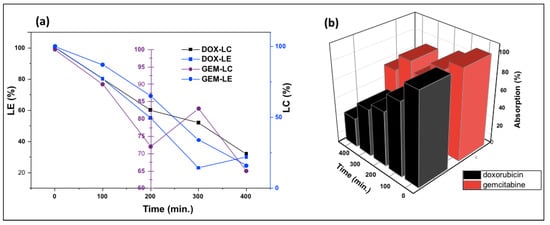
Figure 9.
(a) Loading capacity (LC), loading efficiency (LE), and (b) relative absorption of gemcitabine and doxorubicin PEGylated ZnO-NPs.
3.3. In Vitro Cytotoxic Activity and MTT Assay on MDA-MB-231 Cell Line
Cancer is considered to be the second leading cause of mortality across the globe resulting from uncontrolled proliferation of cells. According to global cancer 2018 statistics, breast cancer is the second most prevalent malignancy among all cancer types [69]. Developing anticancer drugs with enhanced efficacy and minimal side effects poses dynamically a challenging task for the scientific community [70]. Patients suffering from triple-negative breast cancer show poor prognosis and a devious metastatic molecular mechanism that is hardly recognizable [71].
Many plant extracts and green synthesized NPs have been reported to reflect anticancer and antioxidant properties [72]. Furthermore, Aloe vera leaf extract-based ZnO-NPs have been reported to be associated with the inhibition of cellular malignancy [73,74]. Green-synthesized NPs could exert their antitumor potential through the production of reactive oxygen species involved in intracellular signaling, the regulation of cell proliferation, and phagocytosis [75]. The antiproliferative activity of green-synthesized ZnO-NPs and DOX-PEG-ZnO-NPs was determined in vitro on triple-negative breast cancer cell line MDA-MB-231 using an MTT assay. However, the cytotoxic assay of GEM-loaded formulations was not performed due to its low LC and LE. Also, the cells treated by incubation with different samples of ZnO-NPs at various concentrations exhibited a significant level of cytotoxicity. Similar behavior was observed in the case of DOX-ZnO-NPs (Figure 10). However, DOX-PEG-ZnO-NPs exhibited the best results. These results revealed that ZnO-NPs have anticancer potential that can be enhanced by anticancer drug loading with the subsequent PEGylation. Our findings validate the results of previous studies addressing the strong preferential cytotoxicity of green-synthesized ZnO-NPs against cancer cell lines [76].
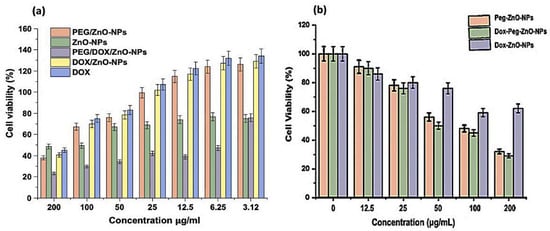
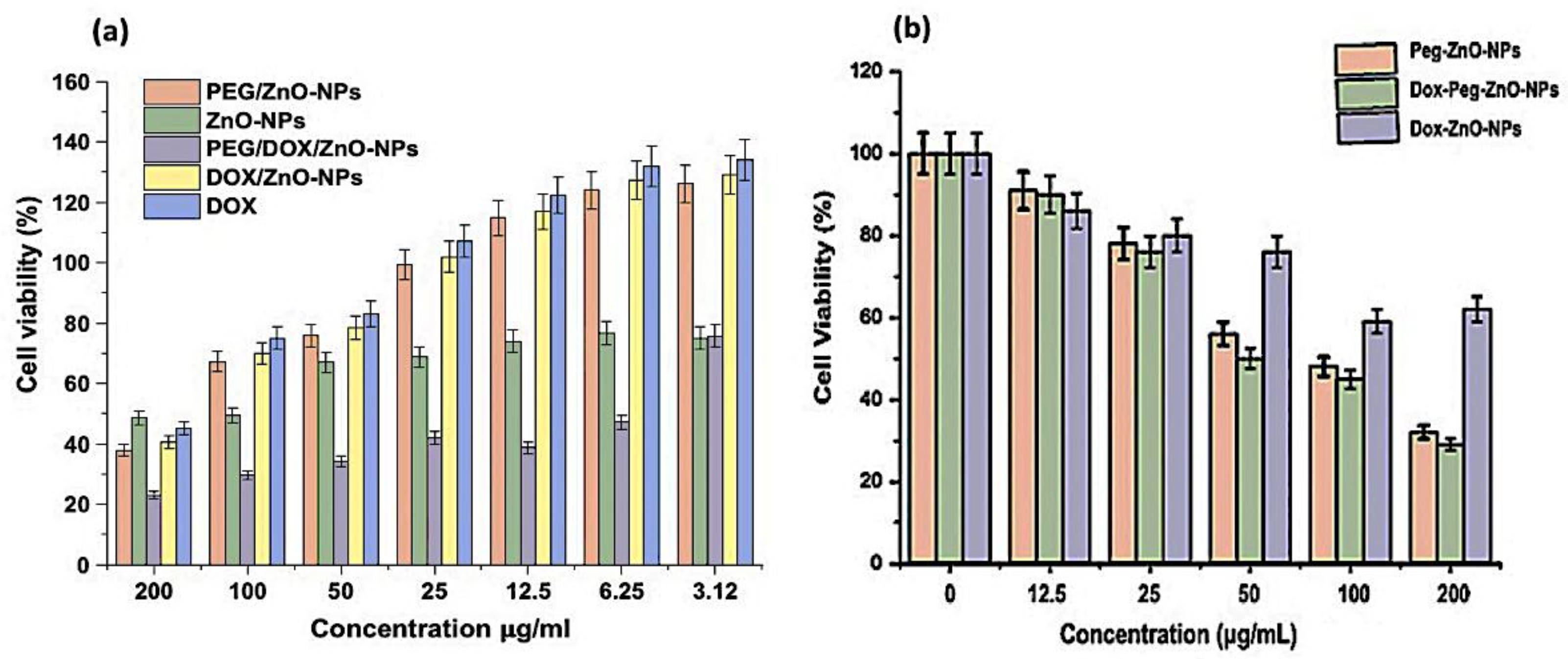
Figure 10.
Anticancer activity of ZnO-NPs as a percentage of apoptosis in cell viability on MDA-MB-231 cancer cell line by MTT assay. (a) Comparison of DOX, ZnO-NPs, PEG-ZnO-NPs, DOX-ZnO-NPs, and DOX-PEG-ZnO-NPs at different concentration of nanoparticles. (b) Comparison of PEG-ZnO-NPs, DOX-ZnO-NPs, and DOX-PEG-ZnO-NPs at different concentration of nanoparticles. The results expressed as the ± SD mean subjected to one-way SPSS version. Experiments were performed in triplicate and repeated for three times and the calculated value of p < 0.05.
The ZnO-NPs generate reactive oxygen species (ROS), which are responsible for the cytotoxic activity exceeding a higher value than the antioxidant potential of the tumor cells [77]. Flavonoid and phenolic compounds, which are encapsulated on the surfaces of the stabilized ZnO-NPs by Aloe vera leaf extract, also exert anticancer effects through the suppression of metastasis and impairment of tumor angiogenesis, thus inhibiting proliferation, inactivating carcinogens, inducing cell arrest, triggering apoptosis, and promoting differentiation and oxidative destruction. Flavonoids also interact with the estrogen binding sites, downregulate kinase signal transduction pathways, and alter gene expression. Aloe vera leaf extract generates small-sized ZnO-NPs that exert potent toxicity effects [78,79,80]. The percentage cell viability of Dox-PEG-ZnO-NPs on MDA-MB-231 at 50 µg/mL was 40% and was reduced to 30% at 200 µg/mL. The Dox-ZnO-NPs showed the highest percentage viability (~60%) at a concentration of 200 µg/mL concentration. The MDA-MB-231 cell killing by Dox-PEG-ZnO-NPs was observed of ~70% at 200 µg/mL as shown in Figure 10. Enhancement of the activity of the nanoparticles is possible due to the dissociation of the encapsulated ZnO-NPs in the acidic tumor microenvironment, which releases Zn2+ ions, enhancing the cellular uptake and retention of DOX. Also, the fairly high, effective, and powerful toxic activity of DOX-PEG-ZnO-NPs against cancer cells may be due to the high loading capacity and efficiency of polyethylene glycol [42,81,82].
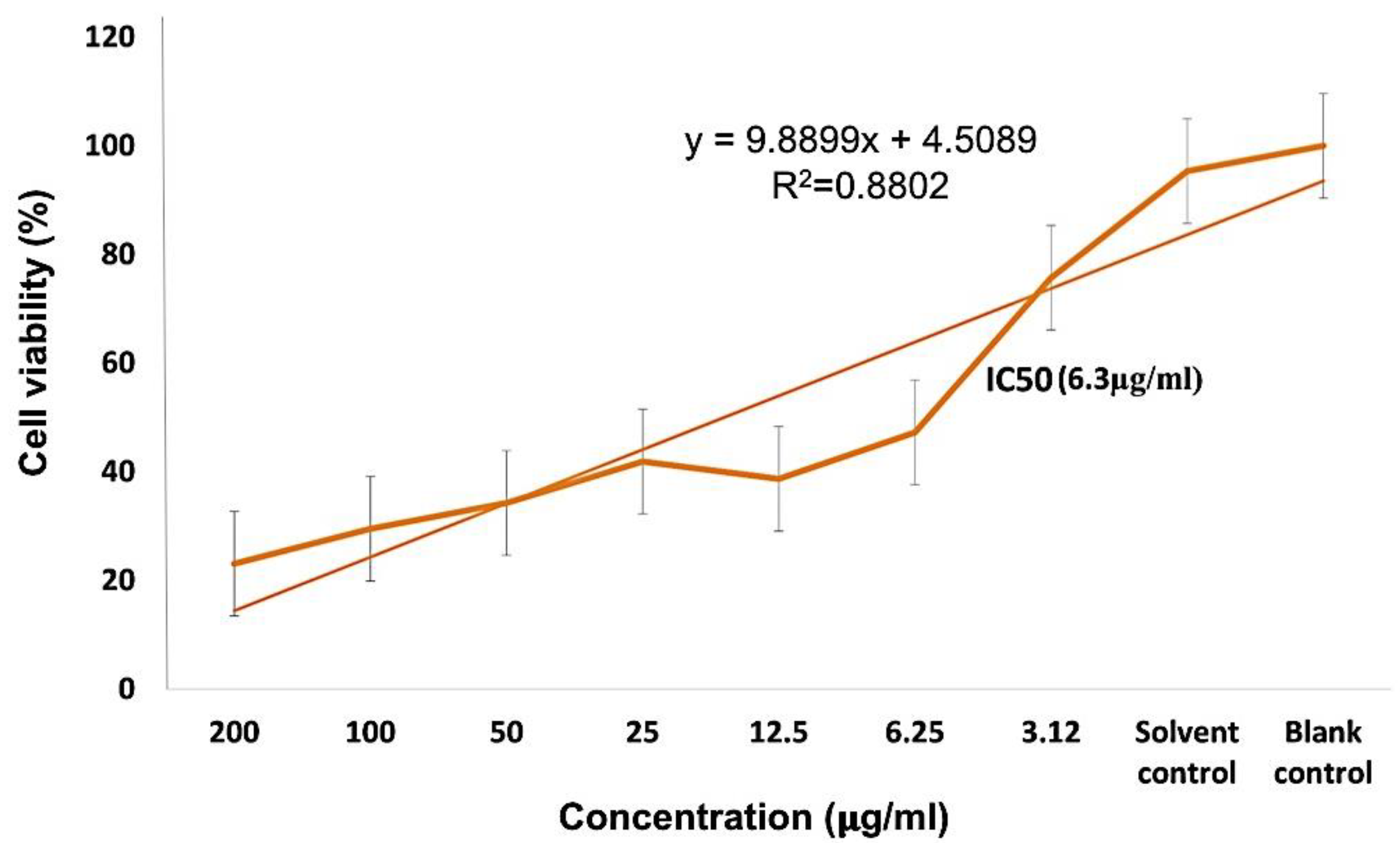
Different concentrations of PEG-ZnO-NPs samples loaded with the anticancer drug treated against the MDA-MB-231 cancer cell line showed an IC50 value of 6.35 µg/mL in the MTT assay, as shown in Figure 10. Comparative study of PEG-ZnO-NPs and non-PEGylated ZnO-NPs showed cytotoxicity in a concentration-dependent manner. When the MDA-MB-231 cell line was treated without drug-loaded PEGylated-ZnO-NPs samples, viability was reduced to 50%, while viability was reduced to 35% with doxorubicin drug-loaded PEGylated ZnO-NPs. These findings indicate that the anticancer drug-loaded PEG-ZnO-NPs have high cytotoxicity. The relative cytotoxic effect on cancer cells between (0–200 µg/mL) concentrations was also examined. It was clear from the results, as shown in Figure 10 and listed in Table 2, that the PEGylated drug-loaded samples showed greater anticancer activity than the ZnO-NPs of calculated IC50 6.35 μg/mL as mentioned and compared with previous related works of the MTT assay of the DOX drug in the literature as listed in Table 2 and presented in Figure 11. It was mentioned in the literature that the size and dose of metal oxide nanoparticles are crucial factors which determine its cytotoxicity. As far as ZnO-NPs antitumor potential is concerned, the intracellular release of dissociated zinc ions with subsequent ROS induction is considered the underlying mechanism even though its precise cytotoxic mode of action is also under debate [83,84]. The IC50 value in the MTT assay describes the concentration of the drug at half inhibition. The IC50 profile of the MDA-MB-231 (TNBC) cancer cell line was estimated by plotting a graph between the concentrations of Dox-PEG-ZnO-NPs against the percentage of cell viability. Table 2 also shows the MTT assay comparison from the literature on different cancer cell lines and half-maximum inhibitory concentration measurements and the potency of biochemical function inhibition. According to the data listed in Table 2, MDA-MB-231, the triple-negative breast cancer cell line used in the present study, showed an IC50 value of 6.35 ± 0.5, which indicates the presence of the cancer cell. The percentage cell viability of PEGylated-ZnO-NPs with doxorubicin drug-loaded MDA-MB-231 at different concentrations shows that it is extremely toxic to cancer cells at 0–200 µg/mL concentration. These interested results need more detailed investigation in future. In our latest study, we proved that PEGylated-ZnO-NPs loaded with doxorubicin have slightly greater toxin then non-PEGylated drug-loaded nanoparticles and PEGylated-ZnO-NPs. The percentage cell viability of the effect of Dox-PEG-ZnO-NPs on MDA-MB-231 at 50 µg/mL was determined as 40% and was reduced to 30% at 200 µg/mL. The Dox-ZnO-NPs showed the highest percentage viability (~ 60%) at a concentration of 200 µg/mL. The MDA-MB-231 cell killing by Dox-PEG-ZnO-NPs was observed to be 70% at 200 µg/mL as shown in Figure 11.

Table 2.
Comparative analysis of (IC50) value in MTT assay on MDA-MB231 cancer cell line (different cancer cell line values are in µg/mL).
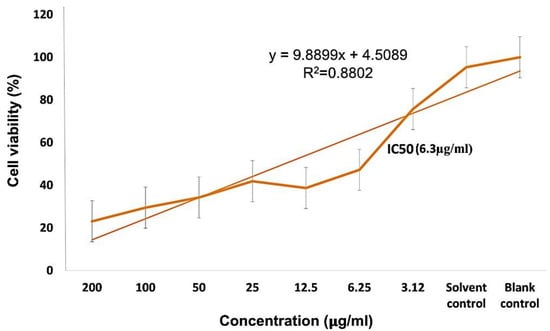
Figure 11.
DOX/PEG-ZnO-NPs sample of metal oxide % cell viability and IC 50(6.3 µg/mL) analysis with a concentration range of 0–200 µg/mL.
4. Conclusions
The biogenic green synthesis process was employed to generate ZnO-NPs using Aloe vera plant extract as a stabilizing and capping agent in the medium. The optimum reaction conditions, easy and cost-effective procedures, and medicinal applications of the green synthesis ZnO-NPs-based drug delivery system are highlighted in our research findings. Literary contributions in the field of medical oncology for effective clinical cancer therapy were also studied. The synthesized ZnO-NPs had particle sizes in the range of 20–40 nm, which is considered ideal for the investigated drug carrier system. The DOX had better loading capacity and loading efficiency than GEM against PEGylated and non-PEGylated ZnO-NPs. Therefore, the DOX-loaded nanoparticles (PEGylated and non-PEGylated), along with the untreated ZnO-NPs, were shortlisted for in vitro analysis, which exhibited potent cytotoxicity against the investigated breast cancer. The antiproliferative activity of DOX/PEG-ZnO-NPs was determined in vitro on triple-negative breast cancer cell line MDA-MB-231 using the MTT assay. On the other hand, the cytotoxic assay of GEM-loaded formulations was not performed due to the its low loading capacity and loading efficiency. Cells incubated with ZnO-NPs at various concentrations exhibited a significant effect of cytotoxicity. A similar trend was observed in the case of the DOX-ZnO-NPs sample. These findings show that ZnO-NPs have anticancer potential that can be enhanced by subsequent PEGylated-ZnO-NPs, with biocompatibility, surface cancer cell targeting, and drug delivery capacity, and can be explored for cancer treatment. Doxorubicin drug-loaded PEGylated-ZnO-NPs exhibited the highest cytotoxicity, with a low concentration threshold for anticancer activity.
Author Contributions
Conceptualization, M.B; methodology, M.B; software W.M.D.; validation, W.M.D.; formal analysis, S.K.; investigation, M.B; resources, M.B; data curation, S.K.; writing—original draft preparation, M.B.; writing—review and editing, W.M.D.; visualization, T.N.; supervision, S.K.; project administration, S.A.S.; funding acquisition, W.M.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the CEMB (Center of excellence in molecular biology) Punjab University Lahore, Pakistan, grant No. HEC NRPU 7657.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Not applicable.
Acknowledgments
The authors thank the CEMB institute and GCU Lahore for financial supports. The COMSAT Institute is acknowledged for the characterization of prepared samples, grant No. HEC NRPU 7657.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lunshof, J.E.; Bobe, J.; Aach, J.; Angrist, M.; Thakuria, J.V.; Vorhaus, D.B.; Hoehe, M.R.; Church, G.M. Personal genomes in progress: From the human genome project to the Personal Genome Project. Dialogues Clin. Neurosci. 2010, 12, 44–57. Available online: www.personalgenomes.org/mission.html (accessed on 5 January 2021).
- Jackson, S.E.; Chester, J.D. Personalised cancer medicine. Int. J. Cancer 2015, 137, 262–266. [Google Scholar] [CrossRef] [PubMed]
- Nagasaka, M.; Gadgeel, S.M. Role of chemotherapy and targeted therapy in early-stage non-small cell lung cancer. Expert Rev. Anticancer. Ther. 2018, 18, 63–70. [Google Scholar] [CrossRef]
- Nevala-Plagemann, C.; Hidalgo, M.; Garrido-Laguna, I. From state-of-the-art treatments to novel therapies for advanced-stage pancreatic cancer. Nat. Rev. Clin. Oncol. 2020, 17, 108–123. [Google Scholar] [CrossRef] [PubMed]
- Tokunaga, M.; Sato, Y.; Nakagawa, M.; Aburatani, T.; Matsuyama, T.; Nakajima, Y.; Kinugasa, Y. Correction to: Perioperative chemotherapy for locally advanced gastric cancer in Japan: Current and future perspectives. Surg. Today 2020, 50, 30–37, doi:10.1007/s00595-019-01896-5; Errarum in 2020, 50, 424, doi:10.1007/s00595-019-01950-2. [Google Scholar]
- Mansoori, B.; Mohammadi, A.; Davudian, S.; Shirjang, S.; Baradaran, B. The different mechanisms of cancer drug resistance: A brief review. Adv. Pharm. Bull. 2017, 7, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Nurgali, K.; Jagoe, R.T.; Abalo, R. Editorial: Adverse effects of cancer chemotherapy: Anything new to improve tolerance and reduce sequelae? Front. Pharmacol. 2018, 9, 245. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.; Liu, K.; Shen, Q.; Li, Q.; Hao, J.; Han, F.; Jiang, R.-W. Reversal of multidrug resistance in cancer by multi-functional flavonoids. Front. Oncol. 2019, 9, 487. [Google Scholar] [CrossRef]
- Yang, Z.; Ma, Y.; Zhao, H.; Yuan, Y.; Kim, B.Y.S. Nanotechnology platforms for cancer immunotherapy. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2020, 12, e1590. [Google Scholar] [CrossRef]
- Shim, H. Bispecific antibodies and antibody-drug conjugates for cancer therapy: Technological considerations. Biomolecules 2020, 10, 360. [Google Scholar] [CrossRef]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.M.; Pineros, M.; Znaor, A.; Bray, F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer 2019, 144, 1941–1953. [Google Scholar] [CrossRef]
- Sauter, E.R. Cancer prevention and treatment using combination therapy with natural compounds. Expert Rev. Clin. Pharmacol. 2020, 13, 265–285. [Google Scholar] [CrossRef]
- Anastasiadi, Z.; Lianos, G.D.; Ignatiadou, E.; Harissis, H.V.; Mitsis, M. Breast cancer in young women: An overview. Updates Surg. 2017, 69, 313–317. [Google Scholar] [CrossRef]
- Subramani, R.; Lakshmanaswamy, R. Complementary and alternative medicine and breast cancer. Prog. Mol. Biol. Transl. Sci. 2017, 151, 231–274. [Google Scholar] [CrossRef]
- Kolak, A.; Kamińska, M.; Sygit, K.; Budny, A.; Surdyka, D.; Kukiełka-Budny, B.; Burdan, F. Primary and secondary prevention of breast cancer. Ann. Agric. Environ. Med. 2017, 24, 549–553. [Google Scholar] [CrossRef]
- Silva, E.F.; Bazoni, R.F.; Ramos, E.B.; Rocha, M.S. DNA-doxorubicin interaction: New insights and peculiarities. Biopolymers 2016, 107, e22998. [Google Scholar] [CrossRef] [PubMed]
- Schwarzenbach, H.; Gahan, P.B. Predictive value of exosomes and their cargo in drug response/resistance of breast cancer patients. Cancer Drug Resist. 2020. [Google Scholar] [CrossRef]
- Benjanuwattra, J.; Siri-Angkul, N.; Chattipakorn, S.C.; Hattipakorn, N.C. Doxorubicin and its proarrhythmic effects: A comprehensive review of the evidence from experimental and clinical studies. Pharmacol. Res. 2020, 151, 104542. [Google Scholar] [CrossRef]
- Najafi, M.; Shayesteh, M.R.H.; Mortezaee, K.; Farhood, B.; Haghi-Aminjan, H. The role of melatonin on doxorubicin-induced cardiotoxicity: A systematic review. Life Sci. 2020, 241, 117173. [Google Scholar] [CrossRef] [PubMed]
- Xinyong, C.; Zhiyi, Z.; Lang, H.; Peng, Y.; Xiaocheng, W.; Ping, Z.; Liang, S. The role of toll-like receptors in myocardial toxicity induced by doxorubicin. Immunol. Lett. 2020, 217, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Stone, L. Gemcitabine reduces recurrence. Nat. Rev. Urol. 2018, 15, 466. [Google Scholar] [CrossRef]
- Smith, S.D. Gemcitabine: End of a chemotherapy’s era? Acta Haematol. 2019, 141, 91–92. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, S.; Cheng, G.; Yu, P.; Chang, J.; Chen, X. Cascade Drug-Release Strategy for Enhanced Anticancer Therapy. Matter 2021, 4, 26–53. [Google Scholar] [CrossRef]
- Zhao, Y.; Lv, F.; Chen, S.; Wang, Z.; Zhang, J.; Zhang, S.; Cao, J.; Wang, L.; Cao, E.; Wang, B.; et al. Caveolin-1 expression predicts efficacy of weekly nab-paclitaxel plus gemcitabine for metastatic breast cancer in the phase II clinical trial. BMC Cancer 2018, 18, 1019. [Google Scholar] [CrossRef] [PubMed]
- Hryciuk, B.; Szymanowski, B.; Romanowska, A.; Salt, E.; Wasąg, B.; Grala, B.; Jassem, J.; Duchnowska, R. Severe acute toxicity following gemcitabine administration: A report of four cases with cytidine deaminase polymorphisms evaluation. Oncol. Lett. 2018, 15, 1912–1916. [Google Scholar] [CrossRef]
- Turco, C.; Jary, M.; Kim, S.; Moltenis, M.; Degano, B.; Manzoni, P.; Nguyen, T.; Genet, B.; Rabier, M.-B.V.; Heyd, B.; et al. Gemcitabine-induced pulmonary toxicity: A case report of pulmonary veno-occlusive disease. Clin. Med. Insights 2015, 9, 75–79. [Google Scholar] [CrossRef]
- Yhee, J.; Son, S.; Lee, H.; Kim, K. Nanoparticle-based combination therapy for cancer treatment. Curr. Pharm. Des. 2015, 21, 3158–3166. [Google Scholar] [CrossRef]
- Ashfaq, U.A.; Riaz, M.; Yasmeen, E.; Yousaf, M.Z. Recent advances in nanoparticle-based targeted drug-delivery systems against cancer and role of tumor microenvironment. Crit. Rev. Ther. Drug Carr. Syst. 2017, 34, 317–353. [Google Scholar] [CrossRef] [PubMed]
- Bregoli, L.; Movia, D.; Gavigan-Imedio, J.D.; Lysaght, J.; Reynolds, J.; Prina-Mello, A. Nanomedicine applied to translational oncology: A future perspective on cancer treatment. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 81–103. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, M.; Alexander, A.; Singh, M.R.; Singh, D.; Saraf, S.; Saraf, S.; Ajazuddin. Understanding the prospective of nano-formulations towards the treatment of psoriasis. Biomed. Pharmacother. 2018, 107, 447–463. [Google Scholar] [CrossRef]
- Muhamad, N.; Plengsuriyakarn, T.; Na-Bangchang, K. Application of active targeting nanoparticle delivery system for chemotherapeutic drugs and traditional/herbal medicines in cancer therapy: A systematic review. Int. J. Nanomed. 2018, 13, 3921–3935. [Google Scholar] [CrossRef]
- Alavi, M.; Hamidi, M. Passive and active targeting in cancer therapy by liposomes and lipid nanoparticles. Drug Metab. Pers. Ther. 2019, 34. [Google Scholar] [CrossRef]
- Lin, G.; Chen, S.; Mi, P. Nanoparticles targeting and remodeling tumor microenvironment for cancer theranostics. J. Biomed. Nanotechnol. 2018, 14, 1189–1207. [Google Scholar] [CrossRef]
- Jo, A.; Zhang, R.; Allen, I.C.; Riffle, J.S.; Davis, R.M. Design and fabrication of streptavidin-functionalized, fluorescently labeled polymeric nanocarriers. Langmuir 2018, 34, 15783–15794. [Google Scholar] [CrossRef]
- Núñez, C.; Estévez, S.V.; Chantada, M.D.P. Inorganic nanoparticles in diagnosis and treatment of breast cancer. J. Biol. Inorg. Chem. 2018, 23, 331–345. [Google Scholar] [CrossRef] [PubMed]
- Jiao, M.; Zhang, P.; Meng, J.; Li, Y.; Liu, C.; Luo, X.; Gao, M. Recent advancements in biocompatible inorganic nanoparticles towards biomedical applications. Biomater. Sci. 2018, 6, 726–745. [Google Scholar] [CrossRef]
- Huang, L.; Drake, V.J.; Ho, E. Zinc. Adv. Nutr. 2015, 6, 224–226. [Google Scholar] [CrossRef] [PubMed]
- Wieringa, F.T.; Dijkhuizen, M.A.; West, C.E. Iron and zinc interactions. Am. J. Clin. Nutr. 2004, 80, 787–788. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Ma, X.; Jin, S.; Xue, X.; Zhang, C.; Wei, T.; Guo, W.; Liang, X.-J. Zinc oxide nanoparticles as adjuvant to facilitate doxorubicin intracellular accumulation and visualize ph-responsive release for overcoming drug resistance. Mol. Pharm. 2016, 13, 1723–1730. [Google Scholar] [CrossRef]
- Racca, L.; Cauda, V. Remotely Activated Nanoparticles for Anticancer Therapy. Nano-Micro Lett. 2021, 13, 1–34. [Google Scholar] [CrossRef]
- Kim, S.; Lee, S.Y.; Cho, H.-J. Doxorubicin-wrapped zinc oxide nanoclusters for the therapy of colorectal adenocarcinoma. Nanomaterials 2017, 7, 354. [Google Scholar] [CrossRef] [PubMed]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug Deliv. Rev. 2016, 99, 28–51. [Google Scholar] [CrossRef]
- Li, C.; Wang, J.; Wang, Y.; Gao, H.; Wei, G.; Huang, Y.; Yu, H.; Gan, Y.; Wang, Y.; Mei, L.; et al. Recent progress in drug delivery. Acta Pharm. Sin. B 2019, 9, 1145–1162. [Google Scholar] [CrossRef]
- Unni, M.; Uhl, A.M.; Savliwala, S.; Savitzky, B.H.; Dhavalikar, R.; Garraud, N.; Arnold, D.P.; Kourkoutis, L.F.; Andrew, J.S.; Rinaldi, C. Thermal decomposition synthesis of iron oxide nanoparticles with diminished magnetic dead layer by controlled addition of oxygen. ACS Nano 2017, 11, 2284–2303. [Google Scholar] [CrossRef]
- Mikhailov, O.V.; Mikhailova, E.O. Elemental silver nanoparticles: Biosynthesis and bio applications. Materials 2019, 12, 3177. [Google Scholar] [CrossRef]
- Sanaeimehr, Z.; Javadi, I.; Namvar, F. Antiangiogenic and antiapoptotic effects of green-synthesized zinc oxide nanoparticles using Sargassum muticum algae extraction. Cancer Nanotechnol. 2018, 9, 1–16. [Google Scholar] [CrossRef]
- Singh, J.; Dutta, T.; Kim, K.-H.; Rawat, M.; Samddar, P.; Kumar, P. ‘Green’ synthesis of metals and their oxide nanoparticles: Applications for environmental remediation. J. Nanobiotechnol. 2018, 16, 1–24. [Google Scholar] [CrossRef]
- Christy, S.R.; Priya, L.S.; Durka, M.; Dinesh, A.; Babitha, N.; Arunadevi, S. Simple combustion synthesis, structural, morphological, optical and catalytic properties of ZnO nanoparticles. J. Nanosci. Nanotechnol. 2019, 19, 3564–3570. [Google Scholar] [CrossRef] [PubMed]
- Mozar, F.S.; Chowdhury, E.H. Impact of PEGylated nanoparticles on tumor targeted drug delivery. Curr. Pharm. Des. 2018, 24, 3283–3296. [Google Scholar] [CrossRef] [PubMed]
- Manikandan, A.; Durka, M.; Selvi, M.A.; Antony, S.A. Aloe vera plant extracted green synthesis, structural and opto-magnetic characterizations of spinel CoxZn1-xAl2O4 nano-catalysts. J. Nanosci. Nanotechnol. 2016, 16, 357–373. [Google Scholar] [CrossRef]
- Hassan, S.S.M.; Azab, W.I.M.E.; Ali, H.R.; Mansour, M.S.M. Green synthesis and characterization of ZnO nanoparticles for photocatalytic degradation of anthracene. Adv. Nat. Sci. Nanosci. Nanotechnol. 2015, 6, 045012. [Google Scholar] [CrossRef]
- Zare, E.; Pourseyedi, S.; Khatami, M.; Darezereshki, E. Simple biosynthesis of zinc oxide nanoparticles using nature’s source, and it’s in vitro bio-activity. J. Mol. Struct. 2017, 1146, 96–103. [Google Scholar] [CrossRef]
- Karnan, T.; Selvakumar, S.A.S. Biosynthesis of ZnO nanoparticles using rambutan (Nephelium lappaceum L.) peel extract and their photocatalytic activity on methyl orange dye. J. Mol. Struct. 2016, 1125, 358–365. [Google Scholar] [CrossRef]
- Bhuyan, T.; Mishra, K.; Khanuja, M.; Prasad, R.; Varma, A. Biosynthesis of zinc oxide nanoparticles from Azadirachta indica for antibacterial and photocatalytic applications. Mater. Sci. Semicond. Process. 2015, 32, 55–61. [Google Scholar] [CrossRef]
- Rasli, N.I.; Basri, H.; Harun, Z. Zinc oxide from aloe vera extract: Two-level factorial screening of biosynthesis parameters. Heliyon 2020, 6, e03156. [Google Scholar] [CrossRef]
- Chabala, L.F.G.; Cuartas, C.E.E.; López, M.E.L. Release behavior and antibacterial activity of chitosan/alginate blends with aloe vera and silver nanoparticles. Mar. Drugs 2017, 15, 328. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Gautam, N.; Mishra, A.; Gupta, R. Heavy metals and living systems: An overview. Indian J. Pharmacol. 2011, 43, 246–253. [Google Scholar] [CrossRef]
- Keshtkar, M.; Dobaradaran, S.; Soleimani, F.; NorooziKarbasdehi, V.; Mohammadi, M.; Mirahmadi, R.; FarajiGhasemi, F. Data on heavy metals and selected anions in the Persian popular herbal distillates. Data Brief 2016, 8, 2352–3409. [Google Scholar] [CrossRef]
- Azizi, S.; Mohamad, R.; Shahri, M.M. Green microwave-assisted combustion synthesis of zinc oxide nanoparticles with Citrullus colocynthis (L.) schrad: Characterization and biomedical applications. Molecules 2017, 22, 301. [Google Scholar] [CrossRef] [PubMed]
- Muniz, F.T.L.; Miranda, M.A.R.; Dos Santos, C.M.; Sasaki, J.M. The Scherrer equation and the dynamical theory of X-ray diffraction. Acta Crystallogr. Sect. A Found. Adv. 2016, 72, 385–390. [Google Scholar] [CrossRef]
- Barad, S.; Roudbary, M.; Omran, A.N.; Daryasari, M.P. Preparation and characterization of ZnO nanoparticles coated by chitosan-linoleic acid; fungal growth and biofilm assay. Bratisl. Med. J. 2017, 118, 169–174. [Google Scholar] [CrossRef]
- Ezealisiji, K.M.; Siwe-Noundou, X.; Maduelosi, B.; Nwachukwu, N.; Krause, R.W.M. Green synthesis of zinc oxide nanoparticles using Solanum torvum (L) leaf extract and evaluation of the toxicological profile of the ZnO nanoparticles–hydrogel composite in Wistar albino rats. Int. Nano Lett. 2019, 9, 99–107. [Google Scholar] [CrossRef]
- Zhang, H.; Ji, Z.; Xia, T.; Meng, H.; Low-Kam, C.; Liu, R.; Pokhrel, S.; Lin, S.; Wang, X.; Liao, Y.-P.; et al. Use of metal oxide nanoparticle band gap to develop a predictive paradigm for oxidative stress and acute pulmonary inflammation. ACS Nano 2012, 6, 4349–4368. [Google Scholar] [CrossRef] [PubMed]
- Zak, A.K.; Majid, W.A.; Mahmoudian, M.; Darroudi, M.; Yousefi, R. Starch-stabilized synthesis of ZnO nanopowders at low temperature and optical properties study. Adv. Powder Technol. 2013, 24, 618–624. [Google Scholar] [CrossRef]
- Punnoose, A.; Dodge, K.; Rasmussen, J.W.; Chess, J.; Wingett, D.; Anders, C. Cytotoxicity of ZnO nanoparticles can be tailored by modifying their surface structure: A green chemistry approach for safer nanomaterials. ACS Sustain. Chem. Eng. 2014, 2, 1666–1673. [Google Scholar] [CrossRef]
- Kc, B.; Paudel, S.N.; Rayamajhi, S.; Karna, D.; Adhikari, S.; Shrestha, B.G.; Bisht, G. Enhanced preferential cytotoxicity through surface modification: Synthesis, characterization and comparative in vitro evaluation of TritonX-100 modified and unmodified zinc oxide nanoparticles in human breast cancer cell (MDA-MB-231). Chem. Cent. J. 2016, 10, 16. [Google Scholar] [CrossRef]
- Kenechukwu, F.C.; Attama, A.A.; Ibezim, E.C.; Nnamani, P.O.; Umeyor, C.E.; Uronnachi, E.M.; Momoh, M.A.; Akpa, P.A.; Ozioko, A.C. Novel intravaginal drug delivery system based on molecularly PEGylated lipid matrices for improved antifungal activity of miconazole nitrate. BioMed Res. Int. 2018, 2018, 3714329. [Google Scholar] [CrossRef] [PubMed]
- Kumari, P.; Ghosh, B.; Biswas, S. Nanocarriers for cancer-targeted drug delivery. J. Drug Target. 2016, 24, 179–191. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Ali, R.; Mirza, Z.; Ashraf, G.M.D.; Kamal, M.A.; Ansari, S.A.; Damanhouri, G.A.; Abuzenadah, A.M.; Chaudhary, A.G.; Sheikh, I.A. New anticancer agents: Recent developments in tumor therapy. Anticancer. Res. 2012, 32, 2999–3005. [Google Scholar]
- Davion, S.M.; Siziopikou, K.P.; Sullivan, M.E. Cytokeratin 7: A re-evaluation of the ‘tried and true’ in triple-negative breast cancers. Histopathology 2012, 61, 660–666. [Google Scholar] [CrossRef]
- Huffman, M.A. Animal self-medication and ethno-medicine: Exploration and exploitation of the medicinal properties of plants. Proc. Nutr. Soc. 2003, 62, 371–381. [Google Scholar] [CrossRef]
- Jiang, J.; Pi, J.; Cai, J. The advancing of zinc oxide nanoparticles for biomedical applications. Bioinorg. Chem. Appl. 2018, 2018, 1062562. [Google Scholar] [CrossRef] [PubMed]
- Ali, K.; Dwivedi, S.; Azam, A.; Saquib, Q.; Al-Said, M.S.; Alkhedhairy, A.A.; Musarrat, J. Aloe vera extract functionalized zinc oxide nanoparticles as nanoantibiotics against multi-drug resistant clinical bacterial isolates. J. Colloid Interface Sci. 2016, 472, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Vinardell, M.P.; Mitjans, M. Antitumor activities of metal oxide nanoparticles. Nanomaterials 2015, 5, 1004–1021. [Google Scholar] [CrossRef] [PubMed]
- Selim, Y.A.; Azb, M.A.; Ragab, I.; El-Azim, M.H.M.A. Green synthesis of zinc oxide nanoparticles using aqueous extract of deverra tortuosa and their cytotoxic activities. Sci. Rep. 2020, 10, 3445. [Google Scholar] [CrossRef]
- El-Shorbagy, H.M.; Eissa, S.M.; Sabet, S.; El-Ghor, A.A. Apoptosis and oxidative stress as relevant mechanisms of antitumor activity and genotoxicity of ZnO-NPs alone and in combination with N-acetyl cysteine in tumor-bearing mice. Int. J. Nanomed. 2019, 14, 3911–3928. [Google Scholar] [CrossRef]
- Radha, M.H.; Laxmipriya, N.P. Evaluation of biological properties and clinical effectiveness of Aloe vera: A systematic review. J. Tradit. Complement. Med. 2015, 5, 21–26. [Google Scholar] [CrossRef]
- Kumar, R.; Singh, A.K.; Gupta, A.; Bishayee, A.; Pandey, A.K. Therapeutic potential of Aloe vera—A miracle gift of nature. Phytomedicine 2019, 60, 152996. [Google Scholar] [CrossRef]
- Gao, Y.; Kuok, K.I.; Jin, Y.; Wang, R. Biomedical applications of Aloe vera. Crit. Rev. Food Sci. Nutr. 2019, 59, S244–S256. [Google Scholar] [CrossRef]
- Wongpinyochit, T.; Uhlmann, P.; Urquhart, A.J.; Seib, F.P. PEGylated silk nanoparticles for anticancer drug delivery. Biomacromolecules 2015, 16, 3712–3722. [Google Scholar] [CrossRef] [PubMed]
- Chung, B.H.; Chung, S.J. Recent advances in pH-sensitive polymeric nanoparticles for smart drug delivery in cancer therapy. Curr. Drug Targets 2018, 19, 300–317. [Google Scholar] [CrossRef]
- Bisht, G.; Rayamajhi, S. ZnO nanoparticles: A promising anticancer agent. Nanobiomedicine 2016, 3, 9. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, J.W.; Martinez, E.; Louka, P.; Wingett, D.G. Zinc oxide nanoparticles for selective destruction of tumor cells and potential for drug delivery applications. Expert Opin. Drug Deliv. 2010, 7, 1063–1077. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).