First Report on the Geologic Occurrence of Natural Na–A Zeolite and Associated Minerals in Cretaceous Mudstones of the Paja Formation of Vélez (Santander), Colombia
Abstract
:1. Introduction
2. Materials and Methods
3. Results
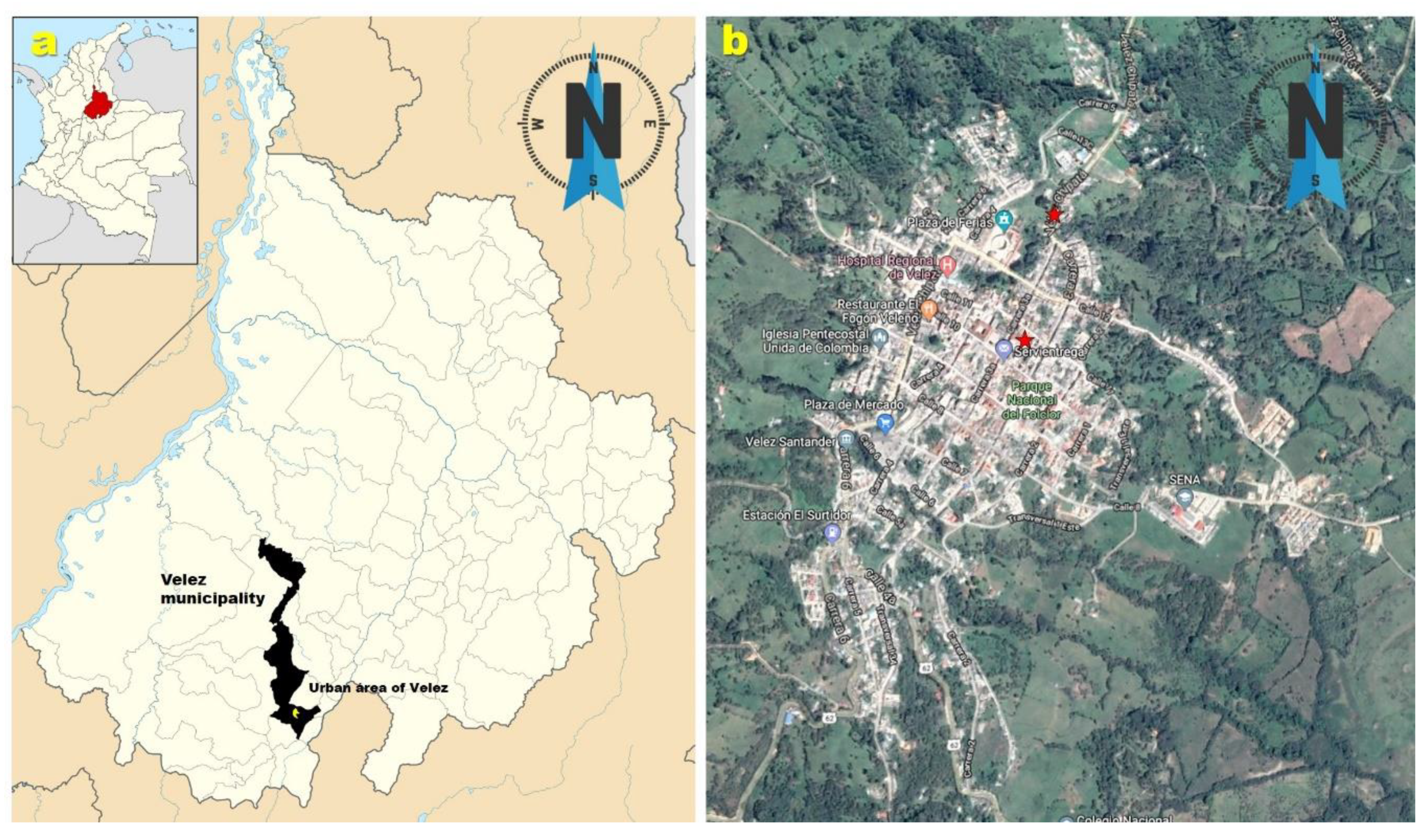
3.1. Field Occurrence
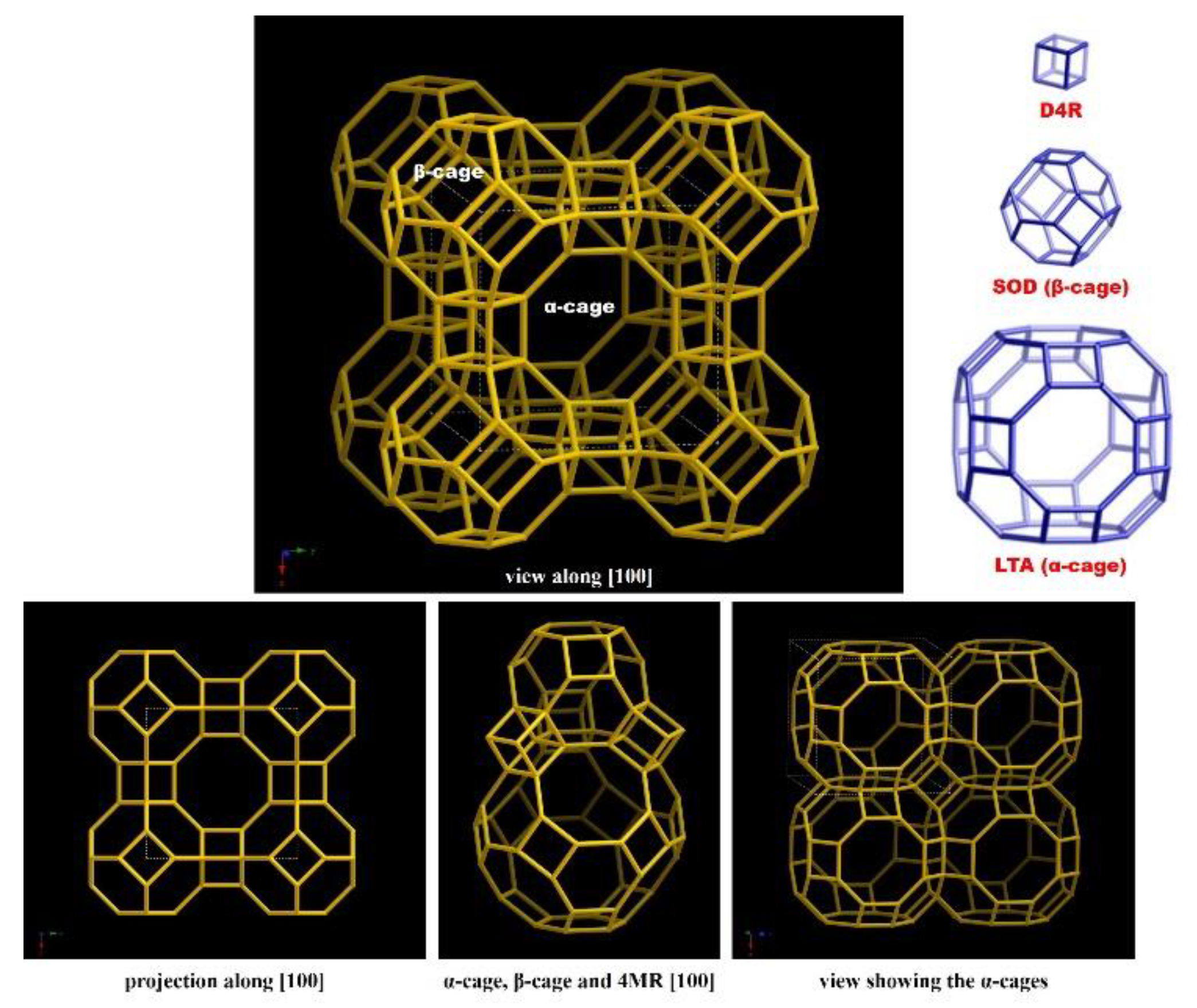
3.2. Framework of Zeolite LTA
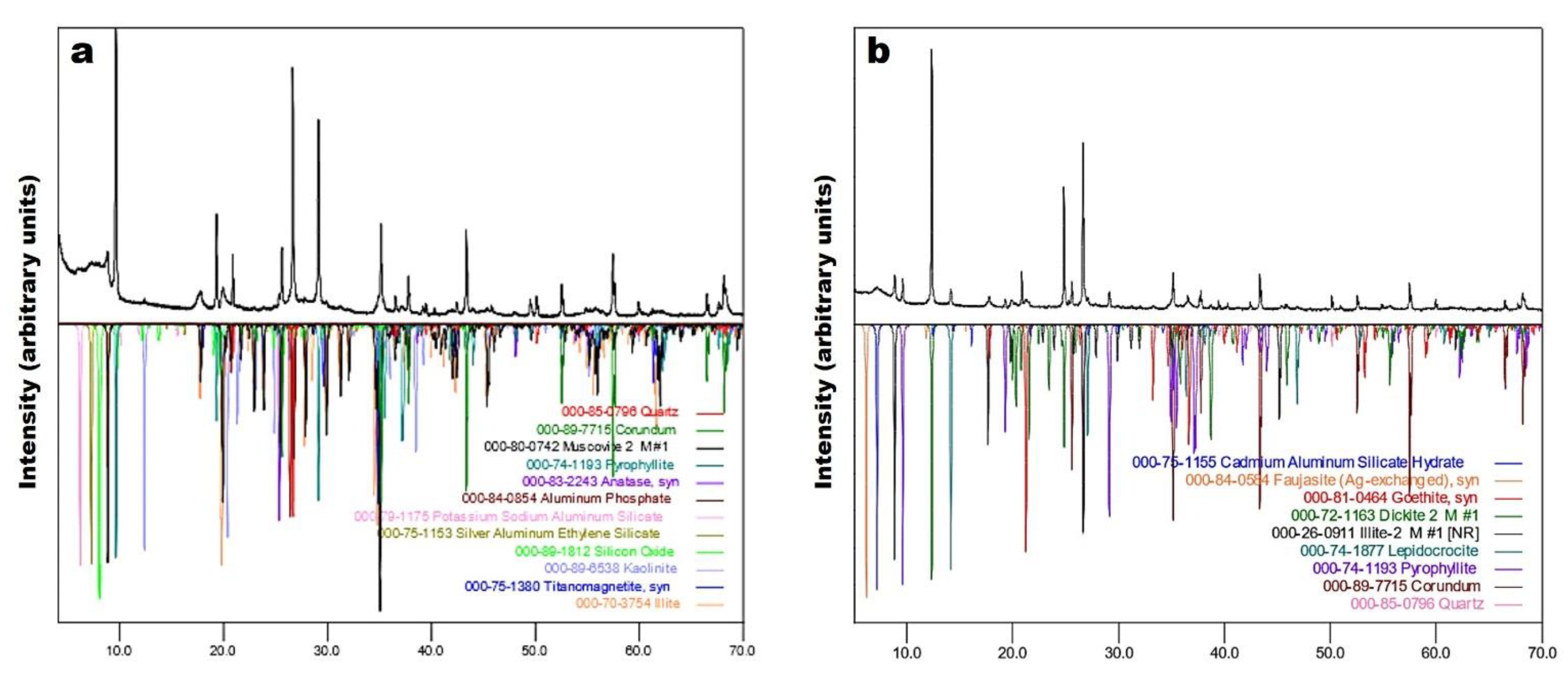
3.3. X-Ray Diffraction
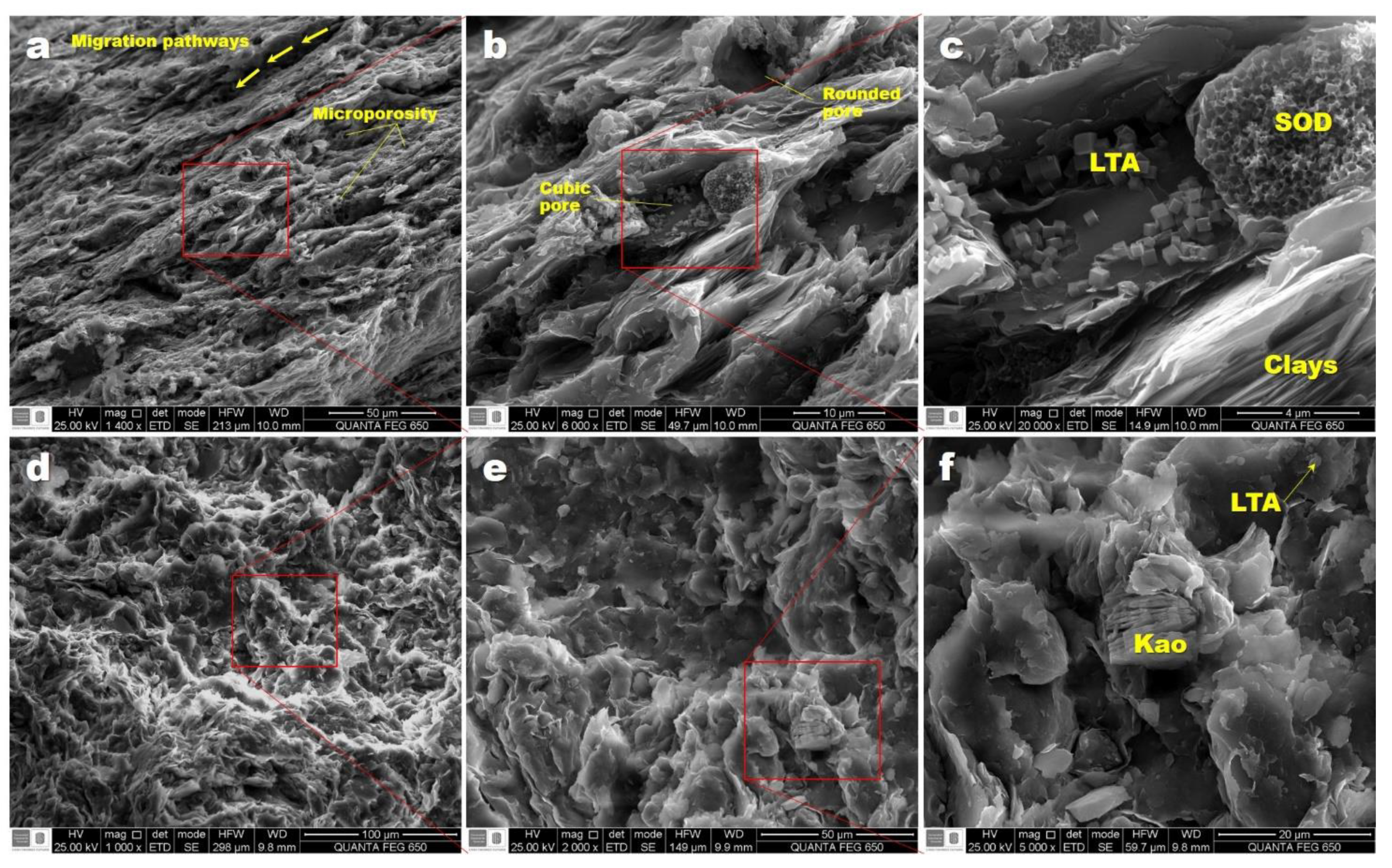
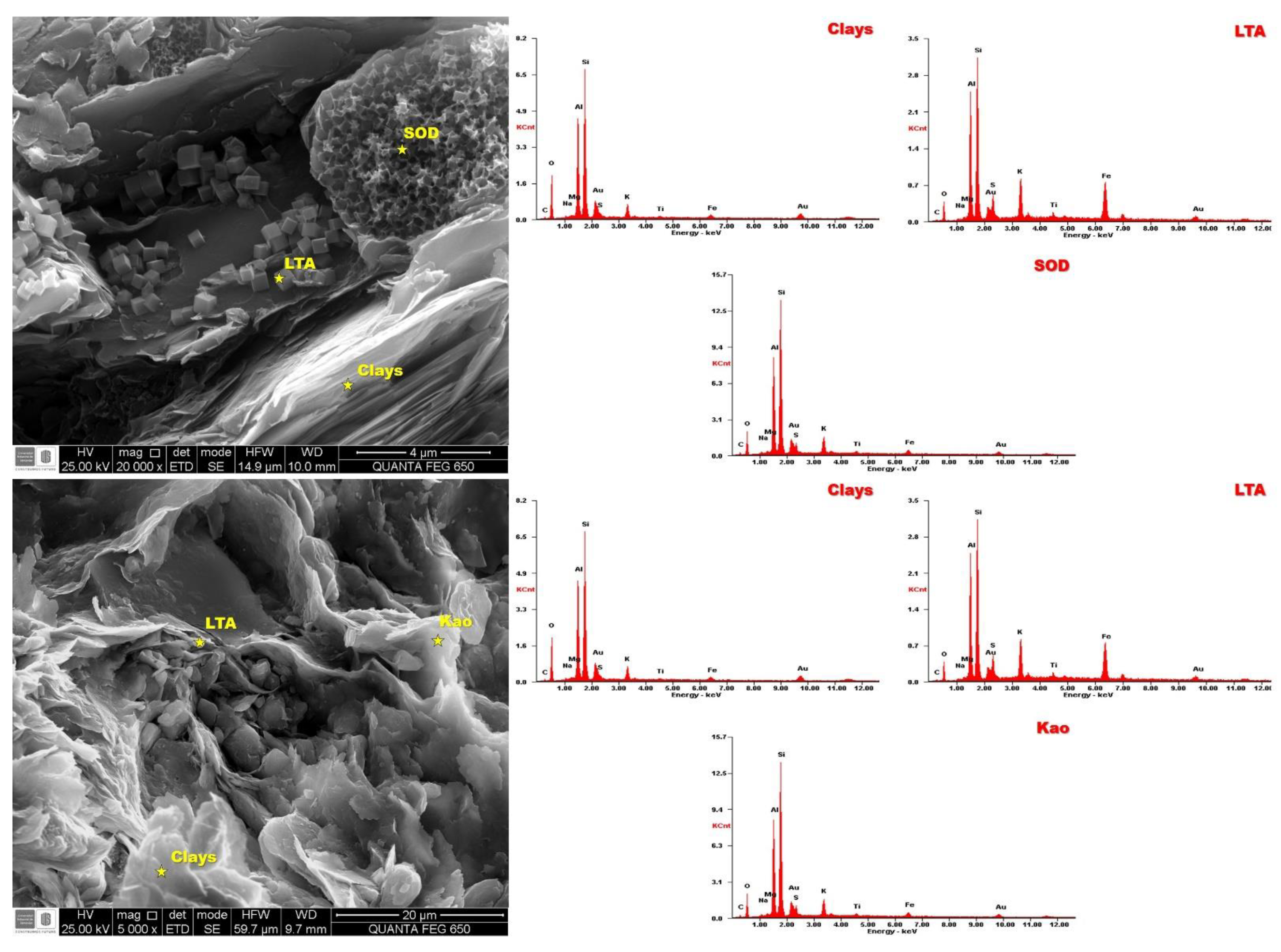
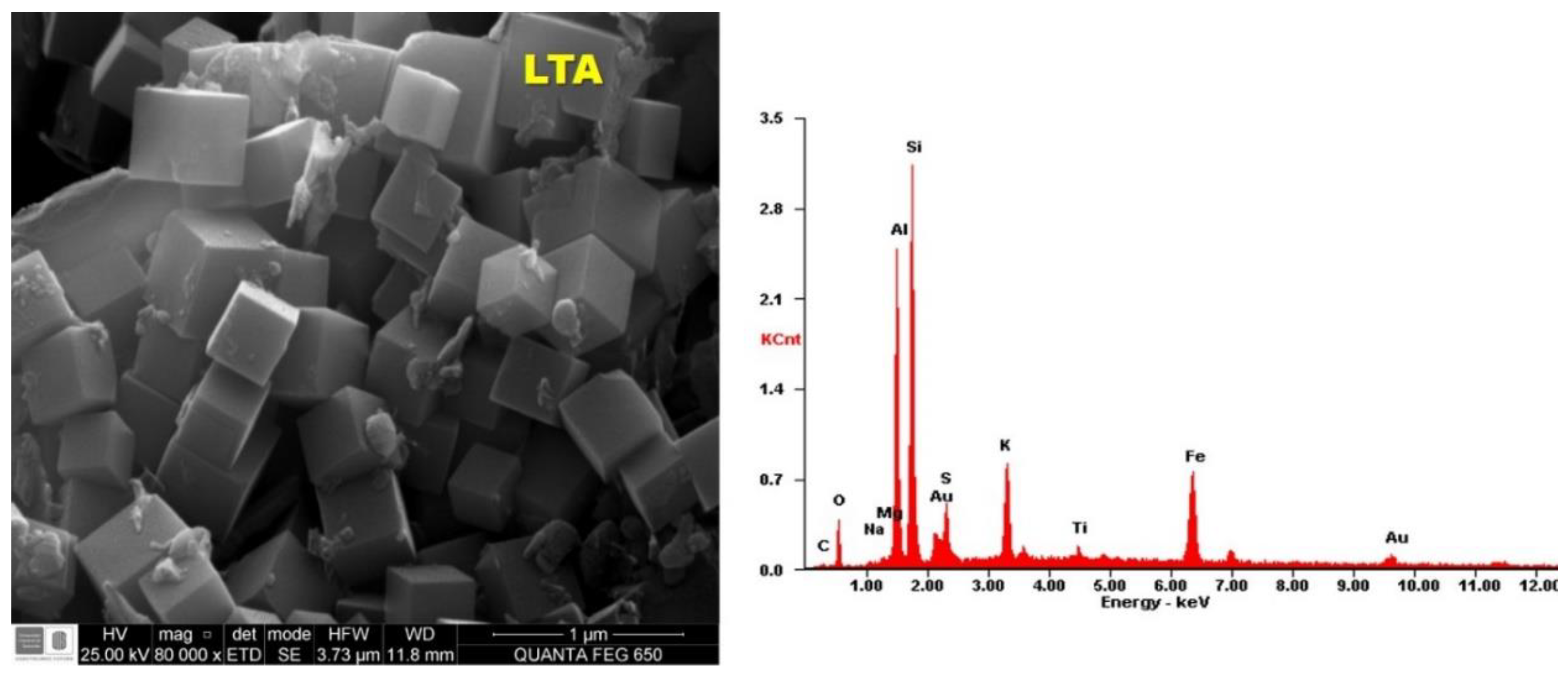
3.4. Scanning Electron Microscopy
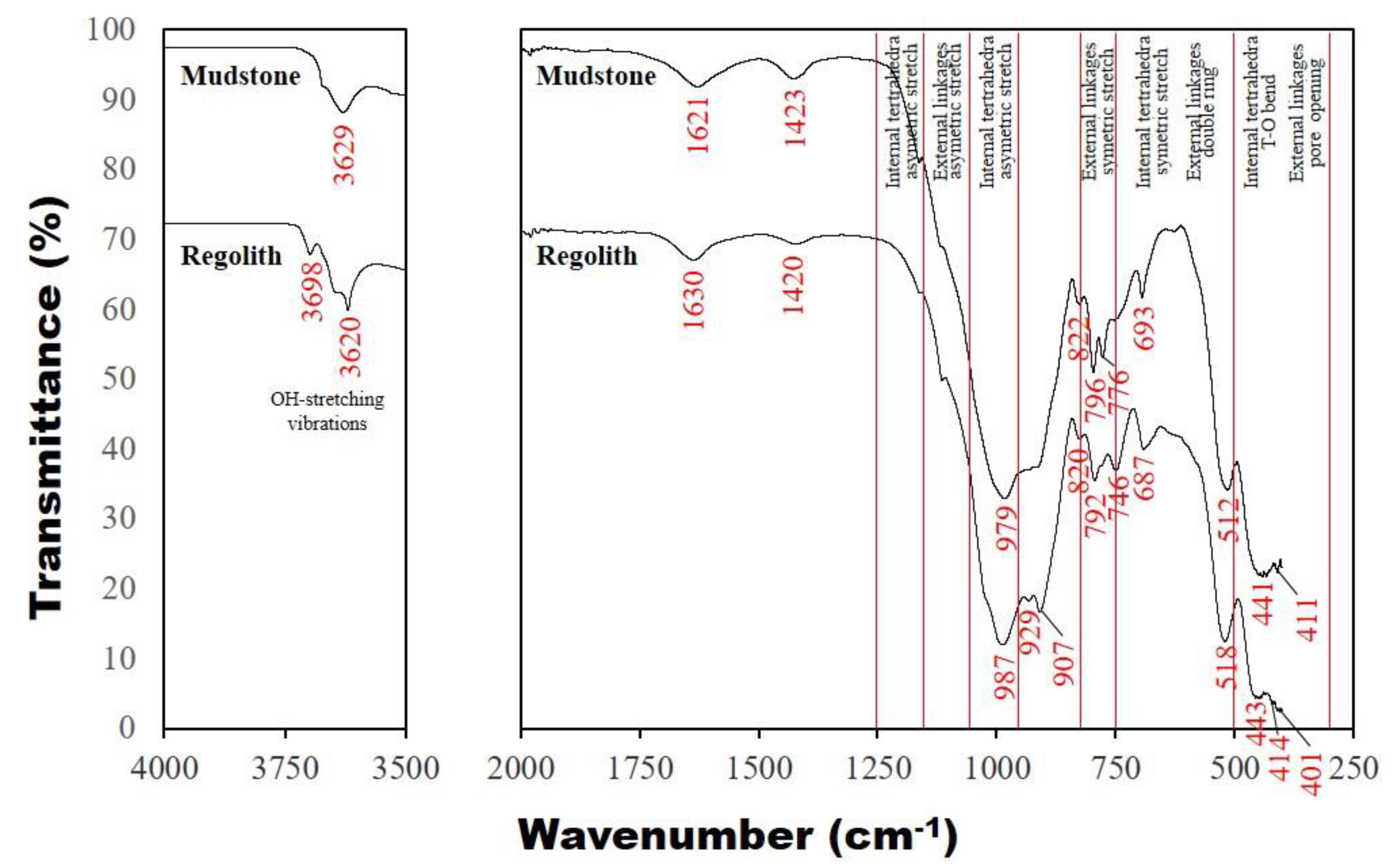
3.5. Fourier Transform Infrared with Attenuated Total Reflection Spectroscopy
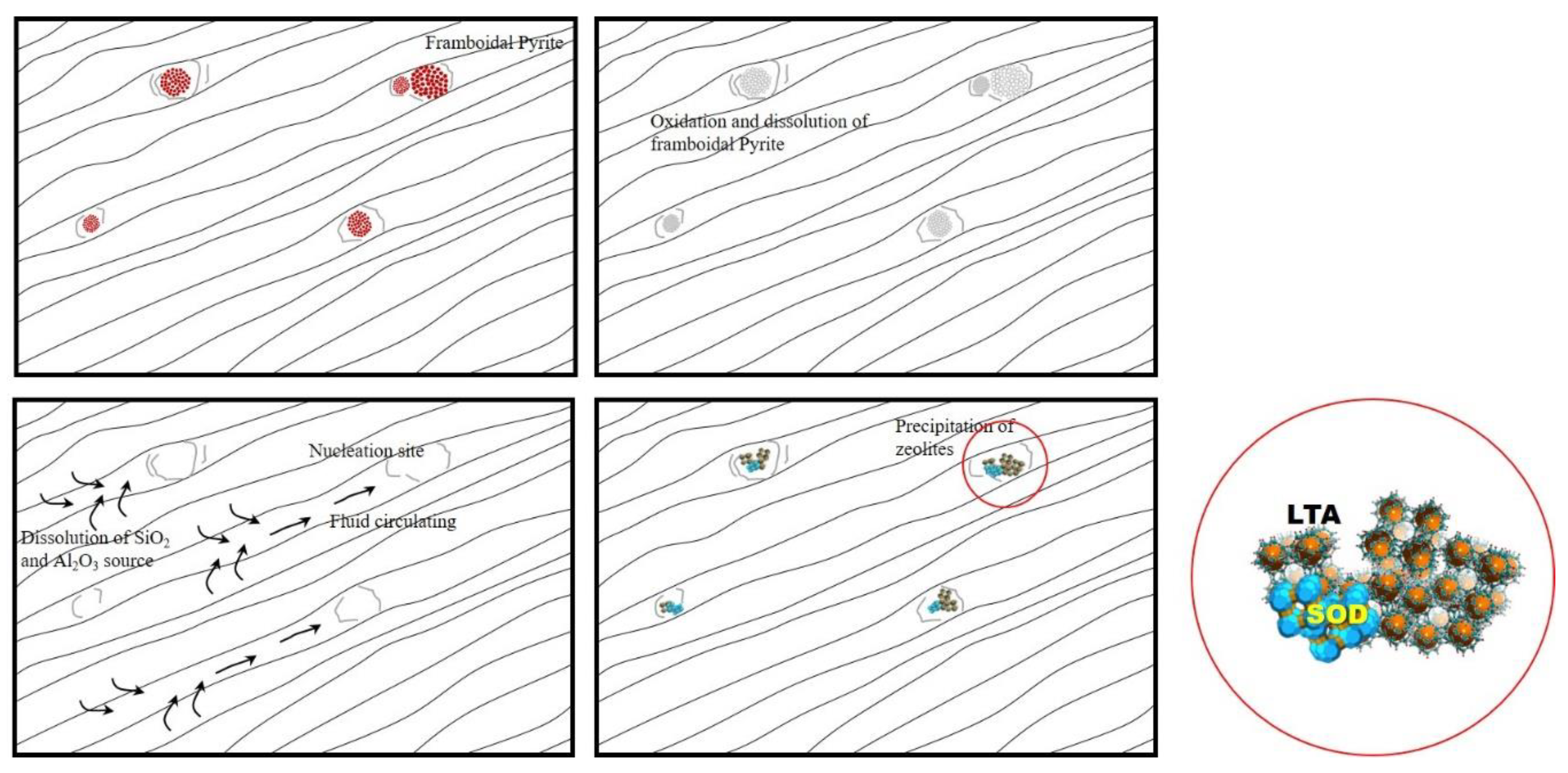
3.6. Discussion on the Mechanism of Formation of the Na–A Zeolite in Mudstone and Regolith
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mumpton, F.A. First reported Occurrence of Zeolites in Sedimentary Rocks of Mexico. Am. Mineral. 1973, 58, 287–290. [Google Scholar]
- Breck, D.W. Zeolite Molecular Sieves: Structure, Chemistry and Use; John Wiley: New York, NY, USA, 1974; 313p. [Google Scholar]
- Gottardi, G.; Galli, E. Natural Zeolites; Springer: Berlin/Heidelberg, Germany, 1985; 409p. [Google Scholar]
- Ming, D.W.; Mumpton, F.A. Zeolites in soils. In Minerals in Soil Environments; Dixon, J.B., Weed, S.B., Eds.; Soil Science Society of America: Madison, WI, USA, 1989; pp. 873–911. [Google Scholar]
- Tsitsishvili, G.; Skhirtladze, N.; Andronikashvili, T.; Tsitsishvili, V.; Dolidze, A. Natural zeolites of Georgia: Occurrences, properties, and application. Prep. Catal. V Sci. Bases Prep. Heterog. Catal. Proc. Fifth Int. Symp. 1999, 125, 715–722. [Google Scholar] [CrossRef]
- Weisenberger, T. Zeolites in fissures of crystalline basement rocks. Ph.D. Thesis, Universitát Freiburg, Freiburg, Germany, 2009. Unpublished. [Google Scholar]
- Boles, J.R.; Coombs, D.S. Zeolite facies alteration of sandstones in the Southland Syncline, New Zealand. Am. J. Sci. 1977, 277, 982–1012. [Google Scholar] [CrossRef]
- Hay, R.L.; Sheppard, R.A. Occurrence of Zeolites in Sedimentary Rocks: An Overview. Rev. Miner. Geochem. 2001, 45, 217–234. [Google Scholar] [CrossRef]
- Langella, A.; Cappelletti, P.; Gennaro, R.D. Zeolites in Closed Hydrologic Systems. Rev. Miner. Geochem. 2001, 45, 235–260. [Google Scholar] [CrossRef]
- Neuhoff, P.S.; Fridriksson, T.; Arnórsson, S. Porosity evolution and mineral paragenesis during low-grade metamorphism of basaltic lavas at Teigarhorn, Eastern Iceland. Am. J. Sci. 1999, 299, 467–501. [Google Scholar] [CrossRef] [Green Version]
- Ballance, P.F.; Waiters, W.A. Hydrothermal alteration, contact metamorphism, and authigenesis in Ferrar Supergroup and Beacon Supergroup rocks, Carapace Nunatak, Allan Hills, and Coombs Hills, Victoria Land, Antarctica. New Zealand J. Geol. Geophys. 2002, 45, 71–84. [Google Scholar] [CrossRef] [Green Version]
- Weisenberger, T.; Selbekk, R.S. Multi-stage zeolite facies mineralization in the Hvalfjördur area, Iceland. Acta Diabetol. 2008, 98, 985–999. [Google Scholar] [CrossRef]
- Walker, G.P.L. Zeolite Zones and Dike Distribution in Relation to the Structure of the Basalts of Eastern Iceland. J. Geol. 1960, 68, 515–528. [Google Scholar] [CrossRef]
- Lagat, J. Hydrothermal alteration mineraology in geotermal fields with case examples from Olkaria Domes Getothermal Field, Kenya. In Proceedings of the Short Course IV on Exploration for Geothermal Resources, organized by UNU-GTP, KenGen and GDC, Lake Naivasha, Kenya, 1–22 November 2009. [Google Scholar]
- Kralj, P.; Rychagov, S.; Kralj, P. Zeolites in volcanic-igneous hydrothermal systems: A case study of Pauzhetka geothermal field (Kamchatka) and Oligocene Smrekovec volcanic complex (Slovenia). Environ. Earth Sci. 2010, 59, 951–956. [Google Scholar] [CrossRef]
- Orlandi, P.; Scortecci, P.B. Minerals of the Elba pegmatites. Mineral. Rec. 1985, 16, 353–364. [Google Scholar]
- Deer, W.A.; Howie, R.A.; Wise, W.S.; Zussman, J. An Introduction to Rock-Forming Minerals; The Geological Society: London, UK, 2004; 696p. [Google Scholar]
- Vincent, M.W.; Ehlig, P.L. Laumontite mineralization in rocks exposed north of San Andreas Fault at Cajon Pass, southern California. Geophys. Res. Lett. 1988, 15, 977–980. [Google Scholar] [CrossRef]
- Weisenberger, T.; Bucher, K. Zeolites in fissures of granites and gneisses of the Central Alps. J. Metamorph. Geol. 2010, 28, 825–847. [Google Scholar] [CrossRef]
- Barrer, R.M. Hydrothermal Chemistry of Zeolites; Academic Press: New York, NY, USA, 1982; 360p. [Google Scholar]
- Szostak, R. Molecular Sieves; Springer: Berlin/Heidelberg, Germany, 1989; p. 359. [Google Scholar]
- Booker, N.A.; Cooney, E.L.; Priestley, A.J. Ammonia removal from sewage using natural Australian zeolite. Water Sci. Technol. 1996, 34, 17–24. [Google Scholar] [CrossRef]
- Dixit, L.; Prasada, T.S.R. New approach to acid catalysis and hydrocarbon—Zeolite interactions. Stud. Surf. Sci. Catal. 1998, 113, 313–319. [Google Scholar]
- Loiola, A.; Andrade, J.; Sasaki, J.; da Silva, L. Structural analysis of zeolite NaA synthesized by a cost-effective hydrothermal method using kaolin and its use as water softener. J. Colloid Interface Sci. 2012, 367, 34–39. [Google Scholar] [CrossRef] [Green Version]
- Nuić, I.; Trgo, M.; Medvidović, N.V. The application of the packed bed reactor theory to Pb and Zn uptake from the binary solution onto the fixed bed of natural zeolite. Chem. Eng. J. 2016, 295, 347–357. [Google Scholar] [CrossRef]
- Liu, G.-H.; Wang, Y.; Zhang, Y.; Xu, X.; Qi, L.; Wang, H. Modification of natural zeolite and its application to advanced recovery of organic matter from an ultra-short-SRT activated sludge process effluent. Sci. Total. Environ. 2019, 652, 1366–1374. [Google Scholar] [CrossRef]
- Pinto, G.C.; Ríos, C.A.; Vargas, L.Y. Comparative study on the use of a natural and modified Ecuadorian zeolites of the Cayo Formation on the remediation of an oil-polluted soil. Ctf–Cienc. Tecnol. Y Futuro 2019, 9, 93–104. [Google Scholar] [CrossRef]
- Tran, Y.T.; Lee, J.; Kumar, P.; Kim, K.-H.; Lee, S.S. Natural zeolite and its application in concrete composite production. Compos. Part. B Eng. 2019, 165, 354–364. [Google Scholar] [CrossRef]
- Vargas, A.M.; Cipagauta, C.C.; Molina, D.R.; Ríos, C.A. A comparative study on diclofenac sodium release from surfactant-modified natural zeolites as a pharmaceutical excipient. Mater. Chem. Phys. 2020, 256, 123644. [Google Scholar] [CrossRef]
- Alabbad, E.A. Efficacy assessment of natural zeolite containing wastewater on the adsorption behaviour of Direct Yellow 50 from; equilibrium, kinetics and thermodynamic studies. Arab. J. Chem. 2021, 14, 103041. [Google Scholar] [CrossRef]
- Rey, V.; Ríos, C.; Vargas, L.; Valente, T. Use of natural zeolite-rich tuff and siliceous sand for mine water treatment from abandoned gold mine tailings. J. Geochem. Explor. 2021, 220, 106660. [Google Scholar] [CrossRef]
- Vogt, E.T.C.; Weckhuysen, B.M. Fluid catalytic cracking: Recent developments on the grand old lady of zeolite catalysis. Chem. Soc. Rev. 2015, 44, 7342–7370. [Google Scholar] [CrossRef] [Green Version]
- Suganuma, S.; Katada, N. Innovation of catalytic technology for upgrading of crude oil in petroleum refinery. Fuel Process. Technol. 2020, 208, 106518. [Google Scholar] [CrossRef]
- Cheng, X.-W.; Wang, J.; Huang, Q.; Long, Y.-C. Modified natural STI zeolite–A potentially useful molecular sieve. Pharmacogenetics 2007, 170, 2080–2085. [Google Scholar] [CrossRef]
- Pitcher, S.; Slade, R.; Ward, N. Heavy metal removal from motorway stormwater using zeolites. Sci. Total. Environ. 2004, 334, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Santos, S.; Machado, R.; Correia, M.J.N.; Carvalho, J.R. Treatment of acid mining waters. Miner. Eng. 2004, 17, 225–232. [Google Scholar] [CrossRef]
- Ríos, C.A. Synthesis of Zeolites from Geological Materials and Industrial Wastes for Potential Application in Environmental Problems. Ph.D. Thesis, University of Wolverhampton, Wolverhampton, UK, 2008. [Google Scholar]
- Wang, S.; Peng, Y. Natural zeolites as effective adsorbents in water and wastewater treatment. Chem. Eng. J. 2010, 156, 11–24. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, W.; Zhang, F.; Low, Z.-X.; Zhong, Z.; Xing, W. Preparation of highly stable porous SiC membrane supports with enhanced air purification performance by recycling NaA zeolite residue. J. Membr. Sci. 2017, 541, 500–509. [Google Scholar] [CrossRef]
- Marinin, D.V.; Brown, G.N. Studies of sorbent/ion-exchange materials for the removal of radioactive strontium from liquid radioactive waste and high hardness groundwaters. Waste Manag. 2000, 20, 545–553. [Google Scholar] [CrossRef]
- Fujii, S.; Horie, N.; Nakaibayashi, K.; Kanematsu, Y.; Kikuchi, Y.; Nakagaki, T. Design of zeolite boiler in thermochemical energy storage and transport system utilizing unused heat from sugar mill. Appl. Energy 2019, 238, 561–571. [Google Scholar] [CrossRef]
- Cardoso, A.M.; Horn, M.B.; Ferret, L.S.; Azevedo, C.M.; Pires, M. Integrated synthesis of zeolites 4A and Na–P1 using coal fly ash for application in the formulation of detergents and swine wastewater treatment. J. Hazard. Mater. 2015, 287, 69–77. [Google Scholar] [CrossRef]
- Hrenovic, J.; Milenkovic, J.; Ivankovic, T.; Rajic, N. Antibacterial activity of heavy metal-loaded natural zeolite. J. Hazard. Mater. 2012, 201–202, 260–264. [Google Scholar] [CrossRef]
- Faras, T.; Ruiz-Salvador, A.R.; Rivera, A. Interaction studies between drugs and a purified natural clinoptilolite. Microporous Mesoporous Mater. 2003, 61, 117–125. [Google Scholar] [CrossRef]
- Cerri, G.; Farina, M.; Brundu, A.; Daković, A.; Giunchedi, P.; Gavini, E.; Rassu, G. Natural zeolites for pharmaceutical formulations: Preparation and evaluation of a clinoptilolite-based material. Microporous Mesoporous Mater. 2016, 223, 58–67. [Google Scholar] [CrossRef]
- Papaioannou, D.; Katsoulos, P.; Panousis, N.; Karatzias, H. The role of natural and synthetic zeolites as feed additives on the prevention and/or the treatment of certain farm animal diseases: A review. Microporous Mesoporous Mater. 2005, 84, 161–170. [Google Scholar] [CrossRef]
- Rouquerol, J.; Avnir, D.; Fairbridge, C.W.; Everett, D.H.; Haynes, J.M.; Pernicone, N.; Ramsay, J.D.F.; Sing, K.S.W.; Unger, K.K. Recommendations for the characterization of porous solids (Technical Report). Pure Appl. Chem. 1994, 66, 1739–1758. [Google Scholar] [CrossRef]
- Google Maps. 2018. Available online: https://www.google.com/maps/?hl=es (accessed on 31 October 2018).
- Reyes-Mendoza, G.A. Evaluación detallada e impacto de rocas lodosas meteorizadas. El caso de Vélez (Santander, Colombia). In Póster y Resumen, en Memorias de la XIII Semana Técnica de Geología, Ingeniería Geológica y Geociencias; Universidad de Caldas: Manizales, Colombia, 2018; 3p. [Google Scholar]
- Lazar, O.R.; Bohacs, K.M.; Macquaker, J.H.S.; Schieber, J.; Demko, T.M. Capturing Key Attributes of Fine-Grained Sedimentary Rocks in Outcrops, Cores, and Thin Sections: Nomenclature and Description Guidelines. J. Sediment. Res. 2015, 85, 230–246. [Google Scholar] [CrossRef] [Green Version]
- Macquaker, J.H.; Adams, A. Maximizing Information from Fine-Grained Sedimentary Rocks: An Inclusive Nomenclature for Mudstones. J. Sediment. Res. 2003, 73, 735–744. [Google Scholar] [CrossRef]
- Reyes-Mendoza, G.A. Las Rocas Lodosas de Vélez: De la Meteorización a Los Deslizamientos; Ponencia oral. Fest Station—UIS Inteligente. Seminario U18 Fest—Ideas para transformar El Mundo; Universidad Industrial de Santander: Bucaramanga, Colombia, 23 September 2019. [Google Scholar]
- Baerlocher, C.; McCusker, L. Database of Zeolite Structures. 2020. Available online: http://www.iza-structure.org/databases/ (accessed on 15 April 2020).
- Schüring, A.; Auerbach, S.M.; Fritzsche, S.; Haberlandt, R. On entropic barriers for diffusion in zeolites: A molecular dynamics study. J. Chem. Phys. 2002, 116, 10890–10894. [Google Scholar] [CrossRef] [Green Version]
- García-Soto, A.R.; Rodríguez-Niño, G.; Trujillo, C.A. Zeolite LTA synthesis: Optimising synthesis conditions by using the modified sequential simplex method Síntesis de Zeolita LTA: Optimización de las condiciones de síntesis, usando el método simplex secuencial modificado. Ing. E Investig. 2013, 33, 22–27. [Google Scholar]
- García, A.L.; López, C.M.; Garcia, L.V.; De Goldwasser, M.R.; Casanova, J.D.C. Improvements in the synthesis of zeolites with low Si/Al ratio from Venezuelan sodium silicate for an environmentally friendly process. Ing. E Investig. 2016, 36, 62–69. [Google Scholar] [CrossRef]
- Heller-Kallai, L.; Lapides, I. Reactions of kaolinites and metakaolinites with NaOH—comparison of different samples (Part 1). Appl. Clay Sci. 2007, 35, 99–107. [Google Scholar] [CrossRef]
- Ríos, C.A.; Williams, C.D.; Fullen, M.A. Nucleation and growth history of zeolite LTA as-synthesized from kaolinite by two different methods. Appl. Clay Sci. 2009, 42, 446–454. [Google Scholar] [CrossRef]
- Flaningen, E.M.; Khatami, H.A.; Szymanski, H.A. Infrared Structural Studies of Zeolite Frameworks. In Molecular Sieve Zeolites, Advances in Chemistry 101; Flanigen, E.M., Sand, L.B., Eds.; American Chemical Society: Washington, DC, USA, 1971; pp. 201–229. [Google Scholar]
- Barnes, M.C.; Addai-Mensah, J.; Gerson, A.R. A methodology for quantifying sodalite and cancrinite phase mixtures and the kinetics of the sodalite to cancrinite phase transformation. Microporous Mesoporous Mater. 1999, 31, 303–319. [Google Scholar] [CrossRef]
- Markovic, S.; Dondur, V.; Dimitrijevic, R. FTIR spectroscopy of framework aluminosilicate structures: Carnegieite and pure sodium nepheline. J. Mol. Struct. 2003, 654, 223–234. [Google Scholar] [CrossRef]
- Byrappa, K.; Suresh-Kumar, B.V. Characterization of Zeolites by Infrared Spectroscopy. Asian J. Chem. 2007, 19, 4933–4935. [Google Scholar]
- Ríos, C.A.; Williams, C.D.; Castellanos, O.M. Nucleation and growth mechanism of sodalite and cancrinite from kaolinite-rich clay under low-temperature hydrothermal conditions. Mater. Res. 2013, 16, 424–438. [Google Scholar]
- Zhao, H.; Deng, Y.; Harsh, J.B.; Flury, M.; Boyle, J.S. Alteration of kaolinite to cancrinite and sodalite by simulated hanford tank waste and its impact on cesium retention. Clays Clay Miner. 2004, 52, 1–13. [Google Scholar] [CrossRef]
- Forero-Onofre, H.; Sarmiento-Rojas, L. La Facies Evaporítica de la Formación Paja; Etayo, F., Laverde, F., Eds.; Proyecto Cretácico; Ingeominas: Bogota, Colombia, 1985; Volume 16, pp. 1–16.
- Rivera, H.A.; Le Roux, J.P.; Sánchez, L.K.; Mariño-Martínez, J.E.; Salazar, C.; Barragán, J.C. Palaeoredox conditions and sequence stratigraphy of the Cretaceous storm-dominated, mixed siliciclastic-carbonate ramp in the Eastern Cordillera Basin (Colombia): Evidence from sedimentary geochemical proxies and facies analysis. Sediment. Geol. 2018, 372, 1–24. [Google Scholar] [CrossRef]
- Passaglia, E.; Vezzalini, G.; Carnevali, R. Diagenetic chabazites and phillipsites in Italy: Crystal chemistry and genesis. Eur. J. Miner. 1990, 2, 827–840. [Google Scholar] [CrossRef]
- Dickinson, W.W.; Grapes, R.H. Authigenic chabazite and implications for weathering in Sirius Group Diamictite, Table Mountain, Dry Valleys, Antartica. J. Seidmentary Res. 1997, 67, 815–820. [Google Scholar]
- Cripps, J.C.; Taylor, R.K. The engineering properties of mudrocks. Q. J. Eng. Geol. Hydrogeol. 1981, 14, 325–346. [Google Scholar] [CrossRef]
- Nicholson, V.R.; Gillham, W.R.; Reardon, J.E. Pyrite oxidation in carbonate-buffered solution: Experimental kinetics. Geochim. Et Cosmochim. Acta 1988, 52, 1077–1085. [Google Scholar] [CrossRef]
- Nicholson, R.V.; Gillham, R.W.; Reardon, E.J. Pyrite oxidation in carbonate-buffered solution: Rate control by oxide coatings. Geochim. Et Cosmochim. Acta 1990, 54, 395–402. [Google Scholar] [CrossRef]
- Chaudhri, A.R.; Mahavir, S. Clay Minerals as Climate Change Indicators—A Case Study. Am. J. Clim. Change 2012, 1, 231–239. [Google Scholar] [CrossRef] [Green Version]
- Ruiz, R.M.; Blanco, C.G.; Pesquera, C.; González, F.; Benito, I.; López, J. Zeolitization of a bentonite and its application to the removal of ammonium ion from waste water. Appl. Clay Sci. 1997, 12, 73–83. [Google Scholar] [CrossRef]
- Baccouche, A.; Srasra, E.; El Maaoui, M. Preparation of Na–P1 and sodalite octahydrate zeolites from interstratified illite–smectite. Appl. Clay Sci. 1998, 13, 255–273. [Google Scholar] [CrossRef]
- Cañizares, P.; Duran, A.; Dorado, F.; Carmona, M. The role of sodium montmorillonite on bounded zeolite-type catalysts. Appl. Clay Sci. 2000, 16, 273–287. [Google Scholar] [CrossRef]
- Gualtieri, A.F. Synthesis of sodium zeolites from a natural halloysite. Phys. Chem. Miner. 2001, 28, 719–728. [Google Scholar] [CrossRef]
- Boukadir, D.; Bettahar, N.; Derriche, Z. Synthesis of zeolites 4A and HS from natural materials. Ann. De Chim. Sci. Des. Mater. 2002, 27, 1–13. [Google Scholar] [CrossRef]
- Shawabkeh, R.; Al-Harahsheh, A.; Hami, M.; Khlaifat, A. Conversion of oil shale ash into zeolite for cadmium and lead removal from wastewater. Fuel 2004, 83, 981–985. [Google Scholar] [CrossRef]
- Shawabkeh, R.; Al-Harahsheh, A.; Al-Otoom, A. Production of zeolite from Jordanian oil shale ash and application for zinc removal from wastewater. Oil Shale 2004, 21, 125–136. [Google Scholar]
- Fernandes-Machado, N.R.C.; Miotto-Bigatão, D.M.M. Synthesis of Na–A and –X zeolites from oil shale ash. Fuel 2005, 84, 2289–2294. [Google Scholar] [CrossRef]
- Fernandes-Machado, N.R.C.; Miotto-Bigatão, D.M.M. Use of zeolites synthesized from oil shale ash for arsenic removal from polluted wáter. Química Nova 2007, 30, 1108–1114. [Google Scholar] [CrossRef]
- Abdmeziem-Hamoudi, K.; Siffert, B. Synthesis of molecular sieve zeolites from a smectite-type clay material. Appl. Clay Sci. 1989, 4, 1–9. [Google Scholar] [CrossRef]
- Kovo, A.S.; Hernandez, O.; Holmes, S.M. Synthesis and characterization of zeolite Y and ZSM-5 from Nigerian Ahoko Kaolin using a novel, lower temperature, metakaolinization technique. J. Mater. Chem. 2009, 19, 6207–6212. [Google Scholar] [CrossRef]
- Hamadi, A.; Nabih, K. Alkali Activation of Oil Shale Ash Based Ceramics. E-J. Chem. 2012, 9, 1373–1388. [Google Scholar] [CrossRef]
- Ngoc, D.T.; Pham, T.H.; Nguyen, K.D.H. Synthesis, characterization and application of nanozeolite NaX from Vietnamese kaolin. Adv. Nat. Sci. Nanosci. Nanotechnol. 2013, 4, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Bao, W.W.; Zou, H.F.; Gan, S.C.; Xu, X.C.; Ji, G.J.; Zheng, K.Y. Adsorption of heavy metal ions from aqueous solutions by zeolite based on oil shale ash: Kinetic and equilibrium studies. Chem. Res. Chin. Univ. 2013, 29, 126–131. [Google Scholar] [CrossRef]
- Zhou, Z.; Jin, G.; Liu, H.; Wu, J.; Mei, J. Crystallization mechanism of zeolite A from coal kaolin using a two-step method. Appl. Clay Sci. 2014, 97–98, 110–114. [Google Scholar] [CrossRef]
- Tong, L.; Luo, H.; Zhang, L.; Zhan, H. Preparation of single phase Na–X zeolite from oil shale ash by melting hydrothermal method. China’s Refract. 2014, 23, 12–17. [Google Scholar]
- Doyle, A.M.; Alismaeel, Z.T.; Albayati, T.M.; Abbas, A.S. High purity FAU-type zeolite catalysts from shale rock for biodiesel production. Fuel 2017, 199, 394–402. [Google Scholar] [CrossRef]
- Bai, S.-X.; Zhou, L.-M.; Chang, Z.-B.; Zhang, C.; Chu, M. Synthesis of Na–X zeolite from Longkou oil shale ash by alkaline fusion hydrothermal method. Carbon Resour. Convers. 2018, 1, 245–250. [Google Scholar] [CrossRef]
- Akolekar, D.; Chaffee, A.; Howe, R.F. The transformation of kaolin to low-silica X zeolite. Zeolites 1997, 19, 359–365. [Google Scholar] [CrossRef]
- Xu, M.; Cheng, M.; Tan, D.; Liu, X.; Bao, X. Growth of zeolite KSO1 on calcined kaolin microspheres. J. Mater. Chem. 1999, 9, 2965–2966. [Google Scholar] [CrossRef]
- Eze, K.A.; Nwadiogbu, J.O.; Nwankwere, E.T. Effect of Acid Treatments on the Physicochemical Properties of Kaolin Clay. Arch. Appl. Sci. Res. 2012, 4, 792–794. [Google Scholar]
- Murat, M.; Amokrane, A.; Bastide, J.P.; Montanaro, L. Synthesis of zeolites from thermally activated kaolinite. Some observations on nucleation and growth. Clay Miner. 1992, 27, 119–130. [Google Scholar] [CrossRef]
- Chandrasekhar, S.; Pramada, P.N. Investigation on the Synthesis of Zeolite NaX from Kerala Kaolin. J. Porous Mater. 1999, 6, 283–297. [Google Scholar] [CrossRef]
- Novembre, D.; Di Sabatino, B.; Gimeno, D.; Pace, C.; Sabatino, D. Synthesis and characterization of Na–X, Na–A and Na–P zeolites and hydroxysodalite from metakaolinite. Clay Miner. 2011, 46, 339–354. [Google Scholar] [CrossRef]
- Sanhueza, V.; Kelm, U.; Cid, R. Synthesis of molecular sieves from Chilean kaolinites: Synthesis of NaA type zeolites. J. Chem. Technol. Biotechnol. 1999, 74, 358–363. [Google Scholar] [CrossRef]
- Belviso, C.; Cavalcante, F.; Lettino, A.; Fiore, S. A and X-type zeolites synthesised from kaolinite at low temperatura. Appl. Clay Sci. 2013, 80–81, 162–168. [Google Scholar] [CrossRef]
- Basaldella, E.I.; Kikot, A.; Tara, J.C. Effect of pellet pore size and synthesis conditions in the in situ synthesis of LSX zeolite. Ind. Eng. Chem. Res. 1995, 34, 2990–2996. [Google Scholar] [CrossRef]
- Covarrubias, C.; García, R.; Arriagada, R.; Yánez, J.; Garland, M.T. Cr(III) exchange on zeolites obtained from kaolin and natural mordenite. Microporous Mesoporous Mater. 2006, 88, 220–231. [Google Scholar] [CrossRef]
- Chorover, J.; Choi, S.; Amistadi, M.K.; Karthikeyan, K.G.; Crosson, G.; Mueller, K.T. Linking Cesium and Strontium Uptake to Kaolinite Weathering in Simulated Tank Waste Leachate. Environ. Sci. Technol. 2003, 37, 2200–2208. [Google Scholar] [CrossRef]
- Buhl, J.C.; Löns, J. Synthesis and crystal structure of nitrate enclathrated sodalite Na8[AlSiO4]6(NO3)2. J. Alloy. Compd. 1996, 235, 41–47. [Google Scholar] [CrossRef]
- Healey, A.; Johnson, G.; Weller, M. The synthesis and characterization of JBW-type zeolites. Microporous Mesoporous Mater. 2000, 37, 153–163. [Google Scholar] [CrossRef]
- Lin, D.-C.; Xu, X.-W.; Zuo, F.; Long, Y.-C. Crystallization of JBW, CAN, SOD and ABW type zeolite from transformation of metakaolin. Microporous Mesoporous Mater. 2004, 70, 63–70. [Google Scholar] [CrossRef]
- Ríos, C.A.; Williams, C.D.; Castellanos, O.M. Synthesis and characterization of zeolites by alkaline activation of kaolinite and industrial by-products (fly ash and natural clinker). Bistua 2006, 4, 60–71. [Google Scholar]
- Ríos, C.A.; Williams, C.D.; Maple, M. Synthesis of zeolites and zeotypes by hydrothermal transformation of kaolinite and metakaolinite. Bistua 2007, 5, 15–26. [Google Scholar]
- Ríos, C.A.; Williams, C.D.; Castellanos, O.M. Synthesis of zeolite LTA from thermally treated kaolinite. Rev. Fac. Ing. 2010, 53, 30–41. [Google Scholar]
- Ríos, C.A.; Williams, C.D. Hydrothermal transformation of kaolinite in the system K2O-SiO2-Al2O3-H2O. DYNA Univ. Nac. Colomb. Medellin 2010, 77, 55–63. [Google Scholar]
- Ríos, C.A.; Williams, C.D.; Roberts, C.L. Synthesis and characterisation of SOD-, CAN- and JBW-type structures by hydrothermal reaction of kaolinite at 200 °C. DYNA Univ. Nac. Colomb. Medellin 2011, 78, 38–47. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ríos-Reyes, C.A.; Reyes-Mendoza, G.A.; Henao-Martínez, J.A.; Williams, C.; Dyer, A. First Report on the Geologic Occurrence of Natural Na–A Zeolite and Associated Minerals in Cretaceous Mudstones of the Paja Formation of Vélez (Santander), Colombia. Crystals 2021, 11, 218. https://doi.org/10.3390/cryst11020218
Ríos-Reyes CA, Reyes-Mendoza GA, Henao-Martínez JA, Williams C, Dyer A. First Report on the Geologic Occurrence of Natural Na–A Zeolite and Associated Minerals in Cretaceous Mudstones of the Paja Formation of Vélez (Santander), Colombia. Crystals. 2021; 11(2):218. https://doi.org/10.3390/cryst11020218
Chicago/Turabian StyleRíos-Reyes, Carlos Alberto, German Alfonso Reyes-Mendoza, José Antonio Henao-Martínez, Craig Williams, and Alan Dyer. 2021. "First Report on the Geologic Occurrence of Natural Na–A Zeolite and Associated Minerals in Cretaceous Mudstones of the Paja Formation of Vélez (Santander), Colombia" Crystals 11, no. 2: 218. https://doi.org/10.3390/cryst11020218
APA StyleRíos-Reyes, C. A., Reyes-Mendoza, G. A., Henao-Martínez, J. A., Williams, C., & Dyer, A. (2021). First Report on the Geologic Occurrence of Natural Na–A Zeolite and Associated Minerals in Cretaceous Mudstones of the Paja Formation of Vélez (Santander), Colombia. Crystals, 11(2), 218. https://doi.org/10.3390/cryst11020218







