Effect of Fe on the Microstructure and Mechanical Properties of Fe/FeAl2O4 Cermet Prepared by Hot Press Sintering
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
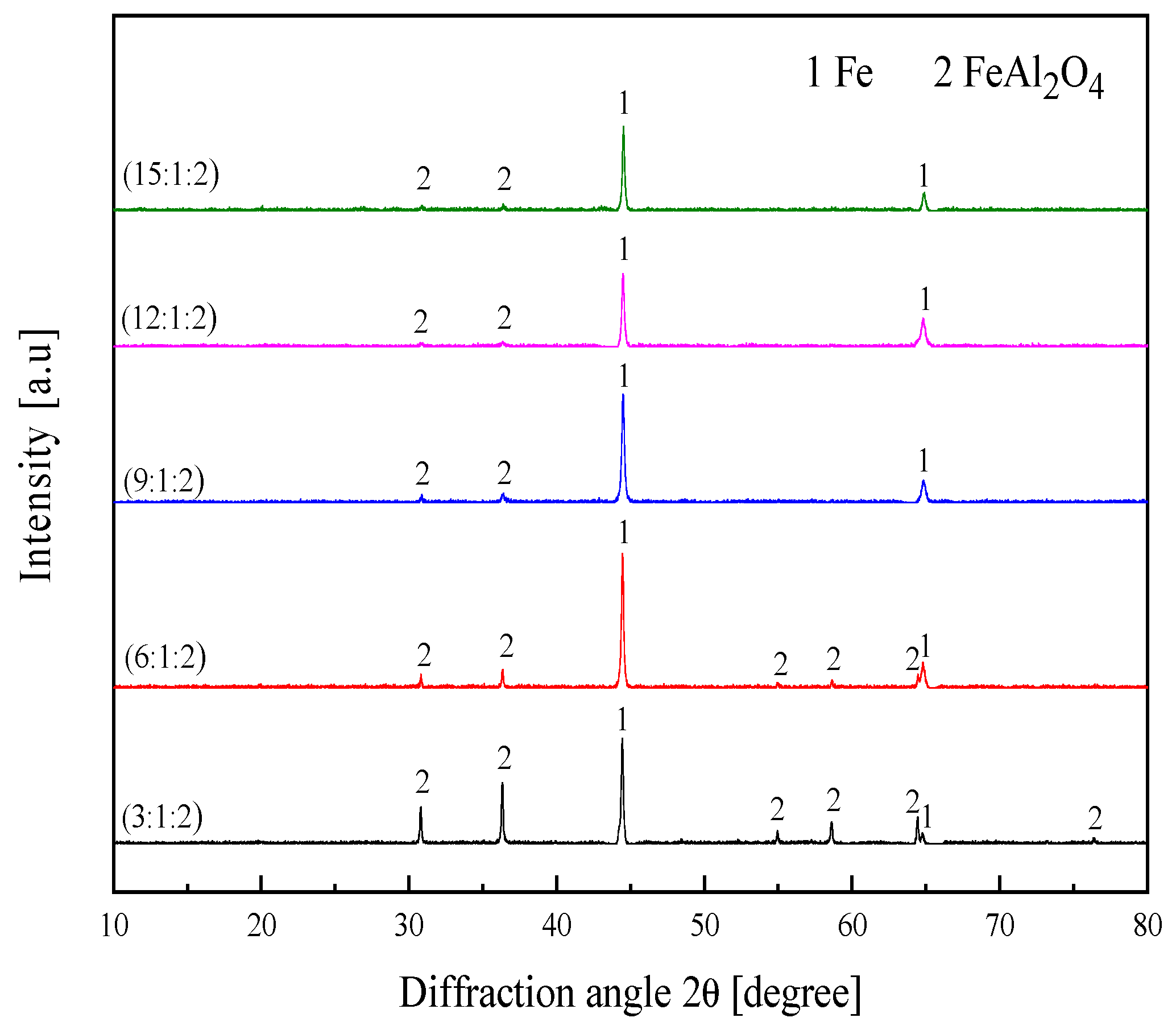
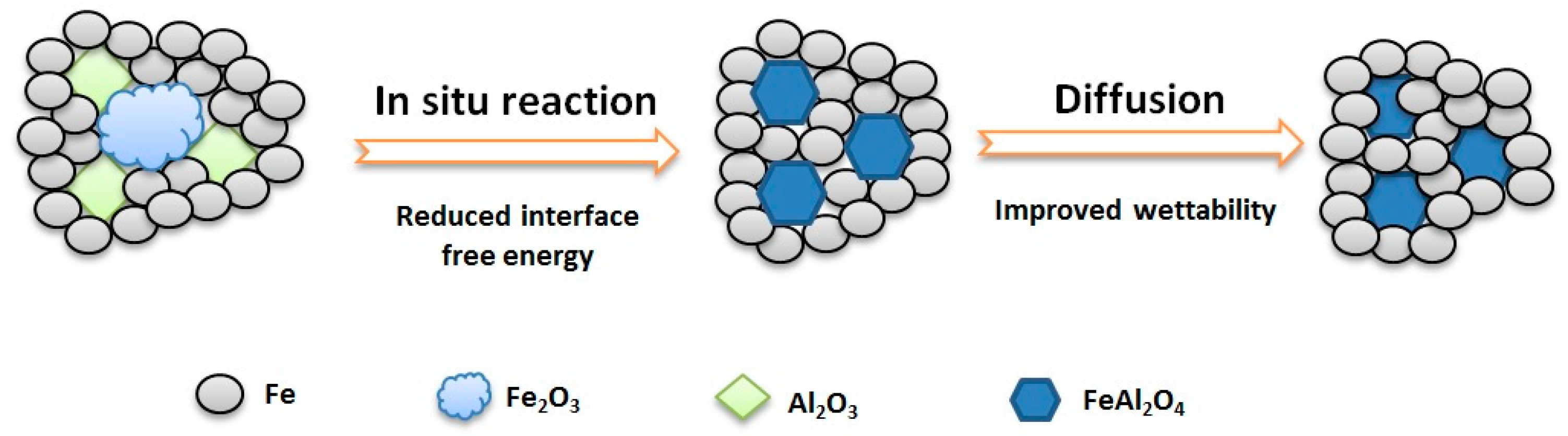
3.1. Preparation Principle of Fe/FeAl2O4 Cermet
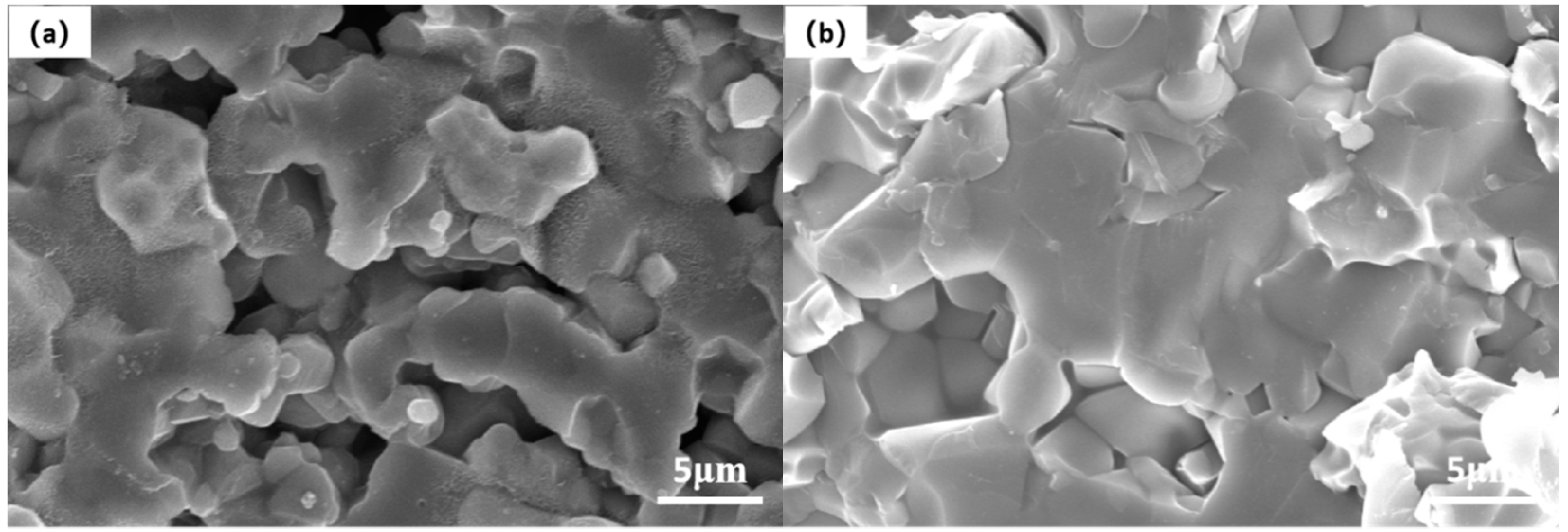
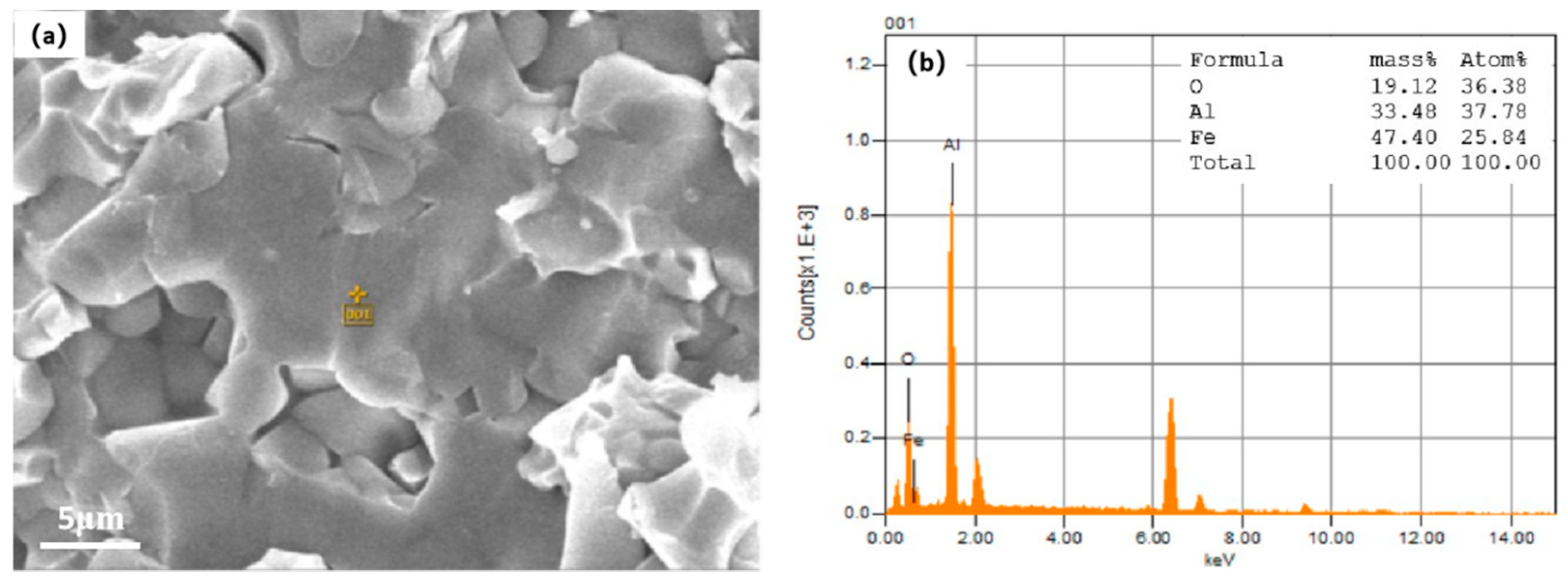
3.2. Effect on Microstructure
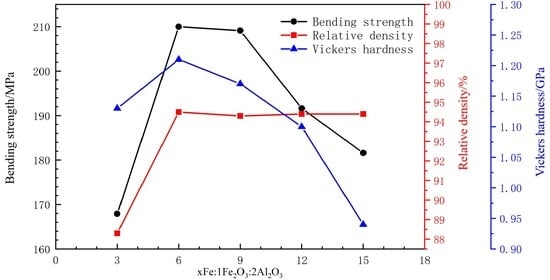
3.3. Effect on Mechanical Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shi, Z.L.; Du, X.K.; Li, C.A. Study on the iron base ceramic metal braking materials for high-speed train. J. China Railw. Soc. 2001, 23, 29–32. [Google Scholar]
- Wang, X.F.; Li, D.S. Iron-cermet friction materials for aircraft application. Mater. Eng. 1999, 3, 27–29. [Google Scholar]
- Rosso, M. Ceramic and metal matrix composites: Routes and properties. J. Mater. Process. Technol. 2006, 175, 364–375. [Google Scholar] [CrossRef]
- Chen, W.P.; Yang, S.F.; Han, M.Y. Research development of ceramic/Fe-based alloy composites. Chin. J. Nonferrous Met. 2010, 20, 257–265. [Google Scholar]
- Reshetenko, T.V.; Avdeeva, L.B.; Khassin, A.A.; Kustova, G.N.; Ushakov, V.A.; Moroz, E.M.; Shmakov, A.N.; Kriventsov, V.V.; Kochubey, D.I.; Pavlyukhin, Y.T. Coprecipitated iron-containing catalysts (Fe-Al2O3, Fe-Co-Al2O3, Fe-Ni-Al2O3) for methane decomposition at moderate temperatures I. Genesis of calcined and reduced catalysts. Appl. Catal. A 2004, 268, 127–138. [Google Scholar] [CrossRef]
- Li, M.C.; Gong, Q.; Yang, Y.Q.; Yu, J.; Zhou, Y.; Zuo, X.Q. Structures and properties of Al2O3/Fe composite honeycombs fabricated by plasticizing powder extrusion. Acta Mater. Compos. Sin. 2016, 33, 2237–2245. [Google Scholar]
- Ma, J.J.; Hou, Y.G.; Li, G.F.; Tian, J.; Zhou, Q.; GAO, Y. Effect of Co on performance and structure of low-temperature Fe-based ceramic/metal composite bonds. Diam. Abras. Eng. 2016, 36, 61–65. [Google Scholar]
- Zhu, L.; Bolot, R.; Liao, H. Formation mechanism of Fe-Al2O3-FeAl2O4 and FeAl coating by reactive plasma spraying under atmosphere and low pressure conditions. Asm Int. 2013, 5, 13–15. [Google Scholar]
- Liu, J.F.; Wang, Z.; Ding, Y.S. Research on the combined properties of Fe/Al2O3 graded coatings. Chin. J. Mater. Res. 2010, 24, 401–405. [Google Scholar]
- Wang, J. Study on wear-resistance of TiC-Fe matrix composite produced by powder metallurgy. Mater. Heat Treat. 2008, 37, 50–52. [Google Scholar]
- Liu, J.W.; Ding, H.F.; Zheng, Z.X. Study on technological parameters and properties of sintered SiCp/Fe composite material. Min. Metall. Eng. 2002, 22, 92–95. [Google Scholar]
- Farid, A.; Guo, S.J.; Jawid, A.; Tian, J. Sintering behavior, microstructure and properties of TiC-FeCr hard alloy. J. Univ. Sci. Technol. Beijing 2007, 14, 89–93. [Google Scholar]
- Srivastava, A.K.; Das, K. Microstructure and abrasive wear study of (Ti, W) C-reinforced high-manganese austenitic steel matrix composite. Mater. Lett. 2008, 62, 3947–3950. [Google Scholar] [CrossRef]
- Bansal, C. Metal-to-ceramic bonding in (Al2O3+Fe) cermet studied by Miissbauer spectroscopy. Bull. Mater. Sci. 1984, 6, 13–16. [Google Scholar] [CrossRef]
- Konopka, K.; Ozieblo, A. Microstructure and the fracture toughness of the Al2O3-Fe composites. Mater. Charact. 2001, 46, 125–129. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, J.F.; Ding, Y.S. Fabrication and Properties of Fe/Al2O3 Composites. Chin. J. Mater. Res. 2012, 26, 206–210. [Google Scholar]
- Konopka, K. Shape, size and distribution of metal particles embedded in a ceramic matrix. Solid State Phenom. 2015, 231, 57–63. [Google Scholar] [CrossRef]
- Gupta, P.; Kumar, D.; Parkash, O.; Jha, A.K. Structural and mechanical behavior of 5% Al2O3 reinforced Fe metal matrix composites (MMC) produced by powder metallurgy (P/M) route. Bull. Mater. Sci. 2013, 36, 859–868. [Google Scholar] [CrossRef]
- Gupta, P.; Kumar, D.; Parkash, O.; Jha, A.K. Sintering and hardness behavior of Fe-Al2O3 metal matrix nanocomposites prepared by powder metallurgy. J. Compos. 2014, 2014, 1–10. [Google Scholar] [CrossRef]
- Garg, P.; Gupta, P.; Kumar, D.; Parkash, O. Structural and mechanical properties of graphene reinforced aluminum matrix composites. J. Mater. Env. Sci. 2016, 7, 1461–1473. [Google Scholar]
- Gupta, P.; Kumar, D.; Parkash, O.; Jha, A.K. Effect of sintering on wear characteristics of Fe-Al2O3 metal matrix composites. Eng. Tribol. 2014, 228, 362–368. [Google Scholar] [CrossRef]
- Li, S.; Wu, W.F.; Chen, W.H.; Liu, J.; Ji, Y.H. Study on preparation and properties of Al2O3-Fe based metal ceramics. Appl. Mech. Mater. 2017, 865, 49–53. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, K.; Yan, H.Y. Reuse of Zinc Kiln Slag to Fire Iron-based Cermet and its Prospect. Multipurp. Util. Miner. Resour. 2020, 4, 8–12. [Google Scholar]
- Wang, C.; Zhang, K.; Yan, H.Y. Strengthening Mechanism of Metal-based and Ceramic-based Cermet. China Ceram. 2020, 56, 1–4. [Google Scholar]
- Aksay, I.A.; Hoge, C.E.; Pask, J.A. Wetting under chemical-equilibrium and nonequilibrium conditions. J. Phys. Chem. 1976, 78, 1178–1183. [Google Scholar] [CrossRef]





| Sample | Fe | Fe2O3 | Al2O3 | Fe:Fe2O3:Al2O3 |
|---|---|---|---|---|
| S1 | 50.0 | 16.7 | 33.3 | 3:1:2 |
| S2 | 66.7 | 11.1 | 22.2 | 6:1:2 |
| S3 | 75.0 | 8.3 | 16.7 | 9:1:2 |
| S4 | 80.0 | 6.7 | 13.3 | 12:1:2 |
| S5 | 83.3 | 5.6 | 11.1 | 15:1:2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, K.; Li, Y.; Wang, C.; Yan, H.; Li, H.; Liang, J.; Dang, J. Effect of Fe on the Microstructure and Mechanical Properties of Fe/FeAl2O4 Cermet Prepared by Hot Press Sintering. Crystals 2021, 11, 204. https://doi.org/10.3390/cryst11020204
Zhang K, Li Y, Wang C, Yan H, Li H, Liang J, Dang J. Effect of Fe on the Microstructure and Mechanical Properties of Fe/FeAl2O4 Cermet Prepared by Hot Press Sintering. Crystals. 2021; 11(2):204. https://doi.org/10.3390/cryst11020204
Chicago/Turabian StyleZhang, Kuai, Yungang Li, Chuang Wang, Hongyan Yan, Hui Li, Jinglong Liang, and Jie Dang. 2021. "Effect of Fe on the Microstructure and Mechanical Properties of Fe/FeAl2O4 Cermet Prepared by Hot Press Sintering" Crystals 11, no. 2: 204. https://doi.org/10.3390/cryst11020204
APA StyleZhang, K., Li, Y., Wang, C., Yan, H., Li, H., Liang, J., & Dang, J. (2021). Effect of Fe on the Microstructure and Mechanical Properties of Fe/FeAl2O4 Cermet Prepared by Hot Press Sintering. Crystals, 11(2), 204. https://doi.org/10.3390/cryst11020204