Abstract
High-quality oxidized pellets are the basis to achieve high-efficiency utilization of vanadium–titanium magnetite (VTM) ores. Bentonite was used as a binder of VTM. The main phase composition of VTM is titanomagnetite and ilmenite. When the amount of bentonite is 1%, the compressive strength and dropping strength of VTM pellets can meet the requirements. To improve metallurgical properties, the pellets need to be roasted. The best conditions for roasting are as follows: calcination temperature of 1523 K and a calcination time of 20 min. The consolidation mechanism, phase transformation, and crystal structure transformation of VTM in the process of oxidation roasting are also explained.
1. Introduction
Vanadium (V), titanium (Ti), and iron (Fe) are important metal elements recognized worldwide. Vanadium-titanium magnetite (VTM) ore is the main source of V, Ti, and Fe in nature with a high comprehensive utilization value [,,]. China is one of the countries which has the richest resources of VTM in the world. At present, the mainstream route for VTM’s comprehensive utilization is the transition from a blast furnace to a basic oxygen furnace (BF→BOF). VTM is mainly smelted in a blast furnace, and the products are high-titanium slag and vanadium-containing molten iron [,,]. In the BF, limestone needs to be added as a solvent, which not only reduces the grade of TiO2 in the blast furnace slag but also forms perovskite with TiO2 []. The generation of perovskite will certainly increase the difficulty of titanium recovery []. As a result, only part of the iron and vanadium can be recovered in the traditional BF-BOF process, while titanium can hardly be extracted []. Moreover, the BF process also has disadvantages such as excessive coke consumption, environmental pollution, and over-sized production equipment. In the past few decades, many scholars have studied the comprehensive utilization of VTM, including fire smelting, direct acid leaching, selective chlorination, and direct reduction [,,]. Among them, direct reduction is considered the most practical and efficient utilization method. There are many direct reduction ironmaking processes in the world, but the gas-based shaft furnace direct reduction ironmaking process is dominant in direct reduction iron production due to its high productivity, low energy consumption, low carbon content, and environmental protection. As the primary charging form for BF, sintering plays an important role in the gas-based shaft furnace direct reduction ironmaking process. Through the sintering process, VTM ore powder is transformed into VTM pellets to improve its permeability and strength []. More importantly, the phase composition of VTM is very complex, whereby iron, vanadium, and titanium closely co-exist in a stable structure, so their reduction is more difficult. To solve this problem, scholars have proposed a method of oxidation sintering of VTM to improve its reduction performance and mechanical properties. In recent years, there have been many reports where VTM pellets are oxidized and sintered to improve reduction performance. VTM is a typical composite mineral resource and its oxidation and sintering mechanism has attracted the attention of scholars. Gan et al. [] conducted a series of studies on the preparation of VTM and explored the oxidation behavior, phase, and microstructure evolution rules of the pellets in the process of thermal processing to optimize the production method of high-quality pellets. Sui [] et al. investigated the gas-based isothermal reduction kinetics of oxidized VTM and relevant parameters including reduction time, gas composition, and reduction temperature in the reduction process at temperatures of 1173–1323 K. Sui et al. [] also investigated the sticking behavior of VTM pellets during gas-based reduction. They not only explored the effects of temperature and gas composition on the sticking index but also pointed out that Ti-bearing tailing is a better anti-sticking coating material than CaO and MgO.
In summary, high-quality oxidized pellets are the basis for efficient gas-based direct reduction. This paper takes China Panxi VTM as the research object and fully analyzes the crystal structure and characteristics of VTM. Bentonite was used as a binder of VTM. The effects of bentonite addition on the compressive strength and falling strength of the pellets of VTM was investigated, and the mechanism by which bentonite increased the pellets’ strength was analyzed. At the same time, this paper also studied the oxidation roasting of VTM pellets and analyzed the effect of roasting temperature and roasting time on the oxidation roasting process and compressive strength of the pellets. In addition, chemical analysis, XRD phase analysis, and SEM analysis were used to study the consolidation mechanism of VTM oxidation pellets. In this way, we explored phase transformation and crystal structure transformation in the process of oxidation roasting and contributed to the theory of VTM pelletizing.
2. Materials and Methods
2.1. Experimental Materials and Methods
The concentrated raw VTM material selected in this paper is produced in Pan-Xi, Sichuan, China, and the additive is bentonite. The pellet preparation process is shown in Figure 1a, and consists of mixing, pelletizing, sieving, strength testing, and drying. The structure diagram of the oxidation roasting device is shown in Figure 1b.
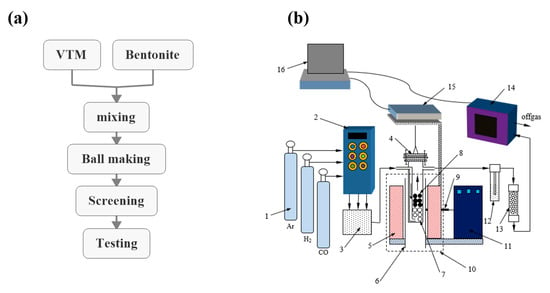
Figure 1.
(a) The preparation process of vanadium–titanium magnetite (VTM) pellets. (b) Schematic of the experimental apparatus: 1—gas cylinder, 2—flow control cabinet, 3—gas mixing chamber, 4—reactor, 5—fever zone, 6—alundum tube, 7—corundum ball, 8—oxidized VTM pellets, 9—thermocouple, 10—tube furnace, 11—temperature controlling cabinet, 12—wash bottle, 13—drying bottle, 14—coal gas analyzer, 15—electronic balance, 16—computer.
The first step of pellet roasting is preheating of the VTM pellets. During the preheating stage, the crystal water evaporates, the magnetite is oxidized to hematite, and the carbonate decomposes. In this paper, the preheating conditions were fixed during the oxidation roasting of VTM. The preheating temperature was selected as 1173 K and the preheating time was 10 min. The specific operation can be stated as follows. First the furnace temperature is increased from room temperature to 1173 K and maintained. Then, a flow rate of 1L/min of air is established. The VTM pellets weighing about 200g are then placed into the corundum crucible to preheat for 10 min in the furnace. After preheating, the furnace temperature is raised to the roasting temperature. The oxidized pellets were then taken out of the furnace according to different roasting times that were tested and cooled to room temperature.
2.2. Characterization
The patterns of X-ray diffraction (XRD) were collected by a powder X-ray diffractometer (Rigaku Dmax-2400, RIGAKU, Japan) with Cu Kα radiation. The surface compositions and valence states of Ti and Fe in VTM were characterized by X-ray photoelectron spectroscopy (AXIS Supra, Kratos Analytical Ltd, UK) with Al Kα radiation. Scanning electron microscopy was performed on a field emission Hitachi S-4800 instrument operating at an accelerating voltage of 10 kV.
3. Results and Discussion
3.1. Basic Characteristics of VTM
The chemical composition of VTM is shown in Table 1. The content of TFe in VTM was 53.7%, the content of TiO2 was 12.6%, and the content of V2O5 was 0.51%. The total content of gangue such as Al2O3, SiO2, MgO, and CaO exceeded 15%, indicating that the raw material of VTM was of a relevantly low grade with a high S content of 1.31%.

Table 1.
Chemical composition of VTM (wt%).
The phase composition and XRD phase analysis spectrum of VTM are shown in Figure 2a,b. The main phase composition of VTM is TTM(titanomagnetite) and ilmenite. Fe in VTM is mainly found in TTM and a small part exists in ilmenite. Ti is closely combined with iron oxides and Ti-containing minerals mainly include TTM and ilmenite. The particle size of the solid solution is extremely fine (0.40–5 μm) and it is not easy to enrich the solid solution by beneficiation. Generally, the TTM which is formed by the solid solution is shown in Figure 2c. The XRD peak of TTM has a slight migration, which should be considered as due to V ions and Mg ions being dissolved in TTM to replace part of Fe2+. For vanadium, no independent mineral of vanadium is found in VTM. The ionic radii of vanadium and iron are similar and they have a high valence which can form strong bonds. Vanadium can be hidden in the spinel structure of TTM during high-temperature crystallization, forming the most stable isomorphic impurities. Therefore, vanadium mainly exists in TTM with isomorphism and cannot be separated by mechanical methods. In summary, the spatial feature is a close symbiosis among Fe3O4—Fe2TiO4—MgO·Al2O3—FeO·TiO2. At the level of chemical structure, iron is contained in Fe3O4, 2FeO·TiO2, and FeO·TiO2 and is easily reduced in Fe3O4 but more difficult in the latter two. Moreover, MgO and VO replace part of FeO, which greatly aggravates the difficulty of reduction
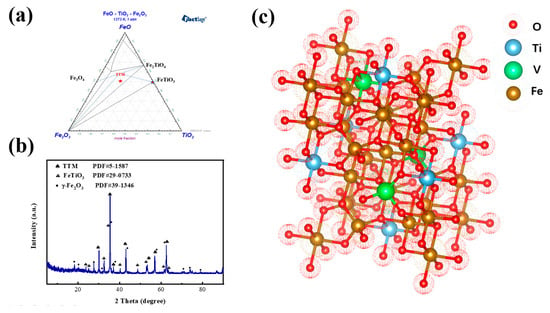
Figure 2.
Characteristics analysis of VTM: (a) phase diagram; (b) xrd spectrum; (c) crystal structure diagram.
The particle size of the VTM ore has a greater impact on pelletization. The most important index for VTM pelletizing is the content of particles less than 45 μm. It is generally believed that the content of the raw material in particles less than 45 μm should be greater than 70%. The particle size analysis of VTM is shown in Figure 3a. The particle size of the VTM is mainly concentrated in 30 μm and the proportion of particles smaller than 45 μm is about 75%, which meets the conditions for pelletizing.
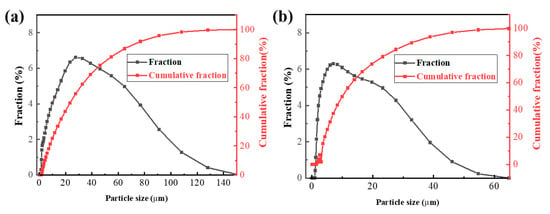
Figure 3.
Particle size distribution analysis: (a) VTM; (b) bentonite.
When preparing VTM pellets it is often necessary to add a small number of hydrophilic substances as binders, such as bentonite, limestone, water glass, and CaCl2. Bentonite is widely used as a binder, so this paper also used bentonite as a binder. The XRF chemical analysis is shown in Table 2. The particle size analysis of bentonite is shown in Figure 3b. The particle size of bentonite is mainly concentrated in 2.5 μm, while the content of bentonite smaller than 45 μm is greater than 95%, which shows that the particle size of bentonite is relatively fine.

Table 2.
Chemical composition of bentonite.
3.2. Influence of Bentonite Addition Amount on the Properties of VTM Pellets
Because VTM pellets need to be screened and transported during the production process, they must have a certain strength to ensure that they are not damaged or broken by external forces. Generally, the method to improve VTM pellet strength is the addition of a certain amount of binder into the VTM. In this way, a closer connection can be made among the VTM particles. However, the addition of a binder is bound to reduce the grade of VTM. Therefore, when the VTM pellet strength reaches the standard, the added amount of bentonite should be as small as possible. The evaluation criteria for VTM pellet strength mainly have three aspects: falling strength, compressive strength, and water content. As shown in Figure 4, the falling strength and compressive strength of pellets increase with the addition of bentonite content. The capillary theory of the consolidation mechanism of wet pellets states that the finer the capillary between the particles, the greater the force between the particles. Bentonite has a fine particle size and high dispersibility. After adding bentonite, the capillary diameter in the pellets is reduced so the strength of the pellets is improved. On the other hand, bentonite becomes colloidal after the pellets absorb and being filled with water, which increases the molecular cohesion between the particles. At the same time, bentonite can also act as a lubricant. When a pellet is impacted by an external force, it can slide, which is beneficial for improving its falling strength. When the added amount of bentonite is 1%, the falling strength of pellets is 3.1 times per pellet. This meets the requirement that the falling strength of pellets should be more than 3 times per pellet. This addition of bentonite also increases the compressive strength of pellets to greater than 9.6N per pellet. This meets the requirement that the compressive strength of pellets should be more than 8.8N per pellet. When the added amount of bentonite is 1%, both the VTM pellets falling strength and the pellet compressive strength meet the requirements, so the added amount of bentonite in the VTM pellets preparation process is set as 1%. At 1% bentonite addition, the moisture content of the pellet was determined to be 8.5%, meeting the requirement that the moisture content of the pellet should be 8–10%.
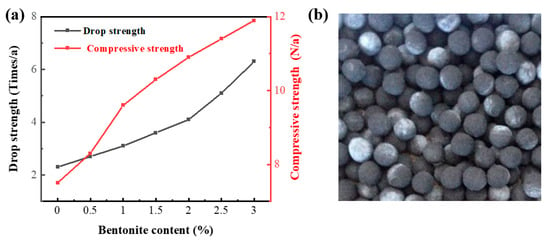
Figure 4.
(a) Relationship between the amount of bentonite added and the falling strength/compressive strength of VTM pellets. (b) Photo of vanadium titanium magnetite pellets prepared with 1% bentonite.
3.3. The Effect of Roasting Temperature and Time on the Sintering Behavior of VTM Pellets
The method for testing compressive strength of VTM oxide pellets can be described as follows. First, use a universal pressure testing machine to test the pellets’ compressive strength. Then, select 12 pellets as samples for each testing time. Remove the maximum and minimum values and calculate the average value of the remaining 10 oxidized pellets. That average value is the compressive strength of the batch of samples. It is generally believed that the compressive strength of oxidized pellets should be greater than 2500 N per pellet. In this part the relationship between the roasting temperature and the compressive strength of the VTM oxide pellets is studied. The roasting time was set to 20 min, and the roasting temperatures were 1373, 1423, 1473, 1523, 1573, 1623, and 1673 K. Figure 5a shows the relationship between the roasting temperature and the compressive strength of the VTM oxide pellets. Generally speaking, the consolidation temperature of VTM is relatively high. This is due to the high content of MgO, Al2O3, and other gangues in VTM. In addition, the intergrowth of ilmenite, gangue, and ilmenite titanium are intercalated between Fe2O3 crystals, hindering the aggregation of Fe2O3 crystals, which will also increases the consolidation temperature of VTM.
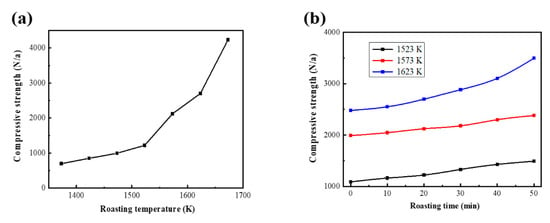
Figure 5.
(a) Relationship between roasting temperature and compressive strength of VTM oxide pellet. (b) Relationship between roasting time and compressive strength of VTM oxide pellets.
The consolidation of VTM is mainly solid-phase consolidation. According to Taman’s theory, the temperature at which the solid matter starts to react is much lower than the melting temperature. The ions, atoms, and molecules (mainly ions in the VTM pellets) on the crystal lattice sites in minerals are mobile, and this migration ability increases with the increase of temperature. This migration can be the exchange of sites within the crystal lattice or it can migrate to the surface of the crystal lattice. After the temperature reaches a certain level, it can also migrate from one crystal to the lattice of adjacent crystals. Because the pellets have finer particles, high dispersibility, high specific surface energy, and serious lattice defects, it has high reactivity. As shown in Figure 5a, when the calcination temperature is 1373 to 1523 K, the compressive strength of the VTM oxide pellets increases slowly. The increase in the compressive strength of the pellets is because the magnetite in the VTM is oxidized to form a crystal bridge between the hematite solid phase through ion migration, which connects the particles so that the pellets possess a certain intensity. However, due to the imperfect internal crystal structure, the strength is not high, resulting in a slower growth rate of the overall compressive strength of the pellets. When the roasting temperature is 1523 to 1623 K, recrystallization and collective recrystallization occur in hematite, and lattice defects are eliminated. In this way, the contact area between particles increases, which makes the connection between particles stronger. Therefore, the growth rate of the compressive strength of VTM oxidized pellets is accelerated in this temperature range. As shown in Figure 6, when the roasting temperature rises to 1673 K, the oxidized pellets of VTM are locally bonded, indicating that ion migration expands to the pellets, and it is also possible that a large amount of liquid phase is formed in the pellets. In short, the consolidation degree of the pellets is further enhanced. Therefore, the compressive strength of VTM oxide pellets can be greatly improved when the calcination temperature is increased from 1623 to 1673 K.
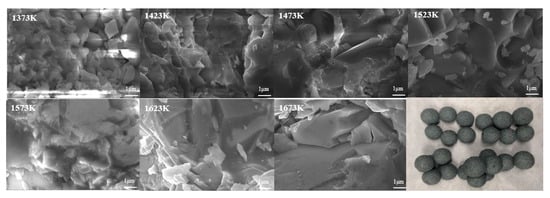
Figure 6.
Cross-sectional SEM image of VTM oxidized pellets from 1373 K to 1673 K.
Figure 6 shows the SEM diagram of the cross-section of VTM oxidized pellets when the roasting temperature is 1373 to 1673 K. When the roasting temperature is 1373 K, granules can be observed obviously in the VTM oxidized pellets, indicating that the consolidation of VTM is very inadequate. Then, with the increase of roasting temperature, the compactness of VTM oxidized pellets gradually increased, indicating that the degree of intergranular fusion in VTM oxidized pellets gradually increased, making the compressive strength of VTM oxidized pellets greater. As shown in Figure 5, when the roasting temperature is 1623 K and the time is 20 min, the compressive strength of VTM oxidized pellets is 2699N/a pellet, which meets the requirements of direct reduction in a gas-based shaft furnace.
A too high roasting temperature requires equipment with very high temperature resistance and will consume a lot of energy. To reduce roasting temperature, this paper explored the relationship between roasting time and the compressive strength of VTM oxidized pellets. The selected roasting temperatures were 1523, 1573, and 1623 K, and the roasting times were 10, 20, 30, 40, and 50 min. When the calcination temperature was 1523 and 1573 K, the compressive strength of the VTM oxidized pellets increased slowly with the extension of the calcination time. Even when the roasting time was extended to 50 min, the compressive strength of VTM oxide pellets still failed to reach 2500N/a pellet. When the calcination temperature was 1623K, with the extension of the calcination time, the compressive strength of the VTM oxidized pellets increased relatively quickly. In actual production, people often hope to strike a balance between roasting temperature and roasting time. The increase in roasting time and roasting temperature will increase the energy consumption of production and the extension of roasting time will reduce production efficiency. Therefore, the calcination conditions were set at a calcination temperature of 1523 K and a calcination time of 20 min. Finally, S is an element that is extremely harmful to the quality of steel, causing the metal to appear hot and brittle and has an adverse effects on thermal processing, welding, and cutting. Therefore, we expect to remove S from raw materials by the oxidation sintering process. S in VTM usually exists in the form of sulfide and sulfate. Under high-temperature conditions, sulfide, sulfate, and oxygen react to form SO2 overflow. Table 3 shows the chemical composition of VTM oxide pellets when the calcination temperature is 1623 K and the calcination time is 20 min. Compared with the original VTM ore, the composition of the VTM pellets increased the Na2O and K2O brought in from the bentonite. Due to the high temperature and oxygen overflow, the content of S was reduced from 1.31% to 0.037%. Therefore, oxidation roasting not only improves the compressive strength of VTM pellets but also achieves the purpose of desulfurization.

Table 3.
Chemical composition of VTM oxide pellets.
3.4. The Phase Transformation Mechanism of VTM Pellets during Oxidation and Sintering
When the calcination temperature is low, the XRD peak of the pellets is almost the same as that of VTM. This indicates that the phase in VTM pellets is stable at low temperatures and no oxidation occurs. When the temperature was increased to 1373 K, the microstructure and phase of iron-containing components and titanium-containing components in vanadium-titanium magnetite changed greatly. XRD patterns of the pellets at different oxidation temperatures are shown in Figure 7a. To investigate the valence state of VTM on the surface, X-ray photoelectron spectroscopy (XPS) was used to analyze the raw material and oxidized samples of VTM. Figure 8 shows XPS patterns of VTM and VTM oxidation pellets. By combining the XPS diagram of VTM raw materials and VTM oxidized pellets, we can see that the valence of titanium ions in the pellets was unchanged in the oxidation process so the roasting process of the VTM oxidation pellets is only the oxidation process of Fe.
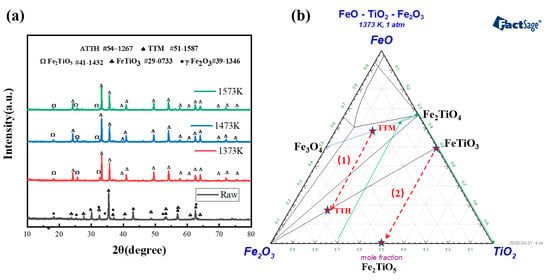
Figure 7.
(a) XRD pattern of VTM oxide pellets after roasting from 1373 to 1573 K. (b) Phase changes during oxidation of VTM pellets.
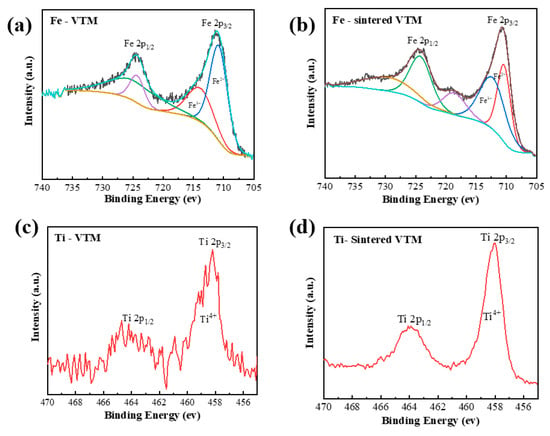
Figure 8.
XPS pattern of Fe and Ti in VTM and after oxidation. (a) Fe-VTM, (b) Fe-sintered VTM, (c) Ti-VTM, (d) Ti-sintered VTM.
The results showed that the main phases in the pellets were TTM and ilmenite when the oxidation roasting temperature was low. With the increase of temperature, the XRD peak characterizing TTM disappeared while the peak of Fe2+ in XPS weakened and the peak of Fe3+ increased. TTH peaks appeared in large numbers. The combination of XRD and XPS showed that the main phase was transformed into TTM and oxidized into TTH. Figure 9a,b show the crystal structures of TTM and TTH. TTM is a cubic lattice and TTH is an orthorhombic six-sided lattice. The lattice structure of TTH is easier to reduce. Combined with the SEM of Figure 6, it can be seen that the calcination has achieved the polymerization and growth of the TTH lattice. The unit cell aggregates and grows and the crystal form changes from a relatively dispersed granular shape to a tight and strong structural interconnection. In addition to TTM, VTM raw materials also have isolated ilmenite (FeTiO3), which will be transformed into Fe2TiO5. The crystal structures of FeTiO3 and Fe2TiO5 are shown in Figure 9c,d. Finally, the oxidation process of VTM is described in the TiO2\FeO\Fe2O3 phase diagram as shown in Figure 7b. The phase transition in the oxidation process of VTM pellets is obtained.
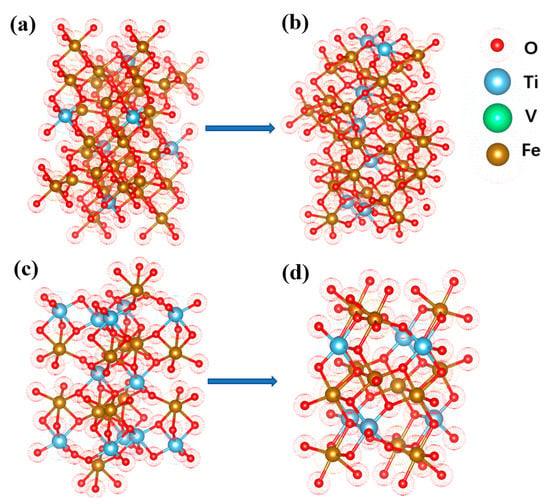
Figure 9.
Schematic diagram of the lattice transformation of the main phases during the VTM oxidation roasting process: (a) TTM; (b) TTH; (c) FeTiO3 (d) Fe2TiO5.
The reaction in route (1) in Figure 7b is as follows:
(Fe2TiO4)x (Fe3O4)1−x +1/4O2=3/2(FeTiO3)2/3x(Fe2O3) 1−2/3x
The reaction in route (2) in Figure 7b is as follows:
2FeTiO3 +1/2O2=Fe2TiO5+TiO2
4. Conclusions
The selected VTM had a fine particle size, and the proportion of particles with sizes of less than 45 μm was more than 70%. In the preparation stage of VTM pellets, the strength of VTM pellets was improved by adding bentonite. When the amount of bentonite is 1%, the compressive strength and dropping strength of VTM pellets can meet the requirements. Roasting temperature and roasting time have a great influence on the compressive strength of the VTM oxidized pellets. The results show that the compressive strength of the VTM oxidized pellets increases with the increase of roasting temperature. When the roasting temperature is 1623 K, the VTM oxidized pellets are more than 2500 N/a pellet, which meets the requirements of direct reduction in a gas-based shaft furnace. The consolidation mechanism of VTM oxidized pellets was studied by XRD phase analysis and SEM analysis and the phase change of pellets during oxidation roasting was verified. Magnetite and ilmenite were oxidized. The structures of ilmenite and ilmenite were opened. Hematite occured in recrystallization and aggregation. At the same time lattice defects were eliminated and crystal development was perfect.
Author Contributions
Conceptualization, X.W.; methodology, W.C.; software, Y.J.; validation, L.L., X.W. and W.C.; formal analysis, Z.D.; investigation, Z.D.; resources, X.W.; data curation, W.C.; writing—original draft preparation, W.C.; writing—review and editing, W.C.; visualization, W.C.; supervision, X.W.; project administration, X.W.; funding acquisition, X.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Key Research and Development Plan of China (2018YFC1901505 and 2018YFC1901503), National Natural Science Foundation of China (51672006), Shanxi Unveiling Bidding Project (20191101007), and the Ministry of Land and Resources Public Welfare Industry Research Project (201511062-02).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tang, W.D.; Yang, S.T.; Xue, X.X. Effect of V2O5 Addition on Oxidation Induration and Swelling Behavior of Chromium-Bearing Vanadium Titanomagnetite Pellets with Simulated Coke Oven Gas Injection into Blast Furnace. ISIJ Int. 2019, 59, 988–997. [Google Scholar] [CrossRef]
- Chen, S.Y.; Chu, M.S. Metalizing reduction and magnetic separation of vanadium titano-magnetite based on hot briquetting. Int. J. Min. Met. Mater. 2014, 21, 225–233. [Google Scholar] [CrossRef]
- Guo, X.F.; Dai, S.J.; Wang, Q.Q. Influence of different comminution flowsheets on the separation of vanadium titano-magnetite. Miner. Eng. 2020, 149, 8. [Google Scholar] [CrossRef]
- Chen, J.W.; Jiao, Y.; Wang, X.D. Thermodynamic studies on gas-based reduction of vanadium titano-magnetite pellets. Int. J. Min. Met. Mater. 2019, 26, 822–830. [Google Scholar] [CrossRef]
- Jiao, K.X.; Chen, C.L.; Zhang, J.L.; Liu, Z.J.; Wang, G.W.; Wang, W.P.; Shao, Q.J. Analysis of titanium distribution behaviour in vanadium-containing titanomagnetite smelting blast furnace. Can. Metall Quart 2018, 57, 274–282. [Google Scholar] [CrossRef]
- Chen, D.S.; Song, B.; Wang, L.N.; Qi, T.; Wang, Y.; Wang, W.J. Solid state reduction of Panzhihua titanomagnetite concentrates with pulverized coal. Miner. Eng. 2011, 24, 864–869. [Google Scholar] [CrossRef]
- Li, G.; Lv, X.; Zheng, Z.; Ling, J.; Qiu, G. Effect of Preformed Calcium Ferrite Addition on Sintering Behavior of Vanadium Titanium Magnetite Ore. Jom-Us 2020, 1–10. [Google Scholar] [CrossRef]
- Bai, Y.Q.; Cheng, S.S.; Bai, Y.M. Analysis of Vanadium-Bearing Titanomagnetite Sintering Process by Dissection of Sintering Bed. J. Iron Steel Res. Int. 2011, 18, 8–36. [Google Scholar] [CrossRef]
- Hu, T.; Lv, X.; Bai, C.; Lun, Z.; Qiu, G. Reduction Behavior of Panzhihua Titanomagnetite Concentrates with Coal. Metallurg. Mater. Trans. B 2013, 44, 252–260. [Google Scholar]
- Jena, B.C.; Dresler, W.; Reilly, I.G. Extraction of titanium, vanadium and iron from titanomagnetite deposits at pipestone lake, Manitoba, Canada. Miner. Eng. 1995, 8, 159–168. [Google Scholar] [CrossRef]
- Sole, K.C. Recovery of titanium from the leach liquors of titaniferous magnetites by solvent extraction—Part 1. Review of the literature and aqueous thermodynamics. Hydrometallurgy 1999, 51, 239–253. [Google Scholar] [CrossRef]
- Chen, J.; Chen, W.; Ji, R.; Jiao, Y.; Wang, X. Kinetic studies on bituminous coal char gasification using CO2 and H2O mixtures. Int. J. Green Energy 2019, 1–8. [Google Scholar] [CrossRef]
- Zhou, M.; Yang, S.; Jiang, T.; Xue, X. Influence of Basicity on High-Chromium Vanadium-Titanium Magnetite Sinter Properties, Productivity, and Mineralogy. Jom-Us 2015, 67, 1203–1213. [Google Scholar] [CrossRef]
- Gan, M.; Sun, Y.F.; Fan, X.H.; Ji, Z.Y.; Lv, W.; Chen, X.L.; Jiang, T. Preparing high-quality vanadium titano-magnetite pellets for large-scale blast furnaces as ironmaking burden. Ironmak. Steelmak. 2020, 47, 130–137. [Google Scholar] [CrossRef]
- Sui, Y.L.; Guo, Y.F.; Jiang, T.; Qiu, G.Z. Reduction kinetics of oxidized vanadium titano-magnetite pellets using carbon monoxide and hydrogen. J. Alloy Compd. 2017, 706, 546–553. [Google Scholar] [CrossRef]
- Sui, Y.L.; Guo, Y.F.; Jiang, T.; Qiu, G.Z. Sticking behaviour of vanadium titano-magnetite oxidised pellets during gas-based reduction and its prevention. Ironmaking & Steelmaking 2017, 44, 185–192. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).