Abstract
Classical nucleation theory (CNT), which was established about 90 years ago, represents the most commonly used theory in describing nucleation processes. For a fluid-to-solid phase transition, CNT states that the solutes in a supersaturated solution reversibly form small clusters. Once a cluster reaches its critical size, it becomes thermodynamically stable and is favored for further growth. One of the most important assumptions of CNT is that the nucleation process is described by one reaction coordinate and all order parameters proceed simultaneously. Recent studies in experiments, computer simulations, and theory have revealed nonclassical features in the early stage of nucleation. In particular, the decoupling of order parameters involved during a fluid-to-solid transition leads to the so-called two-step nucleation mechanism, in which a metastable intermediate phase (MIP) exists in parallel to the initial supersaturated solution and the final crystals. These MIPs can be high-density liquid phases, mesoscopic clusters, or preordered states. In this Special Issue, we focus on the role of the various MIPs in the early stage of crystal nucleation of organic materials, metals and alloys, aqueous solutions, minerals, colloids, and proteins, and thus on various scenarios of nonclassical pathways of crystallization.
1. Role of Local Structure Ordering and Crystal–Liquid Interface in the Nucleation Pathways and Formation of Metastable Intermediates
Nucleation is a fascinating phenomenon that is related to our daily life; it is observed in snowing and raining, in the formulation of pharmaceutical drugs, and even in foods, to name a few examples. We have understood the nucleation event based on classical nucleation theory (CNT) to be the result of competition between thermodynamic driving forces and interface kinetics via density fluctuation. Although CNT has successfully explained the formation of crystal nucleation in many different kinds of liquids, it has often failed to elaborate the nucleation rate and to predict metastable intermediate phases preceding stable phases. These failures have caused researchers to suspect that assumptions in CNT are too simplistic. That is, the spherical shape of crystal nuclei may not be true, the crystal-liquid interface structure may be more complicated than we expected, and the role of local ordering of atoms or molecules may be underestimated.
Such a viewpoint has necessitated more sophisticated theoretical and experimental developments of the nucleation process. In a simulation study, ten Wolde and Frenkel [1] suggested that the order parameter (i.e., density) for nucleation in CNT is possibly divided into two parts, showing a sequential process of density and structural ordering; this two-step nucleation (TSN) process results in taking more various pathways for the nucleation. The precursors for TSN have been reported in many other systems [2,3,4]. As another case showing new insight into nucleation, Tanaka’s two-order-parameter model (i.e., density and bond orientational orders) [5] has shown that nuclei are not of spherical shape; i.e., it has shown the anisotropic property of the interface which may lead to intermediate metastable phases. This is consistent with experimental observations in colloidal liquid [6]. Moreover, the model demonstrated that the formation of stable crystals occurs through the transient formation of crystalline-like nuclei [5], originated by Ostwald [7]. Experimental findings further support this scenario; the structural similarity between a metastable liquid and crystal could significantly reduce crystal-liquid interfacial free energy [8], which could cause the formation of metastable quasicrystals detouring the nucleation pathways from liquid metals [9], aqueous solutions [8,10], and even water under high pressure [11]. Recently, the importance of the local ordering and crystal-liquid interfacial energy have been extended to explain glass-forming ability [12,13]. Furthermore, the influence of interface structure of solvent or hydration on nucleation or aggregation has been recognized and investigated [14,15,16].
These studies require a new perspective of nucleation and manifest that local ordering of liquids and crystal-liquid interface structure play a key role in determining nucleation pathways across the metastable intermediate phases. Therefore, many questions should be addressed with more detailed experimental and theoretical studies: What is the local order of the prenuclei or dense liquid region in the TSN? What is the origin of stability for the prenuclei or dense liquid region? What are the thermophysical characteristics and kinetic behavior of the prenuclei? How do different interfaces between crystals and prenuclei and solution form? How does the interface of the crystal/prenuclei or liquid/prenuclei influence the nucleation rate and the formation of metastable intermediate phases? Further studies need to be pursued for a profound understanding of nucleation beyond CNT, which was recognized by Ostwald [7] more than a century ago.
2. Perspectives of Molecular Simulations for Understanding Nucleation
Indeed, CNT is a macroscopic theory, essentially modeling nucleation only by two parameters to describe the interface and bulk energy as functions of surface area and bulk volume, respectively. Considering the small size of forming nuclei before reaching post-critical size, it is compelling to employ theoretical and modeling methods to shine some light onto molecular-scale details. This is how molecular dynamics simulation approaches became indispensable to state-of-the-art analyses of nucleation and prenucleation phenomena [17].
Over the past 25 years, simulation models and simulation technology have experienced exciting developments. The pioneering studies addressed simplified models that would describe the molecules as spherical particles with hard boundaries or interacting via effective, often Lennard-Jones type, potentials [1,18,19,20,21,22]. On the basis of molecular resolution, these studies paved the way to understanding the interplay of energy and entropy in desolvation phenomena [1], the assessment of phase diagrams [18], and the determination of gradual ordering processes that govern the evolution of forming nuclei [18,19,20,21] and secondary nucleation from seeds [22]. Moreover, such colloidal models were consequently extended to more detailed accounts of explicit molecules. To one end, this involved other shapes like rods and two-particle models to mimic stick-like molecules, hence enabling the analyses of mismatching species at nuclei–solvent interfaces [23,24].
However, a particularly important step forward in computer simulations of nucleation is the development of accurate interaction models with atomic resolution [25]. This enables direct comparability to the experimental setups, including boundary conditions like temperature and pressure [17]. Moreover, the fine details of hydrogen bonding, salt-bridges, and intramolecular deformation allowed mechanistic insights going beyond current experimental capabilities. Seminal studies unraveled the competition of energy and entropy during calcium carbonate precipitation [26,27], hence providing a profound mechanistic account of the nonclassical nucleation of apparently simple inorganic compounds [28].
In parallel to improving the details of the solute and solvent models, a number of dedicated sampling techniques were also pushed forward to extend the scope of exploring (pre)nucleation events in molecular dynamics simulations [17]. While direct molecular dynamics simulations of million-atom systems barely reach the microsecond time scale, smart approaches to focus on events of particular interest have been created. For example, the crossing of energy barriers may be promoted by artificial yet controlled driving forces such that solute–solute coordination numbers are biased to facilitate desolvation [29]. Additionally, Monte Carlo type approaches to particle insertion and docking to forming aggregates help to overcome the time scale problem inherent to diffusion processes in solutions of low solute concentration [27,30].
The current forefront of modeling nucleation processes encompasses the subtle differences of molecular crystal polymorphs, hence offering exciting perspectives on tailoring the formulation of pharmaceutical compounds [31,32]. Moreover, efficient methods to mimic the quantum mechanics of redox reactions facilitate the study of metal ion reduction upon precipitation [33], and combined quantum/molecular mechanics routes allow pH-dependent analyses of proton transfer processes at the crystal/nucleus–solvent interface [34]. On this basis, various mechanisms of two-step nucleation have become accessible, effectively separating solute desolvation, preordering, and, upon increasing aggregate size, the evolution of intramolecular alignment and ripening reactions [17].
3. The Contribution of Proteins to the Understanding of Nucleation
Among other crystallization systems, i.e., small molecules and organic and other macromolecules, proteins (representing here all biological macromolecules and complexes) are also the best representative of systems recalcitrant to crystallize. At least, this is one of the reasons typically found in many articles in which protein crystallization is defined as more an art than a science. However, as we will be able to read within this Special Issue, the inherent complexity of protein molecules has been a key factor in understanding the complexity of the nucleation process. It is also envisaged that the fragile equilibrium between the folded and unfolded state of proteins will contribute to understanding other density fluctuation processes such as the naturally occurring unfolded protein aggregation, directly linked to several diseases [35,36,37] and strongly correlated with the nucleation mechanism we are revising here.
In 1989, Mikol and co-workers observed the formation of aggregates of different sizes in the early stages of lysozyme crystallization using dynamic light scattering [38]. The size of these aggregates increases from dimers to octamers with higher supersaturation [39] according to the CNT. However, the existence of stable aggregates in solution does not necessarily imply their identification as growth units, as pointed out [40]. Niimura et al. (1995) [41] found that the growth of the nuclei occurs from monomeric units even though larger aggregates exist in solution. It was Rosenberger, in 1996 [42], who proposed an analysis of the data in terms of colloid theory, more in line with the possible interactions present in a solution of macromolecules in which the intermolecular distances are of the order of or even inferior to those of the crystal itself. Differential scanning calorimetry [43] and transmission electron microscopy [44] demonstrated the presence of different prenucleation clusters approaching the colloid theory in which a stationary distribution of concentration fluctuations is assumed. Further investigations using magnetic nuclear resonance supported the fact that the formation of nuclei is a transient process and not a steady-state process [45,46]. It then became clear that the CNT fails to explain observed nucleation rates and nucleation kinetics curves or the presence of other phases in solution. From there on, a series of studies have dealt with the aggregation of protein molecules into a variety of condensed phases (clusters, fractal aggregates, etc.) which may or may not produce crystalline material, as has been summarized in several reviews [47,48,49]. The multiple nucleation paths have been exemplified using orthorhombic lysozyme for which both classical and cluster-mediated nucleation pathways were observed [50] and with glucose isomerase which followed the classical theory during the formation of the P2(1)2(1)2 form but nucleated via a one-step or cluster-mediated mechanism when crystallizing in the I222 crystal form [51].
More recently, a broader picture of protein nucleation has been drawn by Houben and co-workers, who used ferritin as a model protein [52]. They described the so-called “gradual ordering” mechanism to distinguish it from the multistep mechanism described by Vans Driessche et al. using glucose isomerase [53]. They suggested that because of the differences in protein structure and therefore their corresponding crystallization conditions, proteins present different desolvation dynamics resulting in a variety of mechanisms of crystallization, which may include the two-step nucleation theory [54]. Although this concept seems new, it was previously described by Duran-Olivencia and co-workers using a theoretical model with two parameters (accounting for changes in the size and inner density of the cluster), demonstrating that the cluster distribution function obtained with their model corroborates the existence of intermediate nucleation states. These precursors are temporarily stabilized by the small-scale kinetics of matter inside the nucleation cluster, highlighting the role of kinetics in nucleation as a unique consequence of a complex energy landscape [55].
4. Connection between the Phase Diagram and the Possible Pathways of Nucleation in Protein and Colloidal Systems
In protein crystallization assays, it is often observed that various metastable intermediate phases, such as clusters, aggregates, and protein-rich liquid phases, appear before nucleation starts. Once crystals are formed, crystal growth consumes these intermediates. While recent progress in the study of the nonclassical nucleation process has provided new insights into the early stage, the exact role of these intermediates in protein crystallization remains unclear.
In the last two decades, it has been established that the effective interactions in protein and colloidal systems, in particular the attractions between particles, are often short-ranged in comparison with their size, which leads to a metastable liquid–liquid phase transition (LLPS) with respect to crystallization. A typical phase diagram is shown in Figure 1, where the gas (G) and liquid (L) phases correspond to a low and high concentration of particles in solution. For systems exhibiting a metastable LLPS, crystal nucleation may follow different pathways.
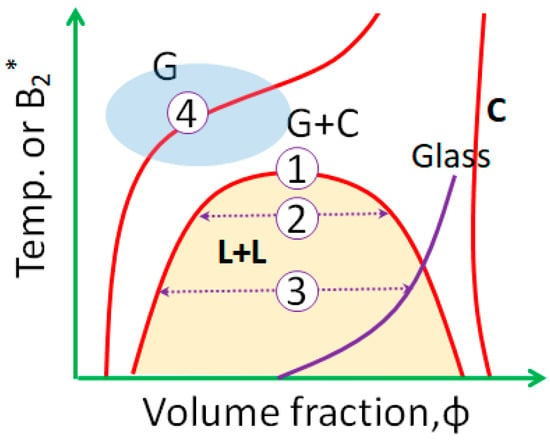
Figure 1.
Schematic concepts of nonclassical pathways of crystallization for a system exhibiting a metastable liquid–liquid phase transition (LLPS) in the phase diagram. Here, G, L, and C are the gas, liquid, and crystal phases; B2* is reduced second virial coefficient. The gas (G) and liquid (L) phases correspond to a low and high concentration of particles in solution.
The pioneering work by ten Wolde and Frenkel in 1997 [1] has inspired research in theory, simulation, and experiment on the understanding of nucleation pathways. Using a model for globular proteins with short-range attractive interactions, their Monte Carlo simulations of the homogeneous crystal nucleation suggested that the presence of a metastable fluid–fluid critical point drastically changes the pathway for nucleation. Close to the critical point, the free-energy barrier is strongly reduced and the nucleation rate increases by many orders of magnitude. Furthermore, the energy landscape indicates that order parameters (density and structure) may develop separately, leading to a two-step nucleation pathway. This two-step nucleation mechanism has been further discussed as a more general process, thus providing a consistent microscopic view of crystal nucleation with a metastable intermediate phase [27,28,47,53,56,57,58,59].
Based on the phase diagram in Figure 1, four different pathways can be identified. Path 1 represents quenching the system into a region near the critical point of LLPS where the density fluctuation leads to the two-step nucleation as initially proposed by ten Wolde [1]. In Path 2, the system is quenched into the two-phase coexistence region where LLPS occurs before nucleation via a spinodal decomposition and both dilute and dense phases are liquid-like. Crystal nucleation inside of the macroscopic dense liquid phase may be difficult but does not conflict with the two-step theory. Path 3 suggests that for a deeper quench, the binodal of the high-density branch is intercepted by the glass line, resulting in a nonequilibrium state of the dense phase. In this case, nucleation within the dense phase is hindered due to the extremely slow dynamics.
Furthermore, outside the LLPS region, near the solubility line, the region marked by the blue ellipse represents another scenario of a nonclassical nucleation pathway (Path 4). This region is often called the nucleation zone or the crystallization gap [60,61,62], where protein clusters with mesoscopic length scale and long lifetime (up to seconds) may serve as nucleation precursors [47,59]. These mesoscopic protein clusters can be either purely liquid-like or exhibit a certain preordering which may further reduce the energy barrier and favor two-step nucleation. Experimental studies following these pathways are discussed in [49].
The pathways discussed above are based on an ideal situation of crystallization, i.e., crystallization via homogeneous nucleation. In practice, very often these metastable phases do not contribute to nucleation directly; instead, they serve as a reservoir, indicating the dominating role of heterogeneous nucleation in these cases. Under this condition, experimentally one observes that once crystals are formed, the crystal growth is favored via vapor deposition from dilute solution, as in the phase diagram the gas–solid transition is thermodynamically stable. This process is analogous to the Bergeron process of ice formation [63]: the Bergeron process for the climate describes ice growth in clouds via vapor deposition at the expense of supercooled water droplets. For this to happen, the temperature must be below the melting point and the liquid state must be supercooled. Due to the fact that vapor saturation relative to ice is lower than that relative to water, ice growth follows the gas–solid phase transition. Experimental examples of protein and colloidal crystallization following the Bergeron process have been recently reported [64,65], suggesting that the Bergeron process indeed provides a unique pathway of crystal growth in the presence of intermediate phases.
5. Outlook
Certainly, in spite of the significant progress, many questions are still open for further investigations. For example, the structural and dynamic evolution of MIPs needs to be understood at the molecular level, and further study should address the role of hydrate water in precursor formation and its release upon crystal nucleation, the local preordering due to specific coordination and oriented attachment, the role of different pathways on crystal polymorph selection, etc. All these issues require further development of simulation and theoretical models and advanced experimental techniques that enable real-time observation in the corresponding pico- to microsecond and nano- to micrometer time and length scales, respectively.
Author Contributions
F.Z., J.A.G., G.W.L. and D.Z. contributed equally. All authors have read and agreed to the published version of the manuscript.
Funding
F.Z. and D.Z. acknowledge financial support of deutsche Forschungsgemeinschaft.
Institutional Review Board Statement
Not Applicable.
Informed Consent Statement
Not Applicable.
Data Availability Statement
Not Applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wolde, P.R.T.; Frenkel, D. Enhancement of Protein Crystal Nucleation by Critical Density Fluctuations. Science 1997, 277, 1975–1978. [Google Scholar] [CrossRef]
- Nielsen, M.N.; Alonni, S.; De Yoreo, J.J. In situ TEM imaging of CaCO₃ nucleation reveals coexistence of direct and indirect pathways. Science 2014, 345, 1158. [Google Scholar] [CrossRef]
- Wang, X.; Chou, I.-M.; Hu, W.; Burruss, R.C. In situ observations of liquid–liquid phase separation in aqueous MgSO4 solu-tions: Geological and geochemical implications. Geochim. Cosmochim. Acta 2013, 103, 1. [Google Scholar] [CrossRef]
- Loh, N.D.; Sen, S.; Bosman, M.; Tan, M.S.F.; Zhong, J.; Nijhuis, C.A.; Král, P.; Matsudaira, P.; Mirsaidov, U. Multistep nucleation of nanocrystals in aqueous solution. Nat. Chem. 2017, 9, 77. [Google Scholar] [CrossRef]
- Kawasaki, T.; Tanaka, H. Formation of a crystal nucleus from liquid. Proc. Natl. Acad. Sci. USA 2010, 107, 14036–14041. [Google Scholar] [CrossRef]
- Gasser, U.; Weeks, E.R.; Schofield, A.; Pusey, P.N.; Weitz, D.A. Real space imaging of nucleation and growth in colloidal crystal-lization. Science 2001, 292, 258–262. [Google Scholar] [CrossRef]
- Ostwald, W. The formation and changes of solids. Z. Phys. Chem. 1897, 22, 289–330. [Google Scholar]
- Nývlt, J. The Ostwald Rule of Stages. Cryst. Res. Technol. 1995, 30, 443–449. [Google Scholar] [CrossRef]
- Kelton, K.F.; Lee, G.W.; Gangopadhyay, A.K.; Hyers, R.W.; Rathz, T.J.; Rogers, J.R.; Robinson, M.B.; Robinson, D.S. First X-ray scattering studies on electrostatically levitated metallic liquids: Demonstrated influence of local icosahedral order on the nu-cleation barrier. Phys. Rev. Lett. 2003, 90, 195504. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Wi, H.S.; Jo, W.; Cho, Y.C.; Lee, H.H.; Jeong, S.Y.; Kim, Y.I.; Lee, G.W. Multiple pathways of crystal nucleation in an ex-tremely supersaturated aqueous potassium dihydrogen phosphate (KDP) solution droplet. Proc. Natl. Acad. Sci. USA 2016, 113, 13618–13623. [Google Scholar] [CrossRef]
- Lee, G.W.; Evans, W.J.; Yoo, C.-S. Crystallization of water in a dynamic diamond-anvil cell: Evidence for ice VII-like local order in supercompressed water. Phys. Rev. B 2006, 74. [Google Scholar] [CrossRef]
- Hu, Y.-C.; Tanaka, H. Physical origin of glass formation from multicomponent systems. Sci. Adv. 2020, 6, eabd2928. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xu, Y.; Hui, X.; Lu, Z.; Li, F.; Chen, G.L.; Lu, J.; Liu, C.T. Metallic Liquids and Glasses: Atomic Order and Global Packing. Phys. Rev. Lett. 2010, 105, 155501. [Google Scholar] [CrossRef]
- Thomä, S.L.J.; Krauss, S.W.; Eckardt, M.; Chater, P.; Zobel, M. Atomic insight into hydration shells around facetted nano-particles. Nat. Commun. 2019, 10, 995. [Google Scholar] [CrossRef]
- Zobel, M.; Neder, R.B.; Kimber, S.A.J. Universal solvent restructuring induced by colloidal nanoparticles. Science 2015, 347, 292–294. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.; Cho, Y.C.; Lee, S.; Lee, Y.-H.; Kim, S.; Kim, Y.; Jo, W.; Duchstein, P.; Zahn, D.; Lee, G.W. Hydration breaking and chemical ordering in a levitated NaCl solution droplet beyond the metastable zone width limit: Evidence for the early stage of two-step nucleation. Chem. Sci. 2020, 12, 179–187. [Google Scholar] [CrossRef]
- Anwar, J.; Zahn, D. Uncovering Molecular Processes in Crystal Nucleation using Molecular Simulation. Angew. Chem. Int. Ed. 2011, 50, 1996–2013. [Google Scholar] [CrossRef]
- Anwar, J.; Boateng, P.K. Computer Simulation of Crystallization from Solution. J. Am. Chem. Soc. 1998, 120, 9600–9604. [Google Scholar] [CrossRef]
- Turci, F.; Schilling, T.; Yamani, M.; Oettel, M. Solid phase properties and crystallization in simple model systems. Eur. Phys. J. Spec. Top. 2014, 223, 421–438. [Google Scholar] [CrossRef]
- Koschke, K.; Limbach, H.J.; Kremer, K.; Donadio, D. Freezing point depression in model Lennard-Jones solutions. Mol. Phys. 2015, 113, 2725–2734. [Google Scholar] [CrossRef]
- Maeda, K.; Miki, T.; Itoh, K.; Arafune, K.; Yamamoto, T.; Fukui, K. Anti-solvent crystallization of a ternary Lennard–Jones mixture performed by molecular dynamics. J. Mol. Liq. 2015, 209, 1–5. [Google Scholar] [CrossRef]
- Anwar, J.; Khan, S.; Lindfors, L. Secondary Crystal Nucleation: Nuclei Breeding Factory Uncovered. Angew. Chem. Int. Ed. 2015, 54, 14681–14684. [Google Scholar] [CrossRef]
- Schilling, T.; Frenkel, D. Self-poisoning of crystal nuclei in hard-rod liquids. J. Phys. Condens. Matter 2004, 16, S2029–S2036. [Google Scholar] [CrossRef]
- Anwar, J.; Boateng, P.K.; Tamaki, R.; Odedra, S. Mode of Action and Design Rules for Additives That Modulate Crystal Nucleation. Angew. Chem. Int. Ed. 2009, 48, 1596–1600. [Google Scholar] [CrossRef]
- Gale, J.D. Empirical potential derivation for ionic materials. Philos. Mag. B 1996, 73, 3–19. [Google Scholar] [CrossRef]
- Raiteri, P.; Gale, J.D. Water Is the Key to Nonclassical Nucleation of Amorphous Calcium Carbonate. J. Am. Chem. Soc. 2010, 132, 17623–17634. [Google Scholar] [CrossRef]
- Wallace, A.F.; Hedges, L.O.; Fernandez-Martinez, A.; Raiteri, P.; Gale, J.D.; Waychunas, G.A.; Whitelam, S.; Banfield, J.F.; De Yoreo, J.J. Microscopic Evidence for Liquid-Liquid Separation in Supersaturated CaCO3 Solutions. Science 2013, 341, 885–889. [Google Scholar] [CrossRef]
- Gebauer, D.; Völkel, A.; Cölfen, H. Stable Prenucleation Calcium Carbonate Clusters. Science 2008, 322, 1819–1822. [Google Scholar] [CrossRef]
- Auer, S.; Frenkel, D. Numerical Simulation of Crystal Nucleation in Colloids. In Advanced Computer Simulation; Springer: Berlin, Germany, 2005; pp. 149–208. [Google Scholar] [CrossRef]
- Kawska, A.; Brickmann, J.; Kniep, R.; Hochrein, O.; Zahn, D. An Atomistic Simulation Scheme for Modeling Crystal Formation from Solution. J. Chem. Phys. 2006, 124, 24513. [Google Scholar] [CrossRef]
- Salvalaglio, M.; Perego, C.; Giberti, F.; Mazzotti, M.; Parrinello, M. Molecular-dynamics simulations of urea nucleation from aqueous solution. Proc. Natl. Acad. Sci. USA 2014, 112, E6–E14. [Google Scholar] [CrossRef]
- Ectors, P.; Duchstein, P.; Zahn, D. From oligomers towards a racemic crystal: Molecular simulation of dl-norleucine crystal nu-cleation from solution. CrystEngComm 2015, 17, 6884–6889. [Google Scholar] [CrossRef]
- Milek, T.; Zahn, D. Molecular Simulation of Ag Nanoparticle Nucleation from Solution: Redox-Reactions Direct the Evolution of Shape and Structure. Nanoletters 2014, 14, 4913–4917. [Google Scholar] [CrossRef]
- Kawska, A.; Duchstein, P.; Hochrein, O.; Zahn, D. Atomistic Mechanism of ZnO Nucleation from Ethanolic Solution: Ion Associ-ation, Proton Transfer and Selforganization. Nanoletters 2008, 8, 2336–2340. [Google Scholar] [CrossRef]
- Siezen, R.J.; Fisch, M.R.; Slingsby, C.; Benedek, G.B. Opacification of gamma-crystallin solutions from calf lens in relation to cold cataract formation. Proc. Natl. Acad. Sci. USA 1985, 82, 1701–1705. [Google Scholar] [CrossRef]
- Magdoff-Fairchild, B.; Chiu, C.C. X-ray diffraction studies of fibers and crystals of deoxygenated sickle cell hemoglobin. Proc. Natl. Acad. Sci. USA 1979, 76, 223–226. [Google Scholar] [CrossRef]
- Ross, C.A.; Poirier, M.A. Protein aggregation and neurodegenerative disease. Nat. Med. 2004, 10, S10–S17. [Google Scholar] [CrossRef]
- Mikol, V.; Hirsch, E.; Giegé, R. Monitoring protein crystallization by dynamic light scattering. FEBS Lett. 1989, 258, 63–66. [Google Scholar] [CrossRef]
- Boué, F.; Lefaucheux, F.; Robert, M.; Rosenman, I. Small angle neutron scattering study of lysozyme solutions. J. Cryst. Growth 1993, 133, 246–254. [Google Scholar] [CrossRef]
- Pusey, M.L. Estimation of the initial equilibrium constants in the formation of tetragonal lysozyme nuclei. J. Cryst. Growth 1991, 110, 60–65. [Google Scholar] [CrossRef]
- Niimura, N.; Minezaki, Y.; Ataka, M.; Katsura, T. Aggregation in supersaturated lysozyme solution studied by time-resolved small angle neutron scattering. J. Cryst. Growth 1995, 154, 136–144. [Google Scholar] [CrossRef]
- Rosenberger, F. Protein crystallization. J. Cryst. Growth 1996, 166, 40–54. [Google Scholar] [CrossRef]
- Igarashi, K.; Azuma, M.; Kato, J.; Ooshima, H. The initial stage of crystallization of lysozyme: A differential scanning calorimetric (DSC) study. J. Cryst. Growth 1999, 204, 191–200. [Google Scholar] [CrossRef]
- Michinomae, M.; Mochizuki, M.; Ataka, M. Electron microscopic studies on the initial process of lysozyme crystal growth. J. Cryst. Growth 1999, 197, 257–262. [Google Scholar] [CrossRef]
- Drenth, J.; Haas, C. Nucleation in protein crystallization. Acta Crystallogr. Sect. D Biol. Crystallogr. 1998, 54, 867–872. [Google Scholar] [CrossRef] [PubMed]
- Haas, C.; Drenth, J. Understanding protein crystallization on the basis of the phase diagram. J. Cryst. Growth 1999, 196, 388–394. [Google Scholar] [CrossRef]
- Vekilov, P.G. Dense Liquid Precursor for the Nucleation of Ordered Solid Phases from Solution. Cryst. Growth Des. 2004, 4, 671–685. [Google Scholar] [CrossRef]
- Buell, A.K. The Nucleation of Protein Aggregates—From Crystals to Amyloid Fibrils. Int. Rev. Cell Mol. Biol. 2017, 329, 187–226. [Google Scholar] [CrossRef]
- Zhang, F. Nonclassical nucleation pathways in protein crystallization. J. Phys. Condens. Matter 2017, 29, 443002. [Google Scholar] [CrossRef]
- Yamazaki, T.; Kimura, Y.; Vekilov, P.G.; Furukawa, E.; Shirai, M.; Matsumoto, H.; Van Driessche, A.E.S.; Tsukamoto, K. Two types of amorphous protein particles facilitate crystal nucleation. Proc. Natl. Acad. Sci. USA 2017, 114, 2154–2159. [Google Scholar] [CrossRef]
- Sleutel, M.; Van Driessche, A.E.S. Nucleation of protein crystals—A nanoscopic perspective. Nanoscale 2018, 10, 12256–12267. [Google Scholar] [CrossRef]
- Houben, L.; Weissman, H.; Wolf, S.G.; Rybtchinski, B. A mechanism of ferritin crystallization revealed by cryo-STEM tomography. Nature 2020, 579, 540–543. [Google Scholar] [CrossRef]
- Van Driessche, A.E.S.; Van Gerven, N.; Bomans, P.H.H.; Joosten, R.R.M.; Friedrich, H.; Gil-Carton, D.; Sommerdijk, N.; Sleutel, M. Molecular nucleation mechanisms and control strategies for crystal polymorph selection. Nature 2018, 556, 89–94. [Google Scholar] [CrossRef]
- Vekilov, P.G. Nucleation. Cryst. Growth Des. 2010, 10, 5007–5019. [Google Scholar] [CrossRef]
- Duran-Olivencia, M.A.; Yatsyshin, P.; Kalliadasis, S.; Lutsko, J.F.; Lutsko, J. General framework for nonclassical nucleation. New J. Phys. 2018, 20, 083019. [Google Scholar] [CrossRef]
- De Yoreo, J.J.; Gilbert, P.U.P.A.; Sommerdijk, N.A.J.M.; Penn, R.L.; Whitelam, S.; Joester, D.; Zhang, H.; Rimer, J.D.; Navrotsky, A.; Banfield, J.F.; et al. Crystallization by particle attachment in synthetic, biogenic, and geologic environments. Science 2015, 349, aaa6760. [Google Scholar] [CrossRef]
- Zhang, T.-H.; Liu, X.Y. Experimental modelling of single-particle dynamic processes in crystallization by controlled colloidal assembly. Chem. Soc. Rev. 2014, 43, 2324–2347. [Google Scholar] [CrossRef]
- Van Meel, J.A.; Page, A.J.; Sear, R.P.; Frenkel, D. Two-step vapor-crystal nucleation close below triple point. J. Chem. Phys. 2008, 129, 204505. [Google Scholar] [CrossRef] [PubMed]
- Sleutel, M.; Van Driessche, A.E.S. Role of clusters in nonclassical nucleation and growth of protein crystals. Proc. Natl. Acad. Sci. USA 2014, 111, E546–E553. [Google Scholar] [CrossRef]
- Asherie, N. Protein crystallization and phase diagrams. Methods 2004, 34, 266–272. [Google Scholar] [CrossRef]
- George, A.; Wilson, W.W. Predicting protein crystallization from a dilute solution property. Acta Crystallogr. Sect. D Biol. Crystallogr. 1994, 50, 361–365. [Google Scholar] [CrossRef]
- Vliegenthart, G.A.; Lekkerkerker, H.N.W. Predicting the gas–liquid critical point from the second virial coefficient. J. Chem. Phys. 2000, 112, 5364–5369. [Google Scholar] [CrossRef]
- Morrison, H.; De Boer, G.; Feingold, G.; Harrington, J.Y.; Shupe, M.D.; Sulia, K. Resilience of persistent Arctic mixed-phase clouds. Nat. Geosci. 2011, 5, 11–17. [Google Scholar] [CrossRef]
- Tsurusawa, H.; Russo, J.; Leocmach, M.; Tanaka, H. Formation of porous crystals via viscoelastic phase separation. Nat. Mater. 2017, 16, 1022–1028. [Google Scholar] [CrossRef] [PubMed]
- Maier, R.; Zocher, G.; Sauter, A.; Da Vela, S.; Matsarskaia, O.; Schweins, R.; Sztucki, M.; Zhang, F.; Stehle, T.; Schreiber, F. Protein Crystallization in the Presence of a Metastable Liquid–Liquid Phase Separation. Cryst. Growth Des. 2020, 20, 7951–7962. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).