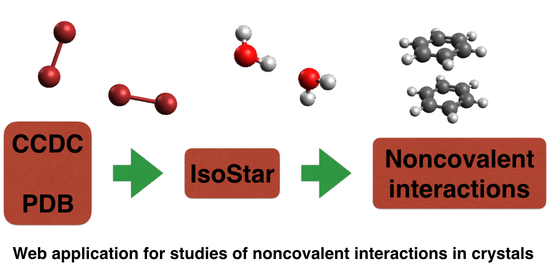
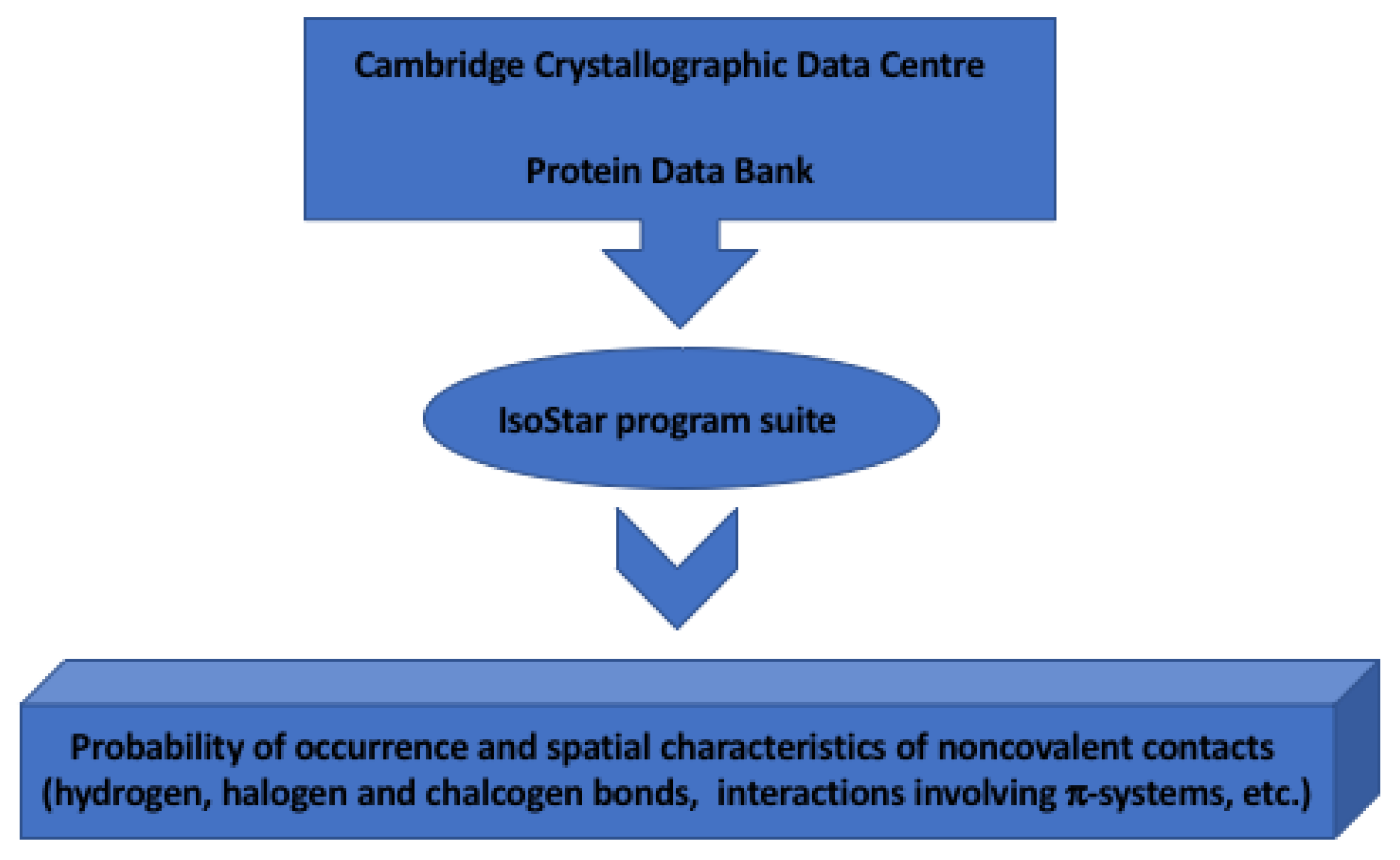
IsoStar Program Suite for Studies of Noncovalent Interactions in Crystals of Chemical Compounds
Abstract
Funding
Conflicts of Interest
References
- Bruno, I.J.; Cole, J.C.; Lommerse, J.P.M.; Rowland, R.S.; Taylor, R.; Verdonk, M.L. IsoStar: A Library of Information About Nonbonded Interactions. J. Comput. Aided Mol. Des. 1997, 11, 525–537. [Google Scholar] [CrossRef] [PubMed]
- Battle, G.M.; Allen, F.H. Learning about Intermolecular Interactions from the Cambridge Structural Database. J. Chem. Educ. 2012, 89, 38–44. [Google Scholar] [CrossRef]
- Aakeröy, C.B.; Sinha, A.S. Chapter 1: Co-crystals: Introduction and Scope. In Co-crystals: Preparation, Characterization and Applications; Aakeröy, C.B., Sinha, A.S., Eds.; The Royal Society of Chemistry: London, UK, 2018; pp. 1–32. [Google Scholar] [CrossRef]
- Sinha, A.S.; Aakeröy, C.B. Design of Molecular Crystals Supramolecular Synthons. In Comprehensive Supramolecular Chemistry II; Elsevier: Amsterdam, The Netherlands, 2017; pp. 3–24. [Google Scholar] [CrossRef]
- Corpinot, M.K.; Bučar, D.-K. A Practical Guide to the Design of Molecular Crystals. Cryst. Growth Des. 2019, 19, 1426–1453. [Google Scholar] [CrossRef]
- Vologzhanina, A.V. Intermolecular Interactions in Functional Crystalline Materials: From Data to Knowledge. Crystals 2019, 9, 478. [Google Scholar] [CrossRef]
- Moghadam, P.Z.; Li, A.; Wiggin, S.B.; Tao, A.; Maloney, A.G.P.; Wood, P.A.; Ward, S.C.; Fairen-Jimenez, D. Development of a Cambridge Structural Database Subset: A Collection of Metal–Organic Frameworks for Past, Present, and Future. Chem. Mater. 2017, 29, 2618–2625. [Google Scholar] [CrossRef]
- Mooibroek, T.J. DFT and IsoStar Analyses to Assess the Utility of σ- and π-Hole Interactions for Crystal Engineering. ChemPhysChem 2021, 22, 141–153. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Novikov, A.S. IsoStar Program Suite for Studies of Noncovalent Interactions in Crystals of Chemical Compounds. Crystals 2021, 11, 162. https://doi.org/10.3390/cryst11020162
Novikov AS. IsoStar Program Suite for Studies of Noncovalent Interactions in Crystals of Chemical Compounds. Crystals. 2021; 11(2):162. https://doi.org/10.3390/cryst11020162
Chicago/Turabian StyleNovikov, Alexander S. 2021. "IsoStar Program Suite for Studies of Noncovalent Interactions in Crystals of Chemical Compounds" Crystals 11, no. 2: 162. https://doi.org/10.3390/cryst11020162
APA StyleNovikov, A. S. (2021). IsoStar Program Suite for Studies of Noncovalent Interactions in Crystals of Chemical Compounds. Crystals, 11(2), 162. https://doi.org/10.3390/cryst11020162