Abstract
(Z)-N′-(4-methoxybenzylidene)benzohydrazide (HL) and its Ni(II) complex (Ni(II)-2L) were synthesized using eco-friendly protocols. The single X-ray crystal structure of Ni(II)-2L was solved. Moreover, the structural properties were evaluated using Fourier transform infrared, proton nuclear magnetic resonance, mass, and Ultraviolet/Visible spectroscopy. The diamagnetic and thermal stability were assessed using magnetic susceptibility and thermogravimetric analysis, respectively. The biological activities of both HL and Ni(II)-2L (62.5–1000 μg/mL) against Gram-positive (Staphylococcus aureus and Streptococcus pyogenes) and Gram-negative (Escherichia coli and Pseudomonas aeruginosa) bacterial and fungal (Candida albicans, Aspergillus niger, and Aspergillus clavatus) species were studied using the minimum inhibitory concentration (MIC) tests method in reference to Gentamycin and Nystatin standard drugs, respectively. The results revealed an affordable, environmentally friendly, and efficient synthetic method of HL using water as a green solvent. The Ni(II)-2L complex crystallized in a distorted square planar, P21/n space group, and one Ni(II) to two bidentate negatively charged ligand ratio. The analysis of biological activity revealed higher activity of the complex against S. aureus and S. pyogenes (bacteria) and A. niger and A. clavatus (fungi) compared to the ligand. However, the highest activity was at a MIC of 62.5 μg/mL for the complex against S. pyogenes and for the ligand against E. coli. Therefore, both HL and Ni(II)-2L could be promising potential antimicrobials and their selective activity could be an additional benefit of these bioactive materials.
1. Introduction
The synthesis of hydrazones and their metal complexes has attracted considerable scientific interest [1]. They are an essential class of biologically active compounds with unique properties, and they exhibit a broad spectrum of physiological and pharmacological activities [2]. However, biological activity is a structure-dependent property. Therefore, various derivatives have been synthesized and adequately investigated. Hydrazones possess an azomethine–NHN = CH- group, formed by the reaction of aldehydes or ketones with hydrazine or hydrazide motifs. They are a special class of Schiff bases, which are known to exhibit a wide range of interesting biological activities, including antioxidant, antimicrobial, anti-inflammatory, and anticonvulsant properties [3,4,5,6,7]. Moreover, the C=O group in the aroylhydrazones (-C=N-NH-CO-) compounds provides the molecule with an additional donor site, making them a more versatile and effective chelating agent with the ability to form a variety of complexes with different transition ions [5,8,9]. Complexation of aroylhydrazones with metal ions results in more reactive materials due to the presence of both imine and carbonyl groups, and it is an emerging class of Schiff base complexes, reported to have useful antimicrobial [10,11,12], antioxidant [13], antitumor [14,15], antidepressant [16], analgesic, anti-inflammatory [17], antitubercular [18,19], anti-convulsant [20], and sensor [21] properties.
N′-(4-methoxybenzylidene)benzohydrazide (MBBH) is a type of aroylhydrazones that possesses both hydrazone and carbonyl groups, which may synergically contribute to their biological activities [22]. Due to their appealing properties, MBBH has been subjected to extensive studies by various researchers [7,13,22,23,24,25,26,27,28]. The crystal structure reported in the literature [27] for MBBH indicates that an (E)-MBBH structure is the preferred configuration. The methods used for MBBH synthesis are varied and use different solvents and reaction conditions. For instance, Periakaruppan et al. [7] used two methods, employing an optimized ratio of chloroacetic acid and malononitrile in water. However, regardless of the synthetic method, its physicochemical and biological activities were adequately investigated. Despite the straightforward methods available for the synthesis of MBBH, green protocols are always needed. Thus, trying to use readily available and eco-friendly media, such as water, is one target for the future of green chemistry [29], for both small- and large-scale productions.
Nickel (Ni) is a ferromagnetic transition metal with an electron configuration of [Ar] 3d8 4s2. Its most common oxidation state is Ni2+, however, compounds with other oxidation states [30] as well as various geometries including tetrahedral and square planar (4-coordinate), trigonal bipyramidal and square pyramidal (5-coordinate), octahedral (6-coordinate), etc., were also known. Besides its implication in a wide variety of metallurgical processes, it plays a well-defined role in biological systems as well [31]. Since nickel amount in individuals is trace, its deficiency is rare [32]. Yet, nickel is known to be essential, at least, for nine classes of enzymes to be active [31], which involved in some catalytic redox and nonredox chemistries.
The coordination chemistry of hydrazide derivatives such as aroylhydrazones with transition metals has interested many authors [22,33,34,35,36,37,38,39]. Nickel(II)-MBBH complex was synthesized by El-Sayed et al. [22]. According to the authors, and depending on the magnetic and spectral analysis, aroylhydrazones can react as a neutral ligand, resulting in octahedral complexes. However, the treatment of such complexes with alkalies leads to their transformation into square planar neutral compounds. Balachandran and George [37] studied the oxidation of some aroylhydrazones, including MBBH, with nickel peroxides and reporting a possible production of 23–41% yields as nickel complexes. However, to the best of our knowledge, no research has been conducted on the crystal structure and biological activities of nickel–MBBH complexes.
Nevertheless, nickel complexes of very close Schiff bases were reported. A square planar structural geometry of nickel-bidentates and an octahedral of nickel-tridentate ones [40,41,42,43,44,45] were common. In addition, the electron density of the amide oxygen and imine nitrogen of aroylhydrazones, involved in coordination with nickel ions, can be controlled by protonation–deprotonation of the amide nitrogen. Thus, structural geometry could be converted from square planar to octahedral within protonation [22,42], making them potentials in various applications including, chemosensors and photo-conductors as well as pharmaceutical drugs for the treatment of, e.g., cancer, schizophrenia, leprosy, etc. [42].
Therefore, the aim of this work was to synthesize MBBH Schiff base (ligand, HL) and its nickel complex (Ni(II)-2L) using optimized, affordable, and eco-friendly methods. Subsequently, Ni(II)-2L was successfully crystallized, and its crystal structure was determined. The structural properties were characterized using various spectral, magnetic, and thermal techniques. Additionally, their biological activities against different types of bacterial and fungal species were compared.
2. Materials and Methods
2.1. Chemicals
p-Anisaldehyde (4-Methoxybenzaldehyde) (pAAD; 99%) was purchased from HPLC Lab Reagent (High Purity Laboratory Chemicals Pvt. Ltd, Sarigam INA, India). N, N-dimethylformamide (DMF, 99.5%), benzoic hydrazide (BHD, 99%), and polyethylene glycol 400 (PEG, Mn ≈ 400 g/mol) were obtained from Spectrochem Pvt. Ltd (Mumbai, India). Absolute ethanol (EtOH, 99.9%), dichloromethane (DCM, 99%), ethyl acetate (EA, 99%), nickel(II) nitrate hexahydrate (Ni(NO3)2∙6H2O, 98%), and ammonium hydroxide (NH4OH) were obtained from SD Fine Chem Limited (Mumbai, India). Double distilled water was used when needed.
2.2. Microorganisms
Microbes were procured from the “Institute of Microbial Technology” (IMTECH), Chandigarh, India, and used for antimicrobial activity studies: the Gram-positive bacteria Staphylococcus aureus (S. aureus (MTCC 96)) and Streptococcus pyogenes (S. pyogenes (MTCC 442)), the Gram-negative bacteria Escherichia coli (E. coli (MTCC 443)) and Pseudomonas aeruginosa (P. aeruginosa (MTCC 1688)), and the fungi Candida albicans (C. albicans (MTCC 227)), Aspergillus niger (A. niger (MTCC 282)), and Aspergillus clavatus (A. clavatus (MTCC 1323)) were used in the study.
2.3. Synthesis of (Z)-N′-(4-Methoxybenzylidene) Benzohydrazide Ligand
For comparison, four different reaction pathways (methods i-iv) were used to synthesize the targeted MBBH Schiff base (from here on, this ligand will be referred to as HL) as shown in Figure 1. The reaction progression was monitored using thin-layer chromatography (TLC), where DCM/EA (2:8 volume ratio) is the mobile phase, and silica gel is the stationary phase. After reaction completion, the reaction mixture was poured into ice-cold water and the precipitated title compound was filtered, dried, and kept until use. However, as detailed below, method (i) is conventional, whereas methods (ii-iv) are new and considerably eco-friendly.
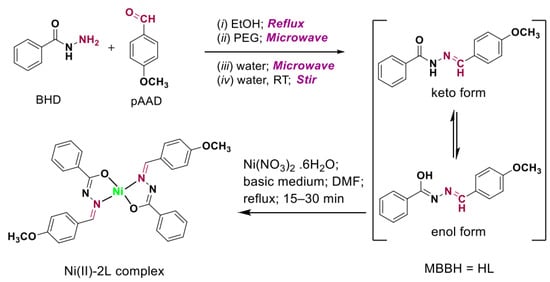
Figure 1.
Schematic presentation of the methods used for the synthesis of anisole Schiff base (HL) and its nickel(II) complex (Ni(II)-2L).
Method (i), reflux method. An equimolar of pAAD and BHD (10 mmol each) was refluxed at 70–80 °C for 2 h in 20 mL absolute ethanol.
Method (ii) and (iii), microwave methods. The reactants pAAD and BHD (10 mmol each) were dissolved in 10 mL of either PEG (method (ii)) or water (method (iii)). Then, they were applied into the microwave reactor for 2 min or 20 s, respectively.
Method (iv), stir method. An aqueous solution of the reactants similar to that used in method (iii) was prepared, but the mixture was stirred at room temperature for 30 s to produce the intended Schiff base (HL).
2.4. Synthesis of (Z)-N′-(4-Methoxybenzylidene) Benzo Hydrazide Ni(II) Complex–(Ni(II)-2L)
A nickel(II) complex of HL (denoted Ni(II)-2L) was synthesized using nickel nitrate salt (Figure 1). In a round-bottom flask, 2 mmol (0.508 g) of HL was dissolved in DMF (15 mL), and a nickel salt (1 mmol, 0.291 g) was added in 5 mL of water. The medium was alkalized with the addition of a few drops of NH4OH. Then, it was heated at ~80 °C under reflux conditions for 30 min. The TLC monitoring test indicated an almost complete reaction in the first 15 min. However, the reaction was continued for 30 min to achieve completeness. Then, it was cooled and left to rest overnight at room temperature. The obtained crystals were filtered, washed with DMF, and dried in vacuo to finally produce orange crystals with an 87% yield. It is worth mentioning that trial synthesis of Ni(II)-2L using EtOH and PEG as reaction mediums was also performed under a similar condition. However, only powder-like microcrystals were observed. Moreover, the microwave-assisted synthesis method using DMF, EtOH, and PEG as reaction mediums (using Ni(II)/ligand mole ratio as 1: 2; the medial was also alkalized using drops of ammonium hydroxide as above) operated for 1.5 min to produce high yields of about 98%. However, only microcrystals were obtained. Thus, only crystals obtained from the reflux method in DMF were used for further studies, including single X-ray analyses.
2.5. Instrumentation
Fourier transform infrared (FTIR) spectra were recorded using a Nicolet iS10 spectrophotometer (Thermo Scientific, Waltham, MA, USA) with a method of attenuated total reflection (ATR; diamond crystal), over a 4000–650 cm−1 region with a spectral resolution of 4 cm−1 and 16 scans per spectrum. Elemental analysis was performed using a Euro EA3000 CHNS-O analyzer (EuroVector S.p.A. (EVISA), Milan, Italy). Proton and carbon-13 nuclear magnetic resonance (1H-, 13C-NMR) spectra were obtained at room temperature using a JEOL ECP400 NMR spectroscope (JEOL Ltd., Akishima, Tokyo, Japan). The mass spectra were recorded using an LC-plus Accu-TOF JMS-T100 LP spectrometer (JEOL Ltd., Akishima, Tokyo, Japan) equipped with a DART ion source (IonSense, Saugus, MA, USA) and operated in the +ve-ion mode. Selected peaks were assigned using Mass Centre software (version 1.3.m). Thermogravimetric analysis/differential thermal analysis (TGA/DTA) curves were obtained using a Shimadzu DTG-60H (Kyoto, Japan) thermal analyzer in a platinum cell and operated with a heating rate of 20 °C/min from 25 to 1000 °C and a nitrogen flow of 50 mL/min. Melting points were measured using a CL-726 digital apparatus (IndiaMART Member Since, India). Electronic spectra were recorded over the range of 200–800 nm at room temperature using a UV-1700 spectrophotometer (Shimadzu, Kyoto, Japan) in a DMSO/water (2:8 v/v) mix solvent. Molar absorptivity (ɛ, M−1 cm−1) of both HL and Ni(II)-2L (0.0003 M) was analyzed according to Peer-Lambert law at room temperature. Magnetic susceptibility measurements were carried out on solid complex at room temperature (297 K) using a Sherwood Scientific magnetic susceptibility balance (MSB) Mark I (Cambridge, UK), calibrated with a sealed standard manganese(II) chloride solution. The magnetic moment was calculated using Equation (1).
where µeff is the effective magnetic moment, xm is the molar susceptibility, and T (K) is the absolute temperature.
2.6. X-ray Crystallography
Diffraction data of the single-crystal structure were collected at 173 K on a Bruker APEX-III photon detector diffractometer equipped with an Oxford Cryosystems, Cryostream 700Plus cryostat. A multilayer monochromator with MoKα radiation (λ = 0.71073 Å) from an Incoatec IμS microsource was used. Data reduction was conducted following standard procedures using the Bruker software package SAINT [46], and absorption and other systematic error corrections were performed using SADABS [47,48]. The structures were solved by direct methods using SHELXS-97 and refined using SHELXL-97 [49]. X-Seed [50] was used as the graphical interface for the SHELX program suite. Hydrogen atoms were placed in calculated positions using riding models. The crystallographic data for the Ni(II)-2L complex are provided in Table 1.

Table 1.
Crystal structure and refinement details for Ni(II)-2L.
2.7. Biological Studies
A minimum inhibitory concentration (MIC) test was performed using the macro-double dilution method [51]. Generally, material solutions of 62.5–1000 µg/mL were prepared in DMSO in labeled test tubes. A measure of 1 mL of nutrient broth or Sabouraud dextrose and 10 μL of bacteria or fungi strains, respectively, were added to each tube. Then, the tubes were incubated at 37 °C for 18–24 h, and after that, they were observed for turbidity or growth. Gentamycin of 0.5–1.0 μg/mL and Nystatin of 100 μg/mL were used as standard antibacterial and antifungal drugs, respectively.
3. Results and Discussion
3.1. Synthesis Protocols
The HL was successfully synthesized from anisole and benzoic hydrazide substrates (Figure 1). The conditions of the applied methods and physical properties of the obtained products can be seen in Table 2. It is evident that, regardless of the method of synthesis, the yields of the ligand were highest when the medium was water, with the reaction being completed within a few seconds. This suggests that methods (iii) and (iv) provide convenient, sufficient, and eco-friendly strategies for the synthesis of HL. However, methods (i) and (ii) resulted in lower yields and required longer reaction durations of up to 2 h. In all cases, ranges of melting points were recorded. However, methods (iii) and (iv) showed a similar melting point of about 156 °C ± 2 °C. The light-brown color of the product of method (ii) could be due to the solvent effect. Moreover, TLC analysis indicated pure products. However, the products from method (iv) were considered more satisfactory for the subsequent experiments with the advantage of being high yielding at room temperature, in a few seconds, with no heating or catalyst required, and using water as a green medium.

Table 2.
Physical properties of the synthesized N′-(4-methoxybenzylidene)benzohydrazide Schiff (HL) base and its nickel(II) complex (Ni(II)-2L).
Crystals of the Ni(II)-2L complex of sufficient sizes (0.21 × 0.20 × 0.18 mm) for single X-ray analysis were acquired in DMF at 80 °C. Noticeably, the fast production of Ni(II)-2L under microwave conditions only resulted in a powder-like precipitate making it less favorable for further experiments conducted herein.
3.2. Analytical Characterization
3.2.1. Spectral Analysis
Observed and estimated weight percentages of the elements C, H, N, and O in both HL and Ni(II)-2L are shown in Table 3. The consistency between the observed and calculated percentage of atoms indicates the successful synthesis of the target compounds, which is further supported by a single X-ray and other analytical methods.

Table 3.
Analytical data of the N′-(4-methoxybenzylidene)benzohydrazide (HL) ligand and its nickel(II) complex (Ni(II)-2L).
Figure 2 shows the FTIR spectra of HL and Ni(II)-2L. In the spectrum of HL, the medium intensity broadband at 3414 cm−1 possibly represents the OH of the iminol form while the weak band at 3181 cm−1 represents NH (amide-iminol tautomerism), which diminished in the Ni(II)-2L spectrum due to coordination [25,52]. The ν(C=O) band in the HL spectra was clear at 1639 cm−1 which disappeared after complexation, indicating enol form. Moreover, the observed shift in the band of ν(C=N) toward lower energies (from 1601 to 1591 cm−1) confirms their contribution in complexation with nickel [22]. The absorption bands of C-H, C=C, C-C, C-N, and C-O are assigned in the spectrum. As metal-organic bonds are usually weak and found in the fingerprint mid-IR region, following them is difficult due to their overlap with skeletal, ring deformation, and bending modes. However, compared with the parent spectra of HL, a new band was observed at 583 cm−1, which could be speculated as a Ni-O bond in Ni(II)-2L. Additionally, the band found at 461 cm−1 in Ni(II)-2L was for Ni-N [53].
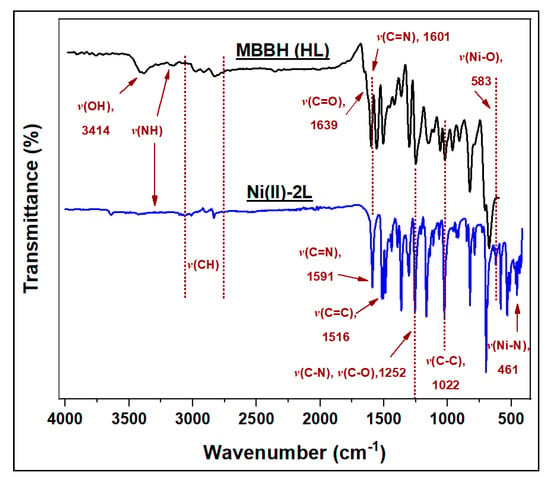
Figure 2.
Fourier transform infrared spectra of the N′-(4-methoxybenzylidene)benzohydrazide (HL) ligand and its nickel(II) complex (Ni(II)-2L).
The structure of the ligand was strictly confirmed using 1H NMR analysis, as shown in Figure 3. The type of H-groups in the molecule was assigned in the spectrum. Furthermore, peak integrations indicated the number of protons in each group. These results are in agreement with those previously reported in the literature [25,54].
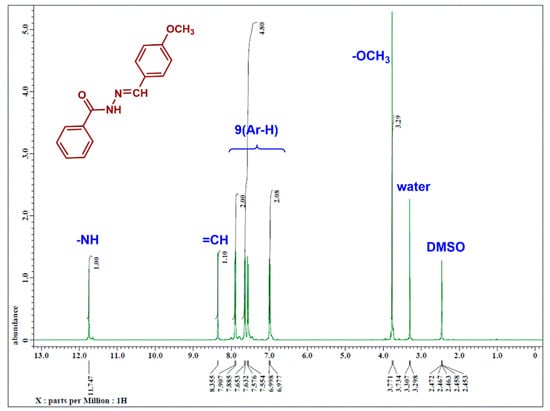
Figure 3.
1H NMR spectrum of N′-(4-methoxybenzylidene) benzohydrazide (HL) in CDCl3.
The mass spectra (obtained in a positive ionization mode, ESI+) and fragmentation pathway of the HL ligand are illustrated in Figure 4. The protonated molecular ion [M+H]+ with an m/z of 255.1137 was the most intense fragment (the base peak), possibly due to the resonance stabilization of the positive charge over the molecule. Fragments with m/z values of 277.0977, 531.2043, and 785.2965 were assigned to the sodium adducts, [M+Na]+, [2M+Na]+, and [3M+Na]+, respectively [55,56,57]. All these results provide evidence of the chemical structure of HL and, thus, its successful synthesis.
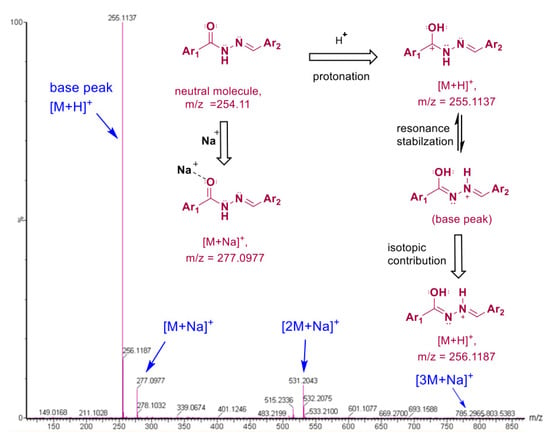
Figure 4.
Mass spectrum (ES+ mode) of N′-(4-methoxybenzylidene)benzohydrazide (HL) Schiff base and its suggested fragmentation pathways.
The electronic spectra of both HL and (Ni(II)-2L) are shown in Figure 5 and Table 4. The spectrum of the ligand revealed a low intense peak at 243 nm and high intense overlapped peaks with maxima at 315 nm and shoulder at ca 296 nm for L–L* transitions (π–π* and n–π*). The spectrum of the complex shows a more or less similar pattern to that of the ligand in the region below 350 nm. However, additional weak bands at lower energies (above 350 nm) possibly responsible for its orange color, were observed. The two bands at 372 and 411 nm could be assigned to the ligand to metal charge transfer (LMCT) transition. The other two bands at 433 and 574 nm were assigned to spin allowed d–d transitions, 1A1g → 1A2g and 1A1g → 1B1g, respectively, which characterize the square planar geometry around Ni(II) ion [58,59,60].
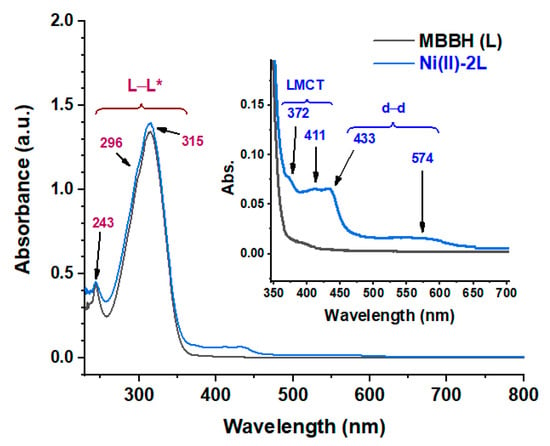
Figure 5.
Electronic spectra of the N′-(4-methoxybenzylidene)benzohydrazide (HL) ligand and its nickel(II) complex (Ni(II)-2L). The inset is a magnification view in the range of 350–700 nm.

Table 4.
Electronic transitions and magnetic moment data of N′-(4-methoxybenzylidene)benzohydrazide (HL) ligand and its nickel(II) complex (Ni(II)-2L).
3.2.2. Magnetic Moment
The magnetic moment measurement of Ni(II)-2L revealed diamagnetic character with an effective magnetic moment of zero (µeff = 0, BM), Table 4. This means that all electrons were paired, indicating square planar coordination, confirming a strong ligand coordinated on its enol form and resulting in a neutral complex. This result is consistent with literature [22,61] and is supported by X-ray crystal structure.
3.2.3. Thermal Analysis
The thermogravimetric analysis of both the HL Schiff base and its nickel(II) complex (Ni(II)-2L) was also performed, under nitrogen atmosphere, for stability assessment. The obtained TGA/DTA thermograms and fragmentation patterns are shown in Figure 6 and Figure 7, respectively, while the data are presented in Table 5. In the thermograms of HL, 9.5% of moisture was lost first at a derivative TGA (DrTGA) of 83 °C, with endothermic peaks at 89 °C. An endothermic peak at DTA of 160 °C with no TGA mass loss was assigned to the HL melting point. However, one step endothermic (DTA 337°C) decomposition of HL is observed at 341 °C (DrTGA) with a mass loss of 90.5% (no mass residue can be detected). Additional endothermic peaks, e.g., at 387 °C and 433 °C, were also detected but not assignable. However, it might be a type of allotropic transformation of carbonaceous materials before final decomposition. The thermal stability of the Ni(II)-2L complex was similar to that of the ligand, meaning that the main decomposition was centered at 341 °C with a mass loss of 42.3%. However, three stages of decomposition were identified. The first stage, below 296 °C, was accompanied by a mass loss of 4.6%, which was estimated as 5.3%, due to the loss of two methyl groups (2(-CH3)). The second stage occurred between 297 °C and 379 °C (with a DrTGA max of 342 °C), with a weight loss of 42.3% due to the elimination of 2[-C7H5NO] fragments, losing the major part of the complex through an endothermic process with DTA max at 340 °C. The third step in the TGA curve indicated a stepwise decomposition above 380 °C, centered at 462 °C, with a mass loss of 30.4% ascribed to the removal of C8H4N2O, leaving a residual mass of 22.8%, which was estimated as 24.1%, assigned to metal carbons (NiC6H6) [62].
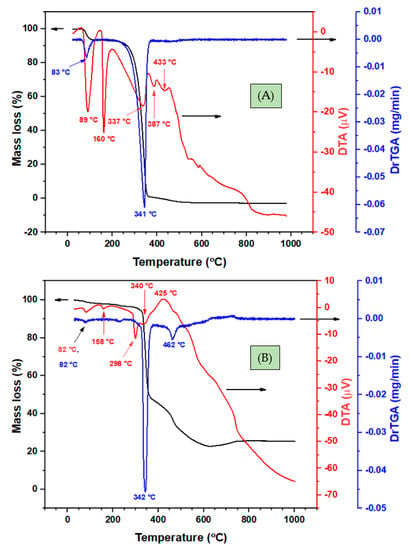
Figure 6.
Thermogravimetric analysis (TGA) (black), derivative (Dr)-TGA (blue), and differential thermal analysis (DTA) (red) curves of (A) the N′-(4-methoxybenzylidene)benzohydrazide (HL) ligand and (B) its nickel complex (Ni(II)-2L).
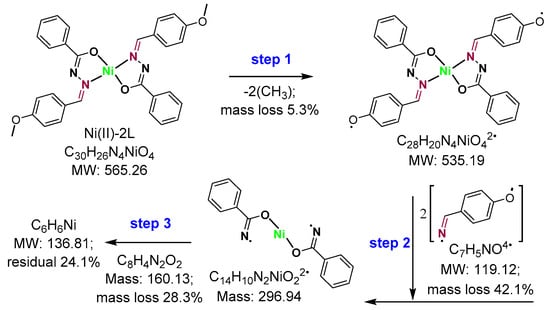
Figure 7.
Schematic presentation of suggested thermogravimetric analysis fragmentation pattern, including the predicted mass-loss percentage.

Table 5.
Thermogravimetric analysis (TGA), derivative (Dr)-TGA, and differential thermal analysis (DTA) data of the N′-(4-methoxybenzylidene)benzohydrazide (HL) Schiff base and Ni(II)-2L.
3.3. Crystal Structure
According to the obtained single-crystal structure data, nickel complex crystallized as a discrete unit in the P21/n space group. The negatively charged ligand bound to Ni(II) ions through the oxygen atom (O1) and the secondary aldimine nitrogen atom (N2). One full Ni(II) ion was bound to two negatively charged ligands in the distorted square planer (hybridization-dsp2) geometry (Figure 8). The overall view along the ac-plane formed by the discrete nickel complex is shown in Figure 9. As the complex is discrete in nature, it is stabilized by the weak van der Waals interactions between the discrete units. No such hydrogen bonding interactions were found in the overall structure. The four donor atoms were provided by the two bidentate chelates ligand, HL. The complex was planar within Ni-O1(and Ni-O1′) of 1.8403(11) and Ni-N (and Ni-N′) of 1.8703(13) Å bonds length, and O1-Ni-N2 (and O1′-Ni-N2′) of 83.70(5) and O1-Ni-N2′ (and O1′-Ni-N2) bond angles around metal. Selected bond lengths and angles are provided in Table 6. However, the complete list of molecular geometry, including bond lengths (Table S1) and bond angles (Table S2) and the crystallographic information files (cif) are available in the supplementary files. The structure reported by Joseph et al. [40] forms 1:1 metal complex with the ligand whereas in our Ni(II)-2L structure the metal to ligand ratio is 1:2. Even though both complexes are based on carbohydrazide derivatives, our Ni(II)-2L structure is without any lattice solvent molecule and the nickel center is in distorted square planer geometry whereas the reported structure has Ni (II) ion in distorted octahedral geometry with lattice N, N′-dimethylformamide molecules. Aroylhydrazones-based square-planar Ni(II) complexes reported by Mondal et al. [42] are somewhat similar to our Ni(II)-2L structure. The reported Ni-O bond distances in the two square-planar complexes are 1.8366 (15) and 1.8283(11), whereas the Ni-N bond distances are 1.8840(16) and 1.8912(14), respectively. These bond distances are almost similar with slight variation as compared to our Ni(II)-2L structure. The presence of NH2 group of anthraniloyl moieties in one of the complexes reported by Mondal and co-workers leads to an intramolecular hydrogen bonding. However, no such intramolecular or intermolecular hydrogen bonding was seen in our Ni(II)-2L case. In addition, there are few other similar such structures are reported in the literature [43,44,45].
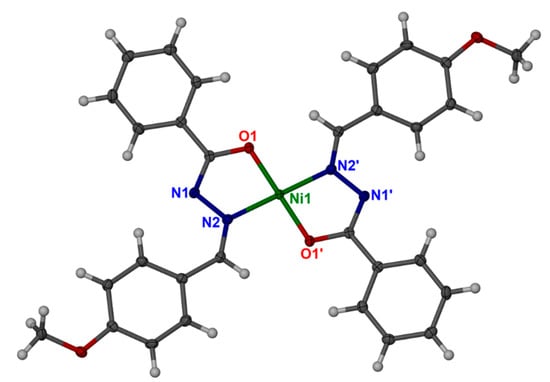
Figure 8.
Coordination mode of Ni(II) ion in the Ni(II)-2L with 50% probability thermal ellipsoid (O1′, N1′ and N2′ are symmetry generated atoms; the symmetry transformation implied for each additional symmetry label atoms: 1-x, -y, 1-z).
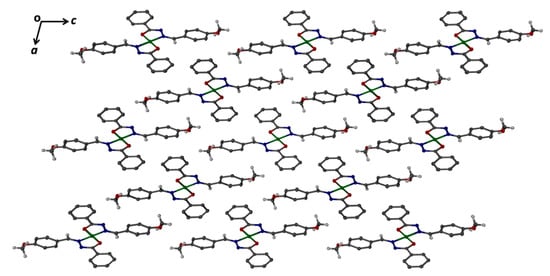
Figure 9.
View showing the arrangement of discrete units of the Ni(II)-2L along ac-plane. Aromatic hydrogen atoms are removed for clarity.

Table 6.
Selected bond length (Å) and bond angles (°) for Ni(II)-2L.
3.4. Biological Activities
The results of the antimicrobial activity tests of both HL and Ni(II)-2L are provided in Table 7. The minimal inhibitory concentration (MIC) values revealed higher activity (lower MIC) of HL against Gram-negative bacteria and lower activity against Gram-positive bacteria. Conversely, the complex activity was higher against Gram-positive bacteria and less active against Gram-negative bacteria. The highest activity (i.e., the lowest MIC) of HL and Ni(II)-2L were against E. coli and S. pyogenes, respectively, and both had a MIC of 62.5 µg/mL. The MIC tests against fungal strains revealed similar activity of the complex against A. niger and A. clavatus with MICs of 500 µg/mL, while it seems inactive against C. albicans. However, HL was more active against C. albicans (MIC of 500 µg/mL) and less active against both A. niger and A. clavatus. Despite the obtained moderate activity of the tested compounds, their activities were still lower than the standard drugs against all microbes under investigation.

Table 7.
Antimicrobial activities of the N′-(4-methoxybenzylidene)benzohydrazide (HL) ligand and its nickel complex (Ni(II)-2L).
The biological properties of bioactive compounds can be rationalized on the basis of their structural differences as well as the cell-wall chemistry of microorganisms. The biological activity of organic compounds is associated with many factors. Some of these factors are the stability, concentration, ability to form hydrogen bonds with active centers of the bacteria cell constituents, and ability to inhibit enzyme function [63]. Moreover, the antimicrobial activity of a complex is governed by various factors, including the chelate effect of the ligand, the type of the donor atoms, the charge, and the geometrical structure of the complex [64]. Both HL and the Ni(II)-2L were comparatively active against bacteria and fungi strains. The ligand can be selectively used for E. coli inhibition; however, the complex is more effective against S. pyogenes. It is worth mentioning that the lipophilicity character of a compound is more favorable in terms of antimicrobial activity. Thus, according to the chelation theory, the ligand activity may be enhanced after coordination [65,66], which explains that a decrease in polarizability of the metal could enhance the lipophilicity of the complexes. This facilitates the compound penetration into the lipid membranes and blocks the metal-binding sites in the enzymes of microorganisms, the action that could disturb the respiration process and protein synthesis, leading to microorganism growth inhibition.
4. Conclusions
An eco-friendly, low-cost, and efficient method for the synthesis of MBBH Schiff base (HL) was established utilizing water as a green solvent. Ni(II)-2L single crystal was synthesized in DMF-based alkaline media with stirring at about 80 °C. Characterization using FTIR, 1HNMR, UV/Vis, mass, and TGA indicated material integrity and successful synthesis. Crystallography showed the distorted square planar geometry of the complex with an ion to ligand ratio of 1:2, organized within the space group of P21/n and monoclinic crystal. The ligand was involved in the crystal structure in its enol form, resulting in a neutral complex as confirmed by a single X-ray analysis. The antimicrobial activity studies indicated that both the ligand and its nickel complex (Ni(II)-2L) are effective, with an overall better activity of the complex compared with the ligand. Interestingly, selective activity of both materials was observed, e.g., Ni(II)-2L was more selective for S. pyogenes (G-positive bacteria) with a MIC of 62.5 μg/m, while it seems that it had no effect on C. albicans (fungi). Similarly, the highest activity of the ligand was against E. coli (62.5 μg/mL) with an overall negligible effect on fungi. Therefore, it might be concluded that HL and Ni(II)-2L are promising antimicrobials with selective properties against targeted species and, thus, deserve further investigation in terms of, e.g., testing other microorganisms and protocols.
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-4352/11/2/110/s1, Crystallographic information files (cif) of the title compound (Ni(II)2L), Table S1: Bond length for the Ni(II)-2L complex, Table S2: Bond angles for the Ni(II)-2L complex.
Author Contributions
Conceptualization, I.A.-Q. and M.F.; formal analysis, A.-B.A.-O. and W.S.S.; funding acquisition, A.A.A.; investigation, I.A.-Q., A.A., A.A.-A., L.A.S.A.-F. and P.L.; methodology, I.A.-Q., L.A.S.A.-F. and P.L.; supervision, M.F.; visualization, A.-B.A.-O. and W.S.S.; writing—original draft, I.A.-Q.; writing—review and editing, A.-B.A.-O. and M.F. All authors have read and agreed to the published version of the manuscript.
Funding
The authors extend their appreciation to the Deanship of Scientific Research at King Saud University for funding this work through Research Group No. RGP-1438-040.
Data Availability Statement
All the data supporting the findings of this study are available within the article and supplementary materials.
Acknowledgments
Prem Lama gratefully acknowledges financial support from the Department of Science and Technology (DST), New Delhi in the form of a DST-INSPIRE Faculty award [DST/INSPIRE/04/2017/000249].
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rajarajan, M.; Senbagam, R.; Vijayakumar, R.; Balaji, S.; Manikandan, V.; Vanangamudi, G.; Thirunarayanan, G. Synthesis, spectral correlations and antimicrobial activities of substituted 4-((E)-2-benzylidenehydrazinyl) benzonitriles. Indian J. Chem. 2016, 55, 197–206. [Google Scholar]
- Chohan, Z.H.; Sherazi, S.K. Biological role of cobalt (II), copper (II) and nickel (II) metal ions on the antibacterial properties of some nicotinoyl-hydrazine derived compounds. Metal-Based Drugs 1997, 4, 69–74. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Belkheiri, N.; Bouguerne, B.; Bedos-Belval, F.; Duran, H.; Bernis, C.; Salvayre, R.; Nègre-Salvayre, A.; Baltas, M. Synthesis and antioxidant activity evaluation of a syringic hydrazones family. Eur. J. Med. Chem. 2010, 45, 3019–3026. [Google Scholar] [CrossRef] [PubMed]
- Deeb, A.; El-Mariah, F.; Hosny, M. Pyridazine derivatives and related compounds. Part 13: Synthesis and antimicrobial activity of some pyridazino [3′,4′:3,4] pyrazolo [5, 1-c]-1,2,4-triazines. Bioorg. Med. Chem. Lett. 2004, 14, 5013–5017. [Google Scholar] [CrossRef]
- Kajal, A.; Bala, S.; Sharma, N.; Kamboj, S.; Saini, V. Therapeutic potential of hydrazones as anti-inflammatory agents. Int. J. Med. Chem. 2014, 2014, 1–11. [Google Scholar] [CrossRef]
- Kaushik, D.; Khan, S.A.; Chawla, G.; Kumar, S. N’-[(5-chloro-3-methyl-1-phenyl-1H-pyrazol-4-yl) methylene] 2/4-substituted hydrazides: Synthesis and anticonvulsant activity. Eur. J. Med. Chem. 2010, 45, 3943–3949. [Google Scholar] [CrossRef]
- Periakaruppan, P.; Abraham, R.; Mahendran, K.; Ramanathan, M. Simple synthesis of hydrazones with quorum quenching activity at room temperature in water. Environ. Chem. Lett. 2018, 16, 1063–1067. [Google Scholar] [CrossRef]
- Cornelissen, J.P.; Van Diemen, J.H.; Groeneveld, L.R.; Haasnoot, J.G.; Spek, A.L.; Reedijk, J. Synthesis and properties of isostructural transition-metal (copper, nickel, cobalt, and iron) compounds with 7,7′,8,8′-tetracyanoquinodimethanide (1-) in an unusual monodentate coordination mode: Crystal structure of bis (3,5-bis (pyridin-2-yl)-4-amino-1,2,4-triazole) bis (7,7′,8,8′-tetracyanoquinodimethanido) copper (II). Inorg. Chem. 1992, 31, 198–202. [Google Scholar]
- Rawat, P.; Singh, R. Synthesis and study on aroylhydrazones having cyanovinylpyrrole. Arab. J. Chem. 2019, 12, 2384–2397. [Google Scholar] [CrossRef]
- Maheswari, R.; Manjula, J. Synthesis, Characterization and Biological Applications of Benzohydrazide derivatives. Int. J. Appl. Res. 2015, 1, 587–592. [Google Scholar]
- Raj, V. Review on the Pharmacological Activities of Hydrazones derivatives. EC Pharm. Sci. 2016, 2, 278–306. [Google Scholar]
- Therese, S.K.; Geethamalika, G. Synthesis, Characterization and Anti Mycobacterial Activity of Novel Hydrazones. Orient. J. Chem. 2017, 33, 335–345. [Google Scholar] [CrossRef]
- Taha, M.; Ismail, N.H.; Jamil, W.; Yousuf, S.; Jaafar, F.M.; Ali, M.I.; Kashif, S.M.; Hussain, E. Synthesis, evaluation of antioxidant activity and crystal structure of 2, 4-dimethylbenzoylhydrazones. Molecules 2013, 18, 10912–10929. [Google Scholar] [CrossRef] [PubMed]
- Wardakhan, W.W.; Sherif, S.M.; Mohareb, R.M.; Abouzied, A.S. The Reaction of Cyanoacetylhdrazine with Furan-2-Aldehyde: Novel Synthesis of Thiophene, Azole, Azine and Coumarin Derivatives and Their Antitumor Evaluation. Int. J. Org. Chem. 2012, 2, 321–331. [Google Scholar] [CrossRef]
- Tyagi, P.; Chandra, S.; Saraswat, B. Ni (II) and Zn (II) complexes of 2-((thiophen-2-ylmethylene) amino) benzamide: Synthesis, spectroscopic characterization, thermal, DFT and anticancer activities. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 134, 200–209. [Google Scholar] [CrossRef]
- Mohareb, R.; El-Sharkawy, K.; Hussein, M.; El-Sehrawi, H. Synthesis of hydrazide-hydrazone derivatives and their evaluation of antidepressant, sedative and analgesic agents. J. Pharm. Sci. Res 2010, 2, 185–196. [Google Scholar]
- Salgın-Gökşen, U.; Gökhan-Kelekçi, N.; Göktaş, Ö.; Köysal, Y.; Kılıç, E.; Işık, Ş.; Aktay, G.; Özalp, M. 1-Acylthiosemicarbazides, 1,2,4-triazole-5 (4H)-thiones, 1,3,4-thiadiazoles and hydrazones containing 5-methyl-2-benzoxazolinones: Synthesis, analgesic-anti-inflammatory and antimicrobial activities. Bioorg. Med. Chem. 2007, 15, 5738–5751. [Google Scholar] [CrossRef]
- Eldehna, W.M.; Fares, M.; Abdel-Aziz, M.M.; Abdel-Aziz, H.A. Design, synthesis and antitubercular activity of certain nicotinic acid hydrazides. Molecules 2015, 20, 8800–8815. [Google Scholar] [CrossRef]
- Joshi, S.; More, Y.; Vagdevi, H.; Vaidya, V.; Gadaginamath, G.; Kulkarni, V. Synthesis of new 4-(2,5-dimethylpyrrol-1-yl)/4-pyrrol-1-yl benzoic acid hydrazide analogs and some derived oxadiazole, triazole and pyrrole ring systems: A novel class of potential antibacterial, antifungal and antitubercular agents. Med. Chem. Res. 2013, 22, 1073–1089. [Google Scholar] [CrossRef]
- Dimmock, J.R.; Vashishtha, S.C.; Stables, J.P. Anticonvulsant properties of various acetylhydrazones, oxamoylhydrazones and semicarbazones derived from aromatic and unsaturated carbonyl compounds. Eur. J. Med. Chem. 2000, 35, 241–248. [Google Scholar] [CrossRef]
- Pegu, R.; Mandal, R.; Guha, A.K.; Pratihar, S. A selective ratiometric fluoride ion sensor with a (2,4-dinitrophenyl) hydrazine derivative of bis (indolyl) methane and its mode of interaction. New J. Chem. 2015, 39, 5984–5990. [Google Scholar] [CrossRef]
- El Sayed, L.; Iskander, M. Coordination compounds of hydrazine derivatives with transition metals—III: The reaction of aroyl hydrazones with Ni (II) and Cu (II) salts. J. Inorg. Nucl. Chem. 1971, 33, 435–443. [Google Scholar] [CrossRef]
- Dimova, V.; Jankulovska, M. QSAR Modeling of Antimicrobial Activity of some p-substituted Aromatic Hydrazones. J. Scient. Ind. Res. 2017, 76, 550–555. [Google Scholar]
- Saleem, H.; Subashchandrabose, S.; Babu, N.R.; Padusha, M.S.A. Vibrational spectroscopy investigation and density functional theory calculations on (E)-N′-(4-methoxybenzylidene) benzohydrazide. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 143, 230–241. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.S.; Lee, D.-U. Syntheses, crystal structure, Hirshfeld surfaces, fluorescence properties, and DFT analysis of benzoic acid hydrazone Schiff bases. Acta A Mol. Biomol. Spectrosc. 2015, 145, 563–574. [Google Scholar] [CrossRef] [PubMed]
- Jankulovska, M.; Čolančeska-Rağenović, K.; Dimova, V.; Spirevska, I.; Makreski, P. Synthesis and characterization of new p-substituted aromatic hydrazones. Org. Chem. An Ind. J. 2012, 8, 326–334. [Google Scholar]
- Gou, J.-X.; Song, M.-Z.; Fan, C.-G.; Yang, Z.-N. (E)-N′-(4-Methoxybenzylidene) benzohydrazide. Acta Cryst. E 2009, 65, 3207–3213. [Google Scholar] [CrossRef]
- Tumkevicius, S.; Mekuskiene, G.; Gefenas, V.; Vainilavicius, P. Substituent effect on proton chemical shifts of amide and azomethine groups of arylidenehydrazides of 5-substituted 2-pyrimidine-carboxylic acids and their aromatic analogs. Chemija 2005, 16, 65–68. [Google Scholar]
- Al-Qadsy, I.; Saeed, W.S.; Al-Odayni, A.-B.; Ahmed Saleh Al-Faqeeh, L.; Alghamdi, A.A.; Farooqui, M. Novel metformin-based schiff bases: Synthesis, characterization, and antibacterial evaluation. Materials 2020, 13, 514. [Google Scholar] [CrossRef]
- Wang, H.; Butorin, S.M.; Young, A.T.; Guo, J. Nickel oxidation states and spin states of bioinorganic complexes from nickel L-edge X-ray absorption and resonant inelastic X-ray scattering. J. Phys. Chem. C 2013, 117, 24767–24772. [Google Scholar] [CrossRef]
- Maroney, M.J.; Ciurli, S. Bioinorganic Chemistry of Nickel. Multidisciplinary Digital Publishing Institute. Inorganics 2019, 7, 131. [Google Scholar] [CrossRef]
- Kumar, S.; Trivedi, A. A review on role of nickel in the biological system. Int. J. Curr. Microbiol. Appl. Sci. 2016, 5, 719–727. [Google Scholar] [CrossRef]
- Iskander, M.; Zayan, S.; Khalifa, M.; El-Sayed, L. Coordination compounds of hydrazine derivatives with transition metals—VI: The reaction of aroylhydrazines with nickel (II), cobalt (II) and copper (II) salts. J. Inorg. Nucl. Chem. 1974, 36, 551–556. [Google Scholar] [CrossRef]
- Wiegand, R.G. The formation of pyridoxal and pyridoxal 5-phosphate hydrazones1. J. Am. Chem. Soc. 1956, 78, 5307–5309. [Google Scholar] [CrossRef]
- Hrušková, K.; Potůčková, E.; Hergeselova, T.; Liptakova, L.; Hašková, P.; Mingas, P.; Kovaříková, P.; Šimůnek, T.; Vávrová, K. Aroylhydrazone iron chelators: Tuning antioxidant and antiproliferative properties by hydrazide modifications. Eur. J. Med. Chem. 2016, 120, 97–110. [Google Scholar] [CrossRef]
- Armstrong, C.M.; Bernhardt, P.V.; Chin, P.; Richardson, D.R. Structural Variations and Formation Constants of First-Row Transition Metal Complexes of Biologically Active Aroylhydrazones. Eur. J. Inorg. Chem. 2003, 2003, 1145–1156. [Google Scholar] [CrossRef]
- Balachandran, K.; George, M. Oxidation with metal oxides—VI: Oxidation of benzoylhydrazones of aldehydes, ketones and 1, 2-diketones with nickel peroxide. Tetrahedron 1973, 29, 2119–2128. [Google Scholar] [CrossRef]
- Iskander, M.; Saddeck, S. Coordination compounds of hydrazine derivatives with transition metals. XIV. Nickel (II) chelates with bidentate aroylhydrazones and their reactions with heterocyclic bases. Inorg. Chim. Acta 1977, 22, 141–147. [Google Scholar] [CrossRef]
- Iskander, M.F.; El-Sayed, L.; Saddeck, S.; Abuo-Taleb, M.A. Coordination compounds of hydrazine derivatives with transition metals. Part 20. Nickel (II) chelates witho-hydroxy ando-methoxy-benzaldehyde aroylhydrazones. An example of coordination isomerism. Trans. Met. Chem. 1980, 5, 168–172. [Google Scholar] [CrossRef]
- Joseph, B.; Sithambaresan, M.; Kurup, M.P.; Ng, S.W. Bis {μ-N-[(E)-4-benzyloxy-2-oxidobenzylidene]-4-nitrobenzenecarbohydrazidato} bis [diaquanickel (II)] dimethylformamide tetrasolvate. Acta Cryst. 2014, 70, m211–m212. [Google Scholar] [CrossRef]
- Ma, J.-X.; Li, Q.-L.; Li, P.-P.; Zhao, J.-X.; Zhao, L. Crystal structure of bis {5-methoxy-2-((E)-((4-((E)-1-(methoxyimino) ethyl) phenyl) imino) methyl) phenolato-κ2N, O} nickel (II), C34H34N4NiO6. Z. Krist.-New Cryst. Struct. 2018, 233, 767–769. [Google Scholar] [CrossRef]
- Mondal, S.; Das, C.; Ghosh, B.; Pakhira, B.; Blake, A.J.; Drew, M.G.; Chattopadhyay, S.K. Synthesis, spectroscopic studies, X-ray crystal structures, electrochemical properties and DFT calculations of three Ni (II) complexes of aroyl hydrazone ligands bearing anthracene moiety. Polyhedron 2014, 80, 272–281. [Google Scholar] [CrossRef]
- Jia, W.P.; Yang, J.G.; Li, F.; Pan, F.Y. Synthesis and Crystal Structure of a Ni (Ⅱ) Complex of 2′-[4-N, N′-(Dimethylaminobenzyl-idene)]-3,5-dihydroxybenzoylhydrazide and Its Spectral Properties. Chin. J. Inorg. Chem. 2009, 1635–1639. [Google Scholar]
- Zhang, S.-P.; Liu, Z.-D.; Chen, S.-S.; Wei, Y.; Shao, S.-C. Synthesis, characterization and crystal structure of bis (p-fluorobenzaldehyde salicylhydrazone) nickel (II). Chin. J. Inorg. Chem. 2007, 23, 1069–1071. [Google Scholar]
- Jia, W.P.; Yang, J.G.; Li, F.; Pan, F.Y. Synthesis and Crystal Structure of Ni (Ⅱ) Complex of 2′-(4-fluorobenzylidene)]-3,5-dihydroxybenzoylhydrazide and Its Spectral Properties. Chin. J. Inorg. Chem. 2008, 24, 627–630. [Google Scholar]
- SAINT Data Reduction Software. Version 6.45. Bruker Analytical X-ray Systems; Bruker AXS Inc.: Madison, WI, USA, 2003. [Google Scholar]
- SADABS, V. 2.05; Bruker AXS Inc.: Madison, WI, USA, 2002.
- Blessing, R.H. An empirical correction for absorption anisotropy. Acta Cryst. 1995, 51, 33–38. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. 2008, 64, 112–122. [Google Scholar] [CrossRef]
- Barbour, L.J. X-Seed—A Software Tool for Supramolecular Crystallography; Elsevier: Amsterdam, The Netherlands, 2001. [Google Scholar]
- Owuama, C.I. Determination of minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) using a novel dilution tube method. Afric. J. Microb. Res. 2017, 11, 977–980. [Google Scholar]
- Arfan, M.; Khan, R.; Tavman, A.; Saba, S. Spectral characterization and crystal structure of 2-amino-N′-[(1Z)-1-(4-chlorophenyl) ethylidene]-benzohydrazide. J. Saud. Chem. Soc. 2016, 20, 40–44. [Google Scholar] [CrossRef]
- Gluvchinsky, P.; Mockler, G.M.; Sinn, E. Nickel (II) complexes of some quadridentate Schiff-base ligands—II. Infrared spectra. Spectro. Acta A Mol. Spect. 1977, 33, 1073–1077. [Google Scholar] [CrossRef]
- Rassem, H.; Nour, A. Synthesis, Spectral Characterization and Crystal Structure of (E)-4-Hydroxy-N-(2-Methoxybenzylidene) Benzohydrazide. Aust. J. Bas. Appl. Sci. 2016, 10, 513–516. [Google Scholar]
- Kruve, A.; Kaupmees, K.; Liigand, J.; Oss, M.; Leito, I. Sodium adduct formation efficiency in ESI source. J. Mass Spect. 2013, 48, 695–702. [Google Scholar] [CrossRef] [PubMed]
- Al-Odayni, A.-B.; Alfotawi, R.; Khan, R.; Saeed, W.S.; Al-Kahtani, A.; Aouak, T.; Alrahlah, A. Synthesis of chemically modified BisGMA analog with low viscosity and potential physical and biological properties for dental resin composite. Dent. Mater. 2019, 35, 1532–1544. [Google Scholar] [CrossRef] [PubMed]
- Demarque, D.P.; Crotti, A.E.; Vessecchi, R.; Lopes, J.L.; Lopes, N.P. Fragmentation reactions using electrospray ionization mass spectrometry: An important tool for the structural elucidation and characterization of synthetic and natural products. Nat. Prod. Rep. 2016, 33, 432–455. [Google Scholar] [CrossRef] [PubMed]
- Iskander, M.F.; El-Sayed, L.; Salem, N.M.; Werner, R.; Haase, W. Synthesis, characterization and magnetochemical studies of dicopper (II) complexes derived from bis (N-salicylidene) dicarboxylic acid dihydrazides. J. Coord. Chem. 2005, 58, 125–139. [Google Scholar] [CrossRef]
- Arafath, M.A.; Al-Suede, F.S.; Adam, F.; Al-Juaid, S.; Khadeer Ahamed, M.B.; Majid, A.M. Schiff base-nickel, palladium, and platinum complexes derived from N-cyclohexyl hydrazine carbothioamide and 3-hydroxy-4-methoxybenzaldehyde: Selective antiproliferative and proapoptotic effects against colorectal carcinoma. Drug. Devel. Res. 2019, 80, 778–790. [Google Scholar] [CrossRef]
- Ejidike, I.P.; Ajibade, P.A. Synthesis, characterization and biological studies of metal (II) complexes of (3E)-3-[(2-{(E)-[1-(2, 4-dihydroxyphenyl) ethylidene] amino} ethyl) imino]-1-phenylbutan-1-one Schiff base. Molecules 2015, 20, 9788–9802. [Google Scholar] [CrossRef]
- Blanchard, S.; Neese, F.; Bothe, E.; Bill, E.; Weyhermüller, T.; Wieghardt, K. Square planar vs tetrahedral coordination in diamagnetic complexes of nickel (II) containing two bidentate π-radical monoanions. Inorg. Chem. 2005, 44, 3636–3656. [Google Scholar] [CrossRef]
- Bouzerafa, B.; Ourari, A.; Aggoun, D.; Ruiz-Rosas, R.; Ouennoughi, Y.; Morallon, E. Novel nickel (II) and manganese (III) complexes with bidentate Schiff-base ligand: Synthesis, spectral, thermogravimetry, electrochemical and electrocatalytical properties. Res. Chem. Int. 2016, 42, 4839–4858. [Google Scholar] [CrossRef]
- Angelusiu, M.V.; Barbuceanu, S.-F.; Draghici, C.; Almajan, G.L. New Cu (II), Co (II), Ni (II) complexes with aroyl-hydrazone based ligand. Synthesis, spectroscopic characterization and in vitro antibacterial evaluation. Eur. J. Med. Chem. 2010, 45, 2055–2062. [Google Scholar] [CrossRef]
- Ahmed, A.H.; Hassan, A.; Gumaa, H.A.; Mohamed, B.H.; Eraky, A.M. Nickel (II)-oxaloyldihydrazone complexes: Characterization, indirect band gap energy and antimicrobial evaluation. Cog. Chem. 2016, 2, 1–14. [Google Scholar] [CrossRef]
- Kumar, D.; Chadda, S.; Sharma, J.; Surain, P. Syntheses, spectral characterization, and antimicrobial studies on the coordination compounds of metal ions with Schiff base containing both aliphatic and aromatic hydrazide moieties. Bioinorg. Chem. Appl. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Mishra, L.; Singh, V.K. Synthesis, structural and antifungal studies of Co (II), Ni (II), Cu (II) and Zn (II) complexes with new Schiff bases bearing benzimidazoles. Indian J. Chem. 1993, 32, 446–449. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).