Influence of the Addition of Ni on as-Cast Microstructure of Duplex Fe-Mn-Al-C Lightweight Steel
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
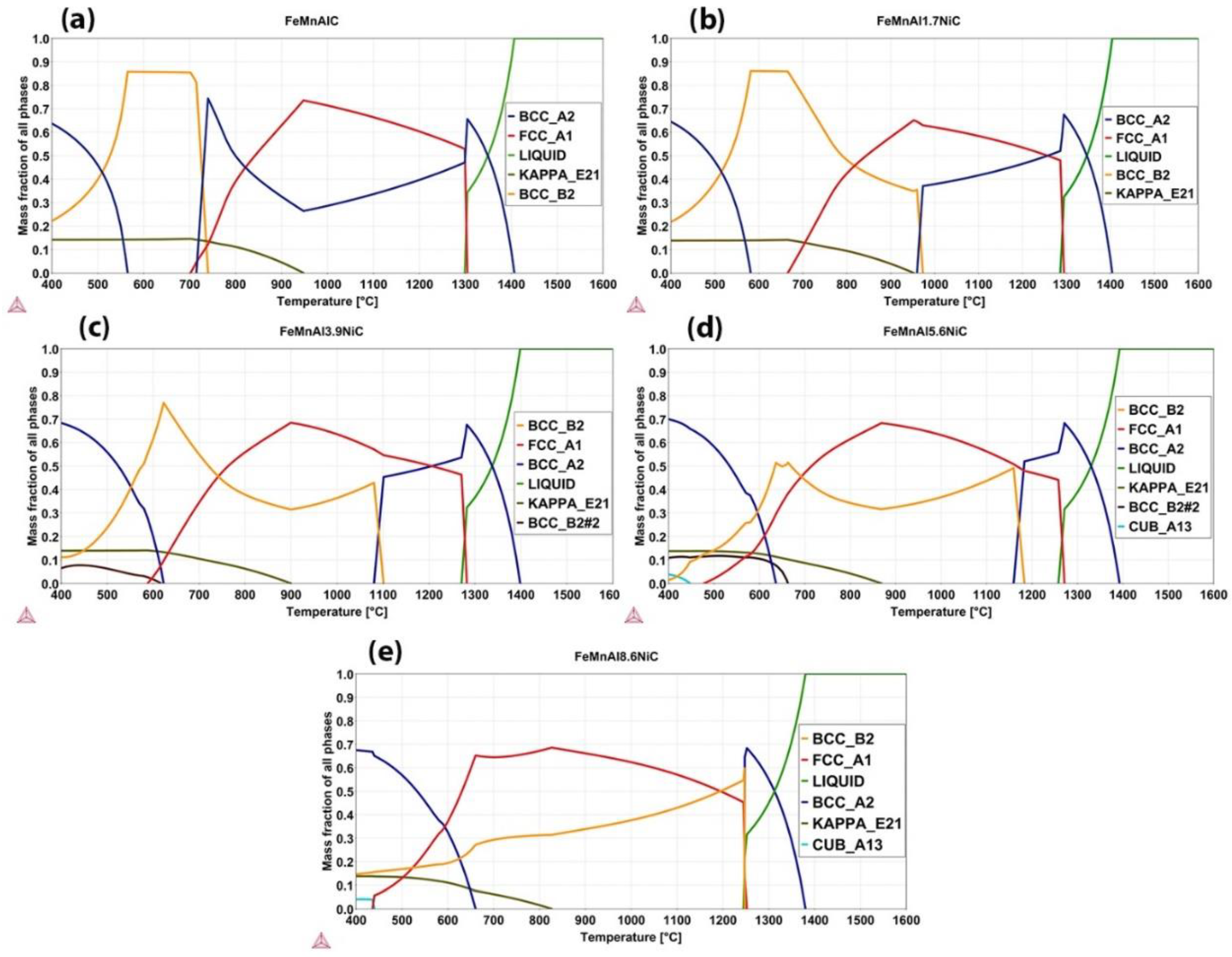
3.1. CALPHAD Calculations
3.2. Optical Microscopy
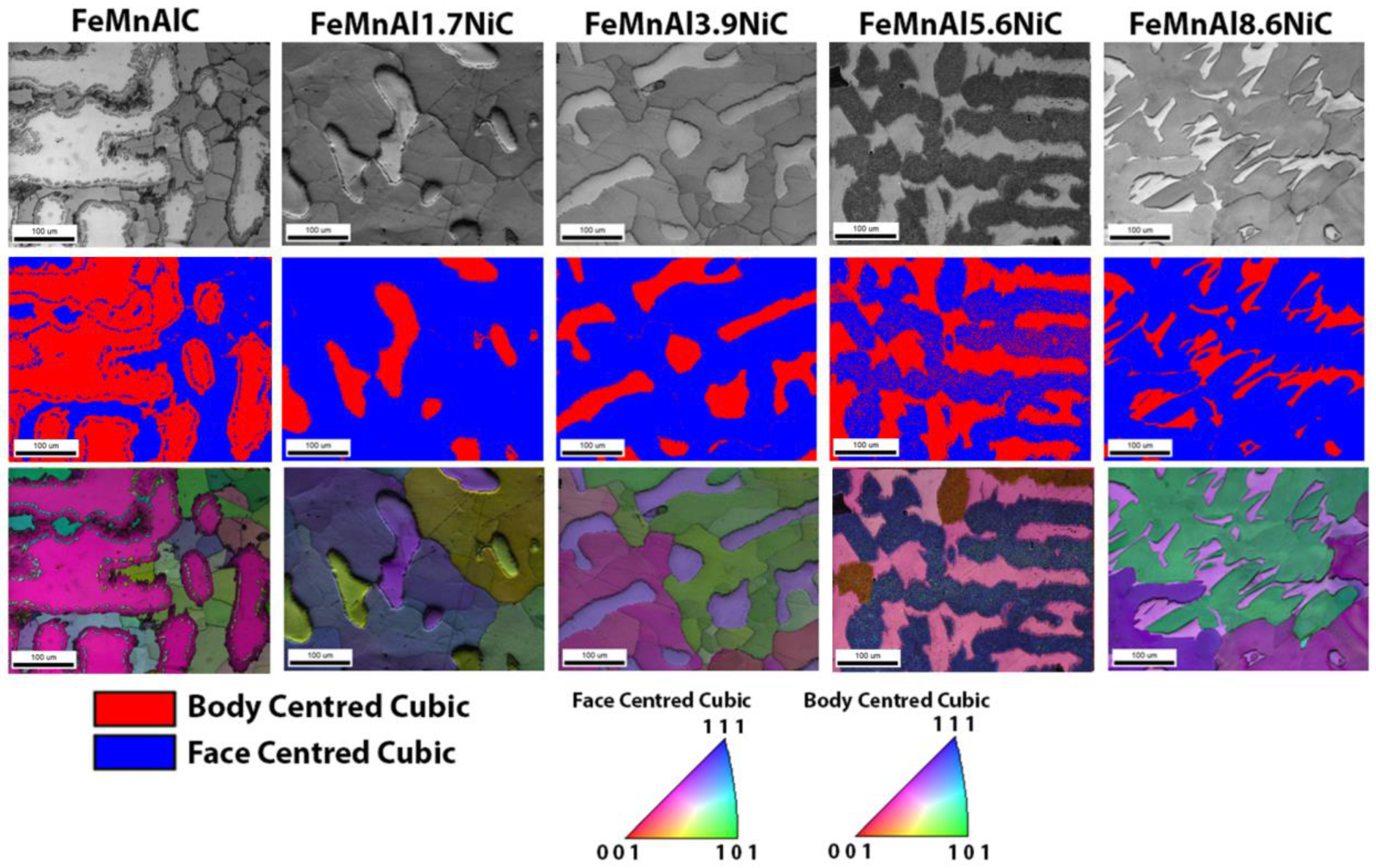
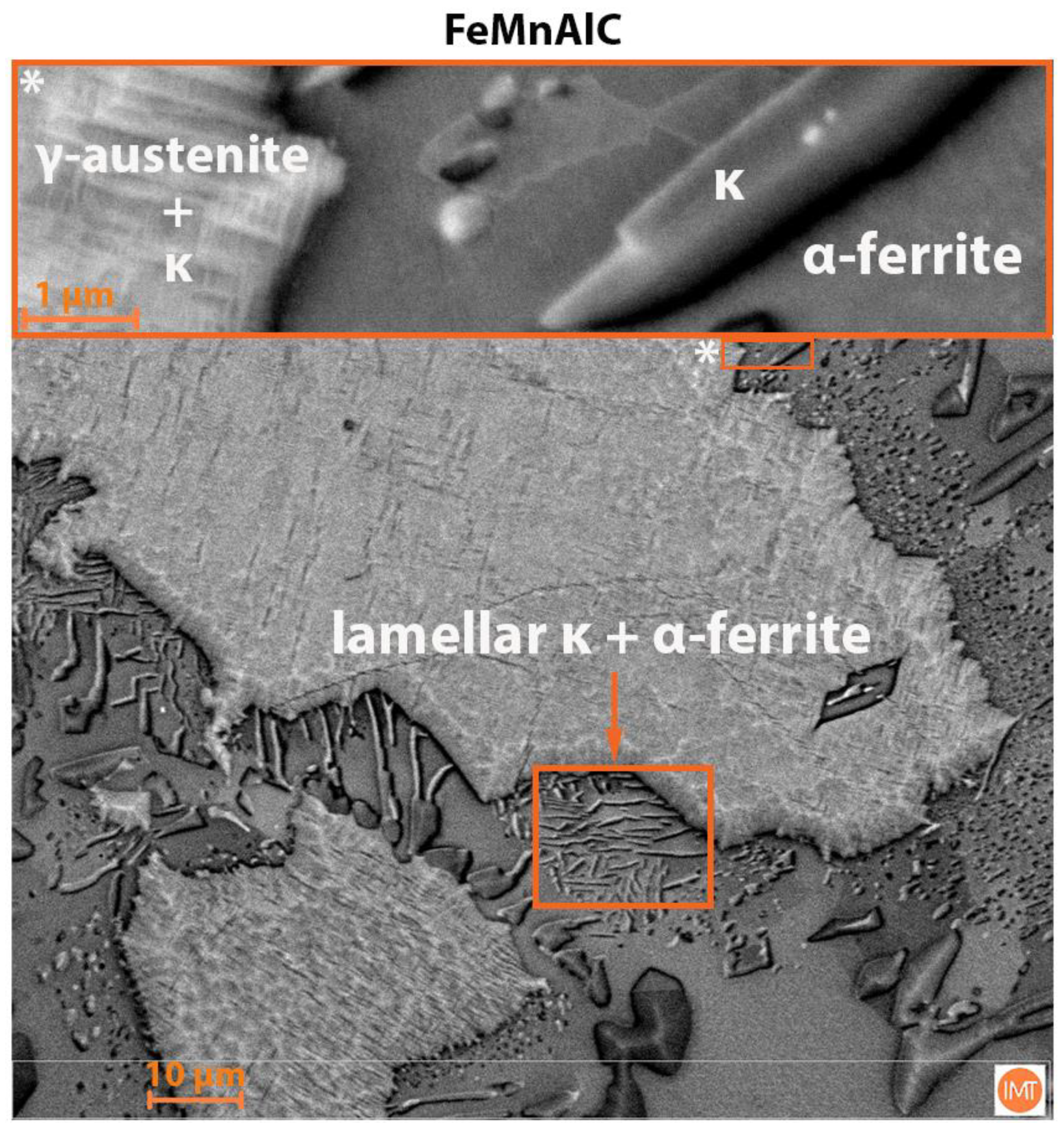
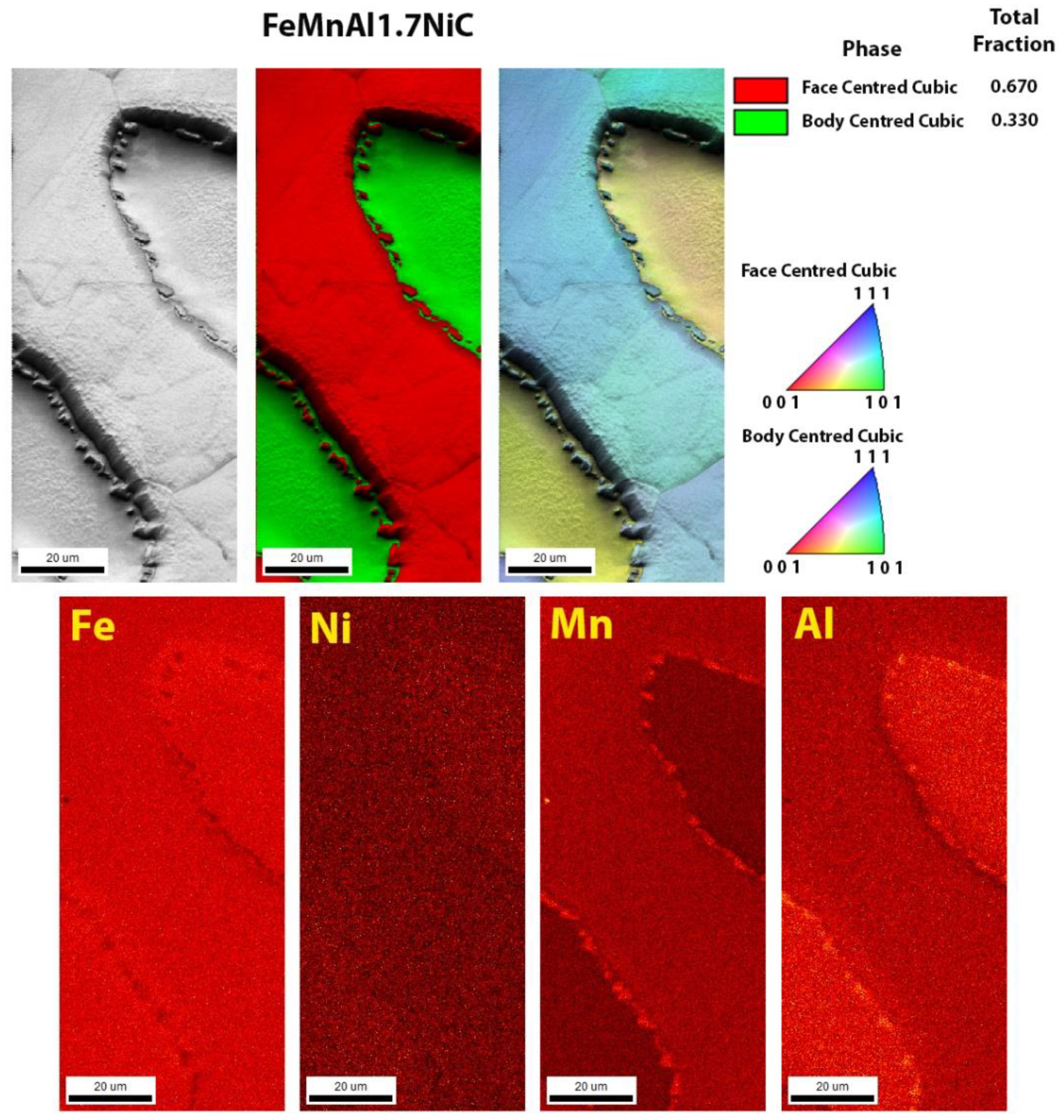
3.3. Scanning Electron Microscopy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ajanovic, A.; Haas, R. The impact of energy policies in scenarios on GHG emission reduction in passenger car mobility in the EU-15. Renew. Sustain. Energy Rev. 2017, 68, 1088–1096. [Google Scholar] [CrossRef]
- Lodi, C.; Seitsonen, A.; Pa, E.; De Gennaro, M.; Huld, T.; Malfettani, S. Reducing CO2 emissions of conventional fuel cars by vehicle photovoltaic roofs. Transp. Res. Part D Transp. Environ. 2018, 59, 313–324. [Google Scholar] [CrossRef]
- Fontaras, G.; Dilara, P. The evolution of European passenger car characteristics 2000–2010 and its effects on real-world CO2 emissions and CO2 reduction policy. Energy Policy 2012, 49, 719–730. [Google Scholar] [CrossRef]
- Tsiakmakis, S.; Fontaras, G.; Ciuffo, B.; Samaras, Z. A simulation-based methodology for quantifying European passenger car fleet CO2 emissions. Appl. Energy 2017, 199, 447–465. [Google Scholar] [CrossRef]
- Hottle, T.; Caffrey, C.; McDonald, J.; Dodder, R. Critical factors affecting life cycle assessments of material choice for vehicle mass reduction. Transp. Res. Part D Transp. Environ. 2017, 56, 241–257. [Google Scholar] [CrossRef]
- Chen, S.; Rana, R.; Haldar, A.; Ray, R.K. Current state of Fe-Mn-Al-C low density steels. Prog. Mater. Sci. 2017, 89, 345–391. [Google Scholar] [CrossRef]
- Kim, Y.G.; Kim, T.W.; Han, J.K.; Chang, R.W. Development of New Austenitic Fe-Mn-Al-C Steels for Automotive Applications. Key Eng. Mater. 1993, 84, 461–471. [Google Scholar] [CrossRef]
- Zuazo, I.; Hallstedt, B.; Lindahl, B.; Selleby, M.; Soler, M.; Etienne, A.; Perlade, A.; Hasenpouth, D.; Cazottes, S.; Kleber, X.; et al. Low-Density Steels: Complex Metallurgy for Automotive Applications. JOM 2014, 66, 1747–1758. [Google Scholar] [CrossRef]
- Rana, R. Low-Density Steels. JOM 2014, 66, 1730–1733. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.; Suh, D.; Kim, N.J. Fe–Al–Mn–C lightweight structural alloys: A review on the microstructures and mechanical properties. Sci. Technol. Adv. Mater. 2013, 14, 014205. [Google Scholar] [CrossRef]
- Howell, R.A.; Van Aken, D.C. A literature review of age hardening Fe-Mn-Al-C alloys. Iron Steel Technol. 2009, 6, 193–212. [Google Scholar]
- Abedi, H.R.; Zarei Hanzaki, A.; Ou, K.L.; Yu, C.H. Substructure hardening in duplex low density steel. Mater. Des. 2017, 116, 472–480. [Google Scholar] [CrossRef]
- Chen, P.; Li, X.; Yi, H. The κ-carbides in low-density fe-mn-al-c steels: A review on their structure, precipitation and deformation mechanism. Metals 2020, 10, 1021. [Google Scholar] [CrossRef]
- Frommeyer, G.; Brox, U. Microstructures and Mechanical Properties of High-Strength Fe-Mn-AI-C Light-Weight TRIPLEX Steels. Steel Res. Int. 2006, 77, 627–633. [Google Scholar] [CrossRef]
- Yoo, J.D.; Park, K.T. Microband-induced plasticity in a high Mn-Al-C light steel. Mater. Sci. Eng. A 2008, 496, 417–424. [Google Scholar] [CrossRef]
- Chumak, I.; Richter, K.W.; Ipser, H. Isothermal sections in the (Fe, Ni)-rich part of the Fe-Ni-Al phase diagram. J. Phase Equilib. Diffus. 2008, 29, 300–304. [Google Scholar] [CrossRef]
- Kim, C.; Hong, H.U.; Jang, J.H.; Lee, B.H.; Park, S.J.; Moon, J.; Lee, C.H. Reverse partitioning of Al from κ-carbide to the γ-matrix upon Ni addition and its strengthening effect in Fe–Mn–Al–C lightweight steel. Mater. Sci. Eng. A 2021, 820, 141563. [Google Scholar] [CrossRef]
- Rahnama, A.; Kotadia, H.; Sridhar, S. Effect of Ni alloying on the microstructural evolution and mechanical properties of two duplex light-weight steels during different annealing temperatures: Experiment and phase-field simulation. Acta Mater. 2017, 132, 627–643. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.; Kim, H.; Kim, N.J. Brittle intermetallic compound makes ultrastrong low-density steel with large ductility. Nature 2015, 518, 77–79. [Google Scholar] [CrossRef]
- Park, G.; Nam, C.H.; Zargaran, A.; Kim, N.J. Effect of B2 morphology on the mechanical properties of B2-strengthened lightweight steels. Scr. Mater. 2019, 165, 68–72. [Google Scholar] [CrossRef]
- Drouven, C.; Song, W.; Bleck, W. Phase-Specific Precipitation of Intermetallic Phases in Fe Al Mn Ni C Duplex Steels. Steel Res. Int. 2019, 90, 1800440. [Google Scholar] [CrossRef]
- Xu, X.; Cheng, H.; Wu, W.; Liu, Z.; Li, X. Stress corrosion cracking behavior and mechanism of Fe-Mn-Al-C-Ni high specific strength steel in the marine atmospheric environment. Corros. Sci. 2021, 191, 109760. [Google Scholar] [CrossRef]
- Burja, J.; Šuler, B.; Nagode, A. Effect of ageing temperature on reverse austenite content in AISI 630 stainless steel. Materwiss. Werksttech. 2019, 50, 405–411. [Google Scholar] [CrossRef]
- Burja, J.; Šuler, B.; Češnjaj, M.; Nagode, A. Effect of intercritical annealing on the microstructure and mechanical properties of 0.1C-13Cr-3Ni martensitic stainless steel. Metals 2021, 11, 392. [Google Scholar] [CrossRef]
- Andersson, J.O.; Thomas, H.; Lars, H.; Pingfang, S.; Bo, S. Thermo-Calc & DICTRA, computational tools for materials science. Calphad 2002, 26, 273–312. [Google Scholar] [CrossRef]
- Thermo-Calc AB. Thermo-Calc Software TCFE10: TCS Steel and Fe-Alloys Database; Thermo-Calc AB: Solna, Sweden, 2019. [Google Scholar]
- Balaško, T.; Burja, J.; Medved, J. Effect of Ni on solidification of duplex low-density steels. J. Therm. Anal. Calorim. 2020, 142, 1605–1611. [Google Scholar] [CrossRef]
- Mapelli, C.; Barella, S.; Gruttadauria, A.; Mombelli, D.; Bizzozero, M.; Veys, X. γ Decomposition in Fe–Mn–Al–C lightweight steels. J. Mater. Res. Technol. 2020, 9, 4604–4616. [Google Scholar] [CrossRef]
- Lu, W.J.; Zhang, X.F.; Qin, R.S. κ-carbide hardening in a low-density high-Al high-Mn multiphase steel. Mater. Lett. 2015, 138, 96–99. [Google Scholar] [CrossRef]



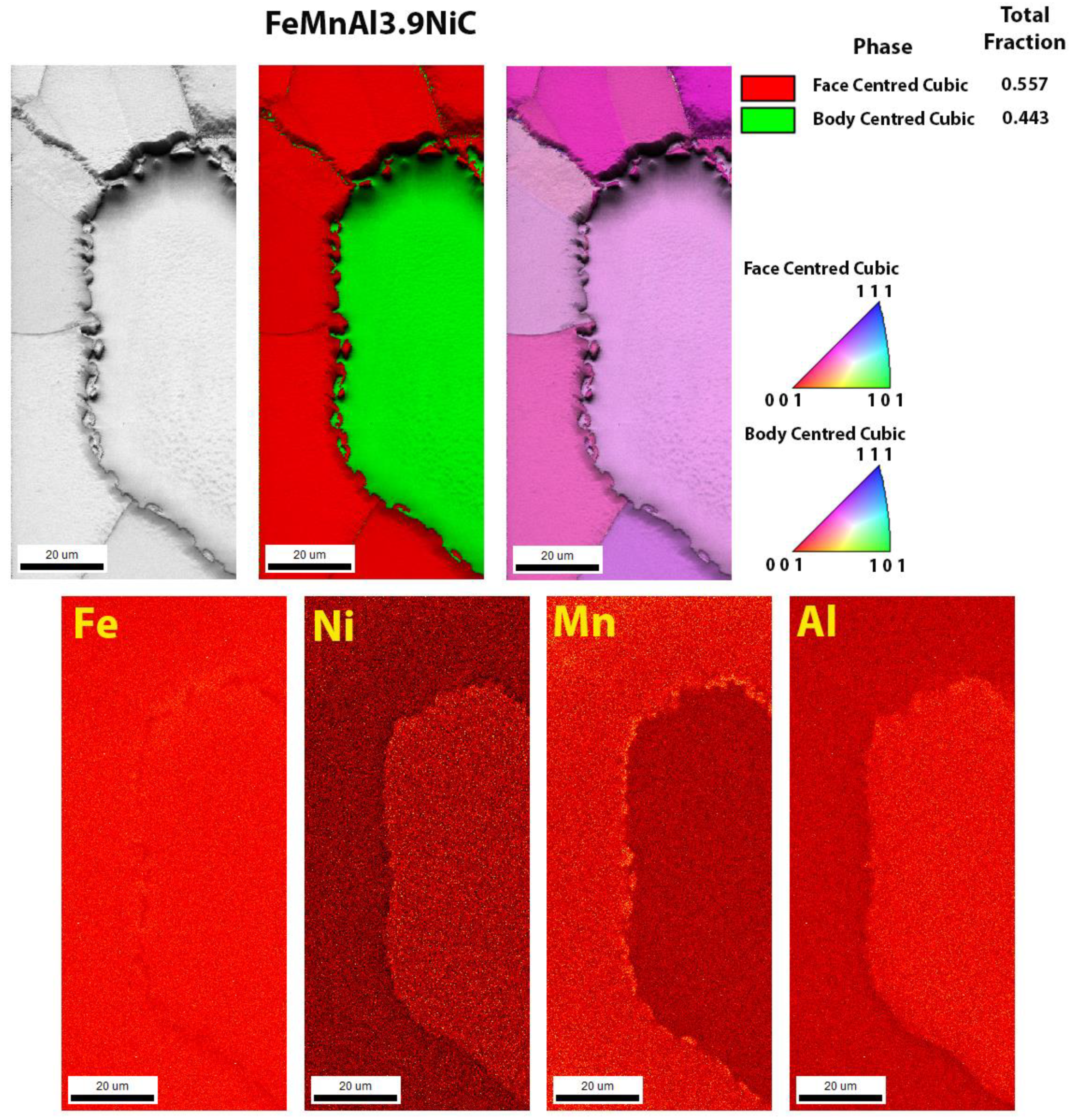
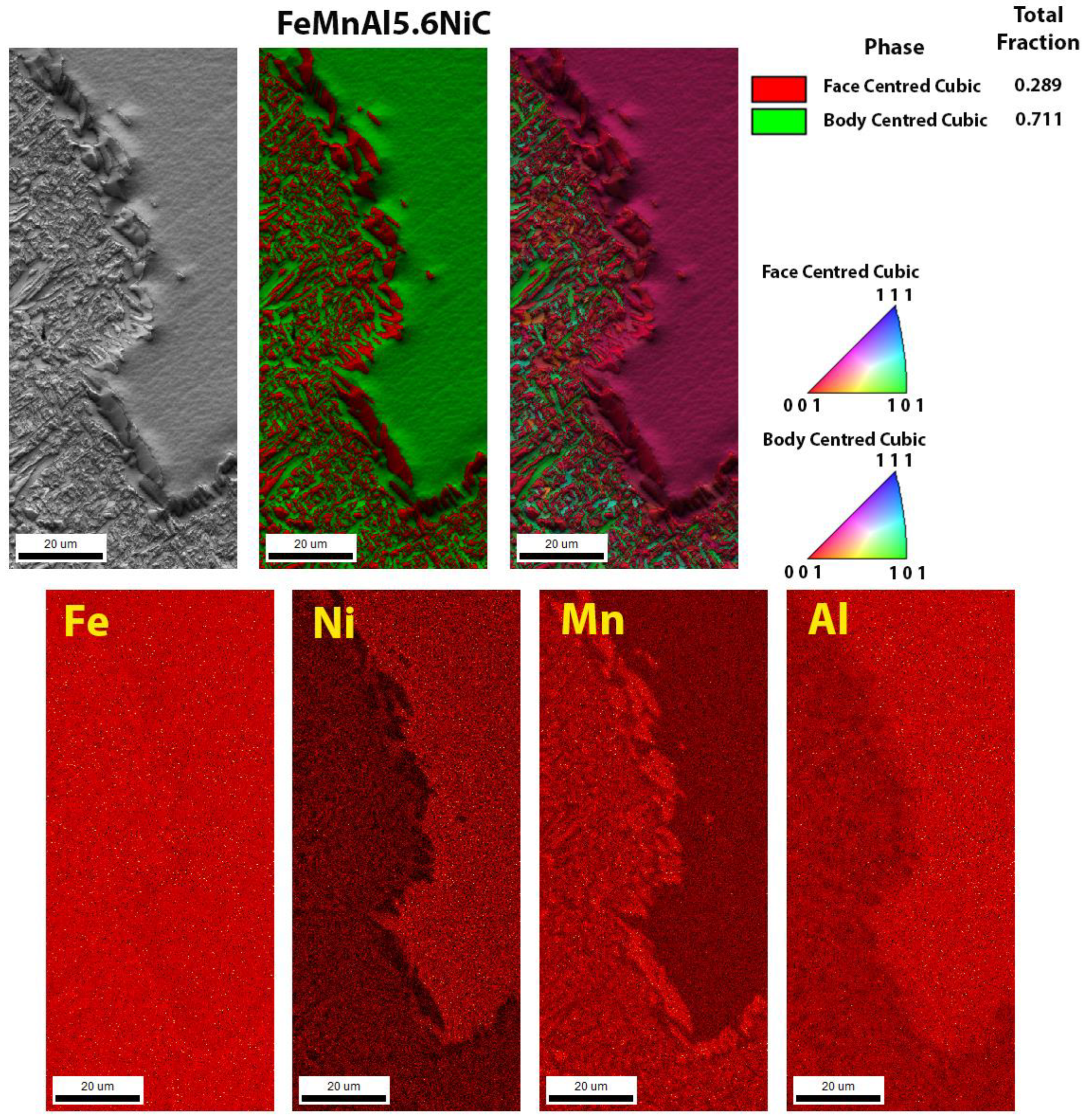

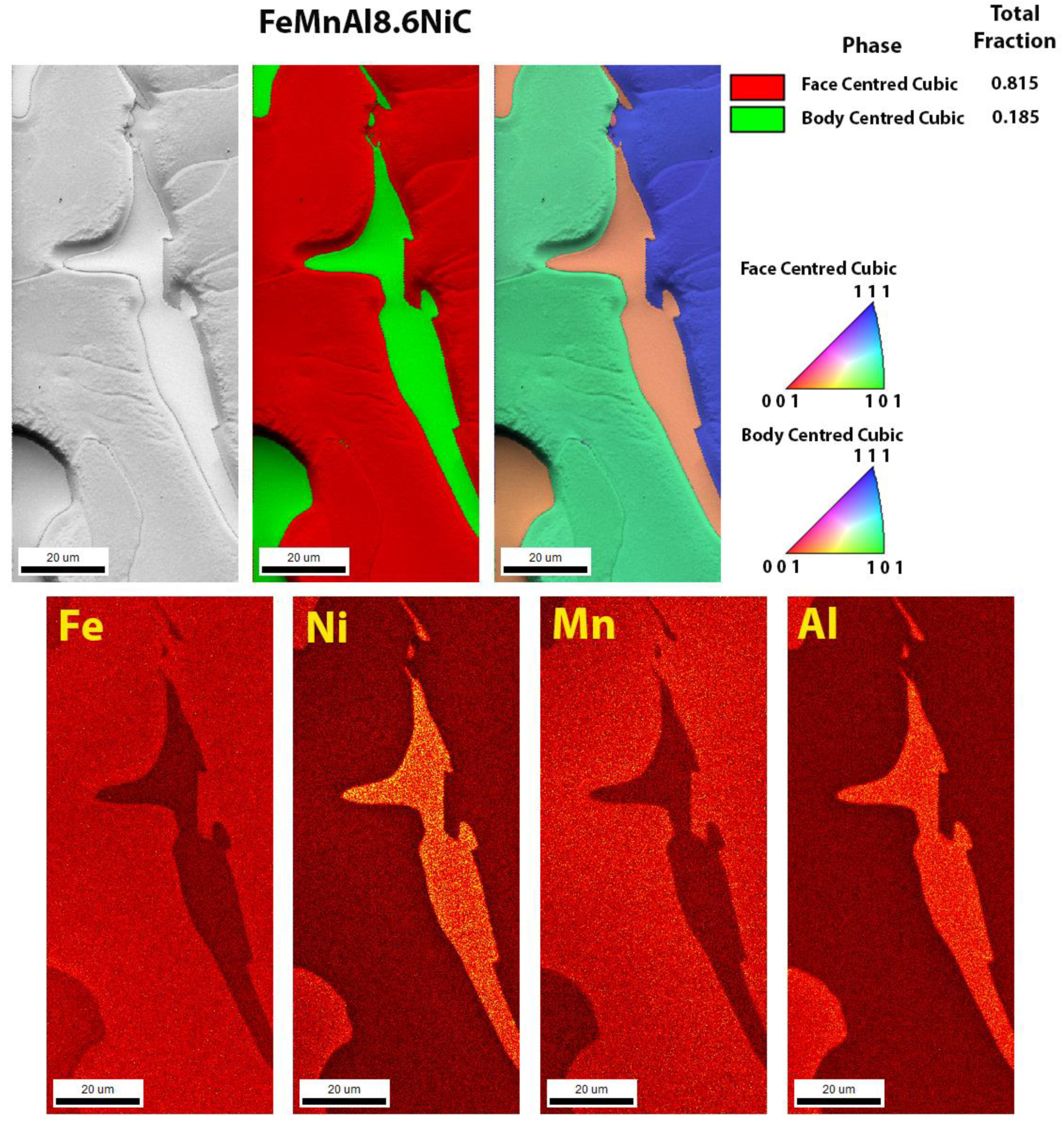
| Sample | C | Si | Cu | Ni | Mn | Al | Fe |
|---|---|---|---|---|---|---|---|
| FeMnAlC | 0.81 | 0.20 | 0.17 | 0.1 | 15.0 | 10.0 | Balance |
| FeMnAl1.7NiC | 0.81 | 0.21 | 0.17 | 1.7 | 14.9 | 10.2 | Balance |
| FeMnAl3.9NiC | 0.83 | 0.19 | 0.17 | 3.9 | 14.8 | 10.0 | Balance |
| FeMnAl5.6NiC | 0.82 | 0.20 | 0.17 | 5.6 | 14.8 | 10.1 | Balance |
| FeMnAl8.6NiC | 0.83 | 0.19 | 0.16 | 8.6 | 14.7 | 10.1 | Balance |
| Temperature/°C | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Sample | Liqidus | Solidus | FCC_A1 | FCC_A1_finish | BCC_A2 | BCC_A2_finish | BCC_B2 | Kappa | BCC_A2 |
| FeMnAlC | 1407 | 1299 | 1304 | 702 | 1407 | 715 | 739 | 947 | 564 |
| FeMnAl1.7NiC | 1404 | 1286 | 1294 | 666 | 1404 | 960 | 973 | 952 | 581 |
| FeMnAl3.9NiC | 1398 | 1271 | 1282 | 588 | 1398 | 1080 | 1101 | 899 | 623 |
| FeMnAl5.6NiC | 1393 | 1258 | 1271 | 478 | 1393 | 1159 | 1183 | 868 | 636 |
| FeMnAl8.6NiC | 1380 | 1245 | 1252 | 436 | 1380 | 1247 | 1248 | 827 | 661 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burja, J.; Šetina Batič, B.; Balaško, T. Influence of the Addition of Ni on as-Cast Microstructure of Duplex Fe-Mn-Al-C Lightweight Steel. Crystals 2021, 11, 1551. https://doi.org/10.3390/cryst11121551
Burja J, Šetina Batič B, Balaško T. Influence of the Addition of Ni on as-Cast Microstructure of Duplex Fe-Mn-Al-C Lightweight Steel. Crystals. 2021; 11(12):1551. https://doi.org/10.3390/cryst11121551
Chicago/Turabian StyleBurja, Jaka, Barbara Šetina Batič, and Tilen Balaško. 2021. "Influence of the Addition of Ni on as-Cast Microstructure of Duplex Fe-Mn-Al-C Lightweight Steel" Crystals 11, no. 12: 1551. https://doi.org/10.3390/cryst11121551
APA StyleBurja, J., Šetina Batič, B., & Balaško, T. (2021). Influence of the Addition of Ni on as-Cast Microstructure of Duplex Fe-Mn-Al-C Lightweight Steel. Crystals, 11(12), 1551. https://doi.org/10.3390/cryst11121551








