Facile Synthesis of Stacked Ni(OH)2 Hexagonal Nanoplates in a Large Scale
Abstract
:1. Introduction
2. Experimental Details
2.1. Synthesis of Ni(OH)2 Hexagonal Nanoplates
2.2. Material Characterization
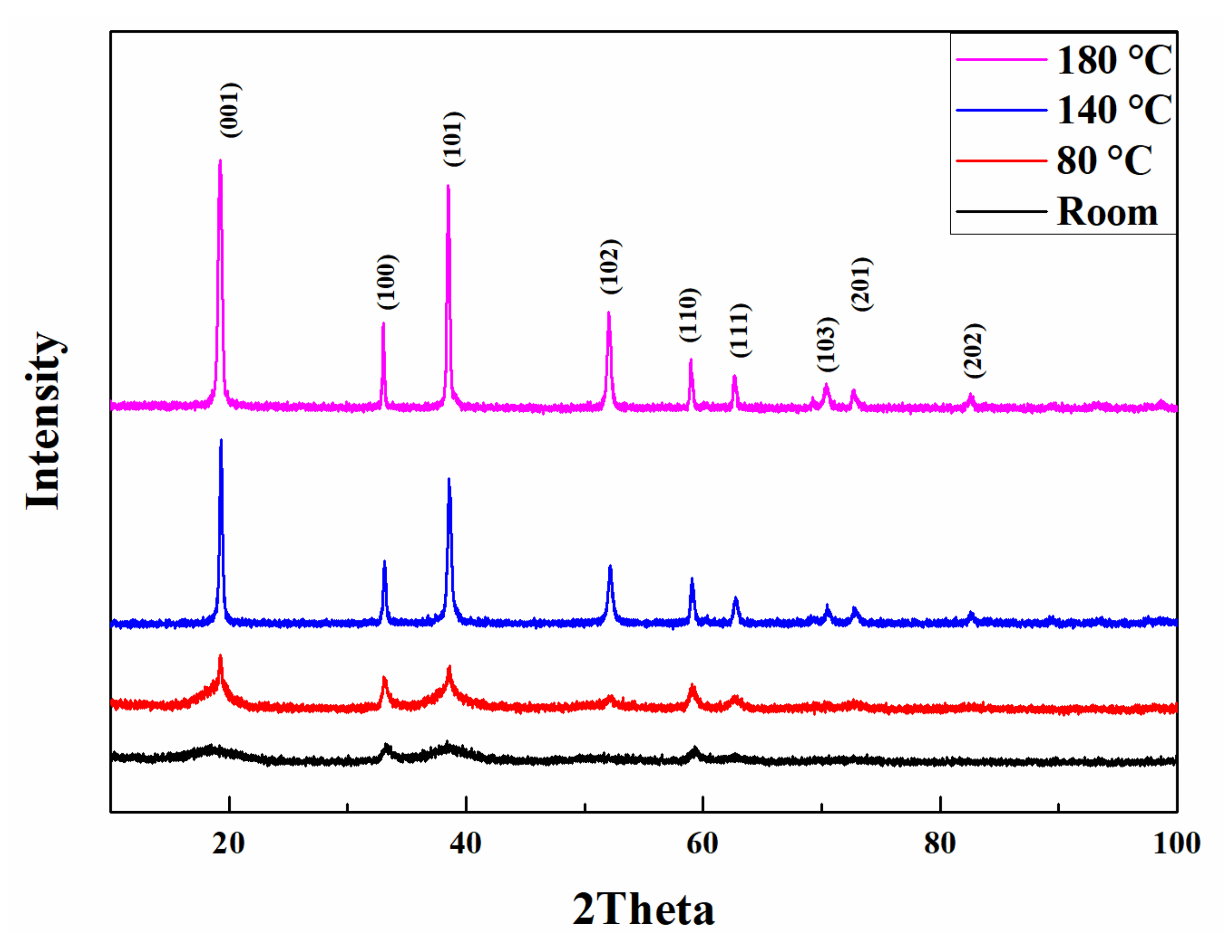
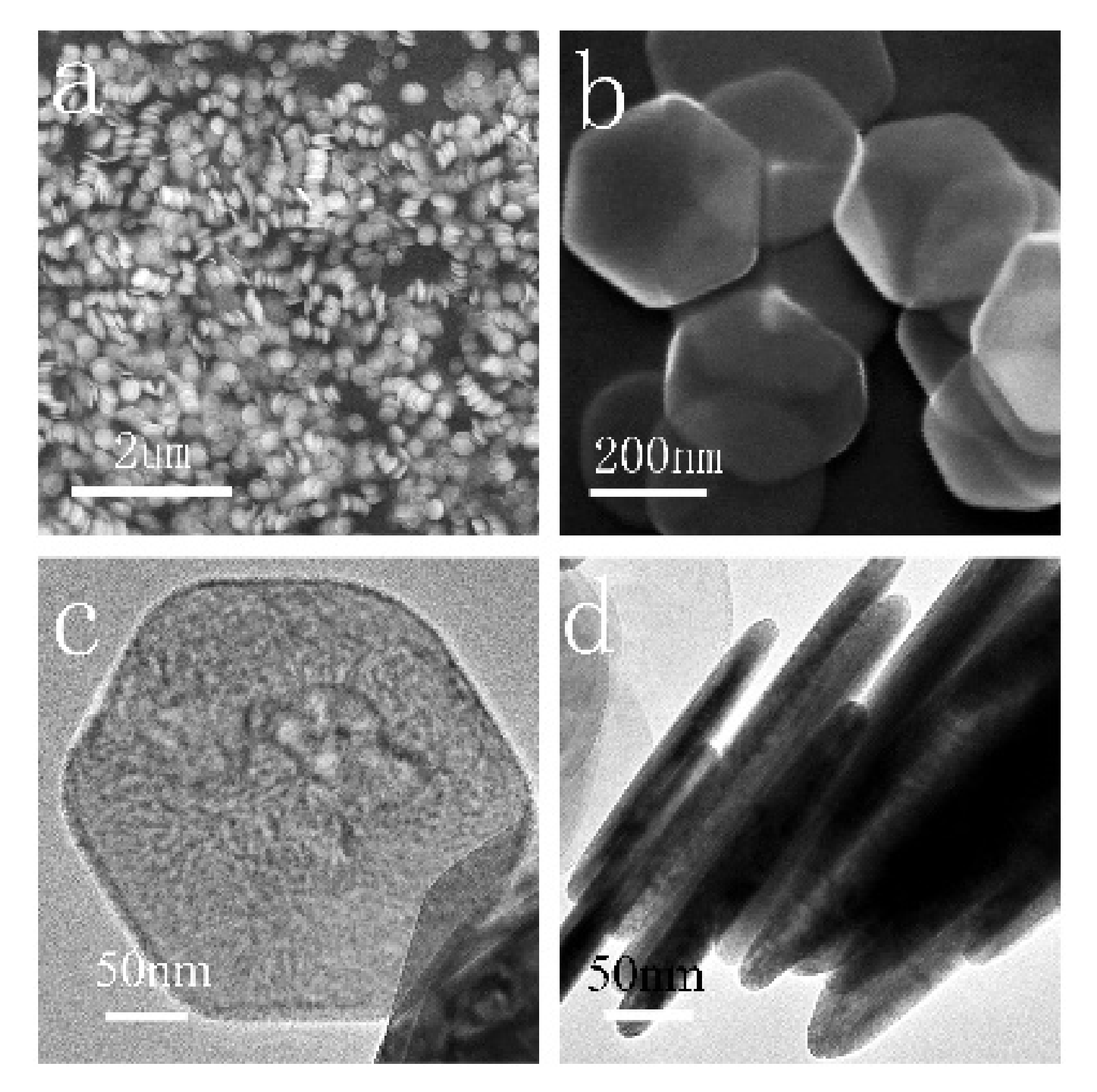
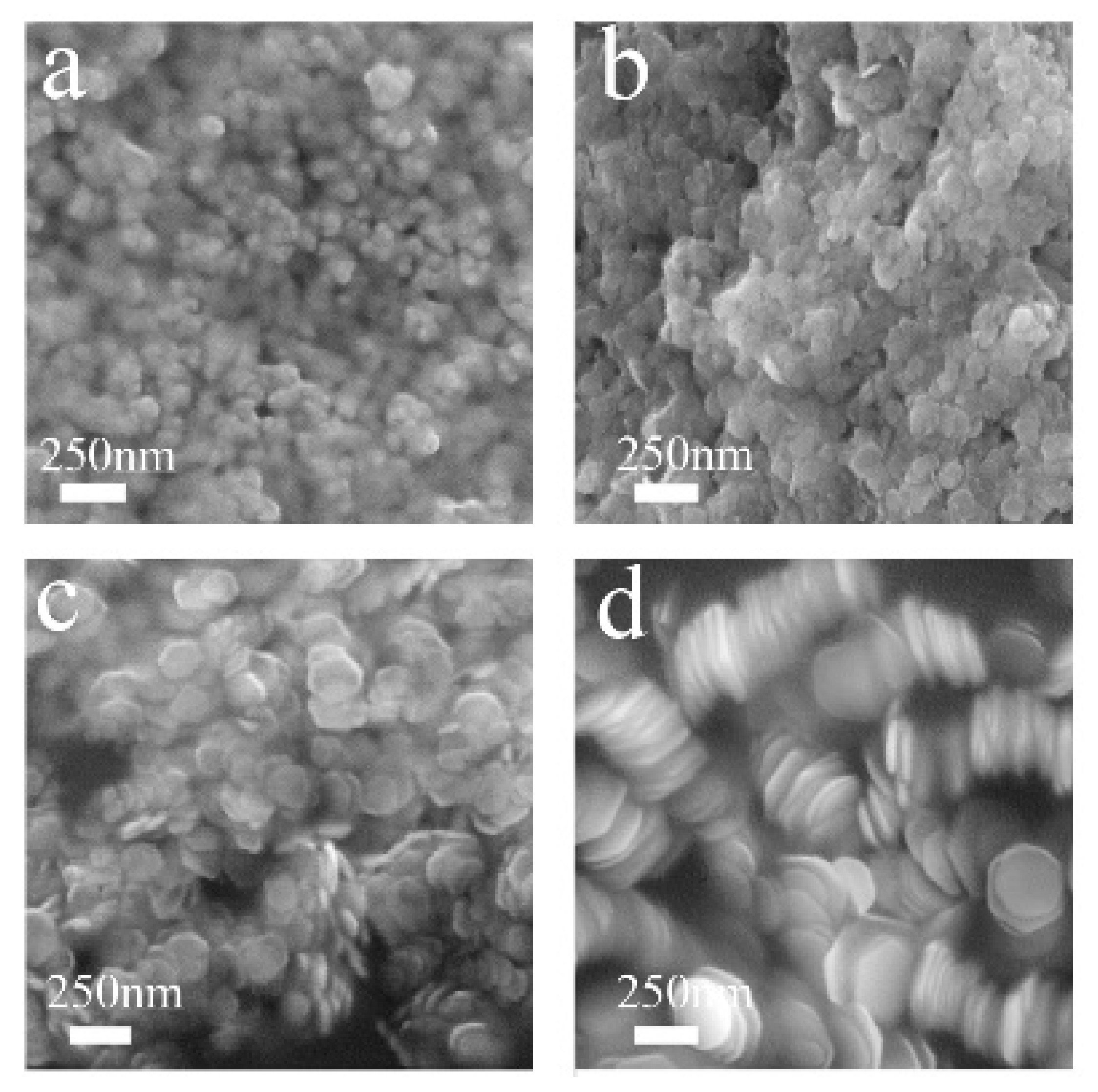
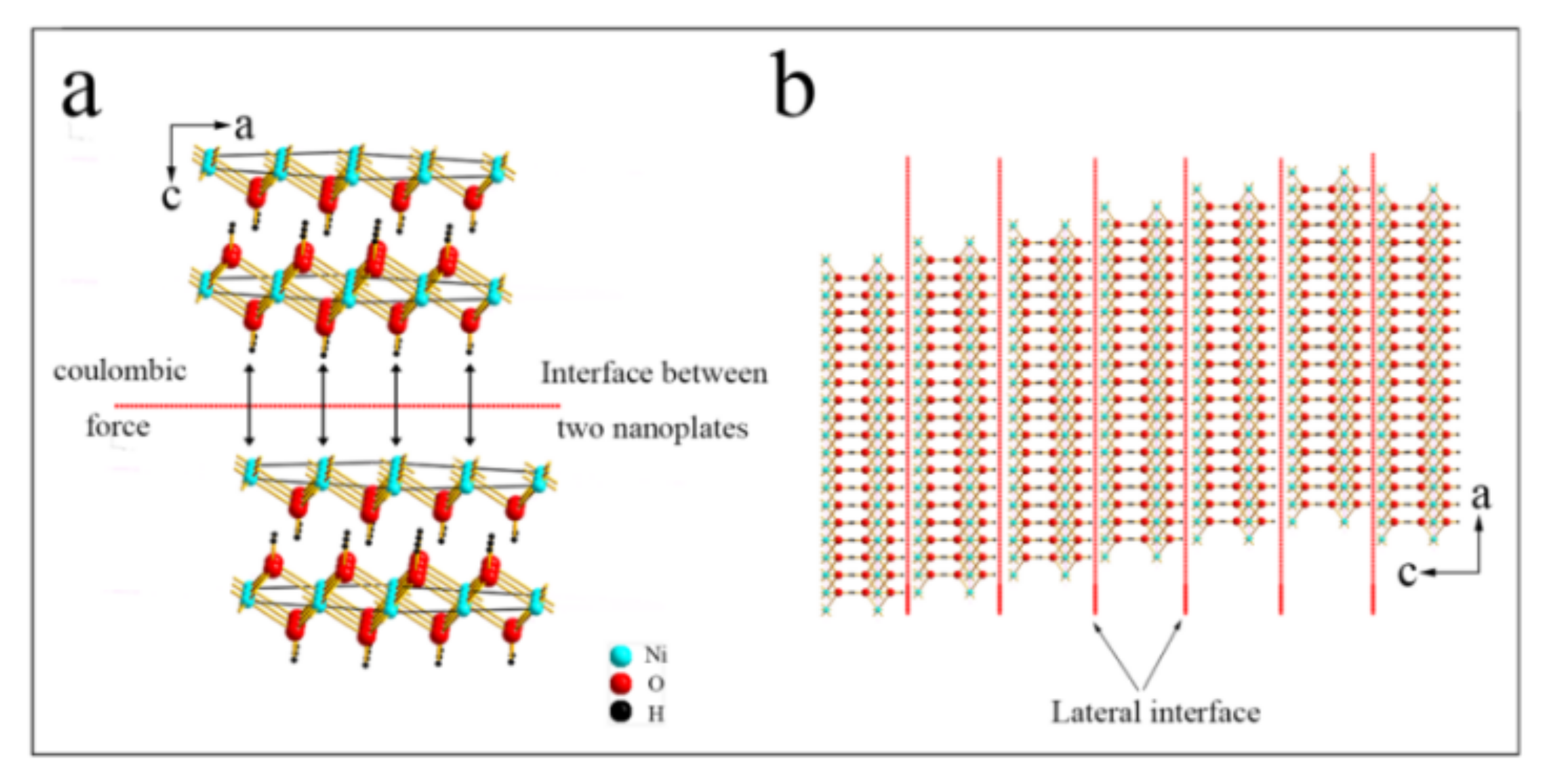
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ede, S.R.; Anantharaj, S.; Kumaran, K.T.; Mishra, S.; Kundu, S. One step synthesis of Ni/Ni(OH)2 nano sheets (NSs) and their application in asymmetric supercapacitors. RSC Adv. 2017, 7, 5898–5911. [Google Scholar] [CrossRef] [Green Version]
- Taniguchi, A.; Fujioka, N.; Ikoma, M.; Ohta, A. Development of nickel/metal-hydride batteries for EVs and HEVs. J. Power Sources 2001, 100, 117–124. [Google Scholar] [CrossRef]
- Cao, M.; He, X.; Chen, J.; Hu, C. Self-assembled nickel hydroxide three-dimensional nanostructures: A nanomaterial for alkaline rechargeable. Cryst. Growth Des. 2007, 7, 170–174. [Google Scholar] [CrossRef]
- Zhang, W.; Jiang, W.; Yu, L.; Fu, Z.; Xia, W.; Yang, M. Effect of nickel hydroxide composition on the electrochemical performance of spherical Ni(OH)2 positive materials for Ni-MH batteries. Int. J. Hydrogen Energy 2009, 34, 473–480. [Google Scholar] [CrossRef]
- Chen, X.; Chen, X.; Zhang, F.; Yang, Z.; Huang, S. One-pot hydrothermal synthesis of reduced graphene oxide/carbon nanotube/α-Ni(OH)2 composites for high performance electrochemical supercapacitor. J. Power Sources 2013, 243, 555–561. [Google Scholar] [CrossRef]
- Dubal, D.; Fulari, V.; Lokhande, C. Effect of morphology on supercapacitive properties of chemically grown β-Ni(OH)2 thin films. Microporous Mesoporous Mater. 2012, 151, 511–516. [Google Scholar] [CrossRef]
- Devaraj, S.; Munichandraiah, N. Effect of Crystallographic Structure of MnO2 on Its Electrochemical Capacitance Properties. J. Phys. Chem. C 2008, 112, 4406–4417. [Google Scholar] [CrossRef]
- Chmiola, J.; Yushin, G.; Dash, R.; Gogotsi, Y. Effect of pore size and surface area of carbide derived carbons on specific capacitance. J. Power Sources 2006, 158, 765–772. [Google Scholar] [CrossRef]
- Ren, X.; Guo, C.; Xu, L.; Li, T.; Hou, L.; Wei, Y. Facile Synthesis of Hierarchical Mesoporous Honeycomb-like NiO for Aqueous Asymmetric Supercapacitors. ACS Appl. Mater. Interfaces 2015, 7, 19930–19940. [Google Scholar] [CrossRef]
- Wu, M.S.; Huang, K.C. Fabrication of nickel hydroxide electrodes with open-ended hexagonal nanotube arrays for high capacitance supercapacitors. Chem. Commun. 2011, 47, 12122–12124. [Google Scholar] [CrossRef]
- Mao, Y.; Li, T.; Guo, C.; Zhu, F.; Zhang, C.; Wei, Y.; Hou, L. Cycling stability of ultrafine β-Ni(OH)2 nanosheets for high capacity energy storage device via a multilayer nickel foam electrode. Electrochim. Acta 2016, 211, 44–51. [Google Scholar] [CrossRef] [Green Version]
- Qi, Y.J.; Qi, H.Y.; Li, J.H.; Lu, C.J. Synthesis, microstructures and UV-vis absorption properties of β-Ni(OH)2 nanoplates and NiO nanostructures. J. Cryst. Growth 2008, 310, 4221–4225. [Google Scholar] [CrossRef]
- Li, X.; Shen, H.; Niu, J.; Li, S.; Zhang, Y.; Wang, H.; Li, L.S. Columnar Self-Assembly of Cu2S Hexagonal Nanoplates Induced by Tin(IV)−X Complex as Inorganic Surface Ligand. J. Am. Chem. Soc. 2010, 132, 12778–12779. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Ma, Z.; Fan, Y.; Shao, G.; Kong, L.; Gao, W. Preparation of size-selective Mn3O4 hexagonal nanoplates with superior electrochemical properties for pseudocapacitors. Phys. Chem. Chem. Phys. 2015, 17, 23017–23025. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, Y.; Xin, Z.; Li, G.; Li, T. Facile Synthesis of Stacked Ni(OH)2 Hexagonal Nanoplates in a Large Scale. Crystals 2021, 11, 1407. https://doi.org/10.3390/cryst11111407
Du Y, Xin Z, Li G, Li T. Facile Synthesis of Stacked Ni(OH)2 Hexagonal Nanoplates in a Large Scale. Crystals. 2021; 11(11):1407. https://doi.org/10.3390/cryst11111407
Chicago/Turabian StyleDu, Yunfei, Zhijie Xin, Guodong Li, and Taotao Li. 2021. "Facile Synthesis of Stacked Ni(OH)2 Hexagonal Nanoplates in a Large Scale" Crystals 11, no. 11: 1407. https://doi.org/10.3390/cryst11111407
APA StyleDu, Y., Xin, Z., Li, G., & Li, T. (2021). Facile Synthesis of Stacked Ni(OH)2 Hexagonal Nanoplates in a Large Scale. Crystals, 11(11), 1407. https://doi.org/10.3390/cryst11111407




