Evaluating the Effect of MgO/Al2O3 Ratio on Thermal Behaviors and Structures of Blast Furnace Slag with Low Carbon Consumption
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Apparatus and Method
3. Results and Discussions
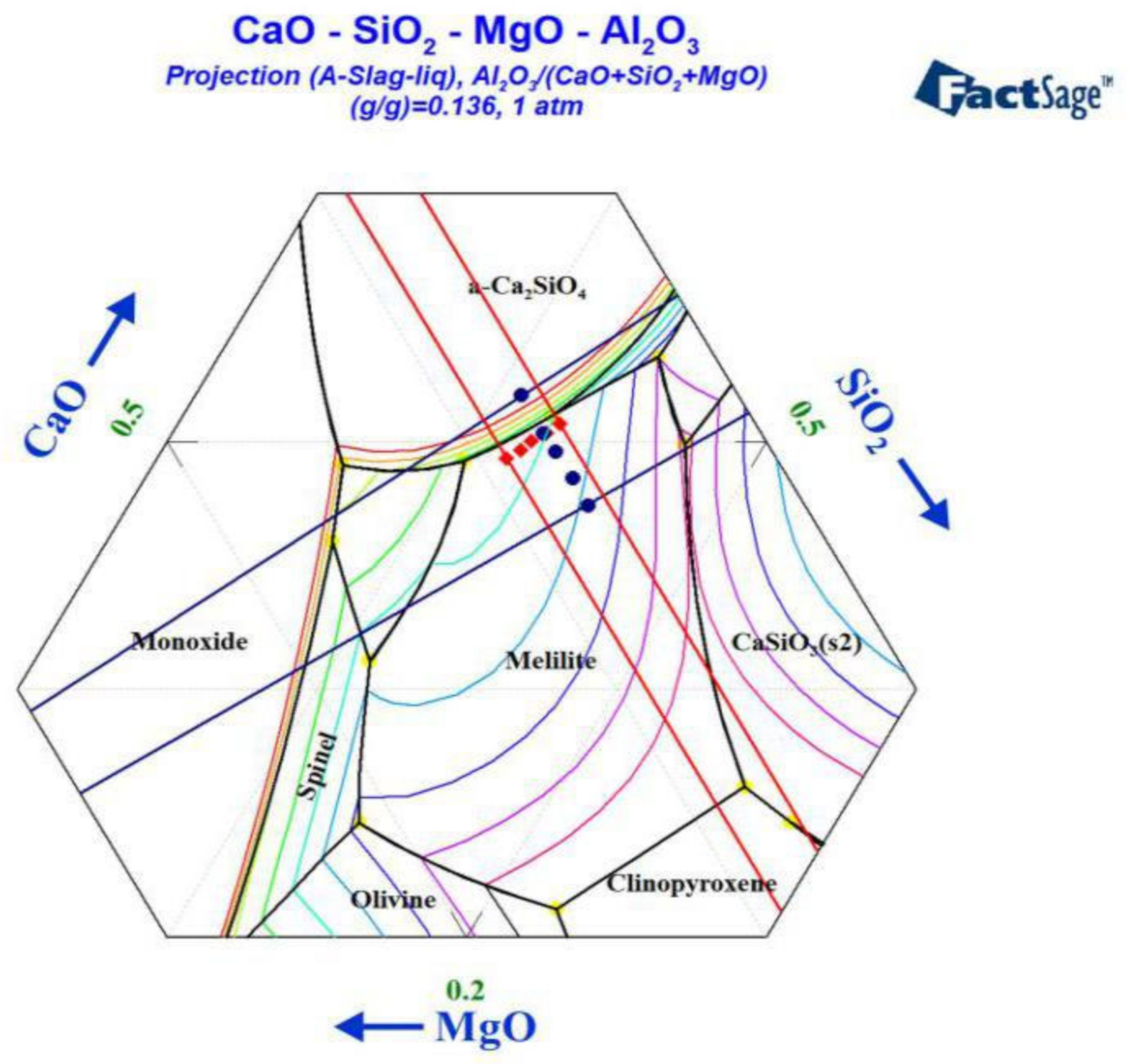
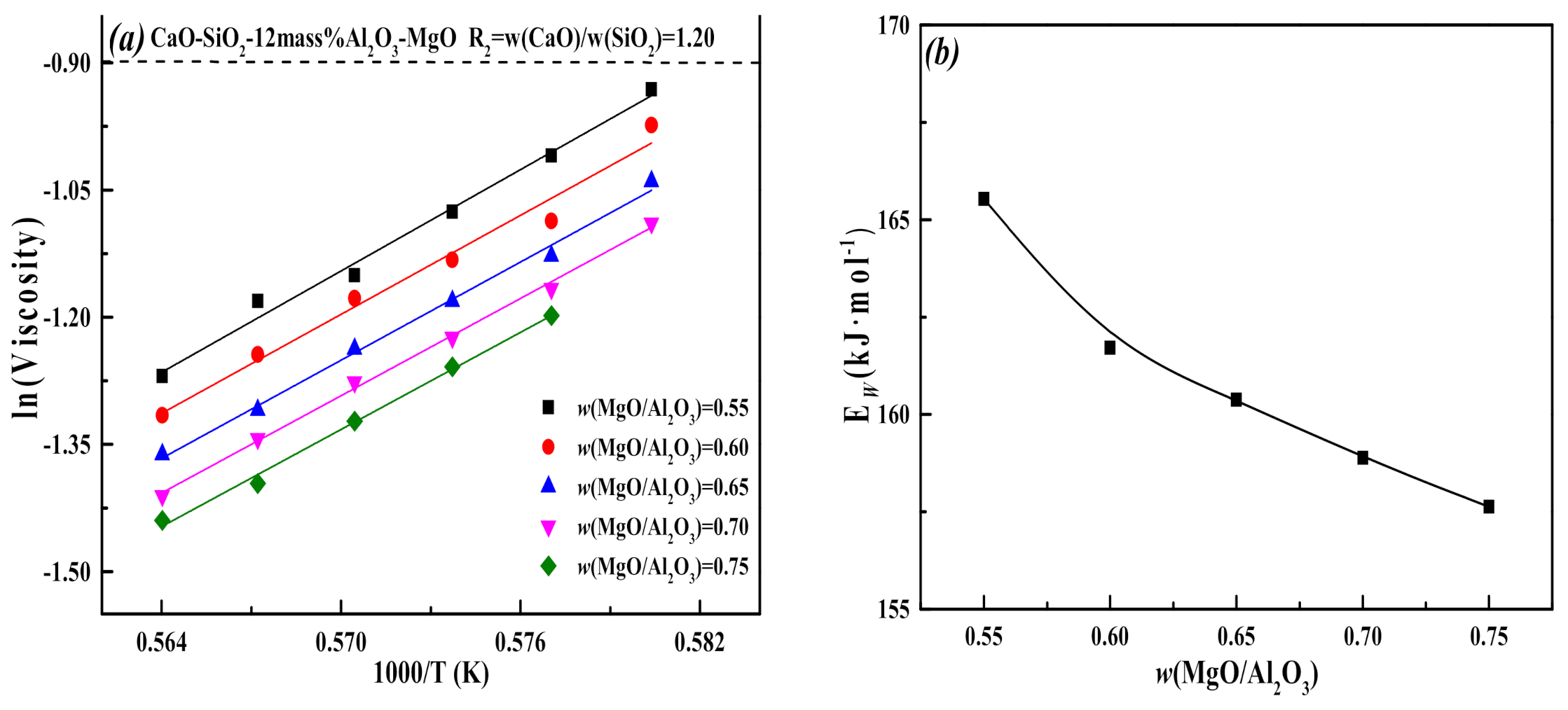
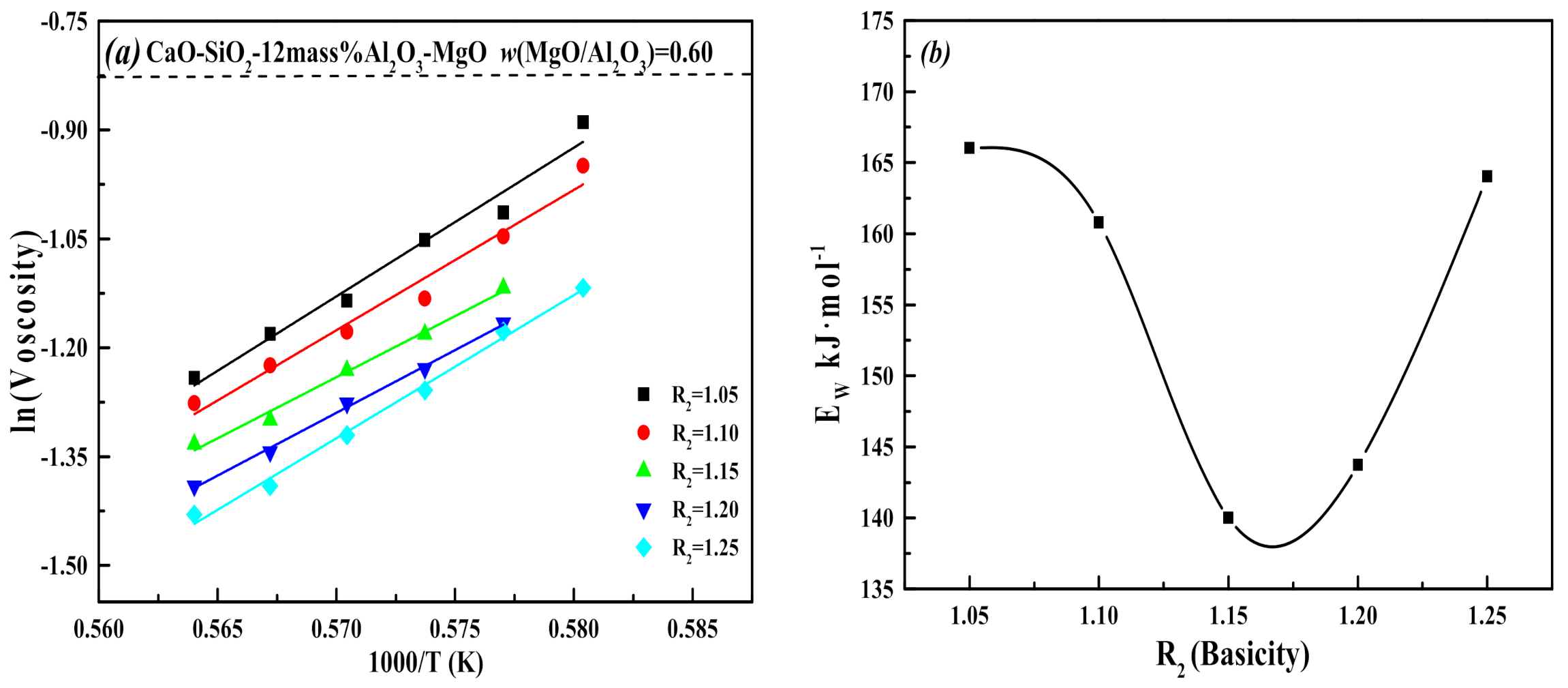
3.1. Effect of MgO/Al2O3 Ratio and Binary Basicity on Slag Viscosity
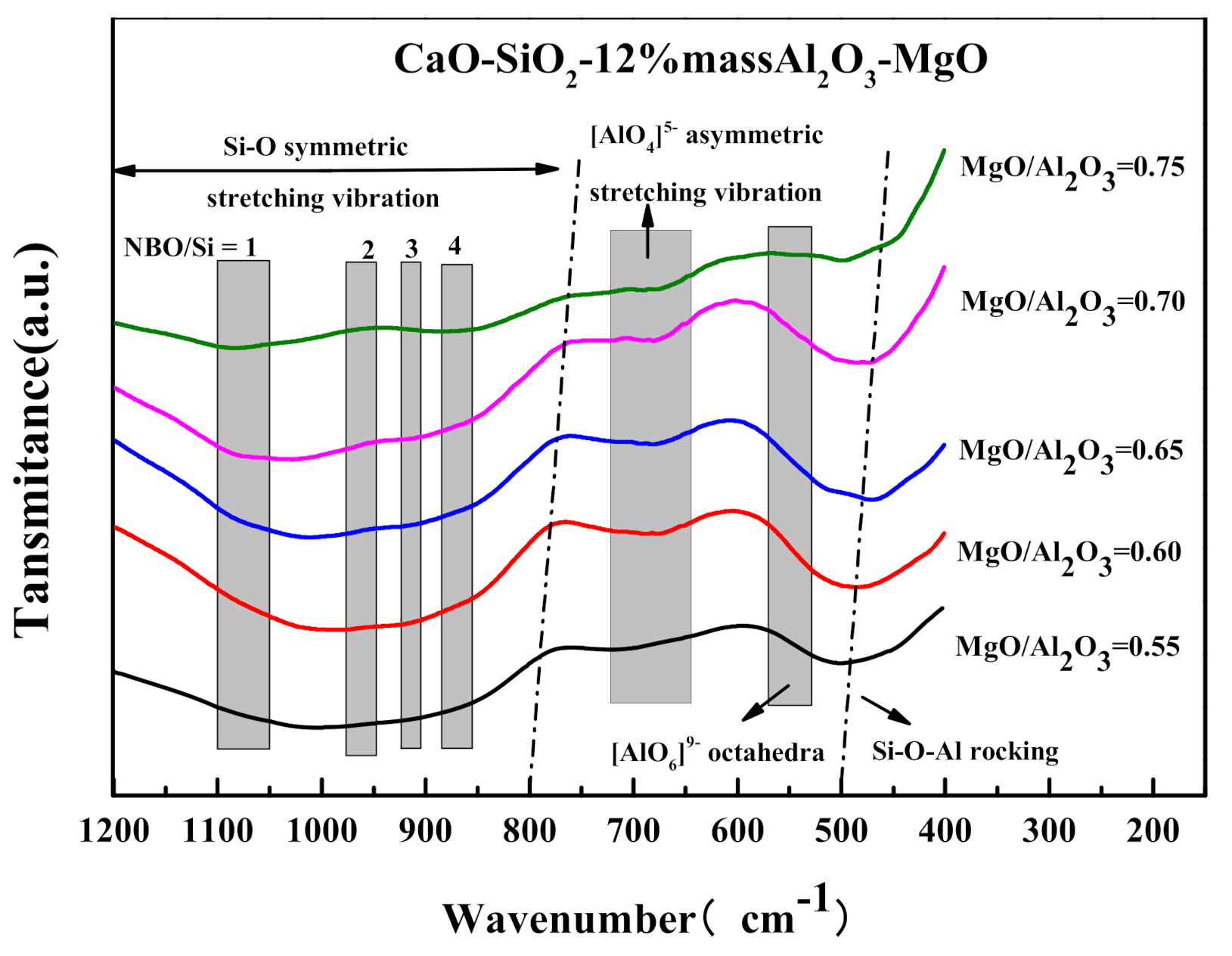
3.2. Effects of MgO/Al2O3 Ratio and Binary Basicity on Slag Thermal Stability
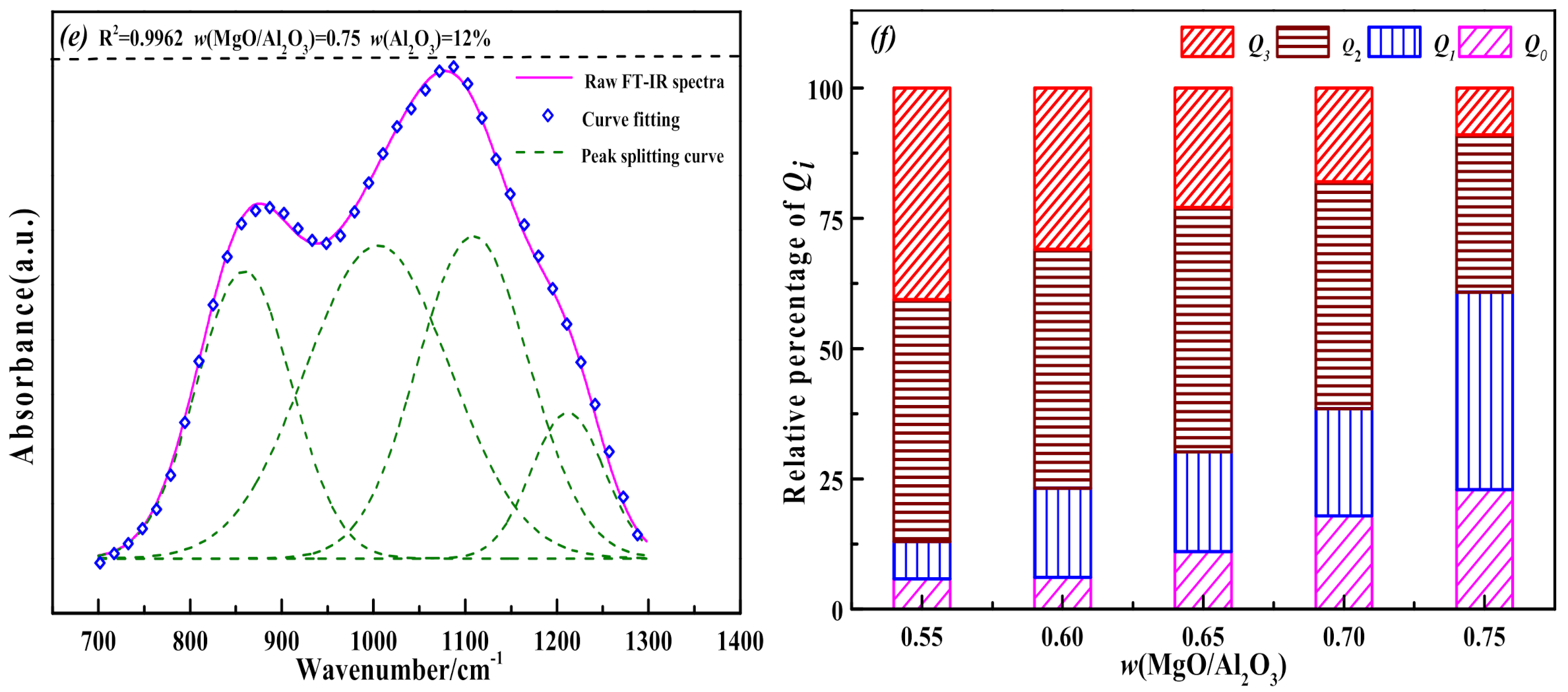
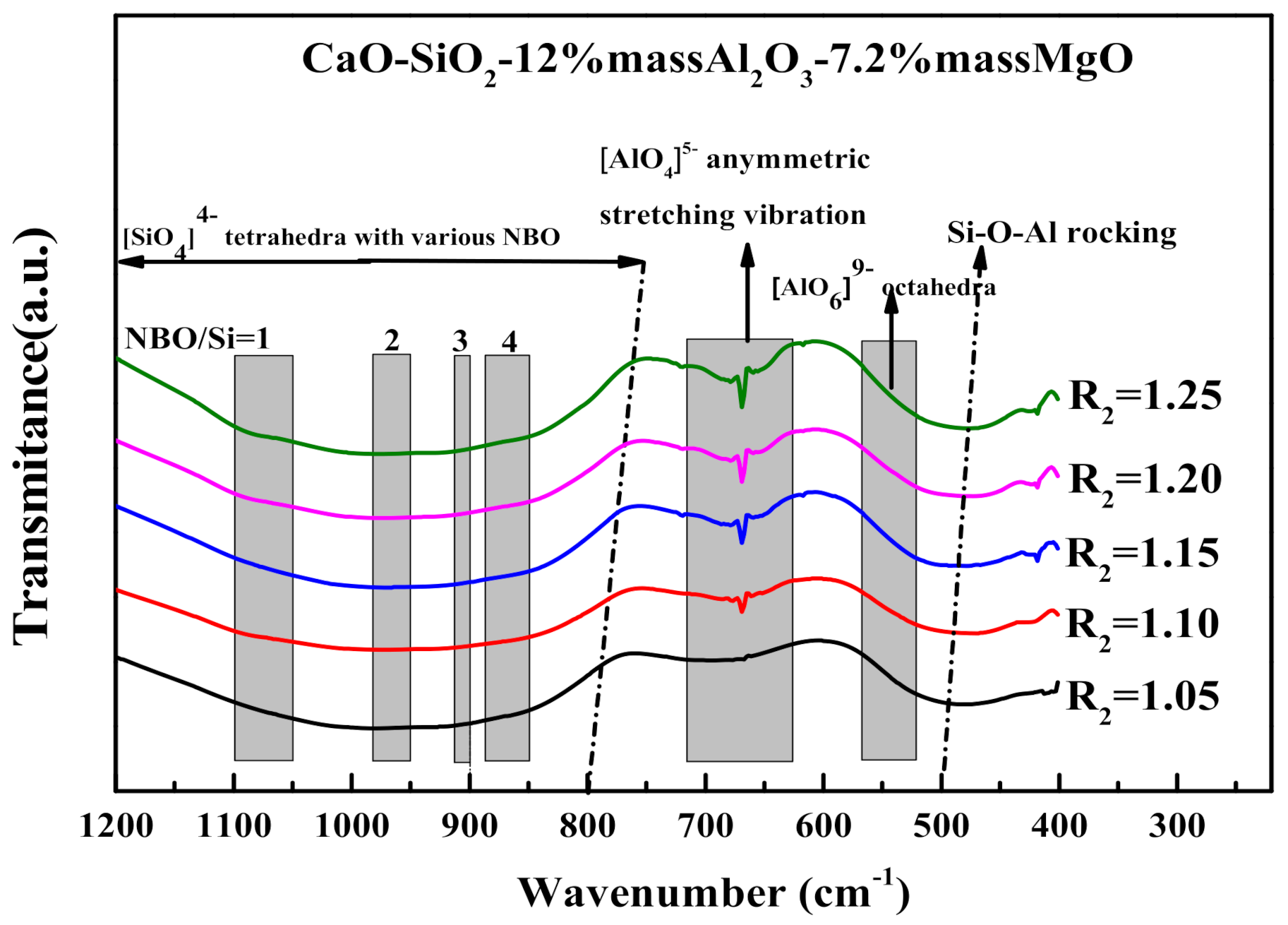
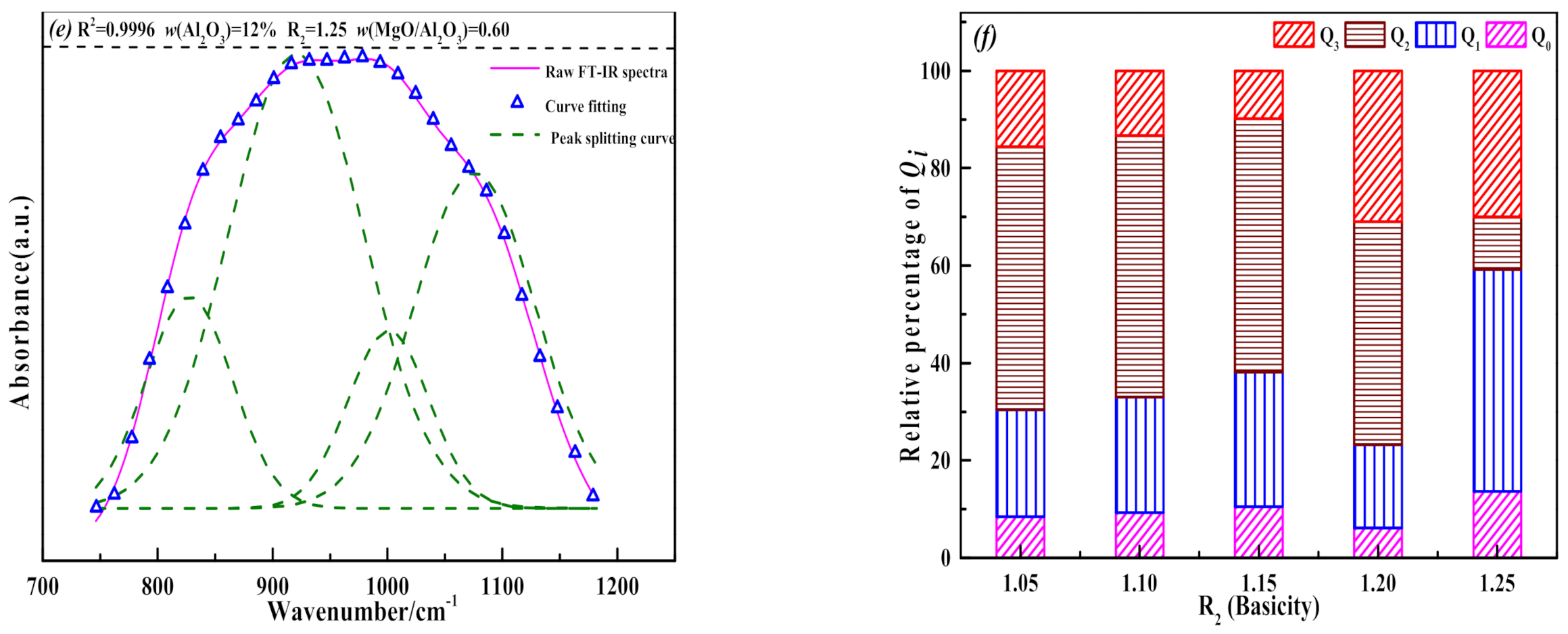
3.3. The Influence of MgO/Al2O3 Ratio and Basicity on the Thermal Stability of Blast Furnace Slag
3.4. Industrial Test Results
4. Conclusions
- The increase of the MgO/Al2O3 ratio and binary basicity in the low-aluminum slag can help reduce the degree of slag polymerization, resulting in a decrease of slag viscosity and viscous flow activation energy, which improves the fluidity and thermal stability of slag.
- When the binary basicity is higher than 1.20, the precipitation of melilite in the slag is inhibited, and the proportion of the high melting point ore phase Ca2SiO4 increases relatively. The slag structure tends to be more complicated, which slows down the growth rate of (Q0 + Q1) in the slag, the Q3 content in the slag increases sharply, and the thermal stability of the slag becomes worse.
- In combination with actual operating conditions and requirements for slag, the MgO/Al2O3 ratio of blast furnace slag should be controlled to 0.60 and the basicity should be no higher than 1.20 under the conditions of this investigation.
- Reducing the MgO/Al2O3 ratio of slag can effectively reduce the amount of blast furnace slag and K based on the results of the industrial tests. The coke rate can be saved as 3.49 kg/t according to the theoretical calculation, which would be a benefit for reducing CO2 emissions and promoting the sustainable development of the ironmaking industry.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mo, J.L.; Zhang, W.R.; Tu, Q.; Yuan, J.H.; Duan, H.B.; Fan, Y.; Pan, J.F.; Zhang, J.; Meng, Z.X. The role of national carbon pricing in phasing out China’s coal power. iScience 2021, 24, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Holappa, L. A General Vision for Reduction of Energy Consumption and CO2 Emissions from the Steel Industry. Metals 2020, 10, 1117–1137. [Google Scholar] [CrossRef]
- Xu, W.Q.; Wan, B.; Zhu, T.Y.; Shao, M.P. CO2 emissions from China’s iron and steel industry. J. Clean. Prod. 2016, 139, 1504–1511. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, Z.; Yin, J.; Su, L. CO2 emission reduction with Chinese Iron & steel industry: Practices, determinants and performance. J. Clean. Prod. 2012, 33, 167–178. [Google Scholar]
- Shen, F.M.; Hu, X.G.; Zheng, H.Y.; Jiang, X.; Gao, Q.J.; Han, H.S.; Long, F. Proper MgO/Al2O3 Ratio in Blast-Furnace Slag: Analysis of Proper MgO/Al2O3 Ratio Based on Observed Data. Metals 2020, 10, 784–792. [Google Scholar] [CrossRef]
- Hidayu, J.N.; Mohd, A.; Bakri, M.A.; Faizul, C.P.; Hasmaliza, M.; Arif, W.W.; Jitrin, C. Influences of SiO2, Al2O3, CaO and MgO in phase transformation of sintered kaolin-ground granulated blast furnace slag geopolymer. J. Mater. Res. Technol-JMRT 2020, 9, 11. [Google Scholar]
- Jiang, X.; Zhang, H.Y.; Zheng, H.Y.; Gao, Q.J.; Shen, F.M. Three-segment control theory of MgO/Al2O3 ratio based on viscosity experiments and phase diagram analyses at 1500 °C. J. Iron Steel Res. Int. 2020, 27, 1–7. [Google Scholar] [CrossRef]
- Talapaneni, T.; Yedla, N.; Pal, S.; Sarkar, S. Experimental and Theoretical Studies on the Viscosity–Structure Correlation for High Alumina-Silicate Melts. Metall. Mater. Trans. B 2017, 48, 1450–1462. [Google Scholar] [CrossRef]
- Jiang, C.H.; Li, K.J.; Zhang, J.L.; Qin, Q.H.; Liu, Z.J.; Sun, M.M.; Wang, Z.M.; Liang, W. Effect of MgO/Al2O3 ratio on the structure and properties of blast furnace slags: A molecular dynamics simulation. Metall. Mater. Trans. B 2018, 50, 367–375. [Google Scholar] [CrossRef]
- Shankar, A.; Görnerup, G.; Lahiri, A.K.; Seetharaman, S. Experimental investigation of the viscosities in CaO−SiO2−MgO−Al2O3 and CaO−SiO2−MgO−Al2O3−TiO2 slags. Metall. Mater. Trans. B 2007, 38, 911–915. [Google Scholar] [CrossRef]
- Feng, C.; Chu, M.S.; Tang, J.; Qin, J.; Li, F.; Liu, Z.G. Effects of MgO and TiO2 on the viscous behaviors and phase compositions of titanium-bearing slag. Int. J. Miner. Metall. Mater. 2016, 23, 868–880. [Google Scholar] [CrossRef]
- Seok, S.H.; Jung, S.M.; Lee, Y.S.; Min, D.J. Viscosity of highly basic slags. ISIJ Int. 2007, 47, 1090–1096. [Google Scholar] [CrossRef] [Green Version]
- Chang, Z.Y.; Zhang, J.L.; Xu, R.Z.; Jiao, K.X.; Bai, X.Q.; Han, W.X. Effect of Al2O3 on viscosity of low alumina slags of Jiusteel and thermodynamics analysis. China Metall. 2018, 28, 6–9. [Google Scholar]
- Zhang, X.; Jiang, T.; Xue, X.X.; Hu, B. Influence of MgO/Al2O3 ratio on voscosity of blast furance slag with high Al2O3 content. Steel Res. Int. 2016, 87, 87–94. [Google Scholar] [CrossRef]
- Kim, H.; Kim, W.H.; Sohn, I.; Min, D.J. The effect of MgO on the viscosity of the CaO–SiO2-20%Al2O3–MgO slag system. Steel Res. Int. 2010, 81, 261–264. [Google Scholar] [CrossRef]
- Jiang, C.H.; Li, K.J.; Zhang, J.L.; Qin, Q.H.; Liu, Z.J.; Liang, W.; Sun, M.M.; Wang, Z.M. Molecular Dynamics Simulation on the Effect of MgO/Al2O3 Ratio on Structure and Properties of Blast Furnace Slag Under Different Basicity Conditions. Metall. Mater. Trans. B 2019, 50, 367–375. [Google Scholar] [CrossRef]
- Sajid, M.; Bai, C.; Aamir, M.; You, Z.; Yan, Z.; Lv, X. Understanding the structure and structural effects on the properties of blast furnace slag (BFS). ISIJ Int. 2019, 59, 1153–1166. [Google Scholar] [CrossRef] [Green Version]
- Dong, X.J.; Sun, H.Y.; She, X.F.; Xue, Q.G.; Wang, J.S. Viscosity and viscosity estimation model of fully liquid slags in TiO2–Al2O3–CaO–SiO2 and TiO2–Al2O3–CaO–SiO2–MgO systems with high TiO2 concentration and low mass ratio of CaO to SiO2. Ironmak. Steelmak. 2014, 41, 99–106. [Google Scholar] [CrossRef]
- Park, H.; Park, J.Y.; Kim, G.H.; Sohn, I. Effect of TiO2 on the viscosity and slag structure in blast furnace type slags. Steel Res. Int. 2012, 83, 150–156. [Google Scholar] [CrossRef]
- Mysen, B.O.; Virgo, D. The solubility behvior of CO2 in melts on the join NaAlSi3O8–CaAl2Si2O8-CO2 at high pressures and temperatures: A Raman-spectroscopic study. Am. Mineral. 1980, 65, 1166–1175. [Google Scholar]
- Gao, Y.M.; Wang, S.B.; Hong, C.; Yang, F. Effects of basicity and MgO content on the viscosity of the SiO2–CaO–MgO-9 wt% Al2O3 slag system. Int. J. Miner. Metall. Mater. 2014, 21, 353–362. [Google Scholar] [CrossRef]
- Ning, X.J.; Li, P.C. Effects of MgO/Al2O3 Ratio and Basicity on the Viscosities of CaO–MgO–SiO2–Al2O3 Slags: Experiments and Modeling. Metall. Mater. Trans. B 2016, 47, 446–457. [Google Scholar]
- Pang, Z.D.; Lv, X.; Ling, J.W.; Jiang, Y.Y.; Yan, Z.M.; Da, J. Blast Furnace Ironmaking Process with Super High TiO2 in the Slag: High-Temperature Structure of the Slag. Metall. Mater. Trans. B 2020, 51, 2348–2357. [Google Scholar] [CrossRef]
- Zheng, H.Y.; Liang, L.S.; Du, J.L.; Zhou, S.F.; Jiang, X.; Gao, Q.J.; Shen, F.M. Mineral Transform and Specific Heat Capacity Characterization of Blast Furnace Slag with High Al2O3 in Heating Process. Steel Res. Int. 2020, 92, 10. [Google Scholar]
- Wang, X.L. Iron and Steel Metallurgy; Metallurgical Industry Press: Beijing, China, 2000; pp. 248–285. [Google Scholar]




| CaO/% | SiO2/% | Al2O3/% | MgO/% | R2 | MgO/Al2O3 |
| 42.72 | 36.21 | 12.11 | 8.96 | 1.18 | 0.74 |
| NO. | MgO/Al2O3 | CaO/% | SiO2/% | Al2O3/% | MgO/% | R2 |
| 1 | 0.55 | 44.40 | 37.00 | 12.00 | 6.60 | 1.20 |
| 2 | 0.60 | 44.07 | 36.73 | 12.00 | 7.20 | 1.20 |
| 3 | 0.65 | 43.75 | 36.45 | 12.00 | 7.80 | 1.20 |
| 4 | 0.70 | 43.42 | 36.18 | 12.00 | 8.40 | 1.20 |
| 5 | 0.75 | 43.09 | 35.91 | 12.00 | 9.00 | 1.20 |
| 6 | 0.60 | 41.39 | 39.41 | 12.00 | 7.20 | 1.05 |
| 7 | 0.60 | 42.32 | 38.48 | 12.00 | 7.20 | 1.10 |
| 8 | 0.60 | 43.22 | 37.58 | 12.00 | 7.20 | 1.15 |
| 9 | 0.60 | 44.07 | 36.73 | 12.00 | 7.20 | 1.20 |
| 10 | 0.60 | 44.89 | 35.91 | 12.00 | 7.20 | 1.25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhan, W.; Liu, Y.; Shao, T.; Han, X.; Pang, Q.; Zhang, J.; He, Z. Evaluating the Effect of MgO/Al2O3 Ratio on Thermal Behaviors and Structures of Blast Furnace Slag with Low Carbon Consumption. Crystals 2021, 11, 1386. https://doi.org/10.3390/cryst11111386
Zhan W, Liu Y, Shao T, Han X, Pang Q, Zhang J, He Z. Evaluating the Effect of MgO/Al2O3 Ratio on Thermal Behaviors and Structures of Blast Furnace Slag with Low Carbon Consumption. Crystals. 2021; 11(11):1386. https://doi.org/10.3390/cryst11111386
Chicago/Turabian StyleZhan, Wenlong, Yi Liu, Tengfei Shao, Xiao Han, Qinghai Pang, Junhong Zhang, and Zhijun He. 2021. "Evaluating the Effect of MgO/Al2O3 Ratio on Thermal Behaviors and Structures of Blast Furnace Slag with Low Carbon Consumption" Crystals 11, no. 11: 1386. https://doi.org/10.3390/cryst11111386






