Cell-Free Biomimetic Mineralization Strategies to Regenerate the Enamel Microstructure
Abstract
:1. Introduction
2. Learning from Enamel
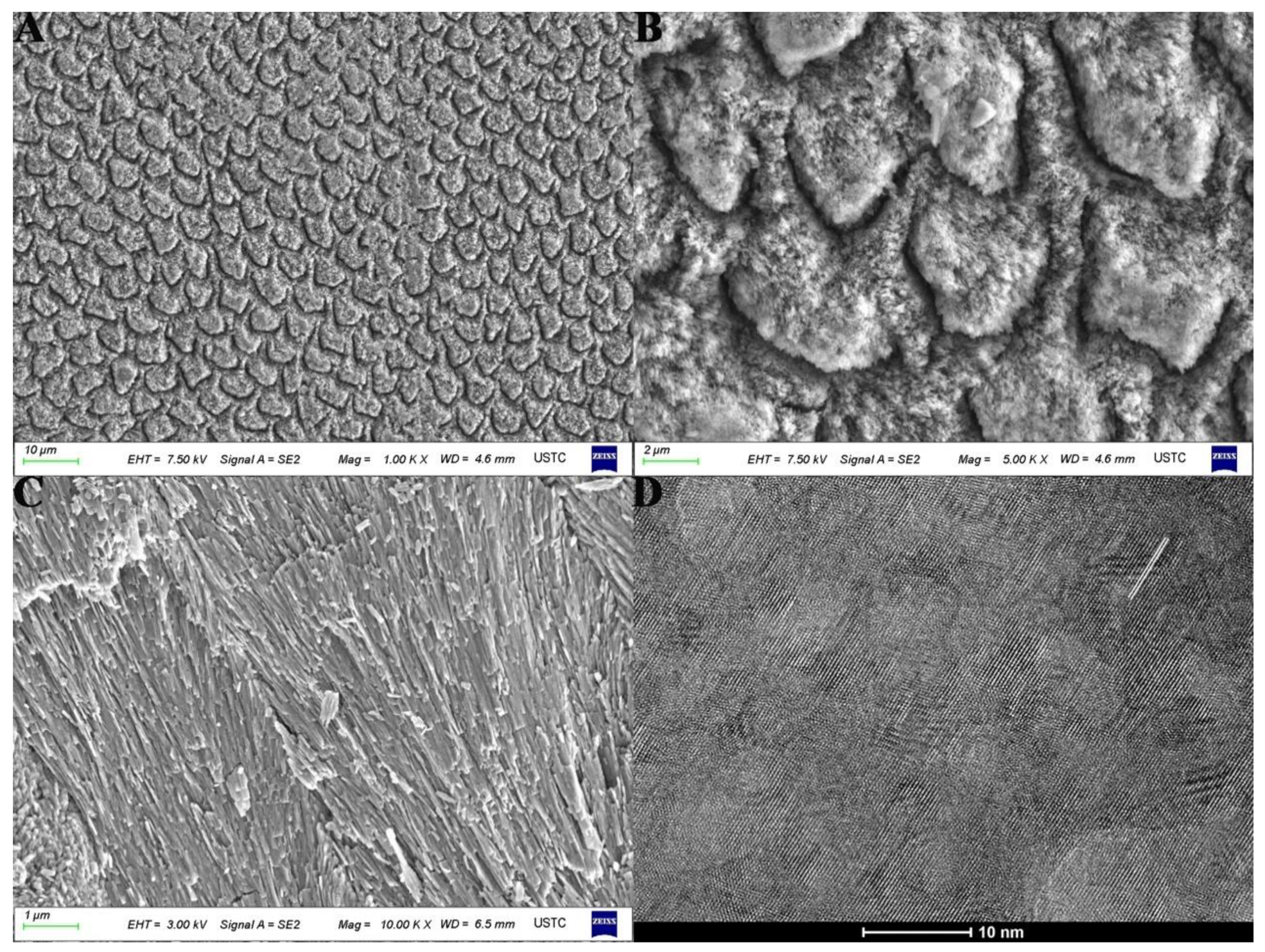
2.1. The Structure of Enamel and Its Mechanical Properties
2.2. The Process of Amelogenesis
2.2.1. The Functional Role of Enamel Protein Matrices
The Function of Amelogenin Protein
The Function of Non-Amelogenin Protein
2.2.2. The Functional Role of Proteinases
3. Organic Matrices Mediated Mineralization
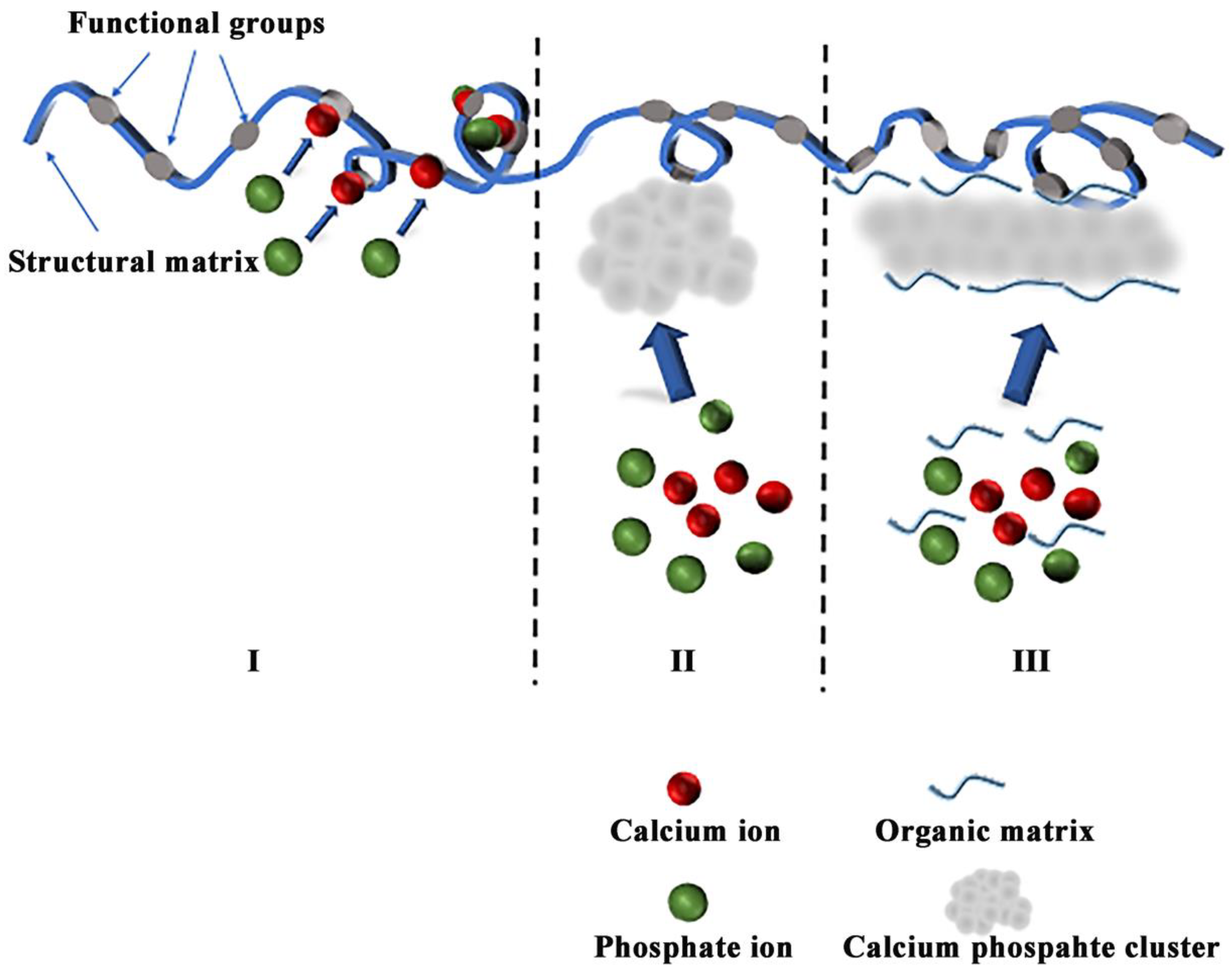
3.1. The Mechanism of Organic Matrices Mediated Mineralization
3.2. Protein Based Organic Matrix-Mediated Mineralization System
3.2.1. Recombinant Amelogenin
3.2.2. Leucine-Rich Amelogenin Peptide (LRAP)
3.2.3. Human Dentine Phosphoprotein (DPP)
3.3. Non-Protein Based Organic Matrix-Mediated Mineralization System
3.3.1. Self-Assembled Peptide
3.3.2. Dendrimers and Their Analog-Mediated Mineralization
3.3.3. Surfactants Mediated Mineralization
Nonionic Surfactant
Ionic Surfactant
3.3.4. EDTA Mediated Mineralization
4. Regeneration of Enamel Microstructure Induced by the Template of the Cation Membrane System
4.1. One-Directional Ca2+ Supply
4.2. Hydrogel Matrix
4.3. Ca2+ and PO4 3− Ionic Inflow, and pH Value
5. Electrolytic Deposition (ELD) Mineralization System
6. The Outlook
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Solaymani, S.; Ghoranneviss, M.; Elahi, S.M.; Shafiekhani, A.; Kulesza, S.; Ţălu, Ş.; Bramowicz, M.; Hantehzadeh, M.; Nezafat, N.B. The relation between structural, rugometric and fractal characteristics of hard dental tissues at micro and nano levels. Microsc. Res. Tech. 2019, 82, 421–428. [Google Scholar] [CrossRef] [PubMed]
- De Dios Teruel, J.; Alcolea, A.; Hernández, A.; Ruiz, A.J.O. Comparison of chemical composition of enamel and dentine in human, bovine, porcine and ovine teeth. Arch. Oral Biol. 2015, 60, 768–775. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Hasegawa, T.; Yamamoto, T.; Hongo, H.; Amizuka, N. Histology of human cementum: Its structure, function, and development. Jpn. Dent. Sci. Rev. 2016, 52, 63–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lacruz, R.S.; Habelitz, S.; Wright, J.T.; Paine, M.L. Dental enamel formation and implications for oral health and disease. Physiol. Rev. 2017, 97, 939–993. [Google Scholar] [CrossRef]
- Kreulen, C.M.; Van’t Spijker, A.; Rodriguez, J.M.; Bronkhorst, E.M.; Creugers, N.H.J.; Bartlett, D.W. Systematic review of the prevalence of tooth wear in children and adolescents. Caries Res. 2010, 44, 151–159. [Google Scholar] [CrossRef]
- Moradian-Oldak, J. Protein-mediated enamel mineralization. Front. Biosci. 2012, 17, 1996. [Google Scholar] [CrossRef] [Green Version]
- Featherstone, J.D.B. Remineralization, the natural caries repair process—The need for new approaches. Adv. Dent. Res. 2009, 21, 4–7. [Google Scholar] [CrossRef]
- Pitts, N.B.; Wefel, J.S. Remineralization/desensitization: What is known? What is the future? Adv. Dent. Res. 2009, 21, 83–86. [Google Scholar] [CrossRef]
- Chen, H.F.T.Z.; Tang, Z.; Liu, J.; Sun, K.; Chang, S.R.; Peters, M.C.; Mansfield, J.F.; Czajka-Jakubowska, A.; Clarkson, B.H. Acellular synthesis of a human enamel-like microstructure. Adv. Mater. 2006, 18, 1846–1851. [Google Scholar] [CrossRef] [Green Version]
- Zou, Z.; Liu, X.; Chen, L.; Lin, K.; Chang, J. Dental enamel-like hydroxyapatite transformed directly from monetite. J. Mater. Chem. 2012, 22, 22637–22641. [Google Scholar] [CrossRef]
- Yamagishi, K.; Onuma, K.; Suzuki, T.; Okada, F.; Tagami, J.; Otsuki, M.; Senawangse, P. A Synthetic enamel for rapid tooth repair. Nature 2005, 433, 819. [Google Scholar] [CrossRef] [PubMed]
- Zhan, J.; Tseng, Y.H.; Chan, J.C.; Mou, C.Y. Biomimetic formation of hydroxyapatite nanorods by a single-crystal-to-single-crystal transformation. Adv. Funct. Mater. 2005, 15, 2005–2010. [Google Scholar] [CrossRef]
- Handa, T.; Anada, T.; Honda, Y.; Yamazaki, H.; Kobayashi, K.; Kanda, N.; Kamakura, S.; Echigo, S.; Suzuki, O. The effect of an octacalcium phosphate co-precipitated gelatin composite on the repair of critical-sized rat calvarial defects. Acta Biomater. 2012, 8, 1190–1200. [Google Scholar] [CrossRef]
- Ethirajan, A.; Ziener, U.; Chuvilin, A.; Kaiser, U.; Cölfen, H.; Landfester, K. Biomimetic hydroxyapatite crystallization in gelatin nanoparticles synthesized using a miniemulsion process. Adv. Funct. Mater. 2008, 18, 2221–2227. [Google Scholar] [CrossRef]
- Jiao, M.J.; Wang, X.X. Electrolytic deposition of magnesium-substituted hydroxyapatite crystals on titanium substrate. Mater Lett. 2009, 63, 2286–2289. [Google Scholar] [CrossRef]
- Lei, C.; Liao, Y.; Feng, Z. Kinetic model for hydroxyapatite precipitation on human enamel surface by electrolytic deposition. Biomed. Mater. 2009, 4, 035010. [Google Scholar] [CrossRef]
- Moradian-Oldak, J. The regeneration of tooth enamel. Dimens. Dent. Hyg. 2009, 7, 12. [Google Scholar] [PubMed]
- Zafar, M.S.; Amin, F.; Fareed, M.A.; Ghabbani, H.; Riaz, S.; Khurshid, Z.; Kumar, N. Biomimetic aspects of restorative dentistry biomaterials. Biomimetics 2020, 5, 34. [Google Scholar] [CrossRef]
- Qasim, S.S.B.; Zafar, M.S.; Niazi, F.H.; Alshahwan, M.; Omar, H.; Daood, U. Functionally graded biomimetic biomaterials in dentistry: An evidence-based update. J. Biomater. Sci. Polym. Ed. 2020, 31, 1144–1162. [Google Scholar] [CrossRef] [PubMed]
- Gandolfi, M.G.; Taddei, P.; Siboni, F.; Modena, E.; Ginebra, M.P.; Prati, C. Fluoride-containing nanoporous calcium-silicate MTA cements for endodontics and oral surgery: Early fluorapatite formation in a phosphate-containing solution. Int. Endod. J. 2011, 44, 938–949. [Google Scholar] [CrossRef]
- Bossù, M.; Saccucci, M.; Salucci, A.; Di Giorgio, G.; Bruni, E.; Uccelletti, D.; Sarto, M.S.; Familiari, G.; Relucenti, M.; Polimeni, A. Enamel remineralization and repair results of Biomimetic Hydroxyapatite toothpaste on deciduous teeth: An effective option to fluoride toothpaste. J. Nanobiotechnol. 2019, 17, 17. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, L.; Chen, W.; Jin, H.; Zhang, Y.; Wu, L.; Shao, H.; Fang, Z.; He, X.; Zheng, S.; et al. Rapid regeneration of enamel-like-oriented inorganic crystals by using rotary evaporation. Mater. Sci. Eng. 2020, 115, 111141. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Sun, Z.; Wang, R.; Abbott, C.; Moradian-Oldak, J. Enamel inspired nanocomposite fabrication through amelogenin su pramolecular assembly. Biomaterials 2007, 28, 3034–3304. [Google Scholar] [CrossRef] [Green Version]
- Kwak, S.Y.; Litman, A.; Margolis, H.C.; Yamakoshi, Y.; Simmer, J.P. Biomimetic enamel regeneration mediated by leucine-rich amelogenin peptide. J. Dent. Res. 2017, 96, 524–530. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, K.M.; Zhai, H.; Zhu, L.; Horst, J.A.; Sitlin, M.; Nguyen, M.; Wagner, M.; Simpliciano, C.; Milder, M.; Chen, C.L.; et al. Amyloid-like ribbons of amelogenins in enamel mineralization. Sci. Rep. 2016, 6, 23105. [Google Scholar] [CrossRef] [Green Version]
- Shafiei, F.; Hossein, B.G.; Farajollahi, M.M.; Fathollah, M.; Marjan, B.; Tahereh, J.K. Leucine-rich amelogenin peptide (LRAP) as a surface primer for biomimetic remineralization of superficial enamel defects: An in vitro study. Scanning 2015, 37, 179–185. [Google Scholar] [CrossRef]
- Le Norcy, E.; Kwak, S.Y.; Wiedemann-Bidlack, F.B.; Beniash, E.; Yamakoshi, Y.; Simmer, J.P.; Margolis, H.C. Leucine-rich ame logenin peptides regulate mineralization in vitro. J. Dent. Res. 2011, 90, 1091–1097. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.L.; Ning, T.Y.; Cao, Y.; Zhang, W.B.; Mei, M.L.; Chu, C.H. A novel self-assembled oligopeptide amphiphile for biomimetic mineralization of enamel. BMC Biotechnol. 2014, 14, 32. [Google Scholar] [CrossRef] [Green Version]
- Philip, N. State of the art enamel remineralization systems: The next frontier in caries management. Caries Res. 2019, 53, 284–295. [Google Scholar] [CrossRef]
- Zhou, L.; Wong, H.M.; Zhang, Y.Y.; Li, Q.L. Constructing an antibiofouling and mineralizing bioactive tooth surface to protect against decay and promote self-healing. ACS Appl. Mater. Interfaces 2019, 12, 3021–3031. [Google Scholar] [CrossRef]
- Zhang, F.; Zhou, Z.H.; Yang, S.P.; Mao, L.H.; Chen, H.M.; Yu, X.B. Hydrothermal synthesis of hydroxyapatite nanorods in the presence of anionic starburst dendrimer. Mater. Lett. 2005, 59, 1422–1425. [Google Scholar] [CrossRef]
- Fowler, C.E.; Li, M.; Mann, S.; Margolis, H.C. Influence of surfactant assembly on the formation of calcium phosphate materials—A model for dental enamel formation. J. Mater. Chem. 2005, 15, 3317–3325. [Google Scholar] [CrossRef]
- Pandya, M.; Diekwisch, T.G.H. Enamel biomimetics-fiction or future of dentistry. Int. J. Oral Sci. 2019, 11, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beniash, E.; Stifler, C.A.; Sun, C.Y.; Jung, G.S.; Qin, Z.; Buehler, M.J.; Gilbert, P.U.P.A. The hidden structure of human enamel. Nat. Commun. 2019, 10, 4383. [Google Scholar] [CrossRef] [Green Version]
- Rivera, C.; Arola, D.; Ossa, A. Indentation damage and crack repair in human enamel. J. Mech. Behav. Biomed. Mater. 2013, 21, 178–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lokappa, S.B.; Chandrababu, K.B.; Moradian-Oldak, J. Tooth enamel protein amelogenin binds to ameloblast cell membrane-mimicking vesicles via its N-terminus. Biochem. Biophys. Res. Commun. 2015, 464, 956–961. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee, K.; Ruan, Q.; Nutt, S.; Tao, J.; De Yoreo, J.J.; Moradian-Oldak, J. Peptide-based bioinspired approach to regrowing multilayered aprismatic enamel. ACS Omega 2018, 3, 2546–2557. [Google Scholar] [CrossRef] [Green Version]
- Beniash, E.; Simmer, J.P.; Margolis, H.C. Structural changes in amelogenin upon self-assembly and mineral interactions. J. Dent. Res. 2012, 91, 967–972. [Google Scholar] [CrossRef] [Green Version]
- Bromley, K.M.; Kiss, A.S.; Lokappa, S.B.; Lakshminarayanan, R.; Fan, D.; Ndao, M.; Evans, J.S.; Moradian-Oldak, J. Dissecting amelogenin protein nanospheres characterization of metastable oligomers. J. Biol. Chem. 2011, 286, 34643–34653. [Google Scholar] [CrossRef] [Green Version]
- Fincham, A.G.; Moradian-Oldak, J.; Simmer, J.P. The structural biology of the developing dental enamel matrix. J. Struct. Biol. 1999, 126, 270–299. [Google Scholar] [CrossRef]
- Iijima, M.; Moradian-Oldak, J. Interactions of amelogenins with octacalcium phosphate crystal faces are dose dependent. Calcif. Tissue Int. 2004, 74, 522–531. [Google Scholar] [CrossRef]
- Iijima, M.; Moriwaki, Y.; Takagi, T.; Moradian-Oldak, J. Effects of bovine amelogenins on the crystal morphology of octacalcium phosphate in a model system of tooth enamel formation. J. Cryst. Growth 2001, 222, 615–626. [Google Scholar] [CrossRef]
- Beniash, E.; Simmer, J.P.; Margolis, H.C. The effect of recombinant mouse amelogenins on the formation and organization of hydroxyapatite crystals in vitro. J. Struct. Biol. 2005, 149, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Fukumoto, S.; Kiba, T.; Hall, B.; Iehara, N.; Nakamura, T.; Longenecker, G.; Krebsbach, P.H.; Nanci, A.; Kulkarni, A.B.; Yamada, Y. Ameloblastin is a cell adhesion molecule required for maintaining the differentiation state of ameloblasts. J. Cell Biol. 2004, 167, 973–983. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, D.; Du, C.; Sun, Z.; Lakshminarayanan, R.; Moradian-Oldak, J. In vitro study on the interaction between the 32 kDa enamelin and amelogenin. J. Struct. Biol. 2009, 166, 88–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deutsch, D.; Leiser, Y.; Shay, B.; Fermon, E.; Taylor, A.; Rosenfeld, E.; Dafni, L.; Charuvi, K.; Cohen, Y.; Haze, A.; et al. The human tuftelin gene and the expression of tuftelin in mineralizing and nonmineralizing tissues. Connect. Tissue Res. 2002, 43, 425–434. [Google Scholar] [CrossRef]
- Jain, P.S.; Damle, S.G.; Dedhia, S.P.; Jetpurwala, A.M.; Gupte, T.S. Evaluation of the association between tuftelin gene polymer phism, Streptococcus mutans, and dental caries susceptibility. J. Indian Soc. Pedod. Prev. Dent. 2020, 38, 381–386. [Google Scholar]
- Ruan, Q.; Moradian-Oldak, J. Amelogenin and enamel biomimetics. J. Mater. Chem. B 2015, 3, 3112–3129. [Google Scholar] [CrossRef]
- Smith, C.E.; Richardson, A.S.; Hu, Y.; Bartlett, J.D.; Hu, J.C.; Simmer, J.P. Effect of Kallikrein 4 Loss on Enamel Mineralization comparison with mice lacking matrix metalloproteinase 20. J. Biol. Chem. 2011, 286, 18149–18160. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Sun, Z.; Ma, R.; Fan, D.; Moradian-Oldak, J. Amelogenin “nanorods” formation during proteolysis by Mmp-20. J. Struct. Biol. 2011, 176, 220–228. [Google Scholar] [CrossRef] [Green Version]
- Kwak, S.Y.; Wiedemann-Bidlack, F.B.; Beniash, E.; Yamakoshi, Y.; Simmer, J.P.; Litman, A.; Margolis, H.C. Role of 20-kDa Amelogenin (P148) Phosphorylation in Calcium Phosphate Formation in vitro. J. Biol. Chem. 2009, 284, 18972–18979. [Google Scholar] [CrossRef] [Green Version]
- Ryu, O.; Hu, J.C.; Yamakoshi, Y.; Villemain, J.L.; Cao, X.; Zhang, C.; Bartlett, J.D.; Simmer, J.P. Porcine kallikrein-4 activation, glycosyl ation, activity, and expression in prokaryotic and eukaryotic hosts. Eur. J. Oral Sci. 2002, 110, 358–365. [Google Scholar] [CrossRef]
- Yuwono, V.M.; Burrows, N.D.; Soltis, J.A.; Penn, R.L. Oriented aggregation: Formation and transformation of mesocrystal inter mediates revealed. J. Am. Chem. Soc. 2010, 132, 2163–2165. [Google Scholar] [CrossRef] [PubMed]
- Penn, R.L.; Soltis, J.A. Characterizing crystal growth by oriented aggregation. CrystEngComm 2014, 16, 1409–1418. [Google Scholar] [CrossRef]
- Al-Ghoul, M.; Issa, R.; Hmadeh, M. Synthesis, size and structural evolution of metal–organic framework-199 via a reaction–dif fusion process at room temperature. CrystEngComm 2017, 19, 608–612. [Google Scholar] [CrossRef]
- Schliehe, C.; Juarez, B.H.; Pelletier, M.; Jander, S.; Greshnykh, D.; Nagel, M.; Meyer, A.; Foerster, S.; Kornowski, A.; Klinke, C.; et al. Ultrathin PbS sheets by two-dimensional oriented attachment. Science 2010, 329, 550–553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cölfen, H.; Mann, S. Higher-order organization by mesoscale self-assembly and transformation of hybrid nanostructures. Angew. Chem. Int. Ed. 2003, 42, 2350–2365. [Google Scholar] [CrossRef] [Green Version]
- Mann, S.; Archibald, D.D.; Didymus, J.M.; Douglas, T.; Heywood, B.R.; Meldrum, F.C.; Reeves, N.J. Crystallization at inorganic-organic interfaces: Biominerals and biomimetic synthesis. Science 1993, 261, 1286–1292. [Google Scholar] [CrossRef] [Green Version]
- Iijima, M. Modification of octacalcium phosphate growth by enamel proteins, fluoride, and substrate materials and influence of morphology on the performance of octacalcium phosphate biomaterials. In Octacalcium Phosphate Biomaterials; Woodhead Publishing: Sawston, UK, 2020; pp. 309–347. [Google Scholar]
- Han, T.; Wang, M.; Cao, C.; Chen, H.; Zhang, G.; Wang, L.; Wang, J. Fluoride or/and aluminum induced toxicity in guinea pig teeth with the low expression of dentine phosphoprotein. J. Biochem. Mol. Toxicol. 2017, 31, e21912. [Google Scholar] [CrossRef]
- Hsu, C.C.; Chung, H.Y.; Yang, J.M.; Shi, W.; Wu, B. Influence of 8DSS peptide on nano-mechanical behavior of human enamel. J. Dent. Res. 2011, 90, 88–92. [Google Scholar] [CrossRef]
- Semino, C.E. Self-assembling peptides: From bio-inspired materials to bone regeneration. J. Dent. Res. 2008, 87, 606–616. [Google Scholar] [CrossRef] [PubMed]
- Brunton, P.A.; Davies, R.P.W.; Burke, J.L.; Smith, A.; Aggeli, A.; Brookes, S.J.; Kirkham, J. Treatment of early caries lesions using biomimetic self-assembling peptides—A clinical safety trial. Br. Dent. J. 2013, 215, E6. [Google Scholar] [CrossRef] [Green Version]
- Kind, L.; Stevanovic, S.; Wuttig, S.; Wimberger, S.; Hofer, J.; Müller, B.; Pieles, U. Biomimetic remineralization of carious lesions by self-assembling peptide. J. Dent. Res. 2017, 96, 790–797. [Google Scholar] [CrossRef]
- Kirkham, J.; Firth, A.; Vernals, D.; Boden, N.; Robinson, C.; Shore, R.C.; Brookes, S.J.; Aggeli, A. Self-assembling peptide scaffolds. promote enamel remineralization. J. Dent. Res. 2007, 86, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Noriega-Luna, B.; Godínez, L.A.; Rodríguez, F.J.; Rodríguez, A.; Zaldívar-Lelo de Larrea, G.; Sosa-Ferreyra, C.F.; Mercado-Curiel, R.F.; Manríquez, J.; Bustos, E. Applications of dendrimers in drug delivery agents, diagnosis, therapy, and detection. J. Nanomater. 2014, 2014, 507273. [Google Scholar] [CrossRef] [Green Version]
- Esfand, R.; Tomalia, D.A. Poly (amidoamine)(PAMAM) dendrimers: From biomimicry to drug delivery and biomedical applications. Drug Discov. Today 2001, 6, 427–436. [Google Scholar] [CrossRef]
- Kesharwani, P.; Banerjee, S.; Gupta, U.; Amin, M.C.I.M.; Padhye, S.; Sarkar, F.H.; Iyer, A.K. PAMAM dendrimers as promising nanocarriers for RNAi therapeutics. Mater. Today 2015, 18, 565–572. [Google Scholar] [CrossRef]
- Niu, Y.; Qu, R.; Chen, H.; Mu, L.; Liu, X.; Wang, T.; Zhang, Y.; Sun, C. Synthesis of silica gel supported salicylaldehyde modified PAMAM dendrimers for the effective removal of Hg (II) from aqueous solution. J. Hazard. Mater. 2014, 278, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Yang, J.; Li, J.; Chen, L.; Tang, B.; Chen, X.; Wu, W.; Li, J. Hydroxyapatite-anchored dendrimer for in situ remineralization of human tooth enamel. Biomaterials 2013, 34, 5036–5047. [Google Scholar] [CrossRef]
- Yang, S.; He, H.; Wang, L.; Jia, X.; Feng, H. Oriented crystallization of hydroxyapatite by the biomimetic amelogenin nanospheres from self-assemblies of amphiphilic dendrons. Chem. Commun. 2011, 47, 10100–10102. [Google Scholar] [CrossRef]
- Bujan, M.; Sikirić, M.; Filipović-Vinceković, N.; Vdović, N.; Garti, N.; Füredi-Milhofer, H. Effect of anionic surfactants on crystal growth of calcium hydrogen phosphate dihydrate. Langmuir 2001, 17, 6461–6470. [Google Scholar] [CrossRef]
- Althues, H.; Kaskel, S. Sulfated zirconia nanoparticles synthesized in reverse microemulsions: Preparation and catalytic properties. Langmuir 2002, 18, 7428–7435. [Google Scholar] [CrossRef]
- Qi, L.; Ma, J.; Cheng, H.; Zhao, Z. Microemulsion-mediated synthesis of calcium hydroxyapatite fine powders. J. Mater. Sci. Lett. 1997, 16, 1779–1781. [Google Scholar] [CrossRef]
- Ye, F.; Guo, H.; Zhang, H. Biomimetic synthesis of oriented hydroxyapatite mediated by nonionic surfactants. Nanotechnology 2008, 19, 245605. [Google Scholar] [CrossRef] [PubMed]
- Vasquez, V.R.; Williams, B.C.; Graeve, O.A. Stability and comparative analysis of AOT/water/isooctane reverse micelle system using dynamic light scattering and molecular dynamics. J. Phys. Chem. B 2011, 115, 2979–2987. [Google Scholar] [CrossRef]
- Yang, S.; Chen, J.; Wang, Z.; Zhang, H.; Zhang, Q. Surfactant-assisted synthesis of oriented hydroxyapatite nanoclusters by reflux method. Mater. Lett. 2013, 96, 177–180. [Google Scholar] [CrossRef]
- Liu, J.; Li, K.; Wang, H.; Zhu, M.; Xu, H.; Yan, H. Self-assembly of hydroxyapatite nanostructures by microwave irradiation. Nanotechnology 2004, 16, 82. [Google Scholar] [CrossRef]
- Ardell, A.J. Non-integer temporal exponents in trans-interface diffusion-controlled coarsening. J. Mater. Sci. 2016, 51, 6133–6148. [Google Scholar] [CrossRef] [Green Version]
- Iijima, M.; Moriwaki, Y. Lengthwise and oriented growth of octacalcium phosphate crystal in polyacrylamide gel in a model system of tooth enamel apatite formation. J. Cryst. Growth 1998, 194, 125–132. [Google Scholar] [CrossRef]
- Iijima, M.; Moriwaki, Y.; Kuboki, Y. Oriented and lengthwise growth of octacalcium phosphate on collagenous matrix in vitro. Connect. Tissue Res. 1997, 36, 51–61. [Google Scholar] [CrossRef]
- Iijima, M.; Kamemizu, H.; Wakamatsu, N.; Goto, T.; Doi, Y.; Moriwaki, Y. Transition of octacalcium phosphate to hydroxyapatite in solution at pH 7.4 and 37 C. J. Cryst. Growth 1997, 181, 70–78. [Google Scholar] [CrossRef]
- Lacruz, R.S.; Nanci, A.; Kurtz, I.; Wright, J.T.; Paine, M.L. Regulation of pH during amelogenesis. Calcif. Tissue Int. 2010, 86, 91–103. [Google Scholar] [CrossRef] [Green Version]
- Zhitomirsky, I. Cathodic electrodeposition of ceramic and organoceramic materials. Fundamental aspects. Adv. Colloid Interface Sci. 2002, 97, 279–317. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Wong, H.M.; McGrath, C.P.; Li, Q.L. In vitro and in vivo evaluation of electrophoresis-aided casein phosphopeptide-amorphous calcium phosphate remineralisation system on pH-cycling and acid-etching demineralised enamel. Sci. Rep. 2018, 8, 8904. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.T.; Mei, M.L.; Li, Q.L.; Cao, C.Y.; Chen, J.L.; Xia, R.; Zhang, Z.H.; Chu, C.H. A direct electric field-aided biomimetic mineralization system for inducing the remineralization of dentin collagen matrix. Materials 2015, 8, 7889–7899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.Y.; Wong, H.M.; McGrath, C.P.; Li, Q.L. Repair of dentine-related lesions without a drill or injection. RSC Adv. 2019, 9, 15099–15107. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.; Schenk, A.; Ihli, J.; Kulak, A.; Hetherington, N.; Tang, C.; Schmahl, W.; Griesshaber, E.; Hyett, G.; Meldrum, F. A critical analysis of calcium carbonate mesocrystals. Nat. Commun. 2014, 5, 4341. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.Y.; Li, Q.L.; Wong, H.M. Cell-Free Biomimetic Mineralization Strategies to Regenerate the Enamel Microstructure. Crystals 2021, 11, 1385. https://doi.org/10.3390/cryst11111385
Zhang YY, Li QL, Wong HM. Cell-Free Biomimetic Mineralization Strategies to Regenerate the Enamel Microstructure. Crystals. 2021; 11(11):1385. https://doi.org/10.3390/cryst11111385
Chicago/Turabian StyleZhang, Yu Yuan, Quan Li Li, and Hai Ming Wong. 2021. "Cell-Free Biomimetic Mineralization Strategies to Regenerate the Enamel Microstructure" Crystals 11, no. 11: 1385. https://doi.org/10.3390/cryst11111385
APA StyleZhang, Y. Y., Li, Q. L., & Wong, H. M. (2021). Cell-Free Biomimetic Mineralization Strategies to Regenerate the Enamel Microstructure. Crystals, 11(11), 1385. https://doi.org/10.3390/cryst11111385






