Synthesis of Heterostructure of ZnO@MOF-46(Zn) to Improve the Photocatalytic Performance in Methylene Blue Degradation
Abstract
:1. Introduction
2. Experiment
2.1. Materials
2.2. Synthesis of Sample
2.2.1. Synthesis of ZnO from Zn-MOFs
2.2.2. Synthesis of ZnO@MOF-46(Zn)
2.2.3. Synthesis of MOF-46(Zn) as a Reference Material
2.3. Characterization of Samples
2.4. Photocatalytic Activity
3. Results and Discussion
3.1. Synthesis of the ZnO Photocatalyst by the Thermal Decomposition of the Metal-Organic Framework
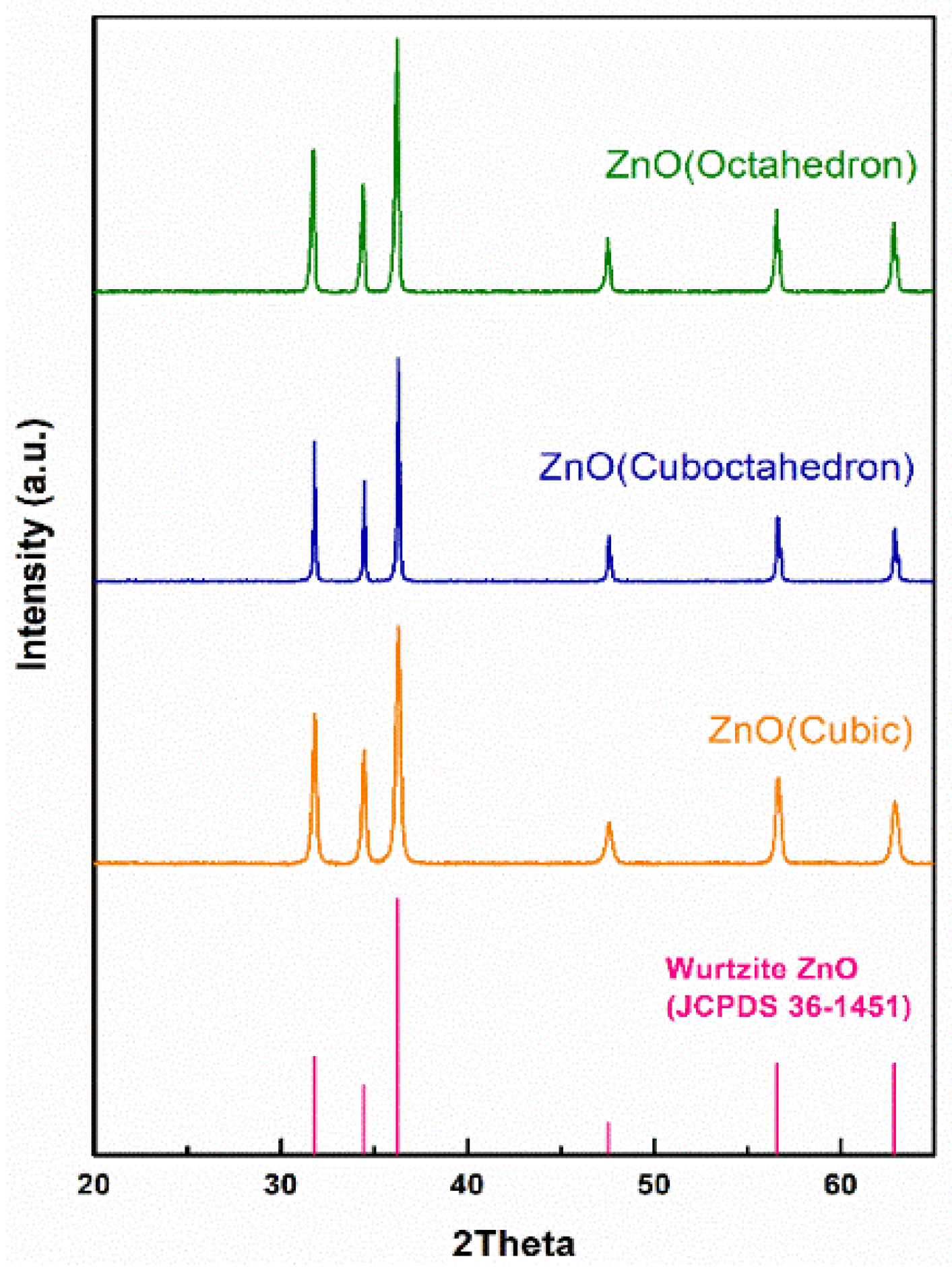
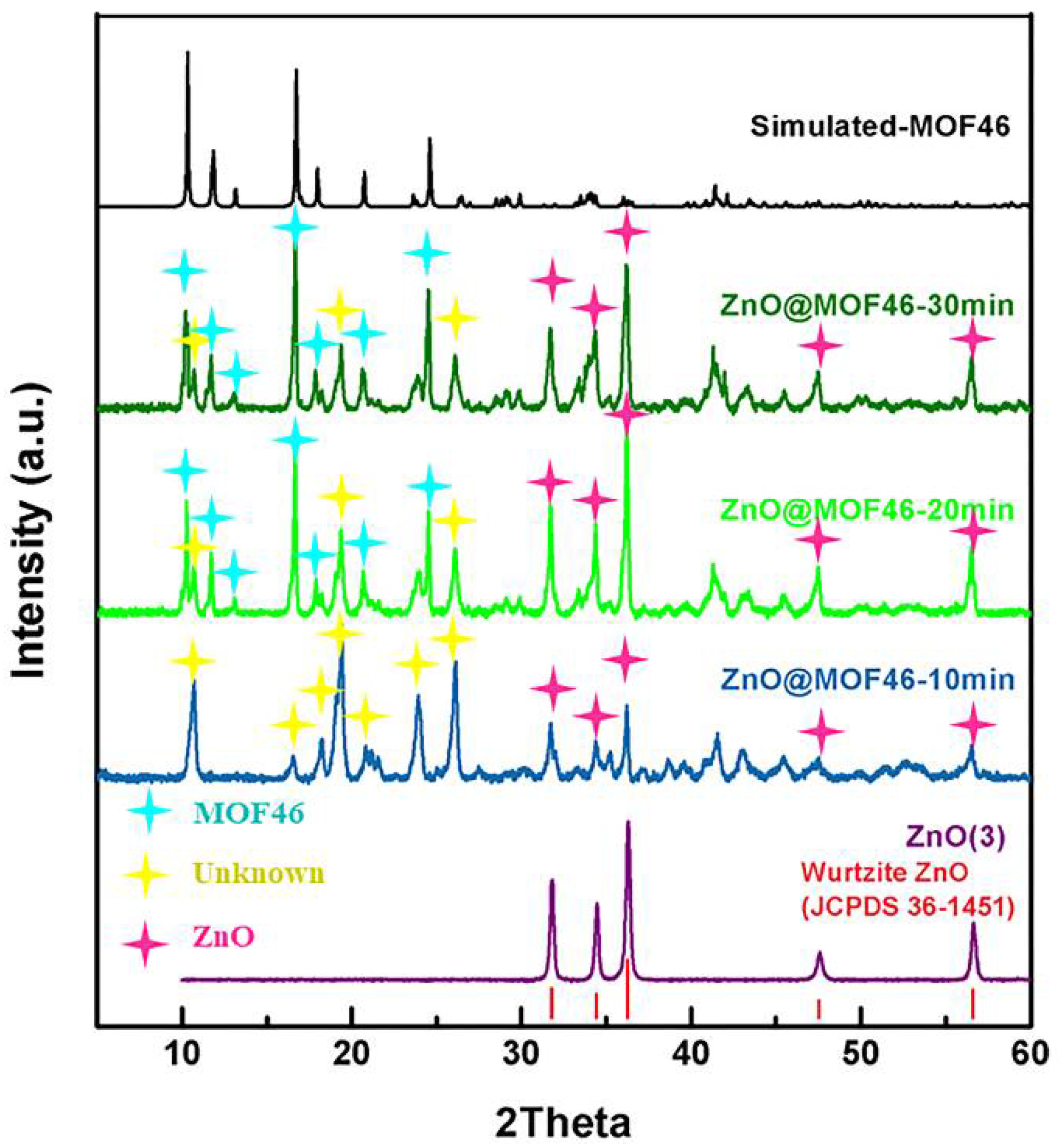
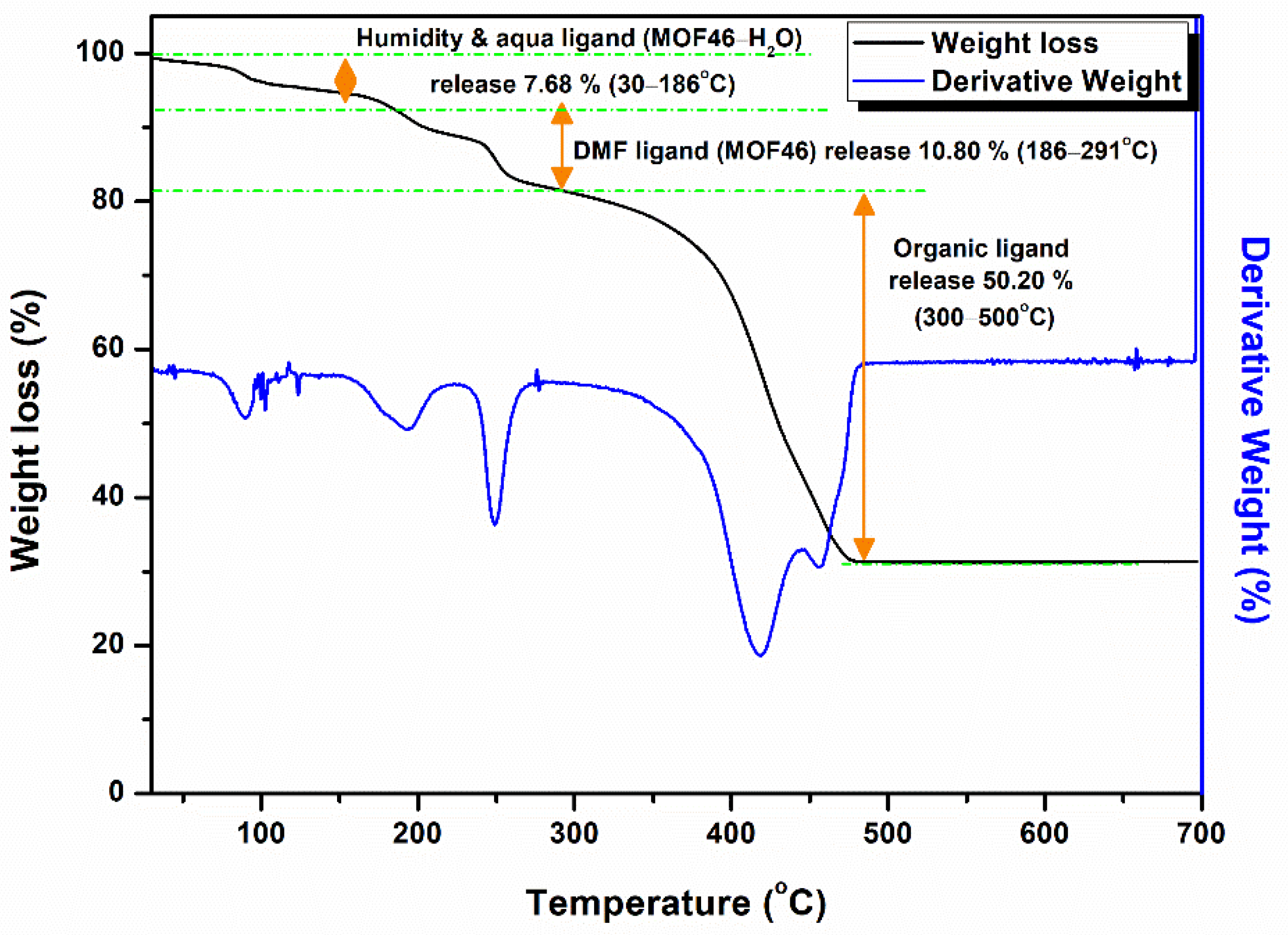
3.1.1. Phase of Samples
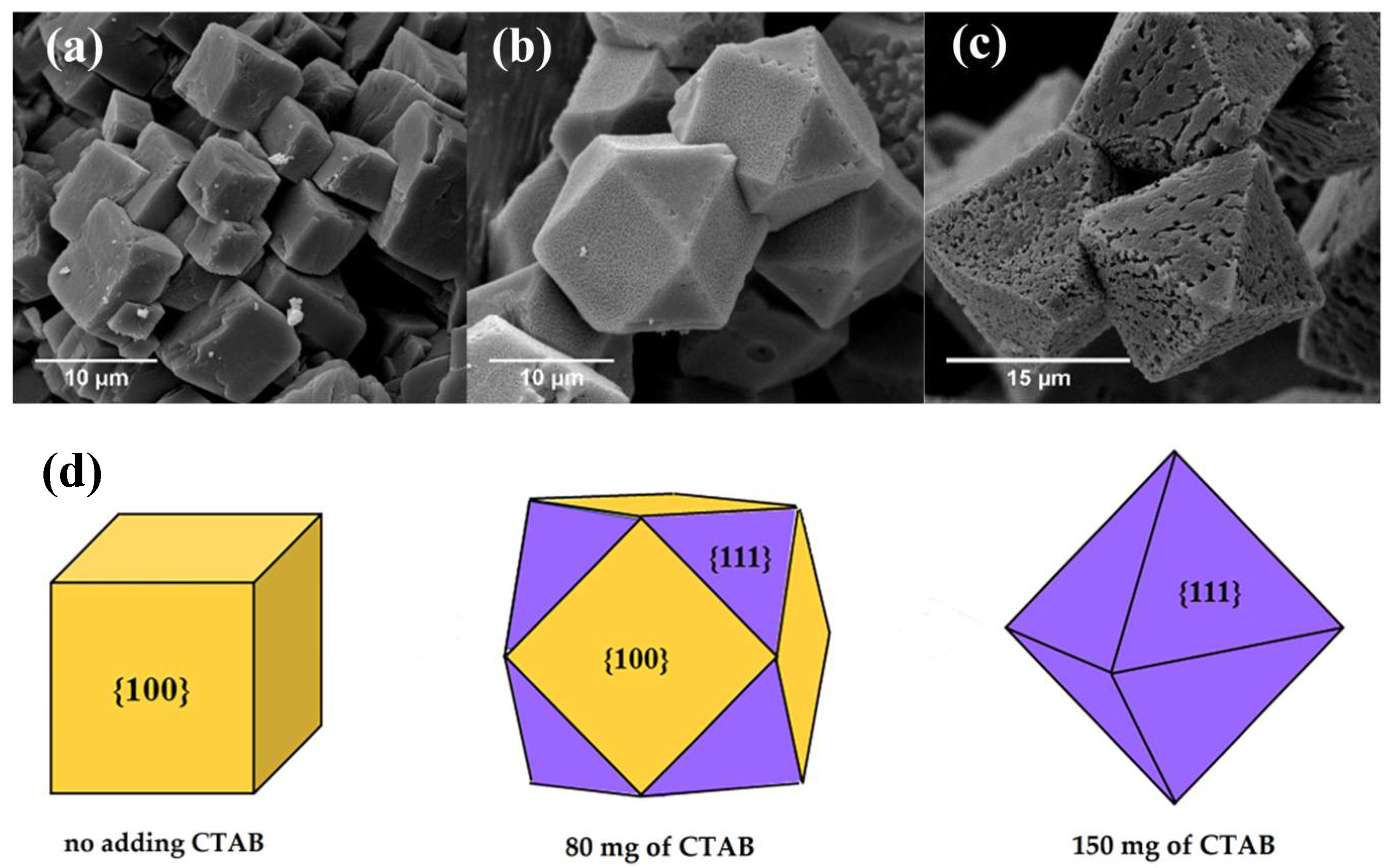
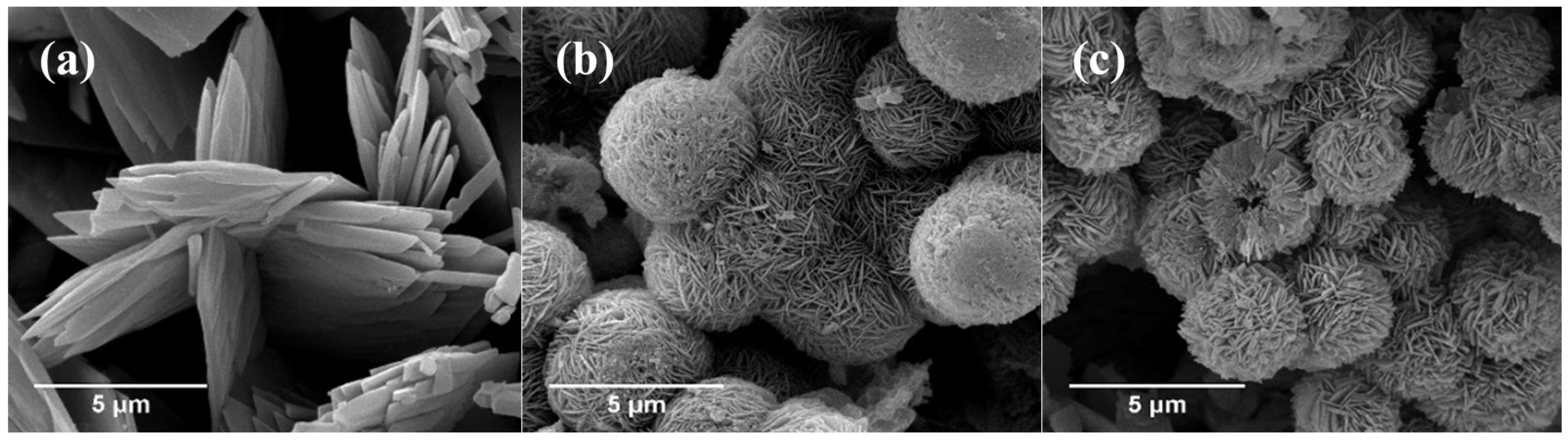
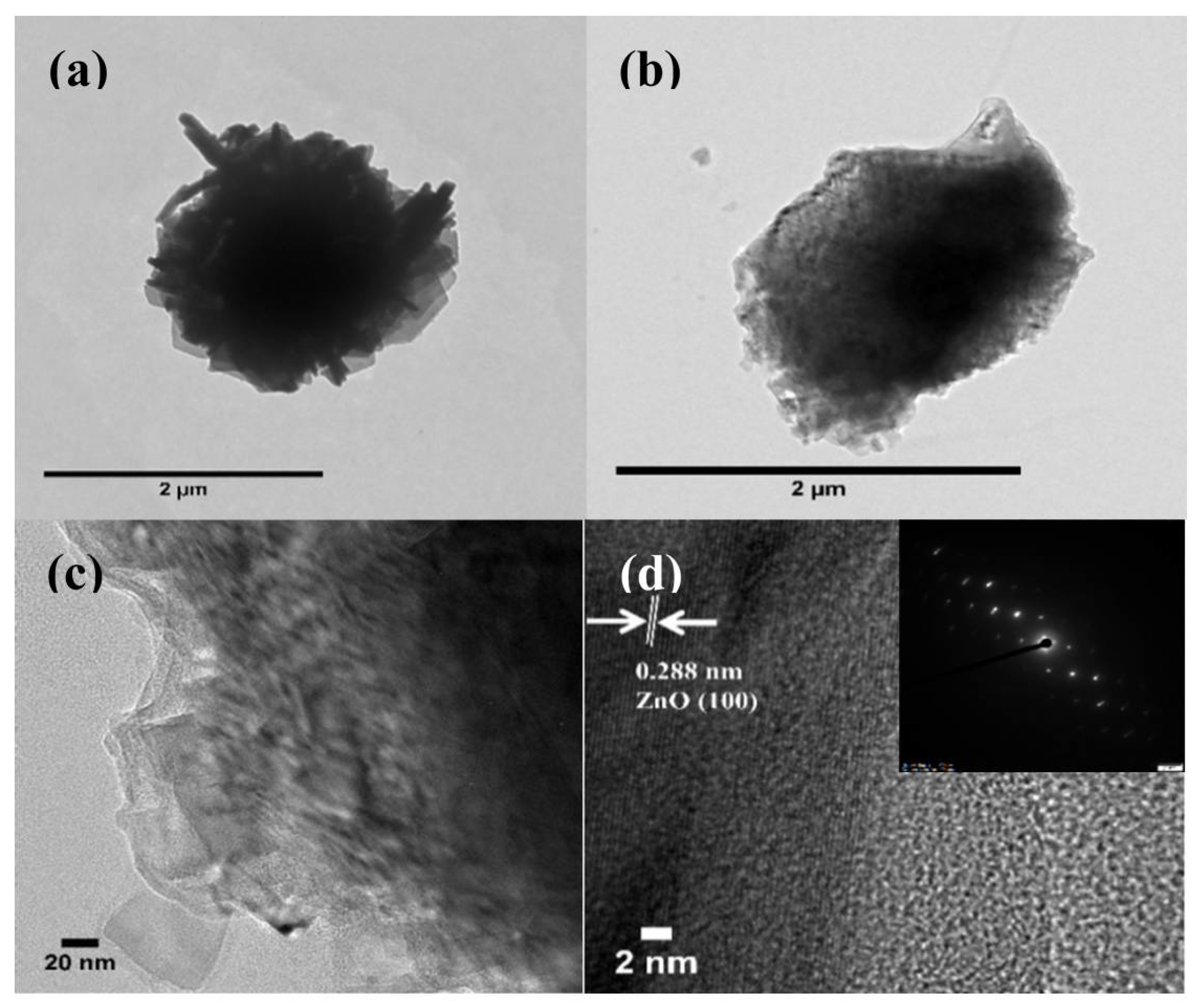
3.1.2. Morphologies of Samples
3.2. Synthesis of ZnO@MOF-46(Zn) Composite Materials for Use as Photocatalysts
3.2.1. Sonication Time
3.2.2. Treatment Time
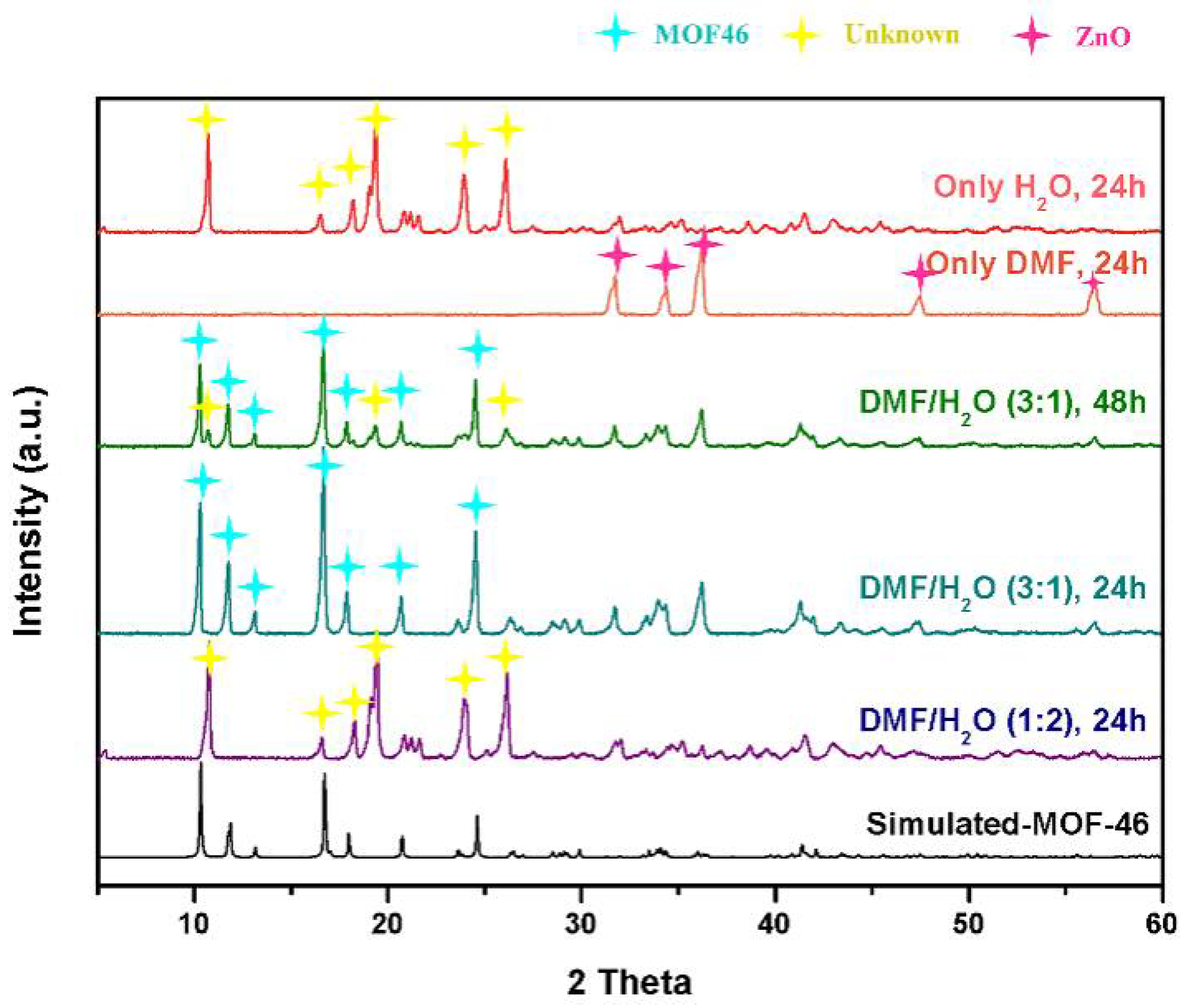
3.2.3. Preparation of MOF-46(Zn) as a Reference Material
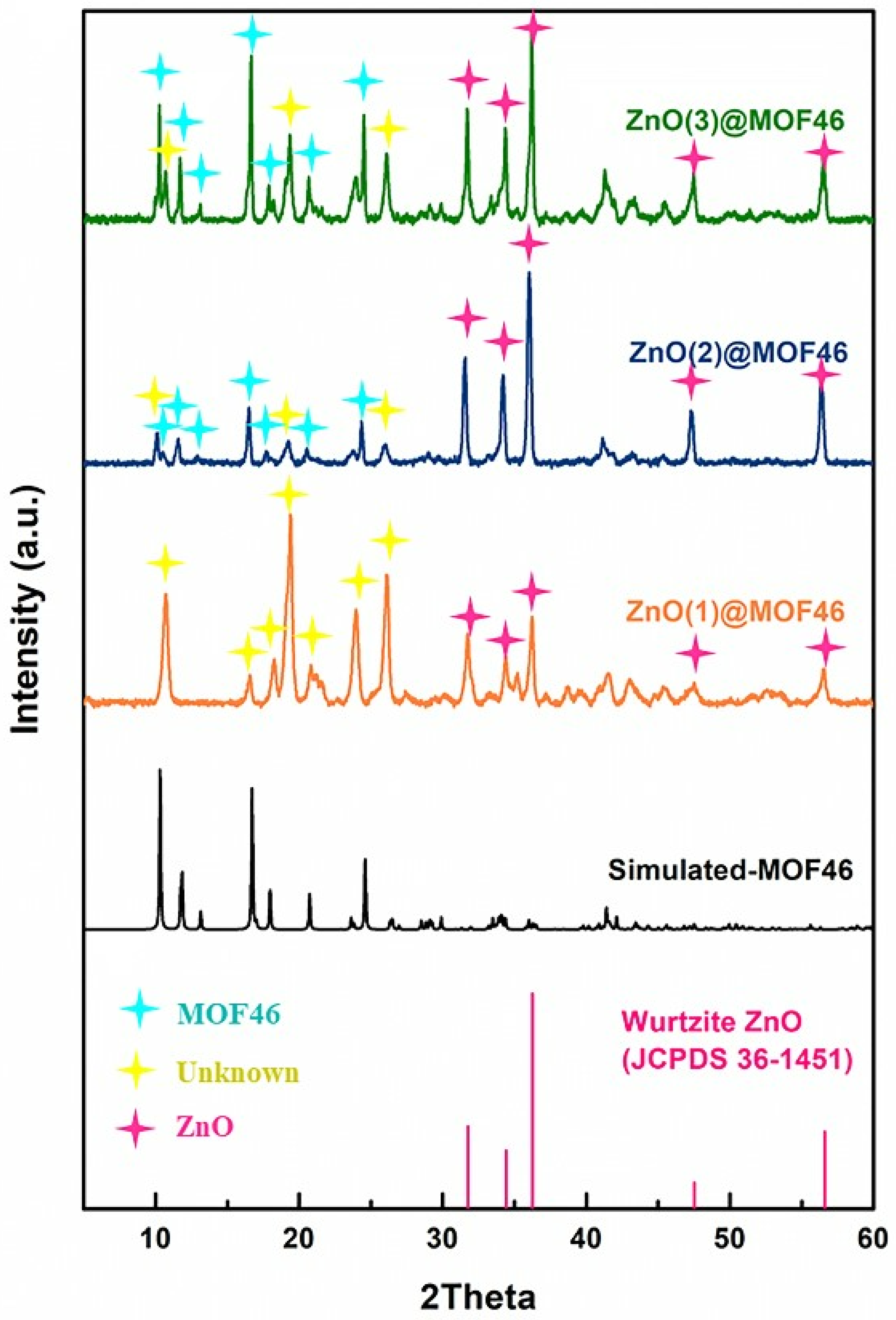
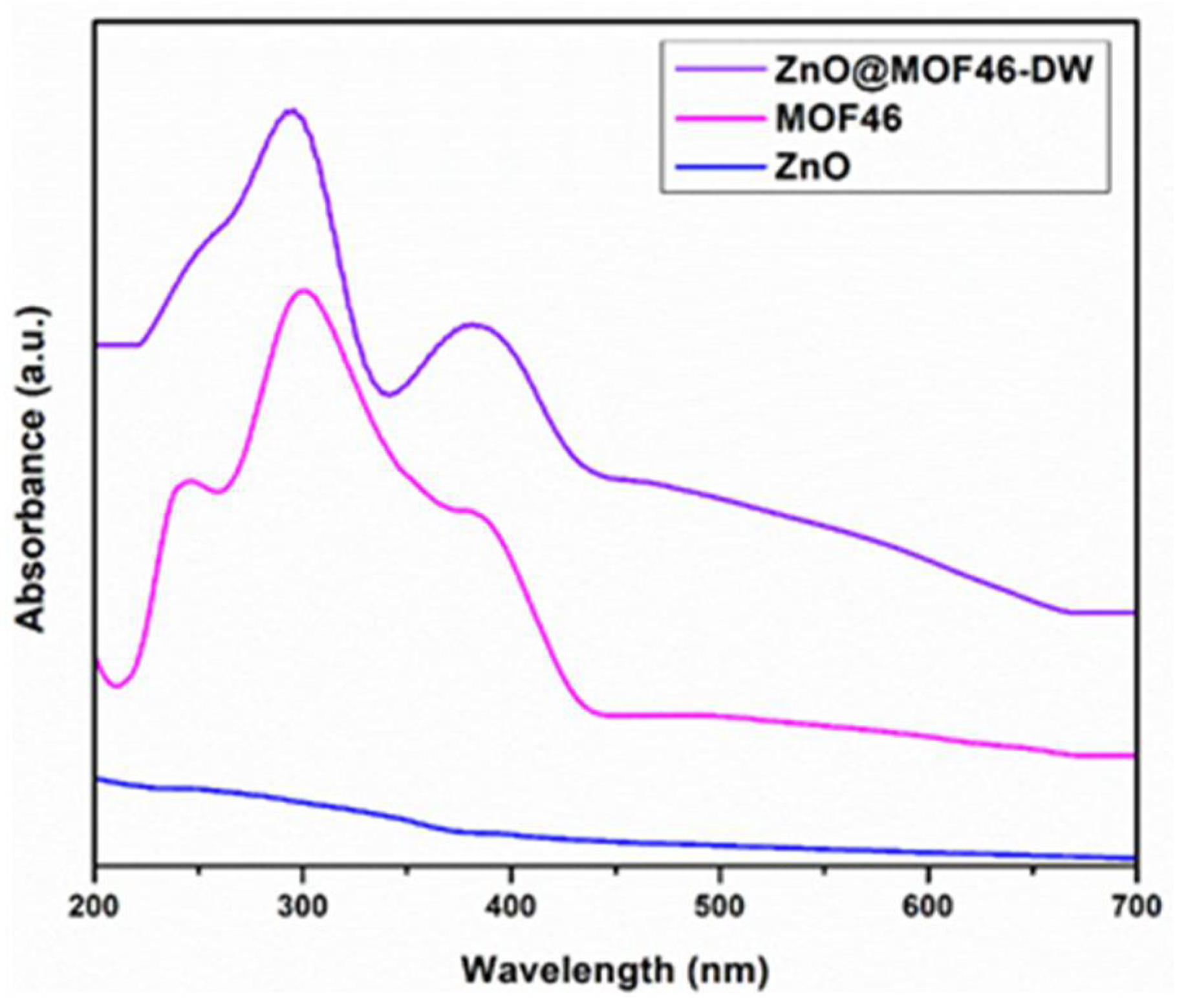
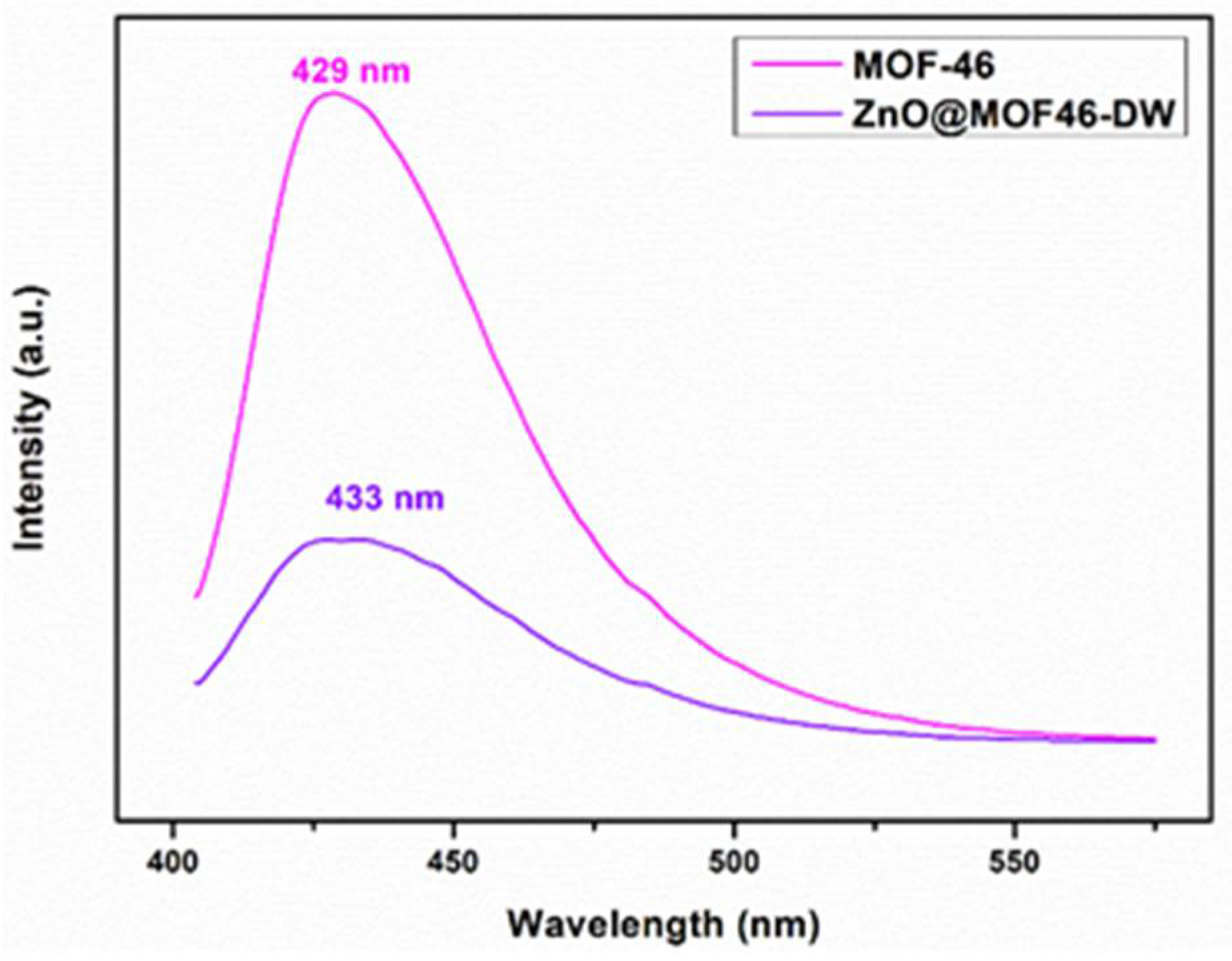
3.3. Structural Confirmation of Samples
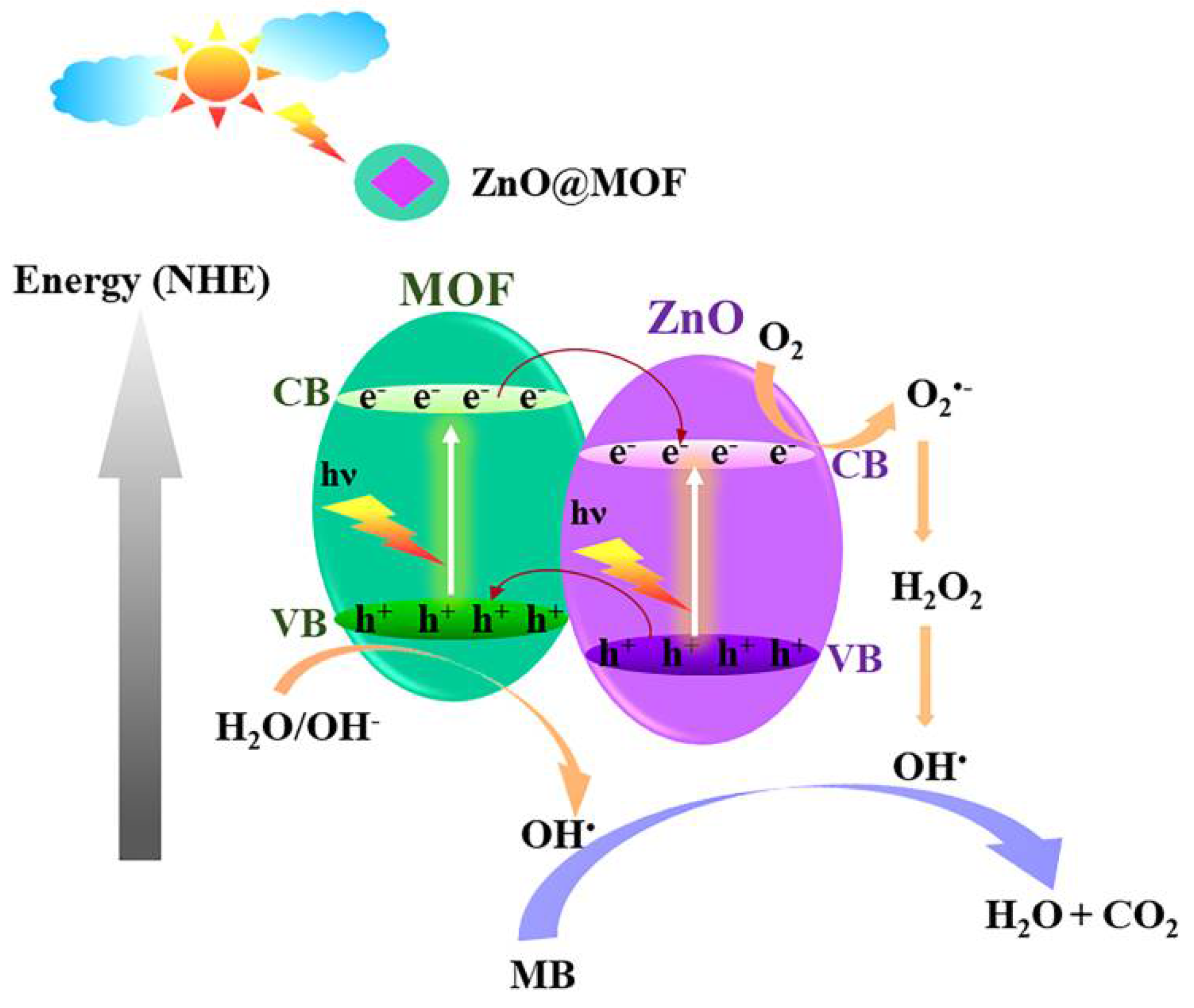
3.4. Photocatalytic Activity of Prepared ZnO and ZnO@MOF-46(Zn)-DW
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nunes, D.; Pimentel, A.; Branquinho, R.; Fortunato, E.; Martins, R. Metal oxide-based photocatalytic paper: A green alternative for environmental remediation. Catalysts 2021, 11, 504. [Google Scholar] [CrossRef]
- Danish, M.S.S.; Estrella, L.L.; Alemaida, I.M.A.; Lisin, A.; Moiseev, N.; Ahmadi, M.; Nazari, M.; Wali, M.; Zaheb, H.; Senjyu, T. Photocatalytic applications of metal oxides for sustainable environmental remediation. Metals 2020, 11, 80. [Google Scholar] [CrossRef]
- Ahuja, P.; Ujjain, S.K.; Kanojia, R.; Attri, P. Transition metal oxides and their composites for photocatalytic dye degradation. J. Compos. Sci. 2021, 5, 82. [Google Scholar] [CrossRef]
- Dong, S.; Cui, L.; Tian, Y.; Xia, L.; Wu, Y.; Yu, J.; Bagley, D.M.; Sun, J.; Fan, M. A novel and high-performance double Z-scheme photocatalyst ZnO-SnO2-Zn2SnO4 for effective removal of the biological toxicity of antibiotics. J. Hazard. Mater. 2020, 399, 123017. [Google Scholar] [CrossRef]
- Alexandrov, A.; Zvaigzne, M.; Lypenko, D.; Nabiev, I.; Samokhvalov, P. Al-, Ga-, Mg-, or Li-doped zinc oxide nanoparticles as electron transport layers for quantum dot light-emitting diodes. Sci. Rep. 2020, 10, 7496. [Google Scholar] [CrossRef] [PubMed]
- Otieno, F.; Airo, M.; Erasmus, R.M.; Quandt, A.; Billing, D.G.; Wamwangi, D. Annealing effect on the structural and optical behavior of ZnO:Eu3+ thin film grown using RF magnetron sputtering technique and application to dye sensitized solar cells. Sci. Rep. 2020, 10, 8557. [Google Scholar] [CrossRef]
- Dey, S.; Nag, S.; Santra, S.; Ray, S.K.; Guha, P.K. Voltage-controlled NiO/ZnO p–n heterojunction diode: A new approach towards selective VOC sensing. Microsyst. Nanoeng. 2020, 6, 35. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Shi, Y.; Shao, H.; Liu, Y.; Zhai, Y. Synthesis and photocatalytic degradation ability evaluation for rhodamine B of ZnO@SiO2 composite with flower-like structure. Water Sci. Technol. 2020, 80, 1986–1995. [Google Scholar] [CrossRef] [PubMed]
- Cerrato, E.; Paganini, M.C.; Giamello, E. Photoactivity under visible light of defective ZnO investigated by EPR spectroscopy and photoluminescence. J. Photochem. Photobiol. 2020, A397, 112531. [Google Scholar] [CrossRef]
- Zhang, L.; Jiang, Y.; Ding, Y.; Povey, M.; York, D. Investigation into the antibacterial behaviour of suspensions of ZnO nanoparticles (ZnO nanofluids). J. Nanopart. Res. 2007, 9, 479–489. [Google Scholar] [CrossRef]
- Sun, Y.; Seo, J.H.; Takacs, C.J.; Seifter, J.; Heeger, A.J. Inverted Polymer Solar Cells Integrated with a Low-Temperature-Annealed Sol-Gel-Derived ZnO Film as an Electron Transport Layer. Adv. Mater. 2011, 23, 1679–1683. [Google Scholar] [CrossRef] [PubMed]
- Al-Gaashani, R.; Radiman, S.; Daud, A.R.; Tabet, N.; Al-Douri, Y. XPS and optical studies of different morphologies of ZnO nanostructures prepared by microwave methods. Ceram. Int. 2013, 39, 2283–2292. [Google Scholar] [CrossRef]
- Manzoor, U.; Siddique, S.; Ahmed, R.; Noreen, Z.; Bokhari, H.; Ahmad, I. Antibacterial, Structural and Optical Characterization of Mechano-Chemically Prepared ZnO Nanoparticles. PLoS ONE 2016, 11, e0154704. [Google Scholar] [CrossRef] [Green Version]
- Chang, T.-H.; Lu, Y.-C.; Yang, M.-J.; Huang, J.-W.; Linda Chang, P.-F.; Hsueh, H.-Y. Multibranched flower-like ZnO particles from eco-friendly hydrothermal synthesis as green antimicrobials in agriculture. J. Clean. Prod. 2020, 262, 121342. [Google Scholar] [CrossRef]
- Buasakun, J.; Srilaoong, P.; Chaloeipote, G.; Rattanakram, R.; Wongchoosuk, C.; Duangthongyou, T. Synergistic effect of ZnO/ZIF8 heterostructure material in photodegradation of methylene blue and volatile organic compounds with sensor operating at room temperature. J. Solid State Chem. 2020, 289, 121494. [Google Scholar] [CrossRef]
- Jais, J. Synthesis of ZnO Nanostructures and their Photocatalytic Activity. J. Appl. Ind. Math. 2013, 1, 32–35. [Google Scholar]
- Brillas, E.; Serrà, A.; Garcia-Segura, S. Biomimicry designs for photoelectrochemical systems: Strategies to improve light delivery efficiency. Curr. Opin. Electrochem. 2021, 26, 100660. [Google Scholar] [CrossRef]
- Reddy, I.N.; Reddy, C.V.; Shim, J.; Akkinepally, B.; Cho, M.; Yoo, K.; Kim, D. Excellent visible-light driven photocatalyst of (Al, Ni) co-doped ZnO structures for organic dye degradation. Catal. Today 2020, 340, 277–285. [Google Scholar] [CrossRef]
- Nguyen, D.T.; Tran, M.D.; Van Hoang, T.; Trinh, D.T.; Pham, D.T.; Nguyen, D.L. Experimental and numerical study on photocatalytic activity of the ZnO nanorods/CuO composite film. Sci. Rep. 2020, 10, 7792. [Google Scholar] [CrossRef]
- Sanchez, C.; Arribart, H.; Giraud Guille, M.M. Biomimetism and bioinspiration as tools for the design of innovative materials and systems. Nat. Mater. 2005, 4, 277–288. [Google Scholar] [CrossRef]
- Serrà, A.; Artal, R.; García-Amorós, J.; Sepúlveda, B.; Gómez, E.; Nogués, J.; Philippe, L. Hybrid Ni@ZnO@ZnS-Microalgae for Circular Economy: A Smart Route to the Efficient Integration of Solar Photocatalytic Water Decontamination and Bioethanol Production. Adv. Sci. 2020, 7, 1902447. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yian, J.; Guo, Y.; Teng, M.; Liu, H.; Li, T.; Lv, P.; Wang, X. ZnO nano-rod arrays synthesized with exposed {0001} facets and the investigation of photocatalytic activity. Crystals 2021, 11, 522. [Google Scholar] [CrossRef]
- Buasakun, J.; Srilaoong, P.; Chainok, K.; Raksakoon, C.; Rattanakram, R.; Duangthongyou, T. Novel zinc(II) coordination polymers (CPs) based on flexible aliphatic carboxylic acids and an N-donor ligand for fluorescence sensors. Inorg. Chim. Acta 2020, 511, 119839. [Google Scholar] [CrossRef]
- Li, Y.; Wen, G. Advances in Metal-Organic Frameworks for Acetylene Storage. Eur. J. Inorg. Chem. 2020, 2020, 2303–2311. [Google Scholar] [CrossRef]
- Chen, S.; Li, Y.; Mi, L. Porous carbon derived from metal organic framework for gas storage and separation: The size effect. Inorg. Chem. Commun. 2020, 118, 107999. [Google Scholar] [CrossRef]
- Uthappa, U.T.; Sriram, G.; Arvind, O.R.; Kumar, S.; Ho Young, J.; Neelgund, G.M.; Losic, D.; Kurkuri, M.D. Engineering MIL-100(Fe) on 3D porous natural diatoms as a versatile high performing platform for controlled isoniazid drug release, Fenton’s catalysis for malachite green dye degradation and environmental adsorbents for Pb2+ removal and dyes. Appl. Surf. Sci. 2020, 528, 146974. [Google Scholar] [CrossRef]
- Zhang, Z.; Xiao, Y.; Lei, Y.; Tang, J.; Qiao, X. Catalytic hydrolysis of β-lactam antibiotics via MOF-derived MgO nanoparticles embedded on nanocast silica. Sci. Total Environ. 2020, 738, 139742. [Google Scholar] [CrossRef]
- Yang, T.; Yu, D.; Wang, D.; Yang, T.; Li, Z.; Wu, M.; Petru, M.; Crittenden, J. Accelerating Fe(III)/Fe(II) cycle via Fe(II) substitution for enhancing Fenton-like performance of Fe-MOFs. Appl. Catal. B Environ. 2021, 286, 119859. [Google Scholar] [CrossRef]
- Yu, D.; Wang, L.; Yang, T.; Yang, G.; Wang, D.; Ni, H.; Wu, M. Tuning Lewis acidity of iron-based metal-organic frameworks for enhanced catalytic ozonation. Chem. Eng. J. 2021, 404, 127075. [Google Scholar] [CrossRef]
- Braun, M.E.; Steffek, C.D.; Kim, J.; Rasmussen, P.G.; Yaghi, O.M. 1,4-Benzenedicarboxylate derivatives as links in the design of paddle-wheel units and metal–organic frameworks. Chem. Commun. 2001, 2532–2533. [Google Scholar] [CrossRef]
- Yang, J.-M.; Liu, Q.; Kang, Y.-S.; Sun, W.-Y. Controlled growth and gas sorption properties of IRMOF-3 nano/microcrystals. Dalton Trans. 2014, 43, 16707–16712. [Google Scholar] [CrossRef] [PubMed]
- Da Trinda, L.G.; Borba, K.M.N.; Trench, A.B.; Zanchet, L.; Teodoro, V.; Pontes, F.M.L.; Longo, E.; Mazzo, T.M. Effective strategy to coupling Zr-MOF/ZnO: Synthesis, morphology and photoelectrochemical properties evaluation. J. Solid State Chem. 2021, 293, 121794. [Google Scholar] [CrossRef]
- Tibodee, A.; Pannak, P.; Akkarachaneeyakorn, K.; Thaweechai, T.; Sirisaksoontorn, W. Use of the graphite intercalation compound to produce low-defect graphene sheets for the photocatalytic enhancement of graphene/TiO2 composites. Mater. Chem. Phys. 2019, 235, 121755. [Google Scholar] [CrossRef]








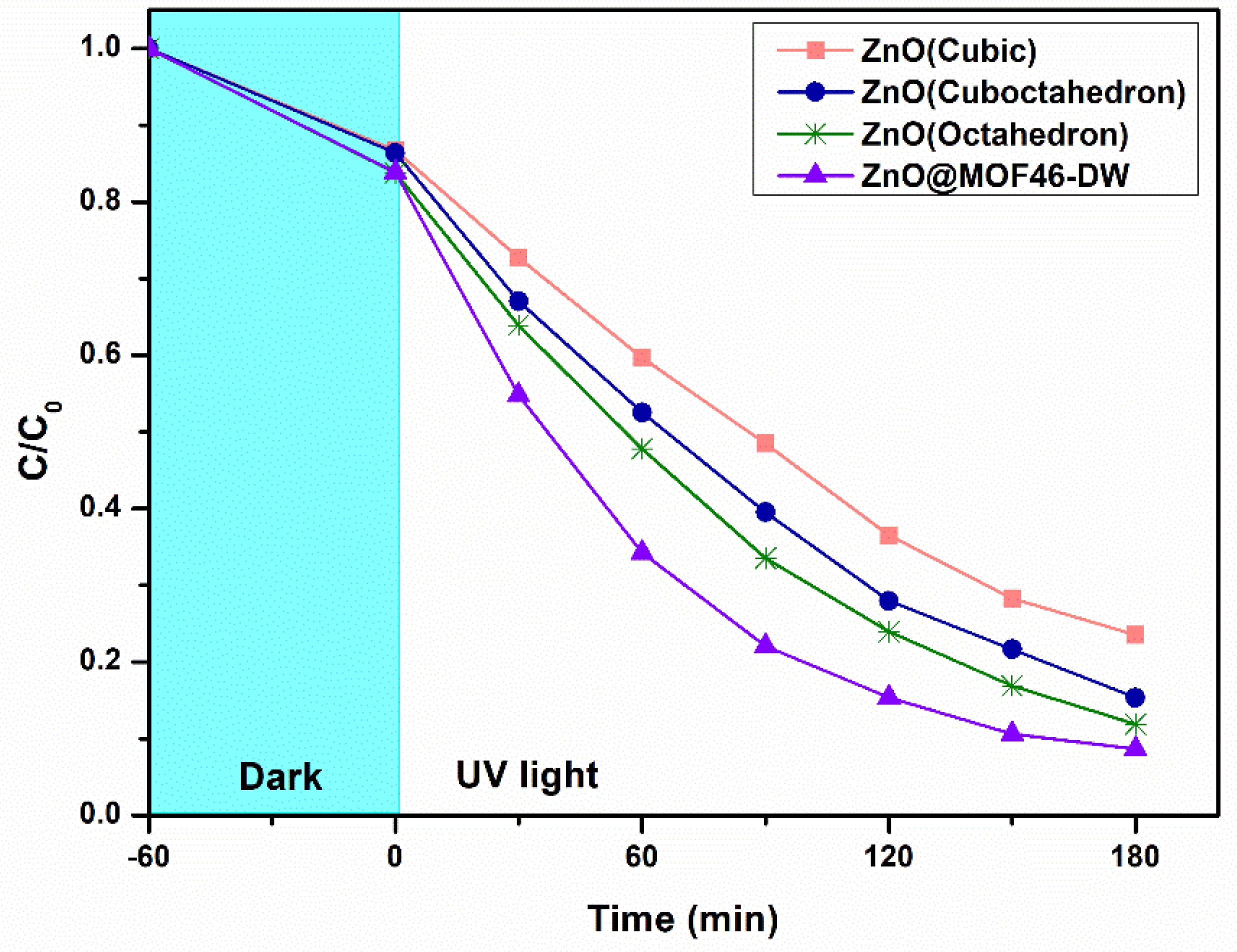

| @ 3 h | Mean @ 1 h | |||||
|---|---|---|---|---|---|---|
| Trial 1 | Trial 2 | Trial 3 | Mean | SD | ||
| ZnO (Cube) | 66.64 | 74.70 | 76.78 | 72.70 | 5.36 | 30.98 |
| ZnO (Cuboctahedron) | 74.25 | 83.36 | 89.52 | 82.38 | 7.69 | 39.35 |
| ZnO (Octahedron) | 81.83 | 85.38 | 90.16 | 85.79 | 4.18 | 42.92 |
| ZnO@MOF-46(Zn)-DW | 89.53 | 90.24 | 90.51 | 90.09 | 0.51 | 61.20 |
| Sample | BET Surface Area (m2/g) | Pore Volume (cm3/g) |
|---|---|---|
| ZnO(1) | 15.76 | 0.114 |
| ZnO(2) | 19.65 | 0.023 |
| ZnO(3) | 7.11 | 0.009 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buasakun, J.; Srilaoong, P.; Rattanakam, R.; Duangthongyou, T. Synthesis of Heterostructure of ZnO@MOF-46(Zn) to Improve the Photocatalytic Performance in Methylene Blue Degradation. Crystals 2021, 11, 1379. https://doi.org/10.3390/cryst11111379
Buasakun J, Srilaoong P, Rattanakam R, Duangthongyou T. Synthesis of Heterostructure of ZnO@MOF-46(Zn) to Improve the Photocatalytic Performance in Methylene Blue Degradation. Crystals. 2021; 11(11):1379. https://doi.org/10.3390/cryst11111379
Chicago/Turabian StyleBuasakun, Jiraporn, Phakinee Srilaoong, Ramida Rattanakam, and Tanwawan Duangthongyou. 2021. "Synthesis of Heterostructure of ZnO@MOF-46(Zn) to Improve the Photocatalytic Performance in Methylene Blue Degradation" Crystals 11, no. 11: 1379. https://doi.org/10.3390/cryst11111379
APA StyleBuasakun, J., Srilaoong, P., Rattanakam, R., & Duangthongyou, T. (2021). Synthesis of Heterostructure of ZnO@MOF-46(Zn) to Improve the Photocatalytic Performance in Methylene Blue Degradation. Crystals, 11(11), 1379. https://doi.org/10.3390/cryst11111379







