Beading Mechanism and Performance of Porous Steel Slag Microbead Abrasive
Abstract
:1. Introduction
2. Sample and Experimental Method
2.1. Sample and Experimental Method
2.2. Thermodynamics Fractal Model
2.3. Sample Analysis
3. Results and Discussion
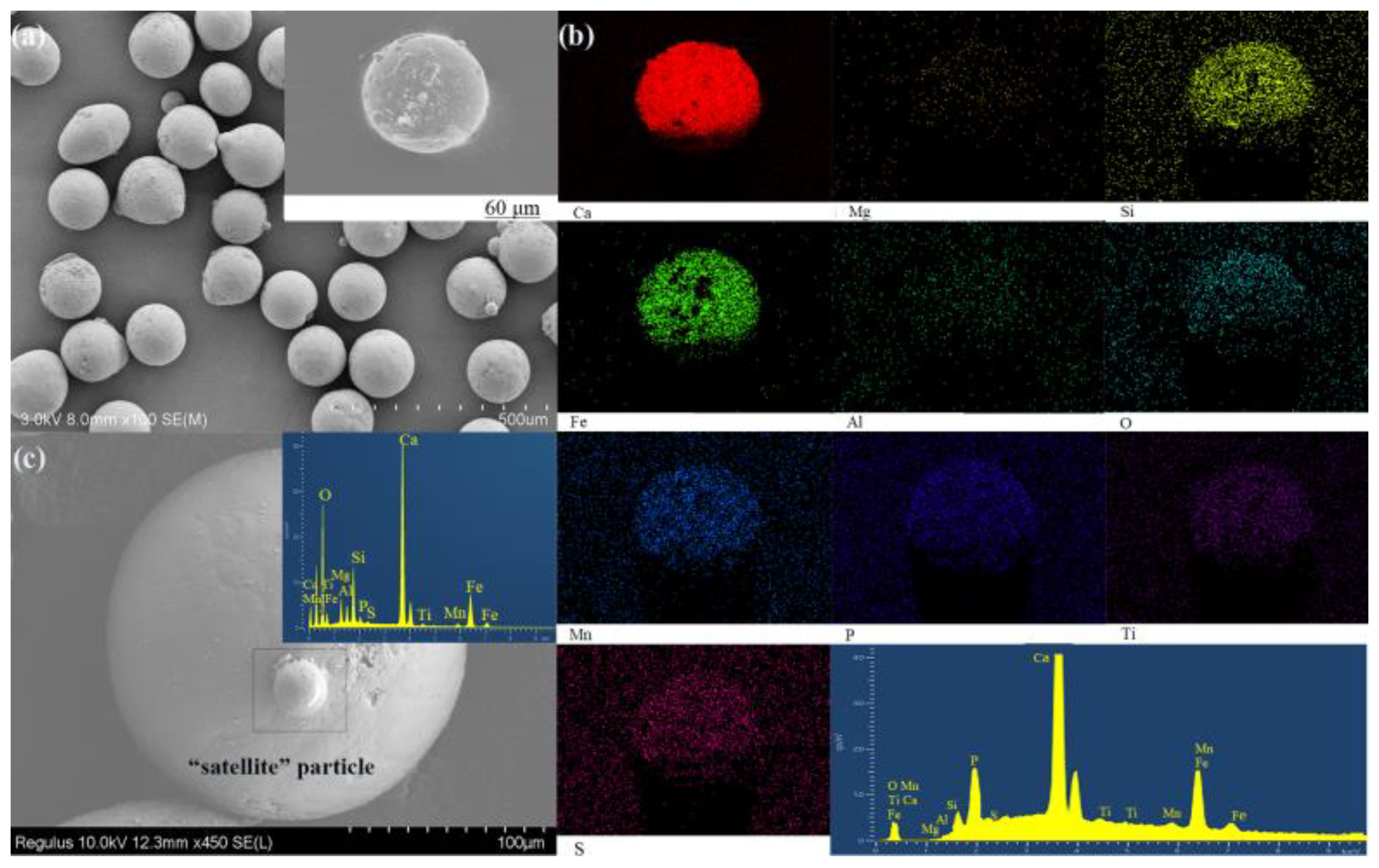
3.1. Microstructure and EDS
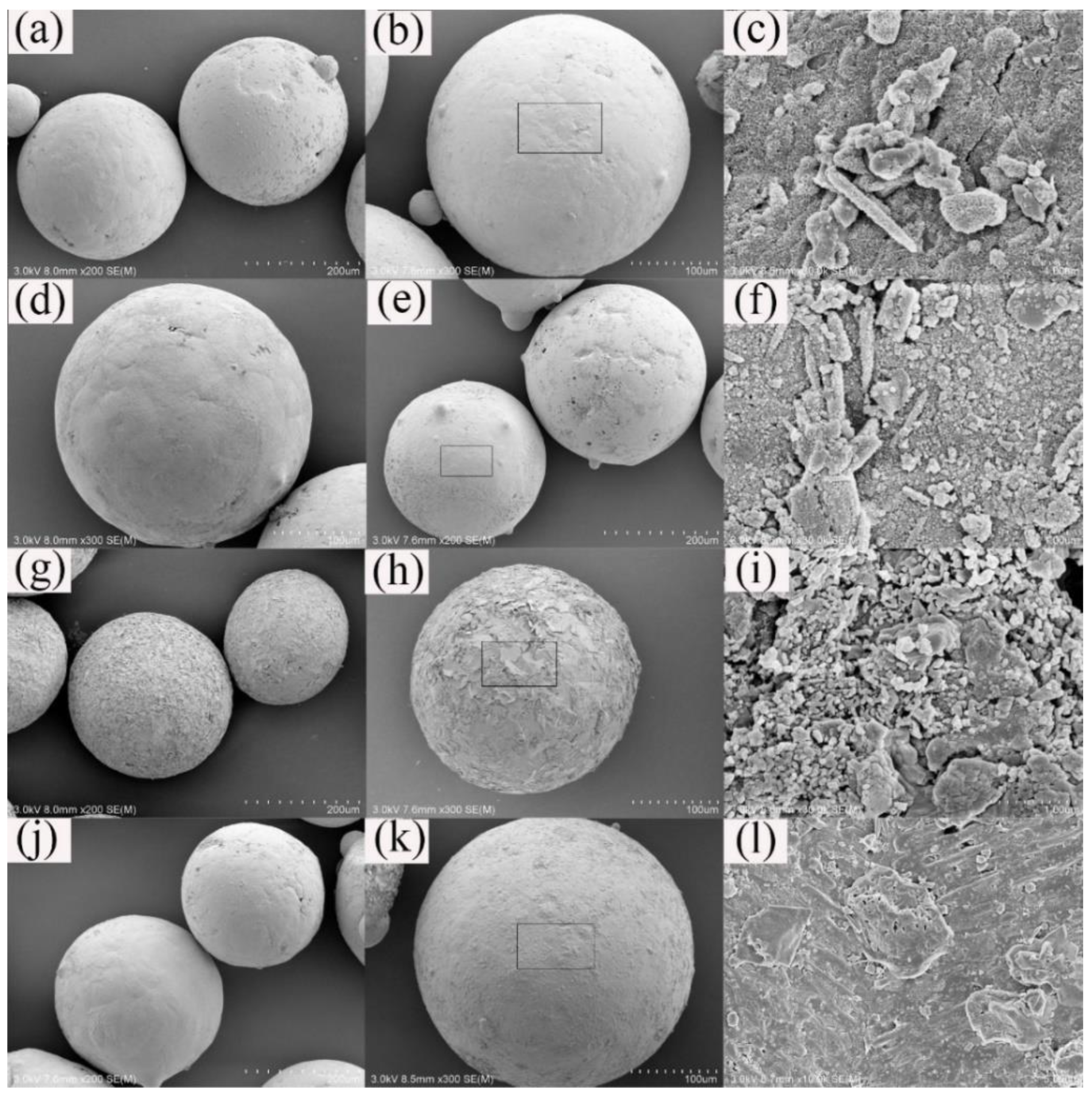
3.1.1. Cross-Section Analysis
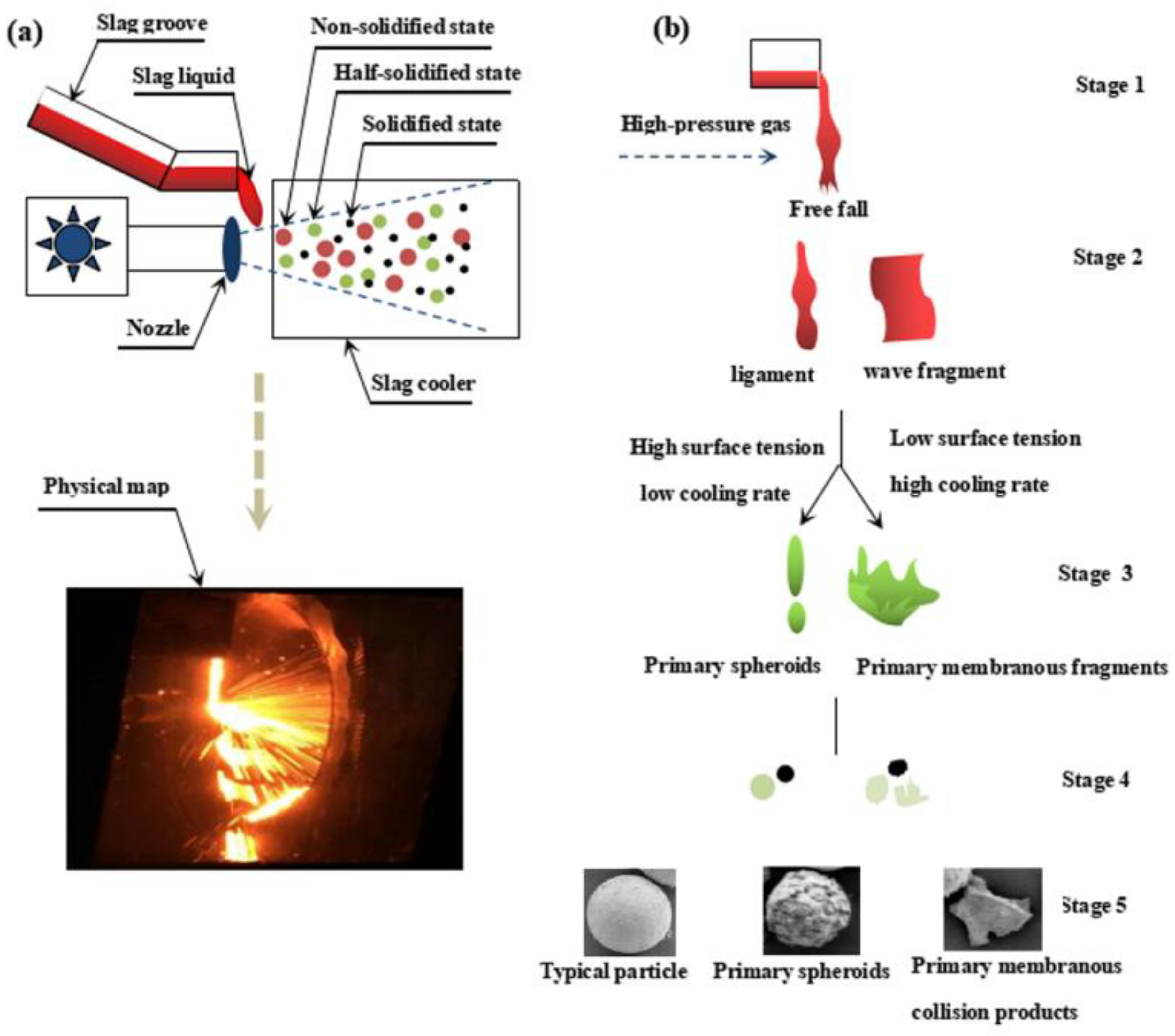
3.1.2. Mechanism
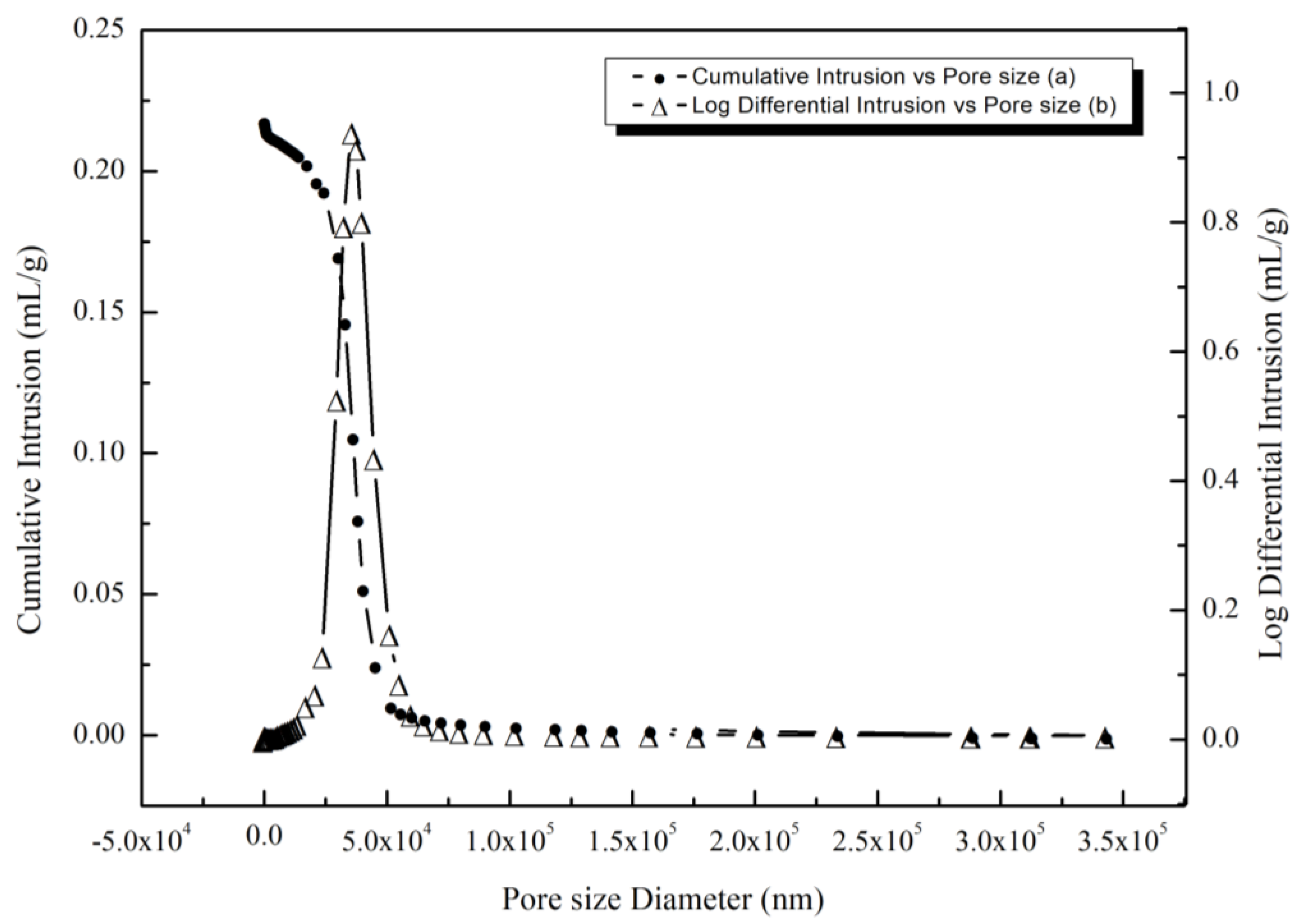
3.2. Characterization of Pore Structure
3.2.1. Mercury Pressure Test
3.2.2. Calculated Result Analysis of Fractal Dimension
3.3. Chemical Stability Analysis
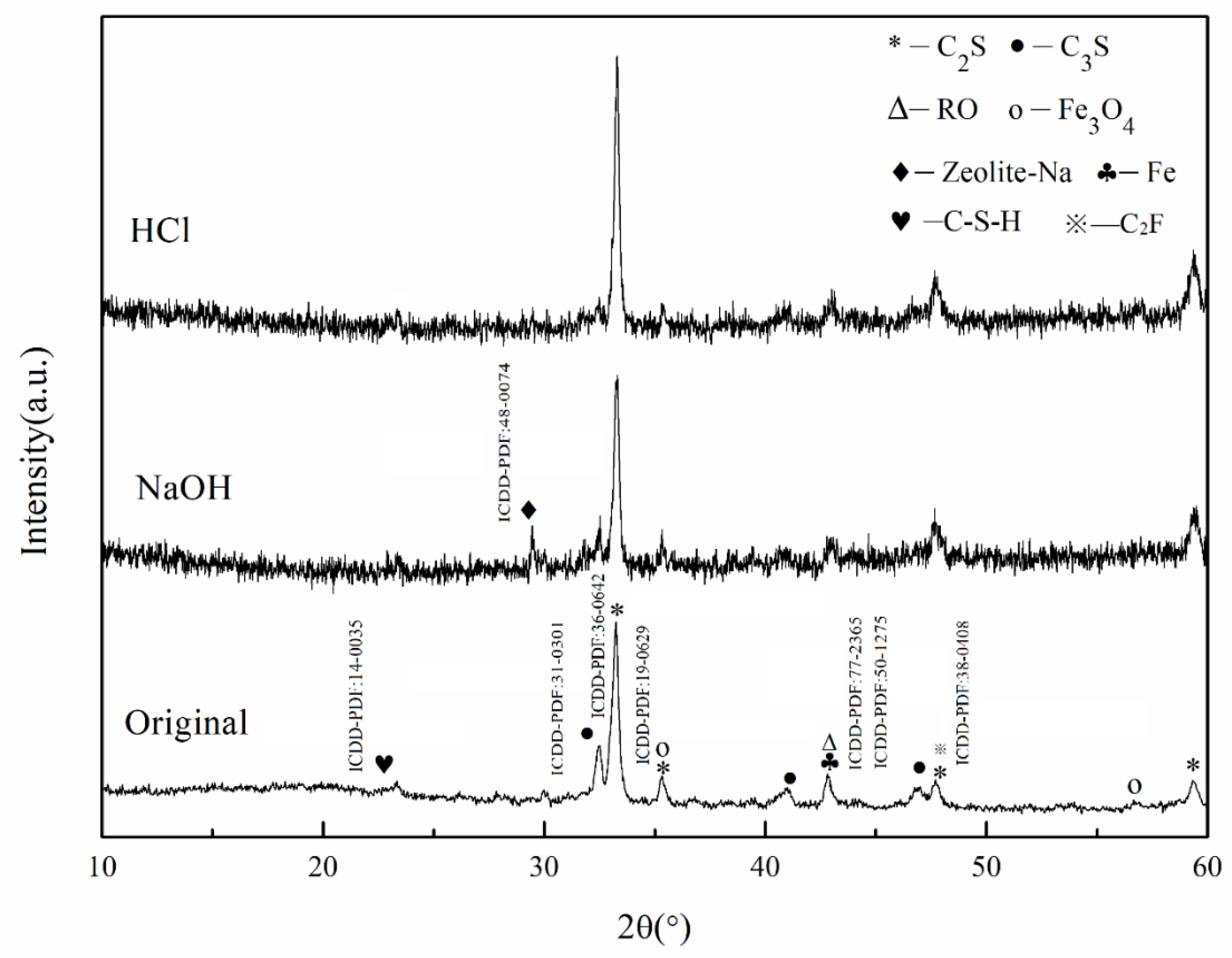
3.3.1. Analysis of Sample Phase Structures after Acid–Alkali Corrosion
3.3.2. Sample Microstructure after Acid–Alkaline Corrosion
3.3.3. Acid–Alkali Resistance Mechanism Analysis
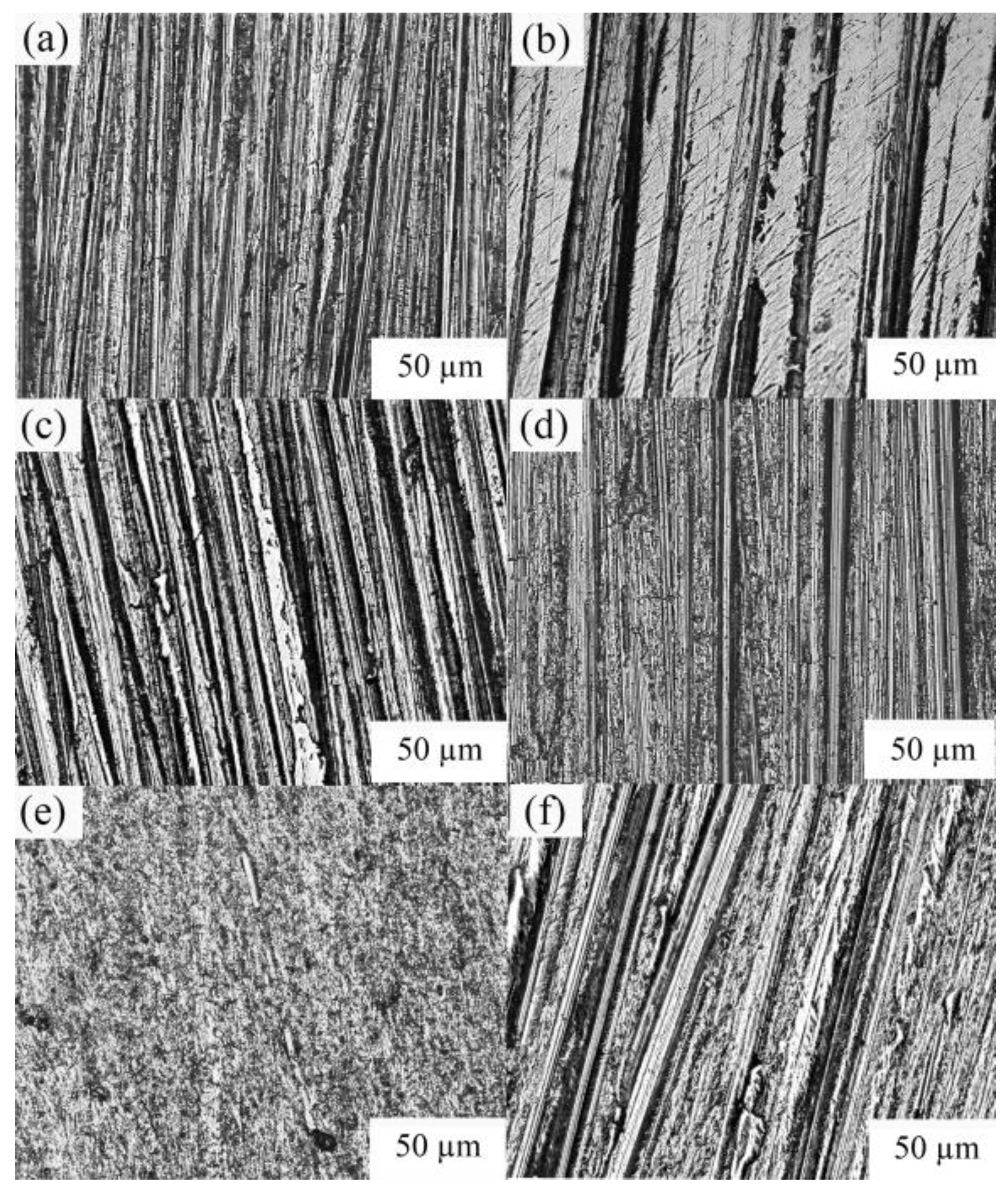
3.4. Analysis of Polishing Properties
4. Conclusions
- In terms of morphological characteristics, the gas-quenching steel slag abrasives present regular monodisperse spherical shapes and the porosity is 42.36%. The mineral morphologies are mainly irregular and major minerals generally include the Ca-Si phase, Ca-Fe phase, Fe-Mg phase, and metallic irons, found by observing the cross-section of the sample.
- The steel slag abrasives with a particle size of about 150 μm microns have stable chemical properties in polishing fluid but their microstructures are changed in acid environments. Villous and rod-like hydration products are generated and both gullies and holes are formed on the sample surface. Nevertheless, an increasing number of structural defects were found on the sample surface in the alkaline environment and many flake product adhered to its surface. A large number of amorphous and compact gelatinization products are generated.
- The gas-quenching steel slag abrasives can effectively improve the surface quality of aluminum alloy and copper alloys due to their superior polishing properties, and their polishing effect on aluminum alloy is better than that on the copper alloy. Hence, these abrasives are applicable to the field of the finishing process. Meanwhile, a new path is provided for the high value-added utilization of steel slags.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, H.G.; Peng, B.; Yue, C.S.; Wu, L. Research progress and prospect of steel slag modification. Environ. Eng. 2020, 38, 133–137. [Google Scholar]
- Liu, C.B.; Peng, B.; Xia, C.; Yue, C.S. The Research Progress of Steel Slag Utilization and Stabilization Technology. Conserv. Util. Miner. Resour. 2018, 6, 145–150. [Google Scholar]
- Ragipani, R.; Bhattacharya, S.; Akkihebbal, S.K. Understanding dissolution characteristics of steel slag for resource recovery. Waste Manag. 2020, 117, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, X.Y.; Liu, Y. Study on Physical Excitation Mechanism of Steel Slag Tailings by XRD and SEM. Spectr. Anal. 2019, 39, 937–994. [Google Scholar]
- Xu, Y.; Wang, Q.L.; Hu, C.H.; Zhang, Z.Z. Research Progress of On-line Reconstruction Technology of Liquid Steel Slag. Multipurp. Util. Miner. Resour. 2019, 2, 1–8. [Google Scholar]
- Zhang, Z.L.; Chen, R.; Men, X.R.; Sun, Y. Phase compositions and microstructure of slag in BOF. J. Mater. Metall. 2019, 18, 37–40. [Google Scholar]
- Wang, H.G.; Wu, L.; Peng, B.; Yue, C.S. Characteristics and Research Progress of Steel Slag Primary Treatment Technology. Sci. Technol. Eng. 2020, 13, 5025–5031. [Google Scholar]
- Hu, S.Y.; Dai, X.T.; Na, X.Z. Treatment Process and Comprehensive Utilization of Steel Slag. Foundry Technol. 2019, 40, 220–224. [Google Scholar]
- Chen, B.; Yang, J.-X.; Ouyang, Z.-Y. Life Cycle Assessment of Internal Recycling Options of Steel Slag in Chinese Iron and Steel Industry. J. Iron Steel Res. Int. 2011, 18, 33–40. [Google Scholar] [CrossRef]
- Cao, L.; Shen, W.; Huang, J.; Yang, Y.; Zhang, D.; Huang, X.; Lv, Z.; Ji, X. Process to utilize crushed steel slag in cement industry directly: Multi-phased clinker sintering technology. J. Clean. Prod. 2019, 217, 520–529. [Google Scholar] [CrossRef]
- Guo, Y.; Xie, J.; Zheng, W.; Li, J. Effects of steel slag as fine aggregate on static and impact behaviours of concrete. Constr. Build. Mater. 2018, 192, 194–201. [Google Scholar] [CrossRef]
- Pasetto, M.; Baliello, A.; Giacomello, G.; Pasquini, E. Sustainable solutions for road pavements: A multi-scale characterization of warm mix asphalts containing steel slags. J. Clean. Prod. 2017, 166, 835–843. [Google Scholar] [CrossRef]
- Yang, Y.; Yin, S.H.; Xu, C.X.; Mao, H.Y. Study on Industrial Waste Materials as Coal Gangue Recycle Fe Deoxidized with High Grade from Steel Slag. Sichuan Build. Mater. 2015, 1, 81–83. [Google Scholar]
- Kumar, D.S.; Sah, R.; Sanyal, S.; Prasad, G. Measurement of metallic iron in steel making slags. Measurement 2019, 131, 156–161. [Google Scholar] [CrossRef]
- Wang, Q.; Yan, P. Hydration properties of basic oxygen furnace steel slag. Constr. Build. Mater. 2010, 24, 1134–1140. [Google Scholar] [CrossRef]
- Calmon, J.L.; Tristão, F.A.; Giacometti, M.; Meneguelli, M.; Moratti, M.; Teixeira, J.E.S.L. Effects of BOF steel slag and other cementitious materials on the rheological properties of self-compacting cement pastes. Constr. Build. Mater. 2013, 40, 1046–1053. [Google Scholar] [CrossRef]
- Duan, S.Y.; Li, X.; Ma, Z.H.; Liao, H.Q. Effect of grinding method on properties of steel slag powder. Mater. Sci. Eng. Powder Metall. 2020, 25, 51–57. [Google Scholar]
- Wang, S.J. Analysis on the utilization status and development trend of steel slag. Heilongjiang Sci. 2019, 10, 160–161. [Google Scholar]
- Tang, Q.J. Research and development of blasting abrasive made of steelmaking slag. Baosteel Tech. Res. 2015, 5, 23–28. [Google Scholar]
- Rao, L.; Chen, G.G.; Zhou, C.H.; Zhou, L. Experimental Research on Steel Slag as Sand-blasting Abrasive. J. Anhui Univ. Technol. 2015, 32, 16–21. [Google Scholar]
- Rao, L.; Chen, G.G.; Zhou, C.H.; Zhou, L. Technical Research on Using Steel Slag as Sandblasting Abrasive. Anhui Metall. 2014, 4, 12–15. [Google Scholar]
- Zhong, P.; Zhou, L.; Chang, L.Z.; Ding, C.L. Feasibility and Application Effect Research of the Wind Quenching Slag as Sand Blasting Abrasive. Surf. Technol. 2014, 2, 49–54. [Google Scholar]
- Zhang, B.; Li, S. Determination of the Surface Fractal Dimension for Porous Media by Mercury Porosimetry. Ind. Eng. Chem. Res. 1995, 34, 1383–1386. [Google Scholar] [CrossRef]
- Mandelbrot, B.B.; Pignoni, R. The Fractal Geometry of Nature; WH Freeman: New York, NY, USA, 1983. [Google Scholar]
- Chen, Y.; Zhang, J.; Wang, B.; Yao, C. Comparative study of IN600 superalloy produced by two powder metallurgy technologies: Argon Atomizing and Plasma Rotating Electrode Process. Vacuum 2018, 156, 302–309. [Google Scholar] [CrossRef]
- Wei, M.; Chen, S.; Liang, J.; Liu, C. Effect of atomization pressure on the breakup of TA15 titanium alloy powder prepared by EIGA method for laser 3D printing. Vacuum 2017, 143, 185–194. [Google Scholar] [CrossRef]
- Wang, L.L.; Zhang, Y.Z.; Ke, H.B.; Long, Y. Numerical simulation of molten slag film breakup during granulation process using air-quenching. J. Mater. Metall. 2020, 19, 87–93. [Google Scholar]
- Long, Y. Study on Granulation Mechanism and Application of Gas Quenching Liquid Steel Slag; Northeastern University: Shenyang, China, 2011. [Google Scholar]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pei, J.; Zhang, Y.; Xing, H.; Ren, Q.; Huo, W.; Wu, J. Beading Mechanism and Performance of Porous Steel Slag Microbead Abrasive. Crystals 2021, 11, 1377. https://doi.org/10.3390/cryst11111377
Pei J, Zhang Y, Xing H, Ren Q, Huo W, Wu J. Beading Mechanism and Performance of Porous Steel Slag Microbead Abrasive. Crystals. 2021; 11(11):1377. https://doi.org/10.3390/cryst11111377
Chicago/Turabian StylePei, Jingjing, Yuzhu Zhang, Hongwei Xing, Qianqian Ren, Wenqing Huo, and Jinhu Wu. 2021. "Beading Mechanism and Performance of Porous Steel Slag Microbead Abrasive" Crystals 11, no. 11: 1377. https://doi.org/10.3390/cryst11111377
APA StylePei, J., Zhang, Y., Xing, H., Ren, Q., Huo, W., & Wu, J. (2021). Beading Mechanism and Performance of Porous Steel Slag Microbead Abrasive. Crystals, 11(11), 1377. https://doi.org/10.3390/cryst11111377




