Gem Elbaite as a Recorder of Pegmatite Evolution: In Situ Major, Trace Elements and Boron Isotope Analysis of a Colour-Zoning Tourmaline Crystal
Abstract
1. Introduction
2. Sample Description
3. Methods
3.1. Major and Trace Element Analysis
3.2. Boron Isotope Analyses
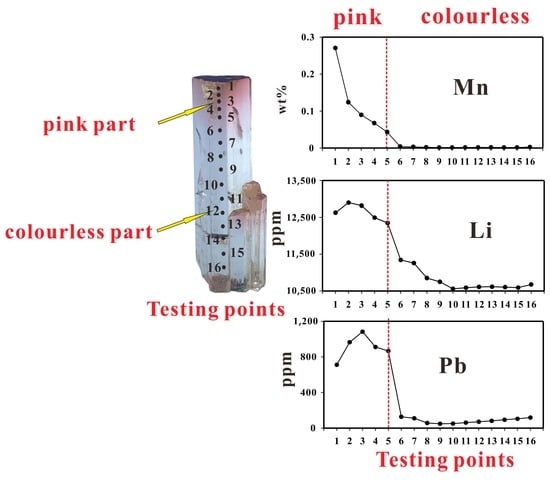
4. Results
5. Discussion
5.1. Composition Variations and Substitution Mechanisms
5.2. Implications for Growth History and Colour Genesis
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Henry, D.J.; Novak, M.; Hawthorne, F.C.; Ertl, A.; Dutrow, B.L.; Uher, P.; Pezzotta, F. Nomenclature of the tourmaline-supergroup minerals. Am. Miner. 2011, 96, 895–913. [Google Scholar] [CrossRef]
- Pezzotta, F.; Laurs, B.M. Tourmaline: The Kaleidoscopic Gemstone. Elements 2011, 7, 333–338. [Google Scholar] [CrossRef]
- Lussier, A.J.; Aguiar, P.M.; Michaelis, V.K.; Kroeker, S.; Herwig, S.; Abdu, Y.; Hawthorne, F.C. Mushroom elbaite from the Kat Chay mine, Momeik, near Mogok, Myanmar. Miner. Mag. 2008, 72, 999–1010. [Google Scholar] [CrossRef]
- Dutrow, B.L.; Henry, D.J. Complexly zoned fibrous tourmaline, Cruzeiro mine, Minas Gerais, Brazil: A record of evolving magmatic and hydrothermal fluids. Can. Miner. 2000, 38, 131–143. [Google Scholar] [CrossRef]
- Lussier, A.J.; Hawthorne, F.C.; Abdu, Y.; Herwig, S.; Michaelis, V.K.; Aguiar, P.M.; Kroeker, S. The crystal chemistry of ‘wheatsheaf’ tourmaline from Mogok, Myanmar. Miner. Mag. 2011, 75, 65–86. [Google Scholar] [CrossRef]
- Filip, J.; Bosi, F.; Novák, M.; Skogby, H. Iron redox reactions in the tourmaline structure: High-temperature treatment of Fe3+-rich schorl. Geochim. Cosmochim. Acta 2012, 86, 239–256. [Google Scholar] [CrossRef]
- London, D. A petrologic assessment of internal zonation in granitic pegmatites. Lithos 2014, 184, 74–104. [Google Scholar] [CrossRef]
- Thomas, R.; Davidson, P. Evidence of a water-rich silica gel state during the formation of a simple pegmatite. Miner. Mag. 2012, 76, 2785–2801. [Google Scholar] [CrossRef]
- Cerny, P. Rare-element granitic pegmatite. Part I: Anatomy and internal evolution of pegmatite deposit. Geosci. Can. 1991, 18, 68–81. [Google Scholar]
- Trumbull, R.B.; Chaussidon, M. Chemical and boron isotopic composition of magmatic and hydrothermal tourmalines from the Sinceni granite–pegmatite system in Swaziland. Chem. Geol. 1999, 153, 125–137. [Google Scholar] [CrossRef]
- Zhang, A.C.; Wang, R.C.; Jiang, S.Y.; Nguyen, H. Chemical and textural features of tourmaline from the spodumene-Subtype Koktokay No.3 pegmatite, Altai, Northwestern China: A record of magmatic to hydrothermal evolution. Can. Miner. 2008, 46, 41–58. [Google Scholar] [CrossRef]
- Chen, B.; Huang, C.; Zhao, H. Lithium and Nd isotopic constraints on the origin of Li-poor pegmatite with implications for Li mineralization. Chem. Geol. 2020, 551, 119769. [Google Scholar] [CrossRef]
- Jolliff, B.L.; Papike, J.J.; Shearer, C.K. Fractionation trends in mica and tourmaline as indicators of pegmatite internal evolution: Bob Ingersoll pegmatite, Black Hills, South Dakota. Geochim. Cosmochim. Acta 1987, 51, 519–534. [Google Scholar] [CrossRef]
- Gadas, P.; Novak, M.; Stanek, J.; Filip, J.; Galiova, M.V. Compositional Evolution of Zoned Tourmaline Crystals from Pockets in Common Pegmatites of the Moldanubian Zone, Czech Republic. Can. Miner. 2012, 50, 895–912. [Google Scholar] [CrossRef][Green Version]
- Hawthorne, F.C.; Dirlam, D.M. Tourmaline the Indicator Mineral: From Atomic Arrangement to Viking Navigation. Elements 2011, 7, 307–312. [Google Scholar] [CrossRef]
- Hong, H.L.; Li, J.; Du, D.W.; Zhong, Z.Q.; Yin, K.; Wang, C.W. Chemical States of Colour-induced Cations in Colourful Tourmaline. J. Gems. Gemmol. 2011, 13, 6–12, (In Chinese with English abstract). [Google Scholar]
- Li, M.; Hong, H.; Yin, K.; Wang, C.; Cheng, F.; Fang, Q. The Chemical States of Color-Induced Cations in Tourmaline Characterized by X-Ray Photoelectron Spectroscopy. J. Spectrosc. 2018, 2018, 3964071. [Google Scholar] [CrossRef]
- Reddy, B.J.; Frost, R.L.; Martens, W.N.; Wain, D.L.; Kloprogge, J.T. Spectroscopic characterization of Mn-rich tourmalines. Vib. Spectrosc. 2007, 44, 42–49. [Google Scholar] [CrossRef]
- Babińska, J.; Dyrek, K.; Pieczka, A.; Sojka, Z. X and Q band EPR studies of paramagnetic centres in natural and heated tourmaline. Eur. J. Miner. 2008, 20, 233–240. [Google Scholar] [CrossRef]
- Rossovskiy, L.N.; Chmyrev, V.M. Distribution patterns of rare-metal pegmatites in the Hindu Kush (Afghanistan). Int. Geol. Rev. 1977, 19, 511–520. [Google Scholar] [CrossRef]
- Morgan, G.B. A spreadsheet for calculating normative mole fractions of endmember species for Na-Ca-Li-Fe2+-Mg-Al tourmalines from electron microprobe data. Am. Miner. 2016, 101, 101–119. [Google Scholar] [CrossRef]
- Liu, Y.S.; Hu, Z.C.; Gao, S.; Günther, D.; Xu, J.; Gao, C.G.; Chen, H.L. In situ analysis of major and trace elements of anhydrous minerals by LA-ICP-MS without applying an internal standard. Chem. Geol. 2008, 257, 34–43. [Google Scholar] [CrossRef]
- Yang, S.Y.; Jiang, S.Y. Chemical and boron isotopic composition of tourmaline in the Xiangshan volcanic–intrusive complex, Southeast China: Evidence for boron mobilization and infiltration during magmatic–hydrothermal processes. Chem. Geol. 2012, 312–313, 177–189. [Google Scholar] [CrossRef]
- Tonarini, S.; Pennisi, M.; Adorni Braccesi, A.; Dini, A.; Ferrara, G.; Gonfiantini, R.; Wiedenbeck, M.; Gröning, M. Intercomparison of Boron Isotope and Concentration Measurements. Part I: Selection, Preparation and Homogeneity Tests of the Intercomparison Materials. Geostand. Newsl. 2003, 27, 21–39. [Google Scholar] [CrossRef]
- Hou, K.J.; Li, Y.H.; Xiao, Y.K.; Liu, F.; Tian, Y.R. In situ boron isotope measurements of natural geological materials by LA-MC-ICP-MS. Chin. Sci. Bull. 2010, 55, 3305–3311. [Google Scholar] [CrossRef]
- Rozhdestvenskaya, I.V.; Setkova, T.V.; Vereshchagin, O.S.; Shtukenberg, A.G.; Shapovalov, Y.B. Refinement of the crystal structures of synthetic nickel- and cobalt-bearing tourmalines. Cryst. Rep. 2012, 57, 57–63. [Google Scholar] [CrossRef]
- Slack, J.F.; Palmer, M.R.; Stevens, B.P.J.; Barnes, R.G. Origin and Significance of Tourmaline-Rich Rocks in the Broken Hill District, Australia. Econ. Geol. 1993, 88, 505–541. [Google Scholar] [CrossRef]
- Ertl, A.; Hughes, J.M.; Prowatke, S.; Ludwig, T.; Brandstatter, F.; Korner, W.; Dyar, M.D. Tetrahedrally coordinated boron in Li-bearing olenite from “mushroom” tourmaline from Momeik, Myanmar. Can. Miner. 2007, 45, 891–899. [Google Scholar] [CrossRef]
- Marks, M.A.W.; Marschall, H.R.; Schühle, P.; Guth, A.; Wenzel, T.; Jacob, D.E.; Barth, M.; Markl, G. Trace element systematics of tourmaline in pegmatitic and hydrothermal systems from the Variscan Schwarzwald (Germany): The importance of major element composition, sector zoning, and fluid or melt composition. Chem. Geol. 2013, 344, 73–90. [Google Scholar] [CrossRef]
- Zhao, H.D.; Zhao, K.D.; Palmer, M.; Jiang, S.Y. In situ elemental and boron isotope variation of tourmaline from the sanfang granite, South China Insights into magmatic-hydrothermal evolution. Chem. Geol. 2019, 504, 190–204. [Google Scholar] [CrossRef]
- van Hinsberg, V.J. Preliminary experimental data on trace-element partitioning between tourmaline and silicate melt. Can. Miner. 2011, 49, 153–163. [Google Scholar] [CrossRef]
- Klemme, S.; Marschall, H.R.; Jacob, D.E.; Prowatke, S.; Ludwig, T. Trace-element partitioning and boron isotope fractionation between white mica and tourmaline. Can. Miner. 2011, 49, 165–176. [Google Scholar] [CrossRef]
- Duchoslav, M.; Marks, M.A.W.; Drost, K.; Mc Cammon, C.; Marschall, H.R.; Wenzel, T.; Markl, G. Changes in tourmaline composition during magmatic and hydrothermal processes leading to tin-ore deposition: The Cornubian Batholish, SW England. Ore Geol. Rev. 2017, 83, 215–234. [Google Scholar] [CrossRef]
- London, D. Pegmatites. Can. Mineral. Spec. Publ. 2008, 10, 368. [Google Scholar]
- Trumbull, R.B.; Beurlen, H.; Wiedenbeck, M.; Soares, D.R. The diversity of B-isotope variations in tourmaline from rare-element pegmatites in the Borborema Province of Brazil. Chem. Geol. 2013, 352, 47–62. [Google Scholar] [CrossRef]
- Weidner, J.R.; Martin, R.F. Phase equilibria of a fluorine-rich leucogranite from the St. Austell pluton, Cornwall. Geochim. Cosmochim. Acta 1987, 51, 1591–1597. [Google Scholar] [CrossRef]
- Ertl, A.; Bačík, P. Considerations about Bi and Pb in the crystal structure of Cu-bearing tourmaline. Minerals 2020, 10, 706. [Google Scholar] [CrossRef]
- Galbraith, C.G.; Clarke, D.B.; Trumbull, R.B.; Wiedenbeck, M. Assessment of Tourmaline Compositions as an Indicator of Emerald Mineralization at the Tsa da Glisza Prospect, Yukon Territory, Canada. Econ. Geol. 2009, 104, 713–731. [Google Scholar] [CrossRef]
- Griffin, W.L.; Slack, J.F.; Ramsden, A.R.; Win, T.T.; Ryan, C.G. Trace elements in tourmalines from massive sulfides deposits and tourmalinites: Geochemical controls and exploration applications. Econ. Geol. 1996, 91, 657–675. [Google Scholar] [CrossRef]
- Shearer, C.K.; Papike, J.J.; Jolliff, B.L. Petrogenetic links among granites and pegmatites in the Harney peak rare-element granite-pegmatite system, Black Hills, South Dakota. Can. Miner. 1992, 30, 785–808. [Google Scholar]
- Martin, R.F.; De Vito, C. The patterns of enrichment in felsic pegmatites ultimately depend on tectonic setting. Can. Miner. 2005, 43, 2027–2048. [Google Scholar] [CrossRef]
- Zhang, H.J.; Tian, S.H.; Wang, D.H.; Li, X.F.; Liu, T.; Zhang, Y.J.; Fu, X.F.; Hao, X.F.; Hou, K.J.; Zhao, Y.; et al. Lithium isotope behavior during magmatic differentiation and fluid exsolution in the Jiajika granite–pegmatite deposit, Sichuan, China. Ore Geol. Rev. 2021, 134, 104139. [Google Scholar] [CrossRef]
- Henderson, P. General geochemical properties and abundances of the rare earth elements. Dev. Geochem. 1984, 2, 1–32. [Google Scholar]
- King, R.W.; Kerrich, R.W. REE distributions in tourmaline: An INAA technique involving pretreatment by B volatilization. Am. Miner. 1988, 33, 424–431. [Google Scholar]
- Jiang, S.Y.; Yu, J.M.; Lu, J.J. Trace and rare-earth element geochemistry in tourmaline and cassiterite from the Yunlong tin deposit, Yunnan, China: Implication for migmatitic–hydrothermal fluid evolution and ore genesis. Chem. Geol. 2004, 209, 193–213. [Google Scholar] [CrossRef]
- Zhao, K.D.; Zhang, L.H.; Palmer, M.R.; Jiang, S.Y.; Xu, C.; Zhao, H.D.; Chen, W. Chemical and boron isotopic compositions of tourmaline at the Dachang Sn-polymetallic ore district in South China: Constraints on the origin and evolution of hydrothermal fluids. Miner. Depos. 2021, 56, 1589–1608. [Google Scholar] [CrossRef]
- Vereshchagin, O.S.; Britvin, S.N.; Wunder, B.; Frank-Kamenetskaya, O.V.; Wilke, F.D.H.; Vlasenko, N.S.; Shilovskikh, V.V.; Bocharov, V.N.; Danilov, D.V. Ln3+ (Ln3+ = La, Nd, Eu, Yb) incorporation in synthetic tourmaline analogues: Towards tourmaline REE pattern explanation. Chem. Geol. 2021, 584, 120526. [Google Scholar] [CrossRef]
- Meyer, C.; Wunder, B.; Meixner, A.; Romer, R.L.; Heinrich, W. Boron-isotope fractionation between tourmaline and fluid: An experimental re-investigation. Contrib. Miner. Petrol. 2008, 156, 259–267. [Google Scholar] [CrossRef]
- Vereshchagin, O.S.; Frank-Kamenetskaya, O.V.; Rozhdestvenskaya, I.V.; Zolotarev, A.A. Incorporation of 3d elements in tourmalines: Structural adjustments and stability. Eur. J. Miner. 2018, 30, 917–928. [Google Scholar] [CrossRef]
- Manning, P.G. Effect of second-nearest-neighbor interaction on Mn3+ absorption in pink and black tourmalines. Can. Miner. 1973, 11, 971–977. [Google Scholar]
- Donnay, G.; Ingamells, C.O.; Mason, B. Buergerite, a new species of tourmaline. Am. Miner. 1966, 51, 198–199. [Google Scholar]
- Li, Y.; Guo, Y. Colorimetry Study on Red Tourmaline Color Genesis. Key Eng. Mater. 2012, 512–515, 657–660. [Google Scholar] [CrossRef]
- Chaudhry, M.N.; Howie, R.A. Lithium tourmalines from the Meldon aplite, Devonshire, England. Miner. Mag. 1976, 40, 747–751. [Google Scholar] [CrossRef][Green Version]
- Krambrock, K.; Pinheiro, M.V.B.; Medeiros, S.M.; Guedes, K.J.; Schweizer, S.; Spaeth, J.M. Investigation of radiation-induced yellow color in tourmaline by magnetic resonance. Nucl. Instrum. Methods Phys. Res. Sect. B 2002, 191, 241–245. [Google Scholar] [CrossRef]
- George, R.R.; Stephanie, M.M. Yellow, Mn-rich elbaite with Mn-Ti intervalence charge transfer. Am. Miner. 1986, 71, 599–602. [Google Scholar]
- Ahn, Y.; Park, J. The Color Formation of Tourmaline in Gemstone by Crystal Field. China Acad. J. Electron. Publ. House 2011, 101–105. [Google Scholar]








| Type | Pink | Colourless | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Spots | 1 | 2 | 3 | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| SiO2 | 37.592 | 37.778 | 37.743 | 38.053 | 38.219 | 38.359 | 37.970 | 37.955 | 38.117 | 38.144 |
| TiO2 | 0.006 | 0.003 | 0.002 | 0.001 | 0.000 | 0.000 | 0.000 | 0.000 | 0.001 | 0.000 |
| Al2O3 | 40.622 | 40.872 | 40.725 | 41.230 | 41.450 | 41.689 | 41.652 | 41.849 | 41.976 | 42.167 |
| FeO | 0.010 | 0.004 | 0.006 | 0.007 | 0.000 | 0.000 | 0.022 | 0.000 | 0.000 | 0.000 |
| MnO | 0.061 | 0.027 | 0.018 | 0.000 | 0.008 | 0.006 | 0.000 | 0.000 | 0.000 | 0.012 |
| MgO | 0.000 | 0.001 | 0.000 | 0.000 | 0.000 | 0.000 | 0.002 | 0.000 | 0.001 | 0.001 |
| CaO | 2.098 | 2.248 | 1.569 | 0.890 | 0.638 | 0.225 | 0.263 | 0.396 | 0.526 | 0.715 |
| Na2O | 1.501 | 1.337 | 1.525 | 1.811 | 1.815 | 1.871 | 1.912 | 1.796 | 1.828 | 1.768 |
| K2O | 0.011 | 0.007 | 0.007 | 0.011 | 0.018 | 0.019 | 0.008 | 0.017 | 0.013 | 0.002 |
| F | 1.383 | 1.106 | 1.111 | 0.970 | 0.961 | 0.798 | 0.712 | 0.802 | 0.869 | 0.884 |
| Li2O * | 2.492 | 2.414 | 2.390 | 2.339 | 2.316 | 2.250 | 2.239 | 2.256 | 2.284 | 2.292 |
| B2O3 * | 11.109 | 11.091 | 11.042 | 11.105 | 11.136 | 11.125 | 11.055 | 11.091 | 11.159 | 11.197 |
| H2O * | 3.177 | 3.302 | 3.283 | 3.372 | 3.387 | 3.460 | 3.477 | 3.446 | 3.438 | 3.444 |
| subTotal | 100.061 | 100.190 | 99.422 | 99.789 | 99.947 | 99.802 | 99.312 | 99.608 | 100.213 | 100.625 |
| O=F | −0.582 | −0.466 | −0.468 | −0.408 | −0.405 | −0.336 | −0.300 | −0.338 | −0.366 | −0.372 |
| Total | 99.479 | 99.724 | 98.954 | 99.381 | 99.542 | 99.466 | 99.013 | 99.271 | 99.847 | 100.253 |
| Structural formula based on 31 total anions | ||||||||||
| Si | 5.905 | 5.918 | 5.946 | 5.959 | 5.969 | 5.987 | 5.957 | 5.940 | 5.934 | 5.918 |
| B | 3.012 | 2.998 | 3.002 | 3.001 | 3.001 | 2.996 | 2.993 | 2.995 | 2.998 | 2.998 |
| Ti | 0.001 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Al | 7.522 | 7.547 | 7.562 | 7.610 | 7.631 | 7.669 | 7.703 | 7.720 | 7.703 | 7.711 |
| Fe | 0.001 | 0.001 | 0.001 | 0.001 | 0.000 | 0.000 | 0.003 | 0.000 | 0.000 | 0.000 |
| Mn | 0.008 | 0.004 | 0.002 | 0.000 | 0.001 | 0.001 | 0.000 | 0.000 | 0.000 | 0.002 |
| Mg | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Ca | 0.353 | 0.377 | 0.265 | 0.149 | 0.107 | 0.038 | 0.044 | 0.066 | 0.088 | 0.119 |
| Na | 0.457 | 0.406 | 0.466 | 0.550 | 0.550 | 0.566 | 0.582 | 0.545 | 0.552 | 0.532 |
| K | 0.002 | 0.001 | 0.001 | 0.002 | 0.004 | 0.004 | 0.002 | 0.003 | 0.003 | 0.000 |
| F | 3.329 | 0.548 | 0.554 | 0.480 | 0.475 | 0.394 | 0.353 | 0.397 | 0.428 | 0.434 |
| Al(T) | 0.083 | 0.082 | 0.052 | 0.040 | 0.029 | 0.013 | 0.043 | 0.060 | 0.066 | 0.082 |
| Al(Y) | 1.439 | 1.465 | 1.510 | 1.571 | 1.601 | 1.656 | 1.660 | 1.659 | 1.637 | 1.629 |
| Al(Z) | 6.000 | 6.000 | 6.000 | 6.000 | 6.000 | 6.000 | 6.000 | 6.000 | 6.000 | 6.000 |
| Li | 1.574 | 1.521 | 1.515 | 1.474 | 1.455 | 1.412 | 1.413 | 1.420 | 1.430 | 1.430 |
| X-site vacancy | 0.187 | 0.215 | 0.268 | 0.299 | 0.340 | 0.392 | 0.373 | 0.385 | 0.358 | 0.349 |
| sum(Z+T+Y+B) | 18.023 | 17.989 | 18.028 | 18.045 | 18.057 | 18.066 | 18.070 | 18.075 | 18.066 | 18.059 |
| Type | Pink | Colorless | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Spots | 1 | 2 | 3 | 4 | 5 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 |
| SiO2 | 38.26 | 38.18 | 37.80 | 38.42 | 38.27 | 39.41 | 39.58 | 39.53 | 39.53 | 39.34 | 39.63 | 39.31 | 39.44 | 39.50 | 39.49 | 39.58 |
| TiO2 | 0.06 | 0.04 | 0.02 | 0.01 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Al2O3 | 43.94 | 44.34 | 44.53 | 44.08 | 44.04 | 43.74 | 43.50 | 44.15 | 44.32 | 44.49 | 44.26 | 44.62 | 44.46 | 44.45 | 44.51 | 44.31 |
| FeO | 0.03 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| MnO | 0.27 | 0.12 | 0.09 | 0.07 | 0.04 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| MgO | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| CaO | 2.32 | 2.13 | 2.32 | 2.23 | 2.29 | 0.91 | 0.78 | 0.31 | 0.16 | 0.22 | 0.25 | 0.26 | 0.37 | 0.37 | 0.44 | 0.52 |
| Na2O | 1.74 | 1.77 | 1.70 | 1.72 | 1.69 | 1.75 | 1.82 | 1.90 | 1.94 | 1.93 | 1.93 | 1.90 | 1.90 | 1.88 | 1.86 | 1.85 |
| K2O | 0.02 | 0.03 | 0.01 | 0.02 | 0.02 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.02 | 0.01 | 0.00 | 0.00 |
| P2O5 | 0.02 | 0.03 | 0.01 | 0.02 | 0.02 | 0.00 | 0.00 | 0.02 | 0.05 | 0.02 | 0.01 | 0.03 | 0.03 | 0.05 | 0.04 | 0.04 |
| B2O3 | 9.84 | 9.80 | 9.96 | 9.99 | 10.21 | 11.12 | 11.17 | 11.13 | 11.06 | 11.12 | 11.06 | 11.06 | 10.99 | 10.96 | 10.91 | 10.93 |
| Li2O | 2.71 | 2.76 | 2.75 | 2.68 | 2.65 | 2.43 | 2.41 | 2.33 | 2.30 | 2.26 | 2.27 | 2.27 | 2.28 | 2.27 | 2.27 | 2.29 |
| Total | 99.21 | 99.20 | 99.20 | 99.24 | 99.24 | 99.38 | 99.29 | 99.37 | 99.37 | 99.39 | 99.42 | 99.45 | 99.48 | 99.50 | 99.51 | 99.54 |
| Be | 61.41 | 54.56 | 53.20 | 53.22 | 56.57 | 58.03 | 64.81 | 64.38 | 63.55 | 73.30 | 59.39 | 53.20 | 52.15 | 48.36 | 54.82 | 40.46 |
| Sc | 19.37 | 13.98 | 10.39 | 8.11 | 5.97 | 3.36 | 2.11 | 1.21 | 1.08 | 0.87 | 1.30 | 0.96 | 1.49 | 0.87 | 1.72 | 1.37 |
| V | 0.79 | 0.03 | 0.09 | 0.05 | 0.04 | 0.07 | 0.01 | 0.00 | 0.03 | 0.04 | 0.00 | 0.27 | 0.35 | 0.01 | 0.15 | 0.28 |
| Cr | 11.36 | 0.44 | 0.56 | 0.00 | 0.00 | 5.60 | 1.13 | 1.88 | 2.99 | 0.00 | 4.03 | 0.00 | 0.00 | 0.58 | 8.25 | 3.88 |
| Co | 0.28 | 0.29 | 0.13 | 0.07 | 0.32 | 0.17 | 0.00 | 0.00 | 31.62 | 0.00 | 0.00 | 18.81 | 0.00 | 0.14 | 103.08 | 0.00 |
| Ni | 1.03 | 0.00 | 0.00 | 2.19 | 0.00 | 0.71 | 0.00 | 0.00 | 1.12 | 0.00 | 0.00 | 0.87 | 0.00 | 0.00 | 0.00 | 0.37 |
| Cu | 10.78 | 7.87 | 8.10 | 7.21 | 6.67 | 6.27 | 5.64 | 4.52 | 5.24 | 4.59 | 4.27 | 3.88 | 4.98 | 4.06 | 5.37 | 4.99 |
| Zn | 6.54 | 5.21 | 1.81 | 1.33 | 2.39 | 1.30 | 0.09 | 0.08 | 0.25 | 1.13 | 1.15 | 0.00 | 2.19 | 0.00 | 2.34 | 0.66 |
| Ga | 305.56 | 351.32 | 357.40 | 359.74 | 369.02 | 431.49 | 443.30 | 459.19 | 472.39 | 477.68 | 486.51 | 487.66 | 507.12 | 502.34 | 509.08 | 511.81 |
| Rb | 0.47 | 0.25 | 0.39 | 0.00 | 0.55 | 0.00 | 0.00 | 0.00 | 0.26 | 0.00 | 0.00 | 0.28 | 0.03 | 0.00 | 0.00 | 0.10 |
| Sr | 40.32 | 14.70 | 9.55 | 6.08 | 5.07 | 1.18 | 1.16 | 0.49 | 0.30 | 0.36 | 0.26 | 0.29 | 0.30 | 0.32 | 0.44 | 0.43 |
| Y | 3.12 | 2.33 | 3.65 | 2.58 | 3.45 | 1.32 | 2.39 | 2.77 | 3.81 | 4.24 | 4.90 | 4.24 | 4.56 | 4.96 | 4.85 | 4.92 |
| Zr | 0.22 | 0.00 | 0.00 | 0.00 | 0.48 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.21 | 0.31 | 0.11 | 0.21 | 0.00 | 0.00 |
| Nb | 1.57 | 1.81 | 1.99 | 2.21 | 1.93 | 0.91 | 1.18 | 1.03 | 1.12 | 1.51 | 1.56 | 2.05 | 2.43 | 2.73 | 3.35 | 3.72 |
| Mo | 0.00 | 0.15 | 0.28 | 0.00 | 0.00 | 0.12 | 0.00 | 0.12 | 0.59 | 0.00 | 0.26 | 0.00 | 0.39 | 0.00 | 0.12 | 0.13 |
| Ag | 0.00 | 0.00 | 0.04 | 0.00 | 0.08 | 0.12 | 0.00 | 0.00 | 0.02 | 0.00 | 0.08 | 0.09 | 0.18 | 0.00 | 0.51 | 0.03 |
| Cd | 2.70 | 3.99 | 4.49 | 3.86 | 5.63 | 8.66 | 6.12 | 7.12 | 10.14 | 7.87 | 6.68 | 6.29 | 5.19 | 2.10 | 6.87 | 5.23 |
| Sn | 64.05 | 78.18 | 66.82 | 61.23 | 51.89 | 34.42 | 31.76 | 35.14 | 35.33 | 34.13 | 42.00 | 38.08 | 39.88 | 42.18 | 46.07 | 48.66 |
| Sb | 5.91 | 5.95 | 5.88 | 5.56 | 5.45 | 3.37 | 2.84 | 1.55 | 1.47 | 1.38 | 0.93 | 1.00 | 1.01 | 0.78 | 1.15 | 1.49 |
| Cs | 0.51 | 0.12 | 0.00 | 0.03 | 0.00 | 0.00 | 0.04 | 0.08 | 0.15 | 0.00 | 0.00 | 0.00 | 0.00 | 0.07 | 0.00 | 0.00 |
| Ba | 0.74 | 0.87 | 0.78 | 0.16 | 0.41 | 0.13 | 0.22 | 0.00 | 0.29 | 0.00 | 0.00 | 0.00 | 0.00 | 0.24 | 0.00 | 0.00 |
| La | 27.30 | 3.76 | 3.58 | 3.08 | 2.75 | 0.15 | 0.03 | 0.00 | 0.04 | 0.00 | 0.02 | 0.01 | 0.00 | 0.02 | 0.05 | 0.04 |
| Ce | 65.35 | 9.51 | 9.66 | 8.77 | 9.02 | 0.30 | 0.15 | 0.03 | 0.03 | 0.00 | 0.00 | 0.00 | 0.00 | 0.05 | 0.01 | 0.04 |
| Pr | 8.81 | 1.51 | 1.42 | 1.25 | 1.51 | 0.03 | 0.04 | 0.04 | 0.03 | 0.02 | 0.01 | 0.00 | 0.00 | 0.02 | 0.00 | 0.02 |
| Nd | 37.80 | 6.21 | 6.04 | 5.08 | 6.91 | 0.57 | 0.09 | 0.05 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.10 | 0.00 | 0.00 |
| Sm | 13.20 | 3.96 | 5.05 | 4.46 | 5.38 | 0.62 | 0.03 | 0.16 | 0.16 | 0.11 | 0.00 | 0.00 | 0.17 | 0.02 | 0.00 | 0.06 |
| Eu | 0.15 | 0.02 | 0.01 | 0.01 | 0.00 | 0.04 | 0.06 | 0.00 | 0.04 | 0.00 | 0.05 | 0.02 | 0.00 | 0.00 | 0.03 | 0.00 |
| Gd | 5.32 | 1.96 | 2.63 | 2.04 | 3.12 | 0.18 | 0.29 | 0.00 | 0.00 | 0.10 | 0.00 | 0.26 | 0.22 | 0.21 | 0.17 | 0.00 |
| Tb | 0.31 | 0.16 | 0.17 | 0.18 | 0.22 | 0.08 | 0.04 | 0.03 | 0.07 | 0.05 | 0.12 | 0.10 | 0.09 | 0.22 | 0.18 | 0.29 |
| Dy | 0.98 | 0.30 | 0.45 | 0.41 | 0.45 | 0.09 | 0.29 | 0.28 | 0.50 | 0.43 | 0.38 | 0.57 | 0.69 | 0.44 | 0.44 | 0.96 |
| Ho | 0.08 | 0.00 | 0.00 | 0.01 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.04 | 0.00 | 0.03 | 0.02 | 0.03 | 0.03 | 0.03 |
| Er | 0.07 | 0.07 | 0.02 | 0.04 | 0.00 | 0.04 | 0.00 | 0.02 | 0.02 | 0.00 | 0.05 | 0.00 | 0.00 | 0.01 | 0.00 | 0.00 |
| Tm | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 | 0.00 | 0.01 |
| Yb | 0.03 | 0.00 | 0.00 | 0.00 | 0.00 | 0.09 | 0.10 | 0.00 | 0.00 | 0.14 | 0.00 | 0.00 | 0.04 | 0.05 | 0.11 | 0.07 |
| Lu | 0.01 | 0.01 | 0.00 | 0.00 | 0.01 | 0.03 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 | 0.05 | 0.00 | 0.00 | 0.02 | 0.02 |
| Hf | 0.00 | 0.05 | 0.00 | 0.00 | 0.00 | 0.16 | 0.02 | 0.05 | 0.00 | 0.00 | 0.00 | 0.11 | 0.00 | 0.00 | 0.08 | 0.00 |
| Ta | 1.41 | 6.86 | 8.88 | 12.15 | 13.06 | 1.60 | 3.07 | 2.37 | 2.78 | 3.71 | 4.73 | 6.39 | 6.39 | 8.25 | 9.06 | 10.53 |
| W | 0.03 | 0.00 | 0.03 | 0.03 | 0.00 | 0.00 | 0.03 | 0.00 | 0.00 | 0.07 | 0.00 | 0.00 | 0.00 | 0.00 | 0.04 | 0.12 |
| Hg | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Tl | 0.02 | 0.05 | 0.08 | 0.06 | 0.01 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | 0.09 | 0.00 | 0.00 | 0.00 | 0.07 | 0.00 |
| Bi | 3914.98 | 3888.80 | 3775.33 | 3652.65 | 3658.22 | 3194.14 | 3880.30 | 3275.29 | 3211.21 | 3038.84 | 2791.76 | 2513.50 | 2285.25 | 2080.23 | 1850.24 | 1765.72 |
| Pb | 711.22 | 964.53 | 1082.71 | 910.99 | 865.68 | 127.72 | 110.35 | 57.57 | 48.84 | 50.41 | 61.59 | 71.58 | 81.88 | 93.98 | 104.58 | 118.57 |
| Th | 91.74 | 69.00 | 63.44 | 54.73 | 47.80 | 22.49 | 38.42 | 27.78 | 39.78 | 48.27 | 52.25 | 59.99 | 59.53 | 58.87 | 57.31 | 55.86 |
| U | 2.42 | 0.90 | 0.72 | 0.40 | 0.31 | 0.03 | 0.11 | 0.03 | 0.05 | 0.05 | 0.06 | 0.01 | 0.07 | 0.00 | 0.01 | 0.06 |
| SUM REE | 159.40 | 27.46 | 29.03 | 25.34 | 29.41 | 2.25 | 1.14 | 0.62 | 0.91 | 0.88 | 0.64 | 1.05 | 1.22 | 1.19 | 1.04 | 1.53 |
| Li+Mn | 1.30 | 1.31 | 1.29 | 1.26 | 1.24 | 1.13 | 1.13 | 1.09 | 1.08 | 1.06 | 1.06 | 1.06 | 1.06 | 1.06 | 1.06 | 1.07 |
| Al+Xvac | 2.62 | 2.70 | 2.75 | 2.66 | 2.67 | 2.78 | 2.74 | 2.86 | 2.90 | 2.92 | 2.87 | 2.93 | 2.89 | 2.89 | 2.89 | 2.85 |
| Type | Spot | δ11B (‰) | 1SD (‰) a |
|---|---|---|---|
| pink | 1 | −11.14 | 0.1 |
| 2 | −11.24 | 0.2 | |
| 3 | −11.41 | 0.1 | |
| 4 | 11.53 | 0.3 | |
| 5 | −11.77 | 0.1 | |
| colourless | 1 | −11.24 | 0.2 |
| 2 | −11.44 | 0.2 | |
| 3 | −11.73 | 0.1 | |
| 4 | −11.43 | 0.3 | |
| 5 | −11.39 | 0.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, B.; Chen, M. Gem Elbaite as a Recorder of Pegmatite Evolution: In Situ Major, Trace Elements and Boron Isotope Analysis of a Colour-Zoning Tourmaline Crystal. Crystals 2021, 11, 1363. https://doi.org/10.3390/cryst11111363
Zheng B, Chen M. Gem Elbaite as a Recorder of Pegmatite Evolution: In Situ Major, Trace Elements and Boron Isotope Analysis of a Colour-Zoning Tourmaline Crystal. Crystals. 2021; 11(11):1363. https://doi.org/10.3390/cryst11111363
Chicago/Turabian StyleZheng, Beiqi, and Meihua Chen. 2021. "Gem Elbaite as a Recorder of Pegmatite Evolution: In Situ Major, Trace Elements and Boron Isotope Analysis of a Colour-Zoning Tourmaline Crystal" Crystals 11, no. 11: 1363. https://doi.org/10.3390/cryst11111363
APA StyleZheng, B., & Chen, M. (2021). Gem Elbaite as a Recorder of Pegmatite Evolution: In Situ Major, Trace Elements and Boron Isotope Analysis of a Colour-Zoning Tourmaline Crystal. Crystals, 11(11), 1363. https://doi.org/10.3390/cryst11111363