Furosemide/Non-Steroidal Anti-Inflammatory Drug–Drug Pharmaceutical Solids: Novel Opportunities in Drug Formulation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
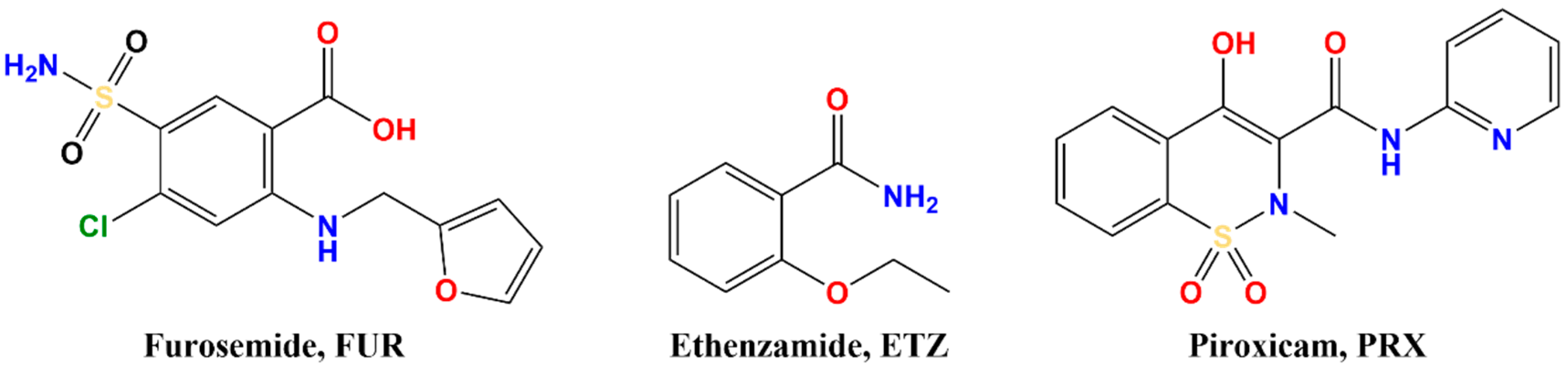
2.2. Coformer Selection
2.3. General Procedure for Mechanochemical Synthesis
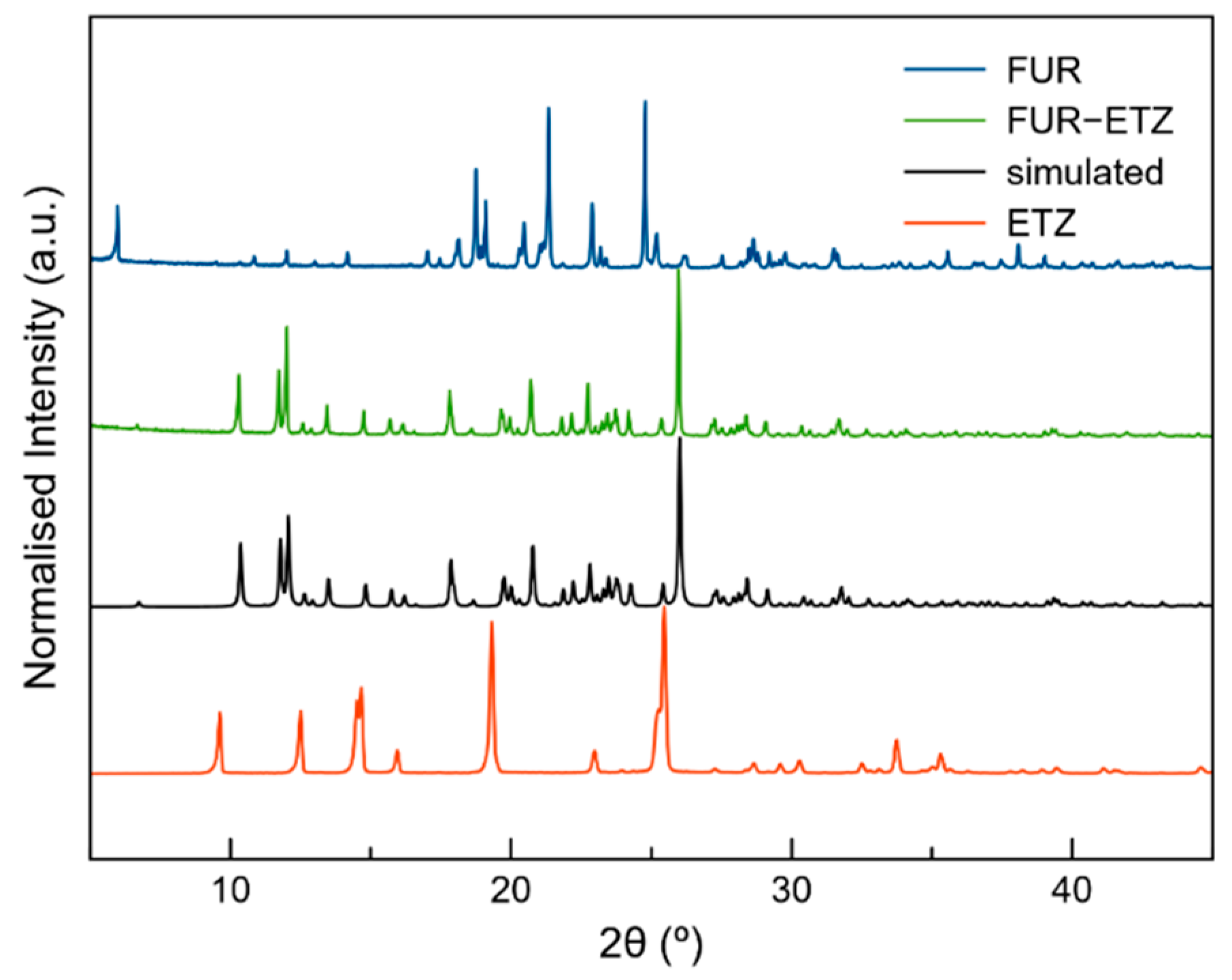
2.4. Powder X-ray Diffraction (PXRD)
2.5. Preparation of Single Crystals
2.6. Single-Crystal X-ray Diffraction (SCXRD)
2.7. Stability Test
2.8. Infrared Spectroscopy
2.9. Thermal Analysis
2.10. Solubility Studies
3. Results and Discussion
3.1. Coformer Selection
3.2. Mechanochemical Synthesis
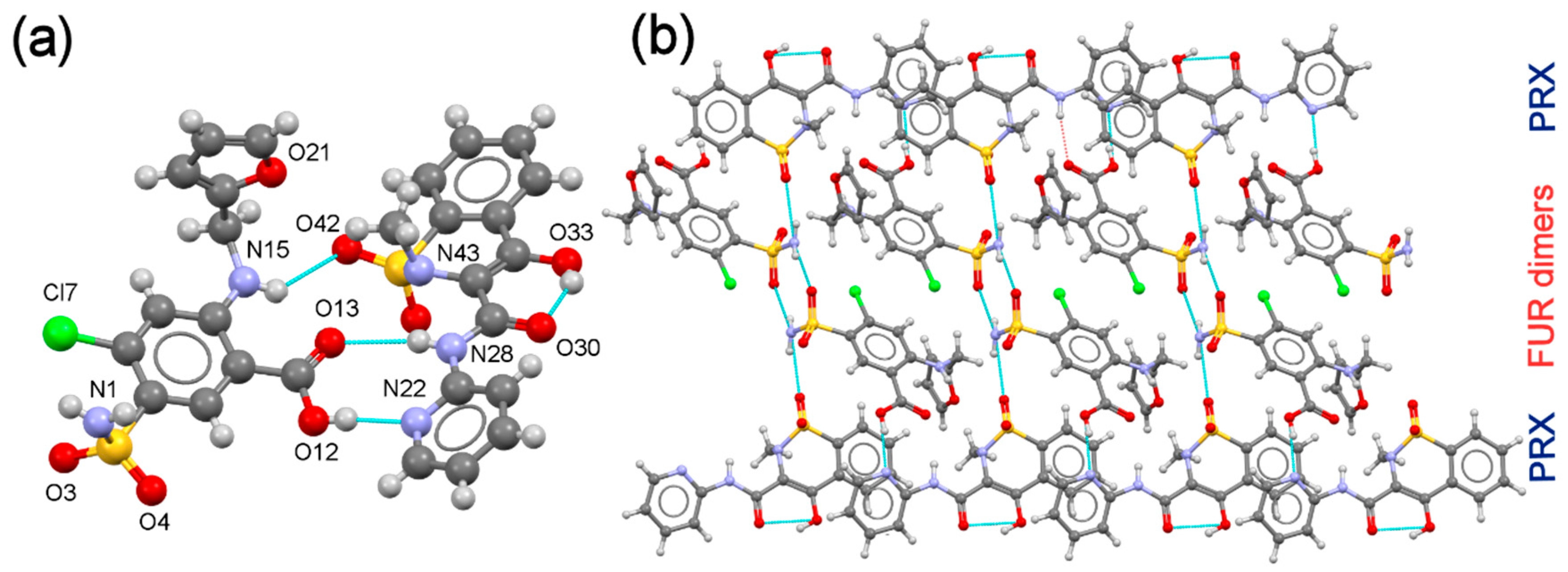
3.3. Structural Studies of Multi-Component Forms
3.4. Fourier Transform Infrared (FT-IR) Spectroscopy
3.5. Thermal Analysis
3.6. Stability Studies
3.7. Equilibrium Solubility
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jackson, E.K. Diuretics. In Goodman and Gilman’s The Pharmacological Basis of Therapeutics; Brunton, L., Lazo, J., Parker, K., Eds.; McGraw-Hill: New York, NY, USA, 2006; pp. 737–770. ISBN 0071422803. [Google Scholar]
- Carone, L.; Oxberry, S.G.; Twycross, R.; Charlesworth, S.; Mihalyo, M.; Wilcock, A. Furosemide. J. Pain Symptom Manag. 2016, 52, 144–150. [Google Scholar] [CrossRef]
- Amidon, G.L.; Lennernäs, H.; Shah, V.P.; Crison, J.R. A Theoretical Basis for a Biopharmaceutic Drug Classification: The Correlation of in Vitro Drug Product Dissolution and in Vivo Bioavailability. Pharm. Res. 1995, 12, 413–420. [Google Scholar] [CrossRef]
- Wisher, D. Martindale: The Complete Drug Reference. 37th Ed. J. Med Libr. Assoc. JMLA 2012, 100, 75–76. [Google Scholar] [CrossRef]
- Grahnén, A.; Hammarlund, M.; Lundqvist, T. Implications of Intraindividual Variability in Bioavailability Studies of Furosemide. Eur. J. Clin. Pharmacol. 1984, 27, 595–602. [Google Scholar] [CrossRef]
- Berry, D.J.; Steed, J.W. Pharmaceutical Cocrystals, Salts and Multicomponent Systems; Intermolecular Interactions and Property Based Design. Adv. Drug Deliv. Rev. 2017, 117, 3–24. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Kumar, S.; Nanda, A. A Review about Regulatory Status and Recent Patents of Pharmaceutical Co-Crystals. Adv. Pharm. Bull. 2018, 8, 355–363. [Google Scholar] [CrossRef]
- Stepanovs, D.; Mishnev, A. Multicomponent Pharmaceutical Cocrystals: Furosemide and Pentoxifylline. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 2012, 68, o488–o491. [Google Scholar] [CrossRef] [PubMed]
- Banik, M.; Gopi, S.P.; Ganguly, S.; Desiraju, G.R. Cocrystal and Salt Forms of Furosemide: Solubility and Diffusion Variations. Cryst. Growth Des. 2016, 16, 5418–5428. [Google Scholar] [CrossRef]
- George, C.P.; Thorat, S.H.; Shaligram, P.S.; Suresha, P.R.; Gonnade, R.G. Drug-Drug Cocrystals of Anticancer Drugs Erlotinib-Furosemide and Gefitinib-Mefenamic Acid for Alternative Multi-Drug Treatment. CrystEngComm 2020, 22, 6137–6151. [Google Scholar] [CrossRef]
- Thorat, S.H.; Sahu, S.K.; Patwadkar, M.V.; Badiger, M.V.; Gonnade, R.G. Drug-Drug Molecular Salt Hydrate of an Anticancer Drug Gefitinib and a Loop Diuretic Drug Furosemide: An Alternative for Multidrug Treatment. J. Pharm. Sci. 2015, 104, 4207–4216. [Google Scholar] [CrossRef] [PubMed]
- Diniz, L.F.; Carvalho, P.S.; Pena, S.A.C.; Gonçalves, J.E.; Souza, M.A.C.; de Souza Filho, J.D.; Bomfim Filho, L.F.O.; Franco, C.H.J.; Diniz, R.; Fernandes, C. Enhancing the Solubility and Permeability of the Diuretic Drug Furosemide via Multicomponent Crystal Forms. Int. J. Pharm. 2020, 587, 119694. [Google Scholar] [CrossRef] [PubMed]
- Abraham Miranda, J.; Garnero, C.; Chattah, A.K.; Santiago De Oliveira, Y.; Ayala, A.P.; Longhi, M.R. Furosemide:Triethanolamine Salt as a Strategy to Improve the Biopharmaceutical Properties and Photostability of the Drug. Cryst. Growth Des. 2019, 19, 2060–2068. [Google Scholar] [CrossRef]
- Wang, X.; Du, S.; Zhang, R.; Jia, X.; Yang, T.; Zhang, X. Drug-Drug Cocrystals: Opportunities and Challenges. Asian J. Pharm. Sci. 2021, 16, 307–317. [Google Scholar] [CrossRef]
- Herchuelz, A.; Derenne, F.; Deger, F.; Juvent, M.; van Ganse, E.; Staroukine, M.; Verniory, A.; Boeynaems, J.M.; Douchamps, J. Interaction between Nonsteroidal Anti-Inflammatory Drugs and Loop Diuretics: Modulation by Sodium Balance. J. Pharmacol. Exp. Ther. 1989, 248, 1175–1181. [Google Scholar]
- Paterson, C.A.; Jacobs, D.; Rasmussen, S.; Youngberg, S.P.; McGuinness, N. Randomized, Open-Label, 5-Way Crossover Study to Evaluate the Pharmacokinetic/Pharmacodynamic Interaction between Furosemide and the Non-Steroidal Anti-Inflammatory Drugs Diclofenac and Ibuprofen in Healthy Volunteers. Int. J. Clin. Pharmacol. Ther. 2011, 49, 477–490. [Google Scholar] [CrossRef]
- Moore, N.; Pollack, C.; Butkerait, P. Adverse Drug Reactions and Drug–Drug Interactions with over-the-Counter NSAIDs. Ther Clin Risk Manag. 2015, 11, 1061–1075. [Google Scholar] [CrossRef]
- Baker, D.E. Piroxicam—Furosemide Drug Interaction. Drug Intell. Clin. Pharm. 1988, 22, 505–506. [Google Scholar] [CrossRef] [PubMed]
- Allen, F.H. The Cambridge Structural Database: A Quarter of a Million Crystal Structures and Rising. Acta Crystallogr. Sect. B Struct. Sci. 2002, 58, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Loschen, C.; Klamt, A. Solubility Prediction, Solvate and Cocrystal Screening as Tools for Rational Crystal Engineering. J. Pharm. Pharmacol. 2015, 67, 803–811. [Google Scholar] [CrossRef]
- Coelho, A.A. TOPAS and TOPAS-Academic: An Optimization Program Integrating Computer Algebra and Crystallographic Objects Written in C++: An. J. Appl. Crystallogr. 2018, 51, 210–218. [Google Scholar] [CrossRef]
- Bruker APEX3. APEX3 V2019.1; Bruker-AXS: Madison, WI, USA, 2019. [Google Scholar]
- Sheldrick, G.M. SHELXT—Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. Sect. A Found. Crystallogr. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Spek, A.L. Structure Validation in Chemical Crystallography. Acta Crystallogr. Sect. D Biol. Crystallogr. 2009, 65, 148–155. [Google Scholar] [CrossRef]
- Macrae, C.F.; Bruno, I.J.; Chisholm, J.A.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Rodriguez-Monge, L.; Taylor, R.; van de Streek, J.; Wood, P.A. Mercury CSD 2.0—New Features for the Visualization and Investigation of Crystal Structures. J. Appl. Crystallogr. 2008, 41, 466–470. [Google Scholar] [CrossRef]
- Horst, J.H.T.; Deij, M.A.; Cains, P.W. Discovering New Co-Crystals. Cryst. Growth Des. 2009, 9. [Google Scholar] [CrossRef]
- Peng, B.; Wang, J.R.; Mei, X. Triamterene–Furosemide Salt: Structural Aspects and Physicochemical Evaluation. Acta Crystallogr. Sect. B: Struct. Sci. Cryst. Eng. Mater. 2018, 74, 738–741. [Google Scholar] [CrossRef]
- Goud, N.R.; Gangavaram, S.; Suresh, K.; Pal, S.; Manjunatha, S.G.; Nambiar, S.; Nangia, A. Novel Furosemide Cocrystals and Selection of High Solubility Drug Forms. J. Pharm. Sci. 2012, 101, 664–680. [Google Scholar] [CrossRef]
- Mishnev, A.; Kiselovs, G. New Crystalline Forms of Piroxicam. Z. Fur Nat.-Sect. C J. Biosci. 2013, 68 B, 168–174. [Google Scholar] [CrossRef]
- Abramov, Y.A.; Loschen, C.; Klamt, A. Rational Coformer or Solvent Selection for Pharmaceutical Cocrystallization or Desolvation. J. Pharm. Sci. 2012, 101, 3687–3697. [Google Scholar] [CrossRef] [PubMed]
- Sangtani, E.; Mandal, S.K.; Sreelakshmi, A.S.; Munshi, P.; Gonnade, R.G. Salts and Cocrystals of Furosemide with Pyridines: Differences in π-Stacking and Color Polymorphism. Cryst. Growth Des. 2017, 17, 3071–3087. [Google Scholar] [CrossRef]
- Srirambhatla, V.K.; Kraft, A.; Watt, S.; Powell, A.V. A Robust Two-Dimensional Hydrogen-Bonded Network for the Predictable Assembly of Ternary Co-Crystals of Furosemide. CrystEngComm 2014, 16, 9979–9982. [Google Scholar] [CrossRef]
- Sangtani, E.; Sahu, S.K.; Thorat, S.H.; Gawade, R.L.; Jha, K.K.; Munshi, P.; Gonnade, R.G. Furosemide Cocrystals with Pyridines: An Interesting Case of Color Cocrystal Polymorphism. Cryst. Growth Des. 2015, 15, 5858–5872. [Google Scholar] [CrossRef]
- Rahal, O.; Majumder, M.; Spillman, M.J.; van de Streek, J.; Shankland, K. Co-Crystal Structures of Furosemide:Urea and Carbamazepine:Indomethacin Determined from Powder x-Ray Diffraction Data. Crystals 2020, 10, 42. [Google Scholar] [CrossRef]
- Ueto, T.; Takata, N.; Muroyama, N.; Nedu, A.; Sasaki, A.; Tanida, S.; Terada, K. Polymorphs and a Hydrate of Furosemide-Nicotinamide 1:1 Cocrystal. Cryst. Growth Des. 2012, 12, 485–494. [Google Scholar] [CrossRef]
- Braga, D.; Maini, L.; Grepioni, F. Mechanochemical Preparation of Co-Crystals. Chem. Soc. Rev. 2013, 42, 7638–7648. [Google Scholar] [CrossRef]
- Delori, A.; Friščić, T.; Jones, W. The Role of Mechanochemistry and Supramolecular Design in the Development of Pharmaceutical Materials. CrystEngComm 2012, 14, 2350. [Google Scholar] [CrossRef]
- Friščić, T.; Childs, S.L.; Rizvi, S.A.A.; Jones, W. The Role of Solvent in Mechanochemical and Sonochemical Cocrystal Formation: A Solubility-Based Approach for Predicting Cocrystallisation Outcome. CrystEngComm 2009, 11, 418–426. [Google Scholar] [CrossRef]
- Verdugo-Escamilla, C.; Alarcón-Payer, C.; Frontera, A.; Acebedo-Martínez, F.J.; Domínguez-Martín, A.; Gómez-Morales, J.; Choquesillo-Lazarte, D. Interconvertible Hydrochlorothiazide–Caffeine Multicomponent Pharmaceutical Materials: A Solvent Issue. Crystals 2020, 10, 1088. [Google Scholar] [CrossRef]
- Babu, N.J.; Cherukuvada, S.; Thakuria, R.; Nangia, A. Conformational and Synthon Polymorphism in Furosemide (Lasix). Cryst. Growth Des. 2010, 10, 1979–1989. [Google Scholar] [CrossRef]
- Heinz, A.; Strachan, C.J.; Gordon, K.C.; Rades, T. Analysis of Solid-State Transformations of Pharmaceutical Compounds Using Vibrational Spectroscopy. J. Pharm. Pharmacol. 2009, 61, 971–988. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.; Tothadi, S.; Chakraborty, S.; Ganguly, S.; Desiraju, G.R. Synthon Identification in Co-Crystals and Polymorphs with IR Spectroscopy. Primary Amides as a Case Study. CrystEngComm 2013, 15, 4640–4654. [Google Scholar] [CrossRef]
- Schultheiss, N.; Newman, A. Pharmaceutical Cocrystals and Their Physicochemical Properties. Cryst. Growth Des. 2009, 9, 2950–2967. [Google Scholar] [CrossRef] [PubMed]
- Perlovich, G. Melting Points of One- and Two-Component Molecular Crystals as Effective Characteristics for Rational Design of Pharmaceutical Systems. Acta Crystallogr. Sect. B Struct. Sci. Cryst. Eng. Mater. 2020, 76, 696–706. [Google Scholar] [CrossRef]
- Khatioda, R.; Bora, P.; Sarma, B. Trimorphic Ethenzamide Cocrystal: In Vitro Solubility and Membrane Efflux Studies. Cryst. Growth Des. 2018, 18, 4637–4645. [Google Scholar] [CrossRef]
- Karataş, A.; Yüksel, N.; Baykara, T. Improved Solubility and Dissolution Rate of Piroxicam Using Gelucire 44/14 and Labrasol. Farmaco 2005, 60, 777–782. [Google Scholar] [CrossRef]





| Compound Name | FUR–ETZ | FUR–PRX |
|---|---|---|
| Formula | C21H22ClN3O7S | C27H24ClN5O9S2 |
| Formula weight | 495.92 | 662.08 |
| Crystal system | Monoclinic | Monoclinic |
| Space group | P21/c | P21/n |
| a/Å | 13.1846 (4) | 9.0971 (4) |
| b/Å | 9.8733 (3) | 23.8637 (10) |
| c/Å | 17.1518 (6) | 13.7806 (6) |
| α/° | 90 | 90 |
| β/° | 95.776 (2) | 99.227 (2) |
| γ/° | 90 | 90 |
| V/Å3 | 2221.41 (12) | 2952.9 (2) |
| Z | 4 | 4 |
| Dc/g cm−3 | 1.483 | 1.489 |
| µ/mm−1 | 0.315 | 3.010 |
| F(000) | 1032 | 1368 |
| Reflections collected | 32,492 | 41,427 |
| Unique reflections | 5104 | 5172 |
| Rint | 0.1392 | 0.0331 |
| Data/restraints/parameters | 5104/0/305 | 5172/0/406 |
| Goodness-of-fit (F2) | 1.002 | 1.032 |
| R1 (I > 2σ(I)) | 0.0584 | 0.0379 |
| wR2 (I > 2σ(I)) | 0.1054 | 0.0959 |
| Packing coefficient | 0.69 | 0.67 |
| Coformer | Hex(kcal/mol) | Ref. for the Corresponding Cocrystal/Salt |
|---|---|---|
| 1,10-phenanthroline * | −5.462215 | [33] |
| 4,4′-bipyridine * | −3.87421 | [34] |
| Piperazine * | −3.85138 | [9] |
| Triamterene | −3.33838 | [29] |
| Pentoxifylline | −3.18718 | [8] |
| Cytosine * | −3.018425 | [30] |
| Caffeine | −2.910815 | [30] |
| Gefitinib | −2.8597 | [11] |
| 4-Aminopyridine * | −2.6074 | [35] |
| Urea * | −2.41366 | [36] |
| Ethenzamide | −2.36084 | This work |
| Erlotinib | −2.3474 | [10] |
| Nicotinamide | −2.13354 | [37] |
| 5-fluorocytosine | −2.101 | [12] |
| 4-toluamide * | −1.95134 | [9] |
| 2,2′-bipyridine * | −1.83637 | [33] |
| 2-picolinamide * | −1.72857 | [9] |
| Anthranilamide * | −1.36987 | [9] |
| Piroxicam | −0.91377 | This work, [31] for acetone solvate |
| Compound | ν(NH2) Sulfonamide | ν(NH) Secondary Amine | ν(C=O) Carboxyl | ν(COO−) Carboxylate | ν(S=O) Sulfonamide |
|---|---|---|---|---|---|
| FUR | (as) 3400 (s) 3351 | 3285 | 1670 | - | (as) 1328 (s) 1139 |
| FUR–ETZ | (as) 3438 (s) 3291 | 3285 | 1670 | - | (as) 1339 (s) 1154 |
| FUR–PRX | (as) 3317 (s) 3230 | 3269 | 1670 | - | (as) 1339 (s) 1154 |
| Solid Form | Equilibrium Solubility at 25 °C (mg/mL) | Extent of Increase Relative to the Solubility of FUR. | Extent of Increase Relative to the Solubility of Coformer. |
|---|---|---|---|
| FUR | 2.40 | - | - |
| FUR–ETZ | 1.24 | ×0.52 | ×41 (ETZ) a |
| FUR–PRX | 1.47 | ×0.61 | ×84 (PRX) b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Acebedo-Martínez, F.J.; Alarcón-Payer, C.; Rodríguez-Domingo, L.; Domínguez-Martín, A.; Gómez-Morales, J.; Choquesillo-Lazarte, D. Furosemide/Non-Steroidal Anti-Inflammatory Drug–Drug Pharmaceutical Solids: Novel Opportunities in Drug Formulation. Crystals 2021, 11, 1339. https://doi.org/10.3390/cryst11111339
Acebedo-Martínez FJ, Alarcón-Payer C, Rodríguez-Domingo L, Domínguez-Martín A, Gómez-Morales J, Choquesillo-Lazarte D. Furosemide/Non-Steroidal Anti-Inflammatory Drug–Drug Pharmaceutical Solids: Novel Opportunities in Drug Formulation. Crystals. 2021; 11(11):1339. https://doi.org/10.3390/cryst11111339
Chicago/Turabian StyleAcebedo-Martínez, Francisco Javier, Carolina Alarcón-Payer, Lucía Rodríguez-Domingo, Alicia Domínguez-Martín, Jaime Gómez-Morales, and Duane Choquesillo-Lazarte. 2021. "Furosemide/Non-Steroidal Anti-Inflammatory Drug–Drug Pharmaceutical Solids: Novel Opportunities in Drug Formulation" Crystals 11, no. 11: 1339. https://doi.org/10.3390/cryst11111339
APA StyleAcebedo-Martínez, F. J., Alarcón-Payer, C., Rodríguez-Domingo, L., Domínguez-Martín, A., Gómez-Morales, J., & Choquesillo-Lazarte, D. (2021). Furosemide/Non-Steroidal Anti-Inflammatory Drug–Drug Pharmaceutical Solids: Novel Opportunities in Drug Formulation. Crystals, 11(11), 1339. https://doi.org/10.3390/cryst11111339