Effects of Specularite on the Preheating and Roasting Characteristics of Fluorine-Bearing Iron Concentrate Pellets
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Methods
2.2.1. Pelletization, Preheating and Roasting Process
2.2.2. Analysis and Characterization
3. Results and Discussion
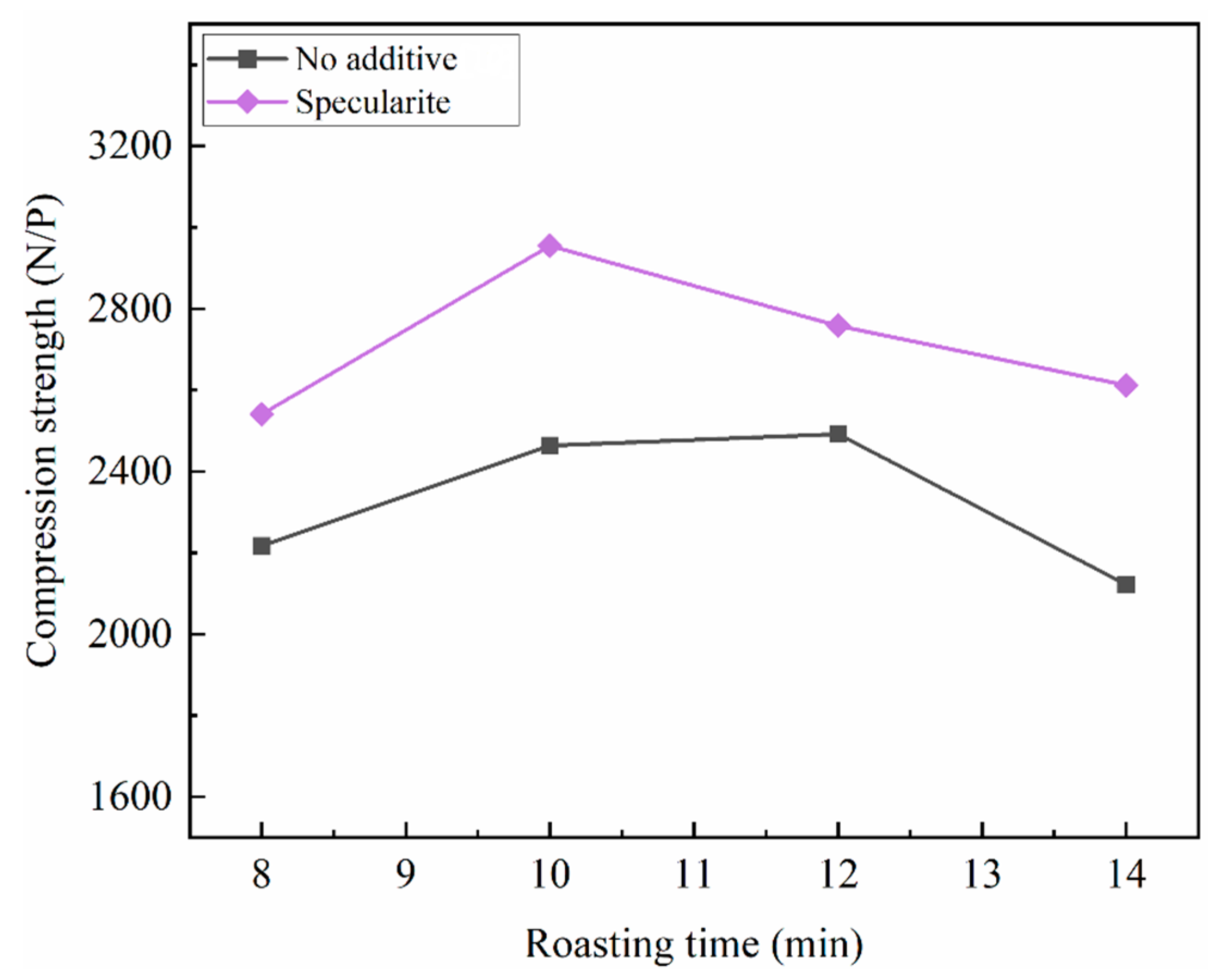
3.1. Preheating and Roasting Properties
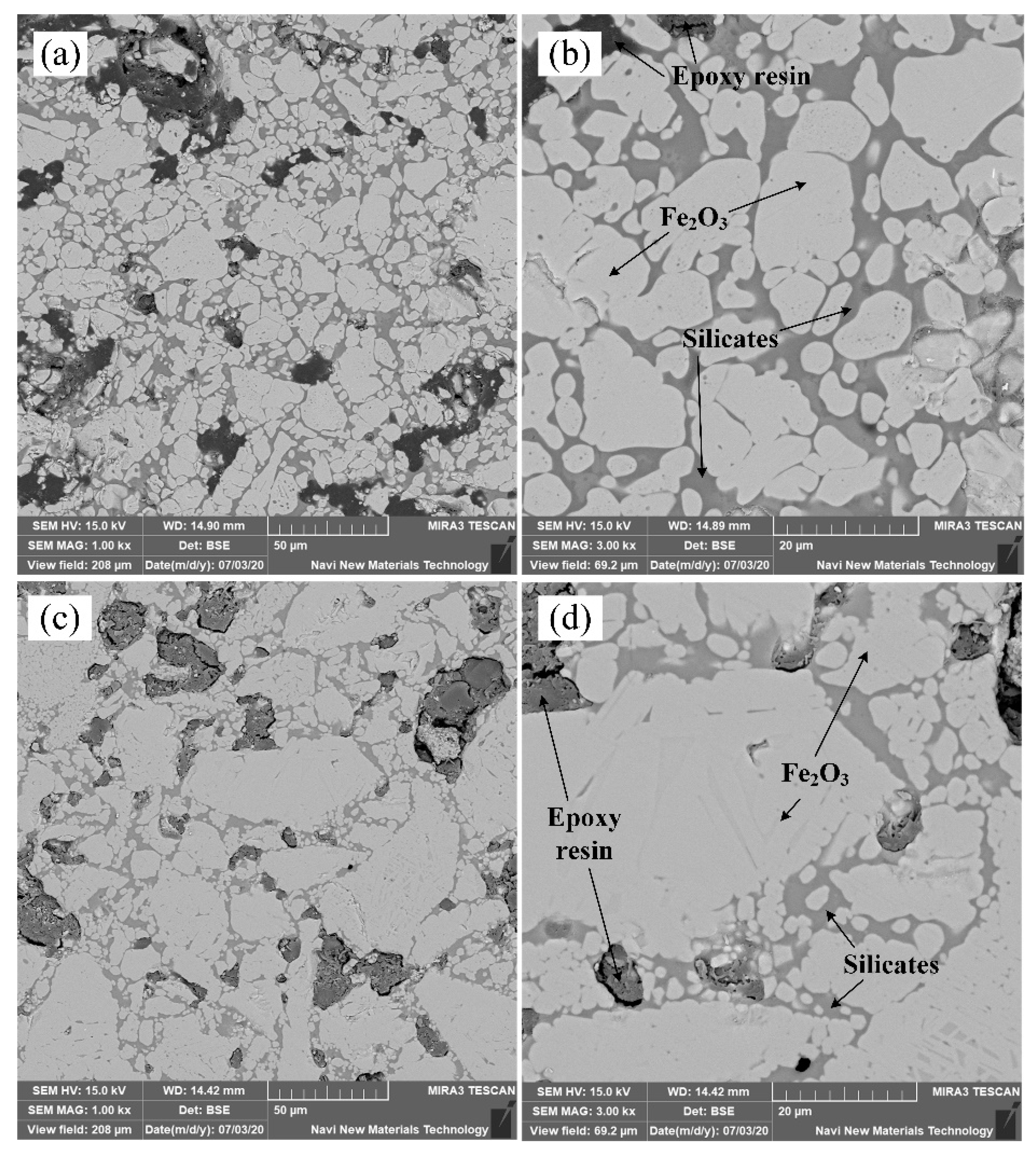
3.2. Microstructure of Roasted Pellets
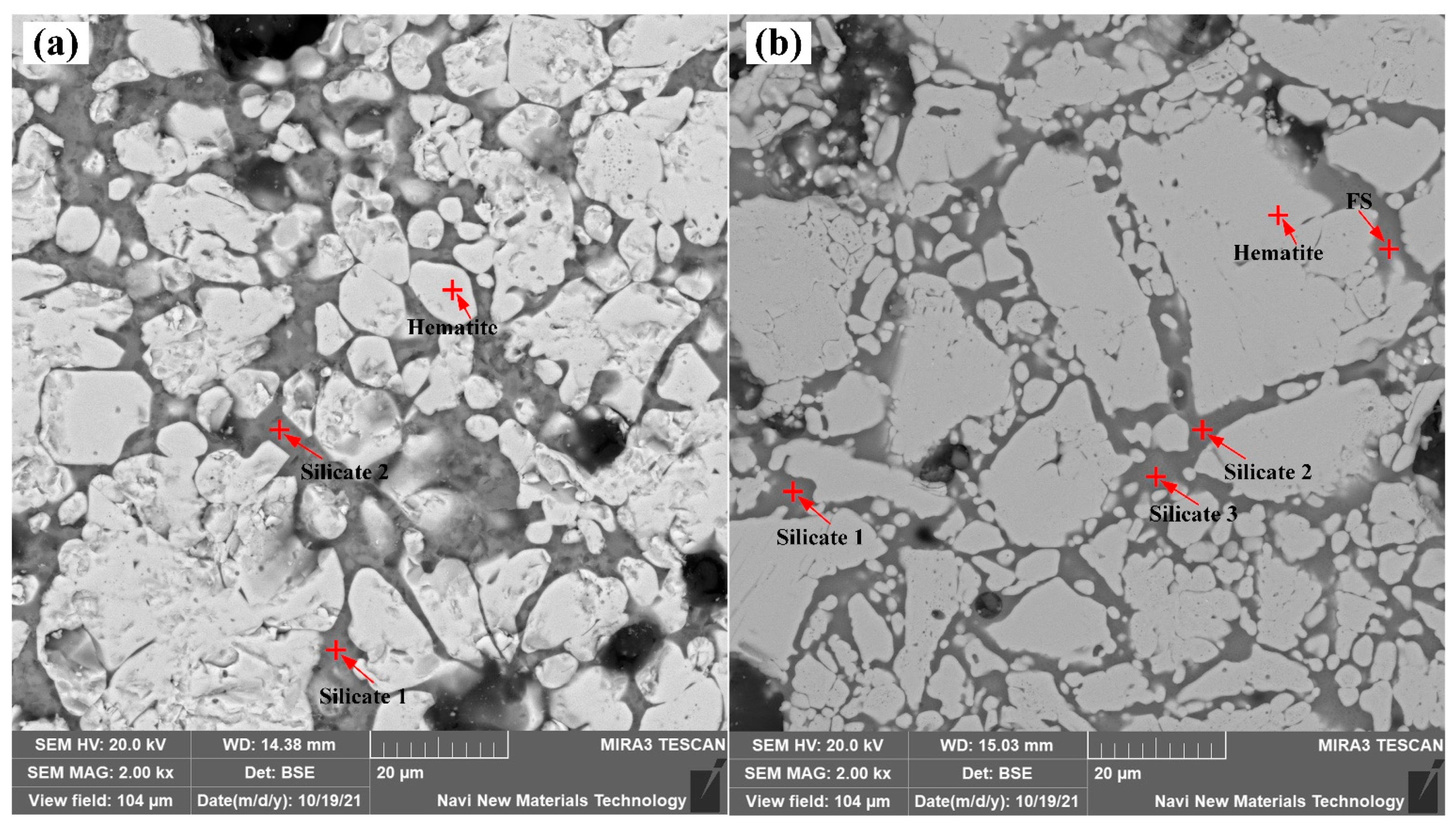
3.3. Element Distribution and Phase Composition Analysis
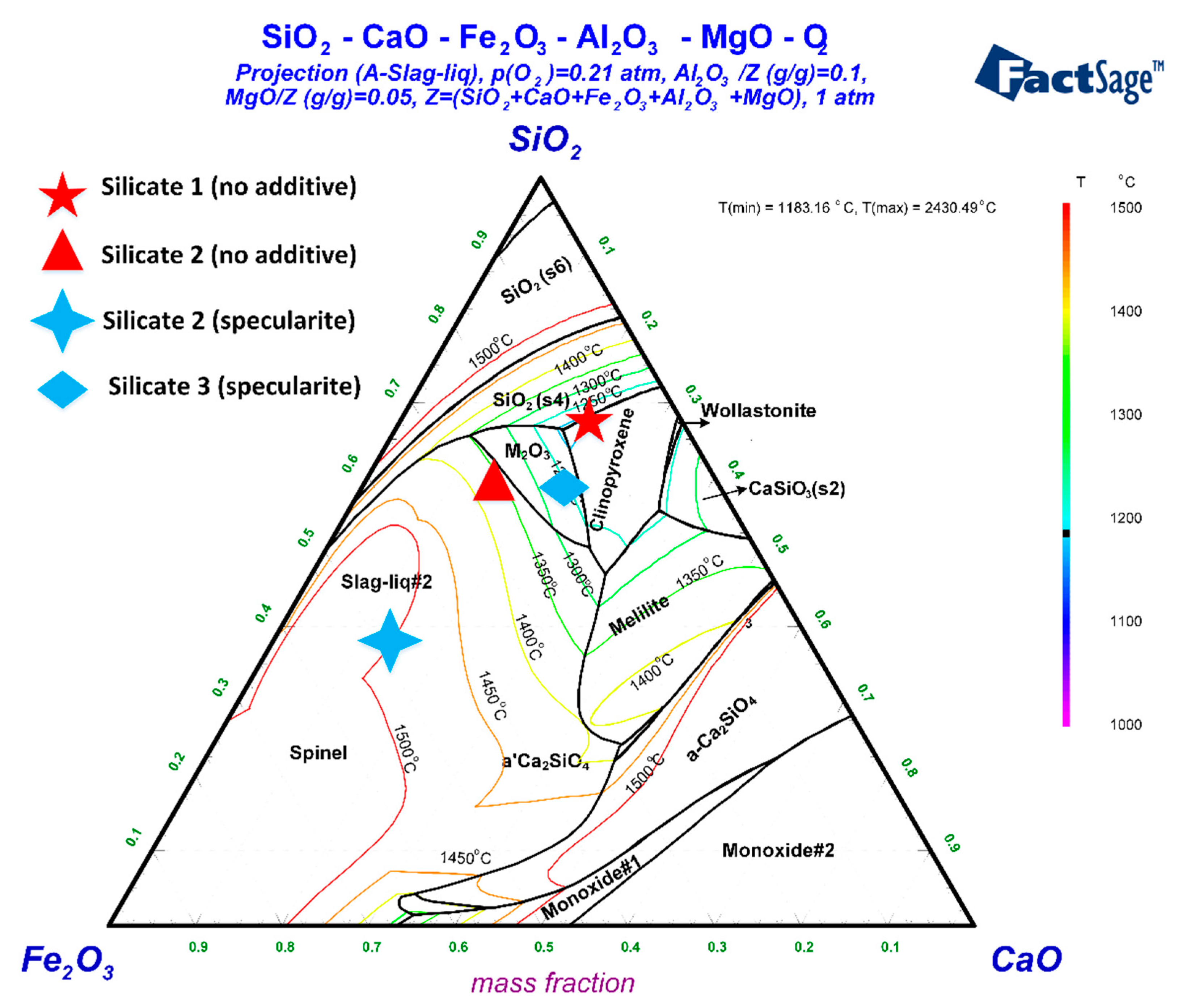
3.4. Phase Diagram Analysis
3.5. Technical and Economic Evaluation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fu, J.; Jiang, T.; Zhu, D. Sintering and Pelletizing; Central South University Technology Press: Changsha, China, 1996; p. 178. [Google Scholar]
- Kawatra, S.K.; Claremboux, V. Iron Ore Pelletization: Part I. Fundamentals. Miner. Process. Extr. Metall. Rev. 2021, 1–16. [Google Scholar] [CrossRef]
- Kawatra, S.K.; Claremboux, V. Iron Ore Pelletization: Part II. Inorganic Binders. Miner. Process. Extr. Metall. Rev. 2021, 1–20. [Google Scholar] [CrossRef]
- Stjernberg, J.; Isaksson, O.; Ion, J.C. The grate-kiln induration machine-history, advantages and drawbacks and outline for the future. J. S. Afr. Inst. Min. Metall. 2015, 115, 137–144. [Google Scholar] [CrossRef] [Green Version]
- Copeland, C.R.; Claremboux, V.; Kawatra, S.K. A Comparison of Pellet Quality from Straight-grate and Grate-kiln Furnaces. Miner. Process. Extr. Metall. Rev. 2019, 40, 218–223. [Google Scholar] [CrossRef]
- Zhao, Y.; Wu, W.; Chen, T.; Song, S. A case study on large-scale production for iron oxide pellets: Ezhou pelletization plant of the BAOWU. Miner. Process. Extr. Metall. Rev. 2018, 39, 211–215. [Google Scholar] [CrossRef]
- Sahu, S.N.; Baskey, P.K.; Barma, S.D.; Sahoo, S.; Meikap, B.C.; Biswal, S.K. Pelletization of synthesized magnetite concentrate obtained by magnetization roasting of Indian low-grade BHQ iron ore. Powder Technol. 2020, 374, 190–200. [Google Scholar] [CrossRef]
- Fan, J.J.; Qiu, G.Z.; Jiang, T.; Guo, Y.F.; Cai, M.X. Roasting Properties of Pellets With Iron Concentrate of Complex Mineral Composition. J. Iron Steel Res. Int. 2011, 18, 1–7. [Google Scholar] [CrossRef]
- Wang, S.; Guo, Y.F.; Zheng, F.Q.; Chen, F.; Yang, L.Z.; Jiang, T.; Qiu, G.Z. Behavior of vanadium during reduction and smelting of vanadium titanomagnetite metallized pellets. Trans. Nonferrous Met. Soc. China 2020, 30, 1687–1696. [Google Scholar] [CrossRef]
- de Moraes, S.L.; Ribeiro, T.R. Brazilian Iron Ore and Production of Pellets. Miner. Process. Extr. Metall. Rev. 2019, 40, 16–23. [Google Scholar] [CrossRef]
- Hu-lin, W. Technical Progress on Iron Making System of Baogang; Science & Technology of Baotou Steel (Group) Corporation: Baotou, China, 2004; pp. 10–16. [Google Scholar]
- Jiang, T.; Hu, Y.M.; Li, Q.A.; Li, G.H.; Yang, Y.B.; Zhang, Y.B.; Guo, Y.F. Mechanisms of composite agglomeration of fluoric iron concentrate. J. Cent. South Univ. Technol. 2010, 17, 1190–1195. [Google Scholar] [CrossRef]
- Pei, C.H. Fluoride Pollution and Its Present Treatment Taken by Ironmaking Plant; Science & Technology of Baotou Steel (Group) Corporation: Baotou, China, 2002; pp. 64–65. [Google Scholar]
- Wang, S.; Guo, Y.F.; Yang, L.; Fu, G.H.; Zheng, F.Q.; Chen, F.; Yang, L.Z.; Jiang, T. Investigation of solidification mechanism of fluorine-bearing magnetite concentrate pellets. Powder Technol. 2018, 332, 188–196. [Google Scholar] [CrossRef]
- Wu, H.L.; Xue, X.X.; Chen, G.; Cui, Y.Y. Research and practice on production of pellets containing fluorine and MgO. Iron Steel 2005, 40, 7–9. [Google Scholar]
- Xu, B.; Xiong, L.; Yang, Y.B.; Jiang, T.; Qian, L.I.; Wang, J.C. Study on fluorine-bearing iron concentrate pellets added with dolomite. Iron Steel 2008, 43, 9–15. [Google Scholar]
- Yang, L.; Wang, S.; Fu, G.; Guo, Y.; Jiang, T. The Preheating and Roasting Properties of Fluorine-Bearing Iron Concentrate Pellets and Main Influence Factors. In 7th International Symposium on High-Temperature Metallurgical Processing; Hwang, J.-Y., Jiang, T., Pistorius, P.C., Alvear, G.R.F., Yücel, O., Cai, Y., Zhao, B., Gregurek, D., Seshadri, V., Eds.; Springer: Berlin, Germany, 2016; pp. 369–375. [Google Scholar] [CrossRef]
- Lv, Z.Y. Experimental Research on Pellet Containing Fluorine and MgO; Science & Technology of Baotou Steel (Group) Corporation: Baotou, China, 2008; pp. 4–6. [Google Scholar]
- Wang, S.; Guo, Y.F.; Zheng, F.Q.; Chen, F.; Yang, L.Z. Improvement of roasting and metallurgical properties of fluorine-bearing iron concentrate pellets. Powder Technol. 2020, 376, 126–135. [Google Scholar] [CrossRef]
- China National Standardization Management Committee. GB/T 27692-2011, Acidity Iron Pellet for Blast Furnace; Standard Press of China: Beijing, China, 2011. [Google Scholar]
- Zhou, Y.L.; Zhang, Y.B.; Liu, B.B.; Li, G.H.; Jiang, T. Effect of modified humic acid binder on pelletization of specularite concentrates. J. Cent. South Univ. 2015, 22, 1247–1255. [Google Scholar] [CrossRef]
- Zhu, D.Q.; Guo, Z.Q.; Pan, J.; Zhang, F. Improving sintering performance of brazilian specularite bearing mixture by separated granulation sintering process. J. Cent. South Univ. (Sci. Technol.) 2014, 45, 3719–3726. [Google Scholar]
- He, X.; Sun, G.; Jing, W. Practice of adding specularite in pellet production. Sinter. Pelletiz. 2014, 39, 35–40. [Google Scholar]
- Jiang, T.; Zhang, Y.B.; Huang, Z.C.; Li, G.H.; Fan, X.H. Preheating and roasting characteristics of hematite–magnetite (H–M) concentrate pellets. Ironmak. Steelmak. 2013, 35, 21–26. [Google Scholar] [CrossRef]
- Zheng, G. Chemical analysis method standards and proficiency testing of laboratory for iron ore. Metall. Anal. 2015, 35, 37–44. [Google Scholar]
- Pouchou, J.L.; Pichoir, F.; Boivin, D. XPP procedure applied to quantitative EDS x-ray analysis in the SEM. In Proceedings of the 25th Annual Conference of Microbeam Analysis Society, Seattle, WA, USA, 12–18 August 1990; pp. 120–126. [Google Scholar]
- Bale, C.W.; Bélisle, E.; Chartrand, P.; Decterov, S.A.; Eriksson, G.; Gheribi, A.E.; Hack, K.; Jung, I.H.; Kang, Y.B.; Melançon, J. FactSage thermochemical software and databases, 2010–2016. Calphad-Comput. Coupling Phase Diagr. Thermochem. 2016, 54, 35–53. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.M.; Li, J. Crystal rule of Fe2O3 in oxidized pellet. J. Cent. South Univ. (Sci. Technol.) 2007, 38, 70–73. [Google Scholar]
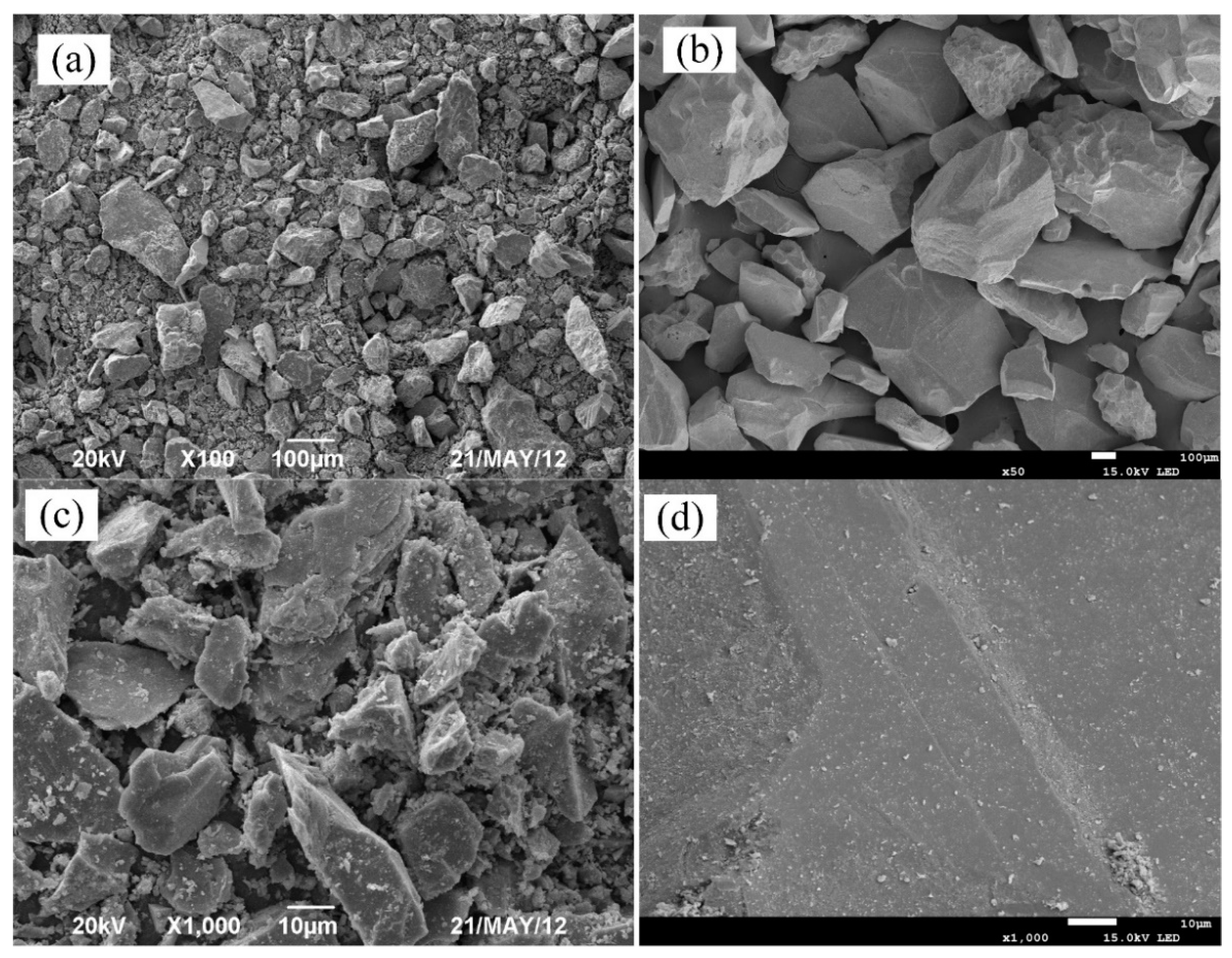
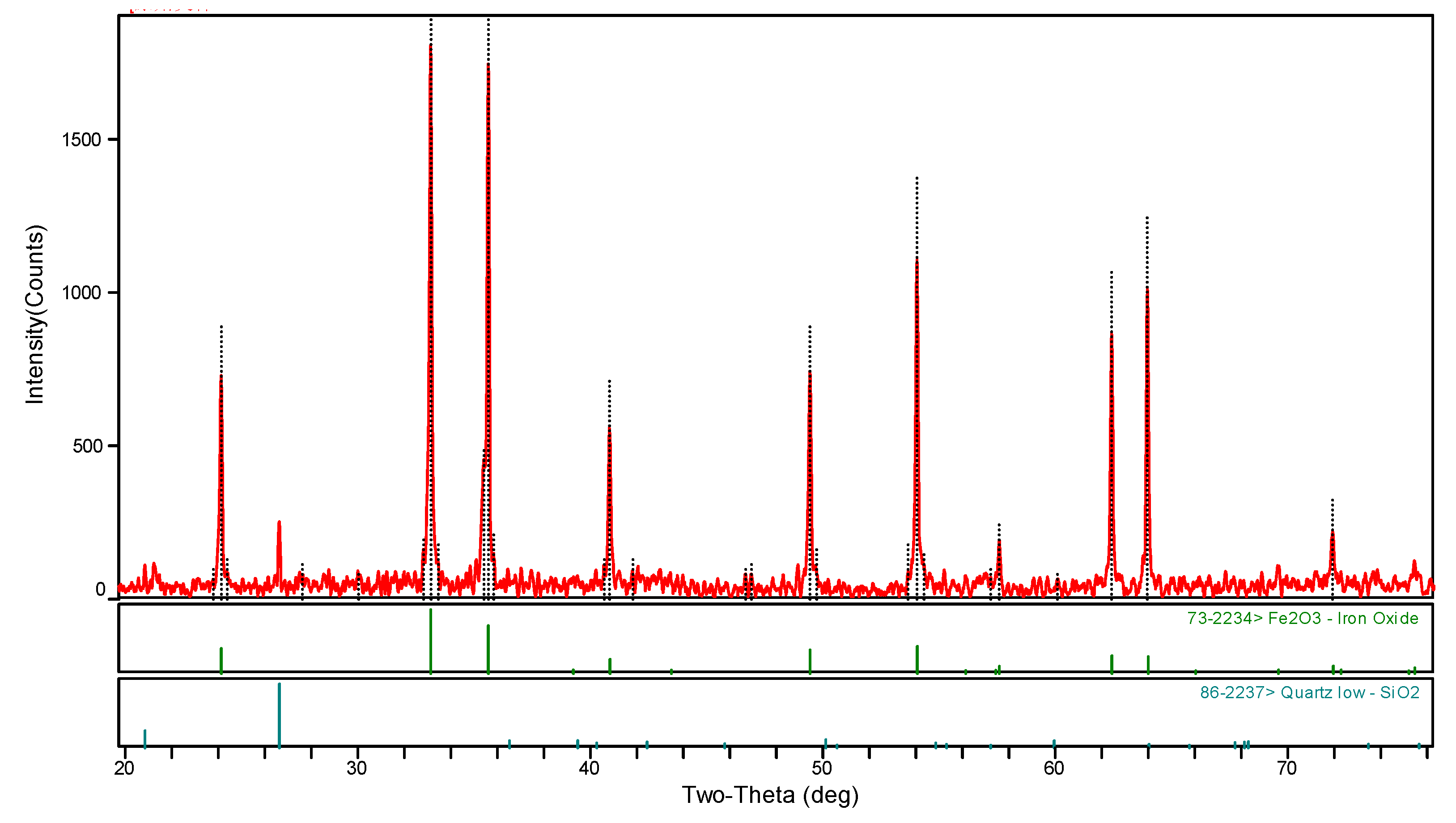
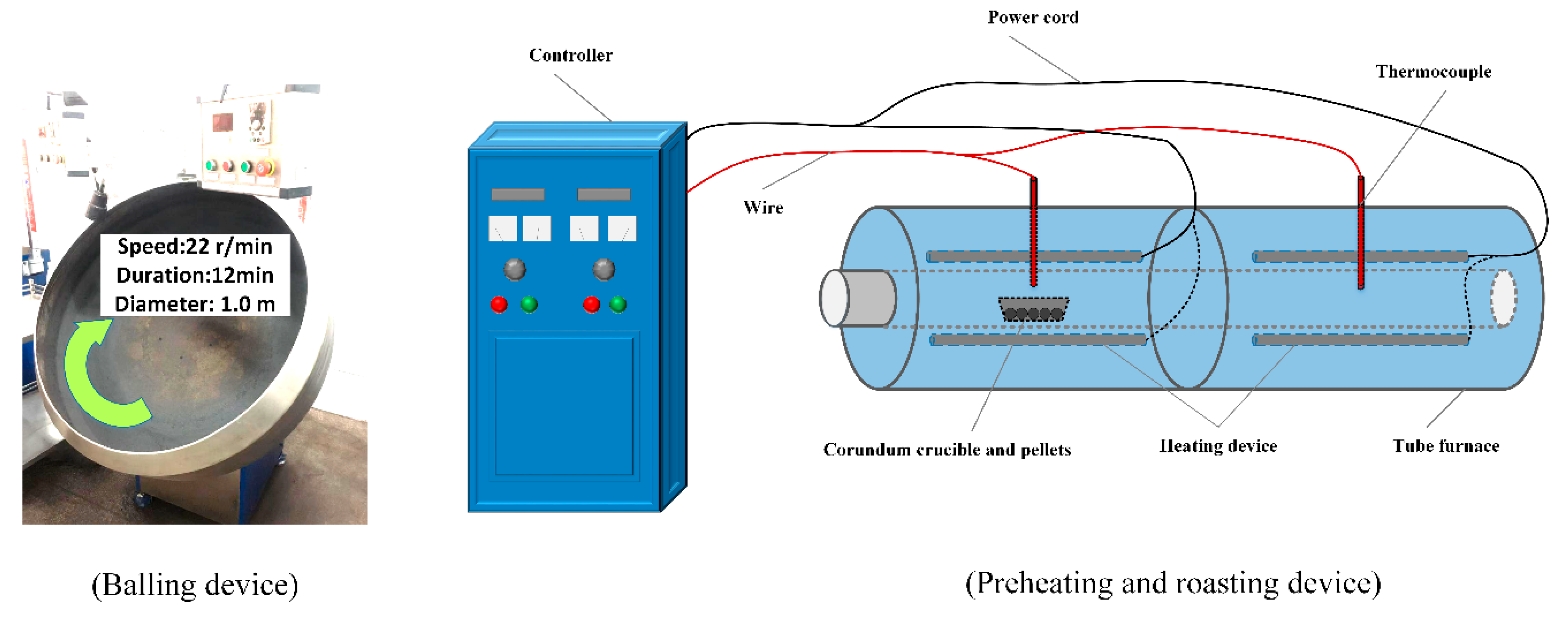



| Kind | TFe | FeO | SiO2 | Al2O3 | CaO | MgO | F | K2O | Na2O | S |
|---|---|---|---|---|---|---|---|---|---|---|
| FIC | 61.9 | 26.4 | 4.9 | 1.7 | 3.0 | 0.5 | 1.6 | 0.3 | 0.2 | 0.1 |
| Bentonite | 2.4 | 0.2 | 54.6 | 9.9 | 5.2 | 2.7 | - | 0.9 | 1.7 | 0.1 |
| Specularite | 65.9 | - | 2.7 | 0.2 | 0.1 | - | - | - | - | - |
| <0.074 mm | 0.074–0.125 mm | 0.125–0.25 mm | 0.25–0.5 mm | 0.5–1 mm | 1–3 mm |
|---|---|---|---|---|---|
| 2.6 | 48.4 | 5.4 | 30.3 | 8.7 | 2.1 |
| Item | Chinese Standard | Method |
|---|---|---|
| Total Fe | GB/T6730.5-2007 | Titanium(III) chloride reduction |
| FeO | GB/T6730.8-1986 | Potassium dichromate volumetris method |
| Si | GB/T6730.10-1986 | Gravimetric method |
| Al | GB/T6730.11-2007 | EDTA titrimetric method |
| S | GB/T6730.61-2005 | High-frequency combustion with infrared absorption method |
| F | GB/T6730.28-2006 | Ion selective electrode method |
| K and Na | GB/T6730.49-1986 | Flame atomic absorption spectrophotometric method |
| Mg and Ca | GB/T6730.13-2007 | EGTA-CyDTA titrimetric method |
| Specularite/% | Preheating Temperature/°C | Preheating Time/min | Roasting Temperature/°C | Roasting Time/min | Compression Strength/(N/P) |
|---|---|---|---|---|---|
| 0 | 920 | 16 | 1080 | 10 | 2463 |
| 5% | 900 | 14 | 1080 | 10 | 2470 |
| 10% | 900 | 14 | 1080 | 10 | 2955 |
| 15% | 900 | 14 | 1080 | 10 | 2471 |
| Roasted Pellets | Phase | Chemical Composition/wt.% | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Fe2O3 | CaO | SiO2 | Al2O3 | MgO | CaF2 | K2O | Na2O | ||
| No additive | Hematite | 98.2 ± 1.3 | 0.2 ± 0 | 0.2 ± 0.1 | 1.1 ± 0.3 | 0.1 ± 0 | 0 | 0 | 0 |
| Silicate 1 | 6.9 ± 1.5 | 12.5 ± 0.9 | 41.9 ± 6.9 | 9.5 ± 1.4 | 5.0 ± 1.1 | 21.1 ± 5.9 | 1.7 ± 0.2 | 1.3 ± 0.1 | |
| Silicate 2 | 19.2 ± 5.5 | 9.2 ± 1.8 | 40.6 ± 2.6 | 9.2 ± 0.6 | 5.1 ± 0.5 | 11.7 ± 1.5 | 1.7 ± 0.4 | 1.3 ± 0.1 | |
| Adding specularite | Hematite | 97.0 ± 1.1 | 0.4 ± 0.2 | 0.6 ± 0.5 | 0.7 ± 0.2 | 0.1 ± 0.1 | 0 | 0 | 0.0 ± 0.1 |
| FS | 84.5 ± 4.3 | 2.7 ± 0.7 | 7.1 ± 2.1 | 3.2 ± 1.0 | 0.8 ± 0.2 | 0 | 0.5 ± 0.2 | 0.4 ± 0.1 | |
| Silicate 1 | 64.9 ± 4.6 | 5.2 ± 0.4 | 17.4 ± 3.9 | 6.4 ± 1.6 | 1.6 ± 0.3 | 1.8 ± 1.8 | 1.4 ± 0.3 | 0.8 ± 0.2 | |
| Silicate 2 | 38.9 ± 7.8 | 10.9 ± 1.5 | 29.9 ± 3.9 | 8.0 ± 1.2 | 3.3 ± 0.5 | 5.6 ± 2.5 | 1.9 ± 0.5 | 0.9 ± 0.2 | |
| Silicate 3 | 11.1 ± 3.0 | 14.1 ± 2.4 | 37.2 ± 1.8 | 10.3 ± 0.0 | 3.6 ± 0.2 | 14.4 ± 0.6 | 2.7 ± 0.2 | 1.3 ± 0.2 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Jiang, Y.; Guo, Y.; Chen, F.; Yang, L. Effects of Specularite on the Preheating and Roasting Characteristics of Fluorine-Bearing Iron Concentrate Pellets. Crystals 2021, 11, 1319. https://doi.org/10.3390/cryst11111319
Wang S, Jiang Y, Guo Y, Chen F, Yang L. Effects of Specularite on the Preheating and Roasting Characteristics of Fluorine-Bearing Iron Concentrate Pellets. Crystals. 2021; 11(11):1319. https://doi.org/10.3390/cryst11111319
Chicago/Turabian StyleWang, Shuai, Ying Jiang, Yufeng Guo, Feng Chen, and Lingzhi Yang. 2021. "Effects of Specularite on the Preheating and Roasting Characteristics of Fluorine-Bearing Iron Concentrate Pellets" Crystals 11, no. 11: 1319. https://doi.org/10.3390/cryst11111319
APA StyleWang, S., Jiang, Y., Guo, Y., Chen, F., & Yang, L. (2021). Effects of Specularite on the Preheating and Roasting Characteristics of Fluorine-Bearing Iron Concentrate Pellets. Crystals, 11(11), 1319. https://doi.org/10.3390/cryst11111319










