Carbonation of High-Ca Fly Ashes under Flue Gas Conditions: Implications for Their Valorization in the Construction Industry
Abstract
:1. Introduction
2. Test materials and Methods
2.1. Test Materials
2.2. Methods
3. Results and Discussion
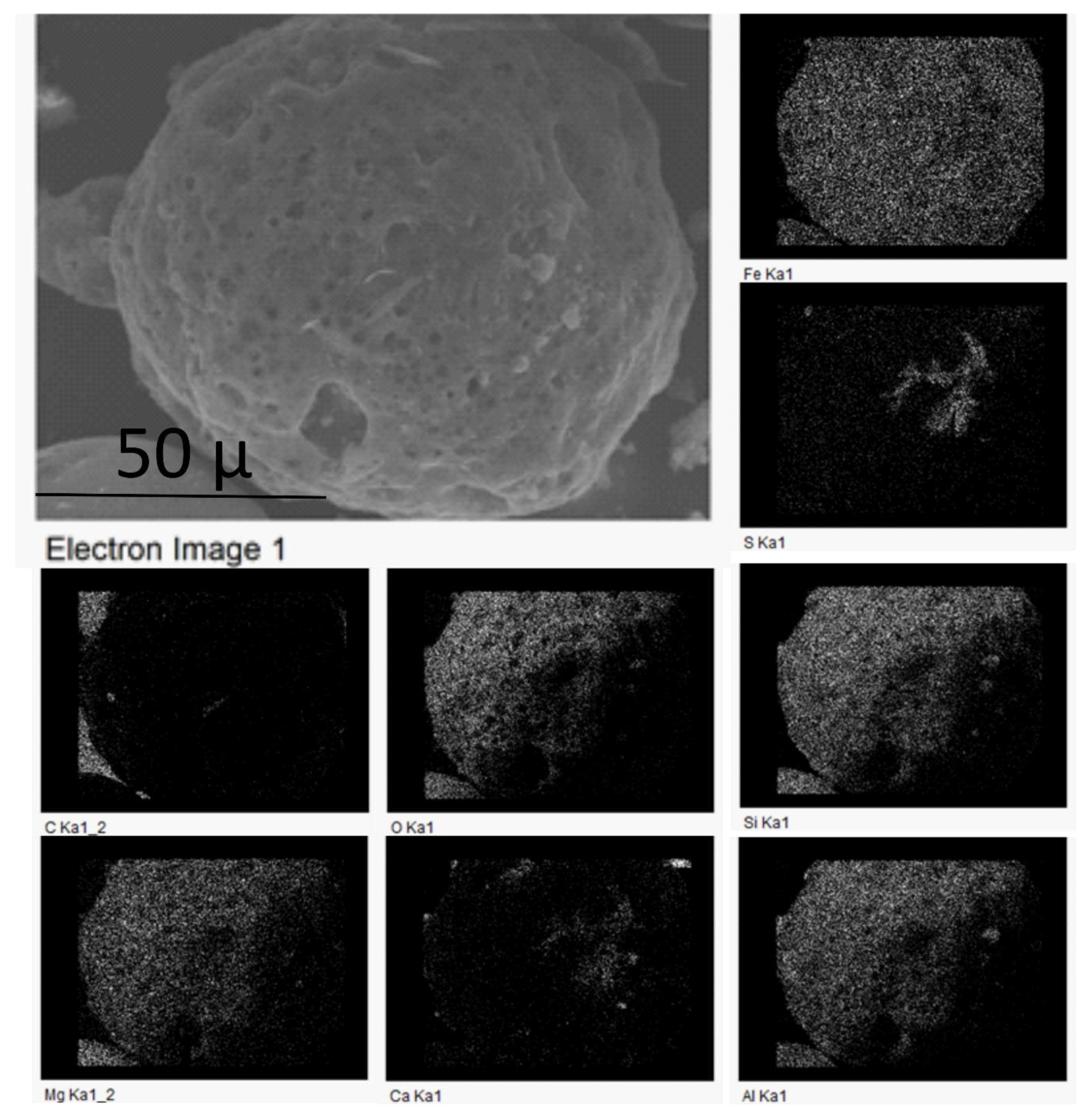
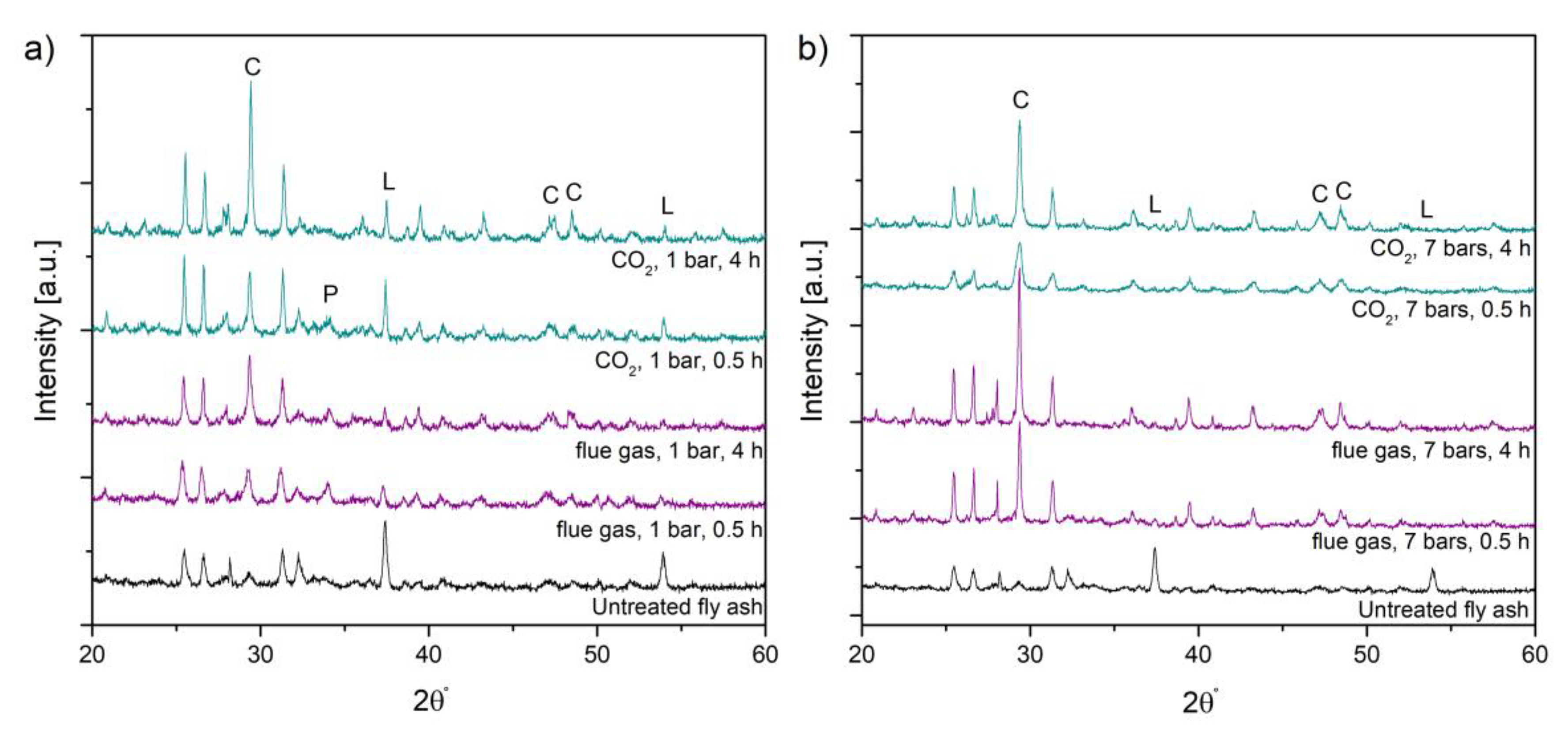
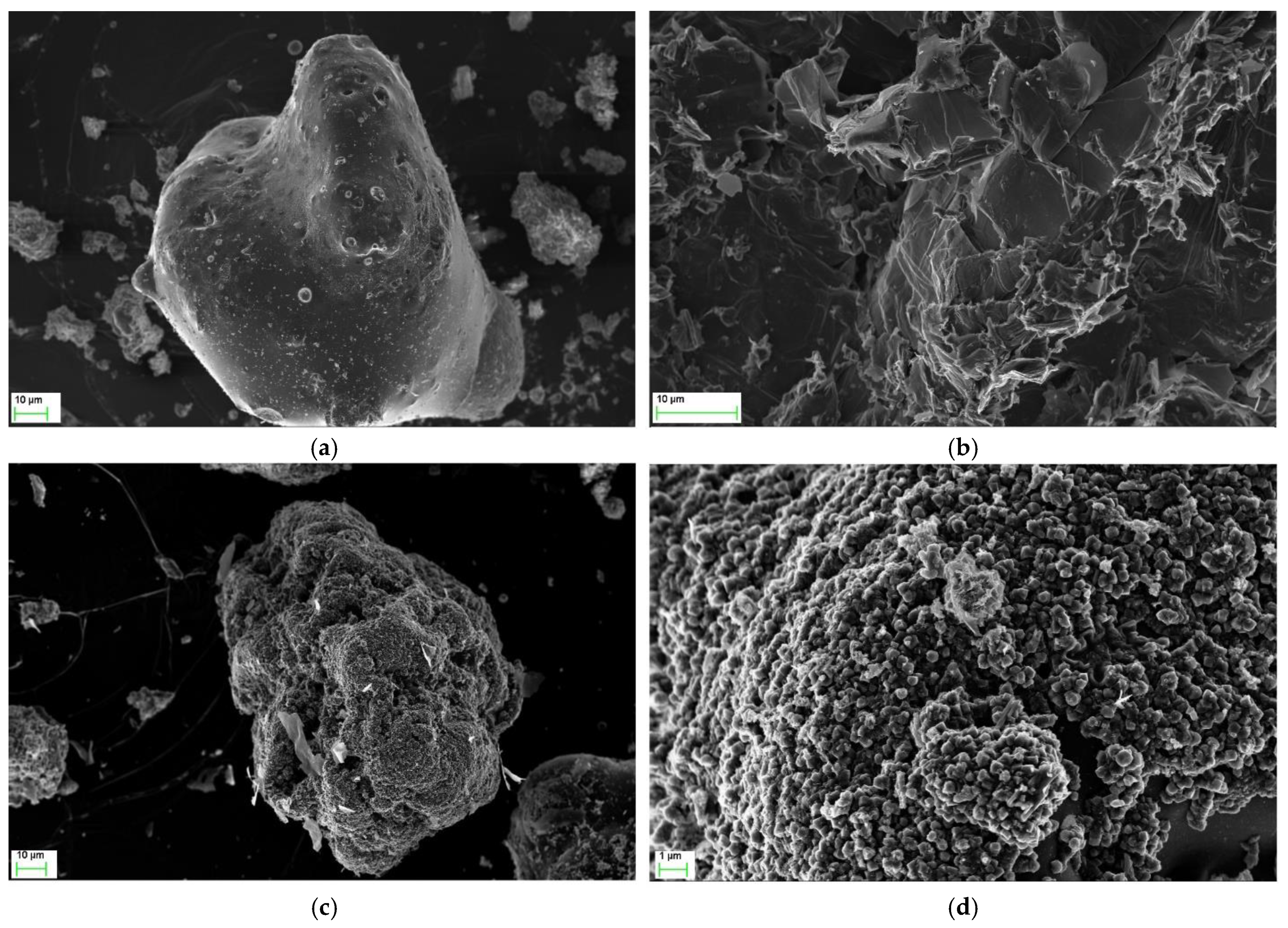
3.1. Analysis of the Material before and after Experiment
3.2. Carbonation Efficiency
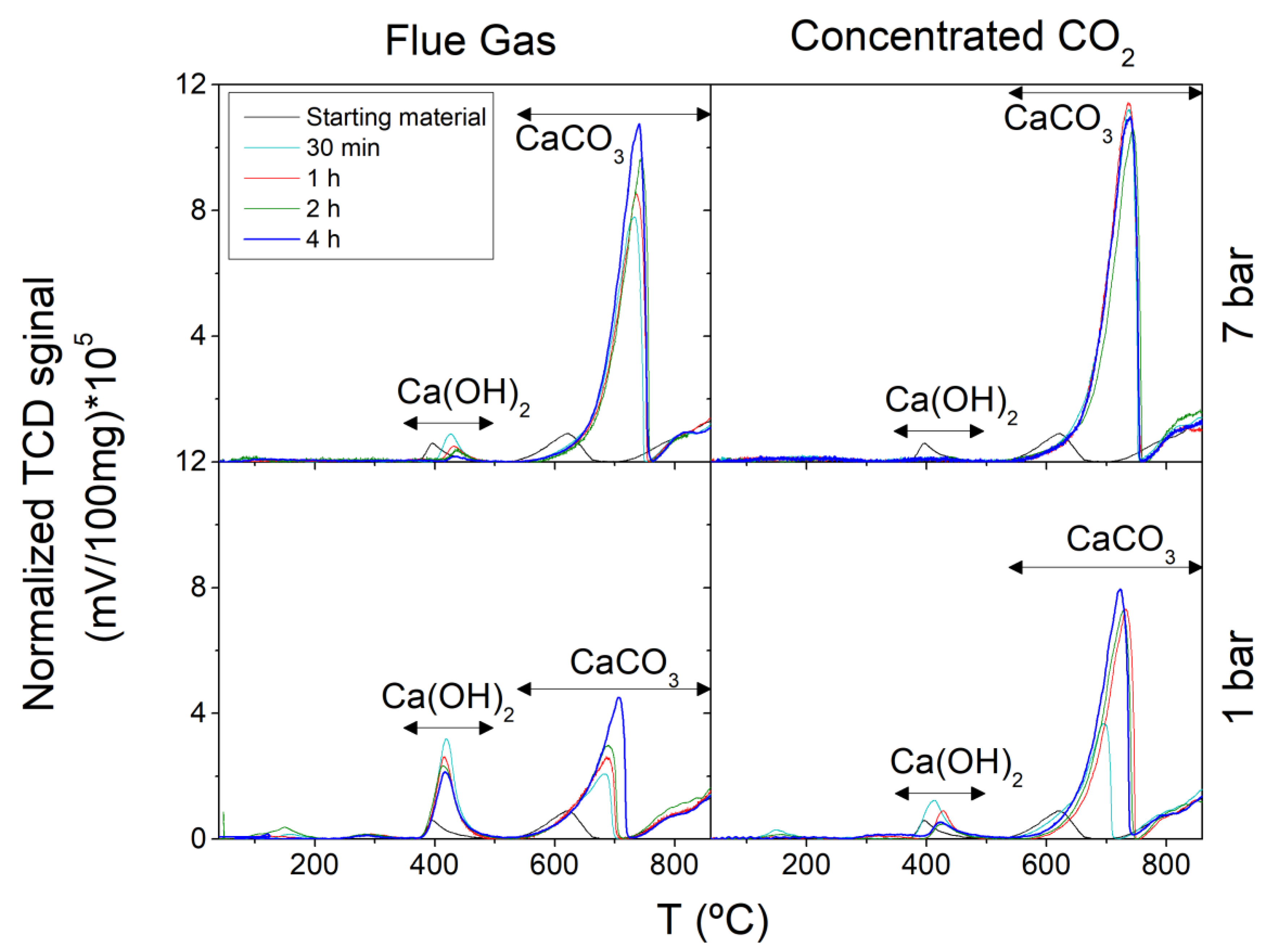
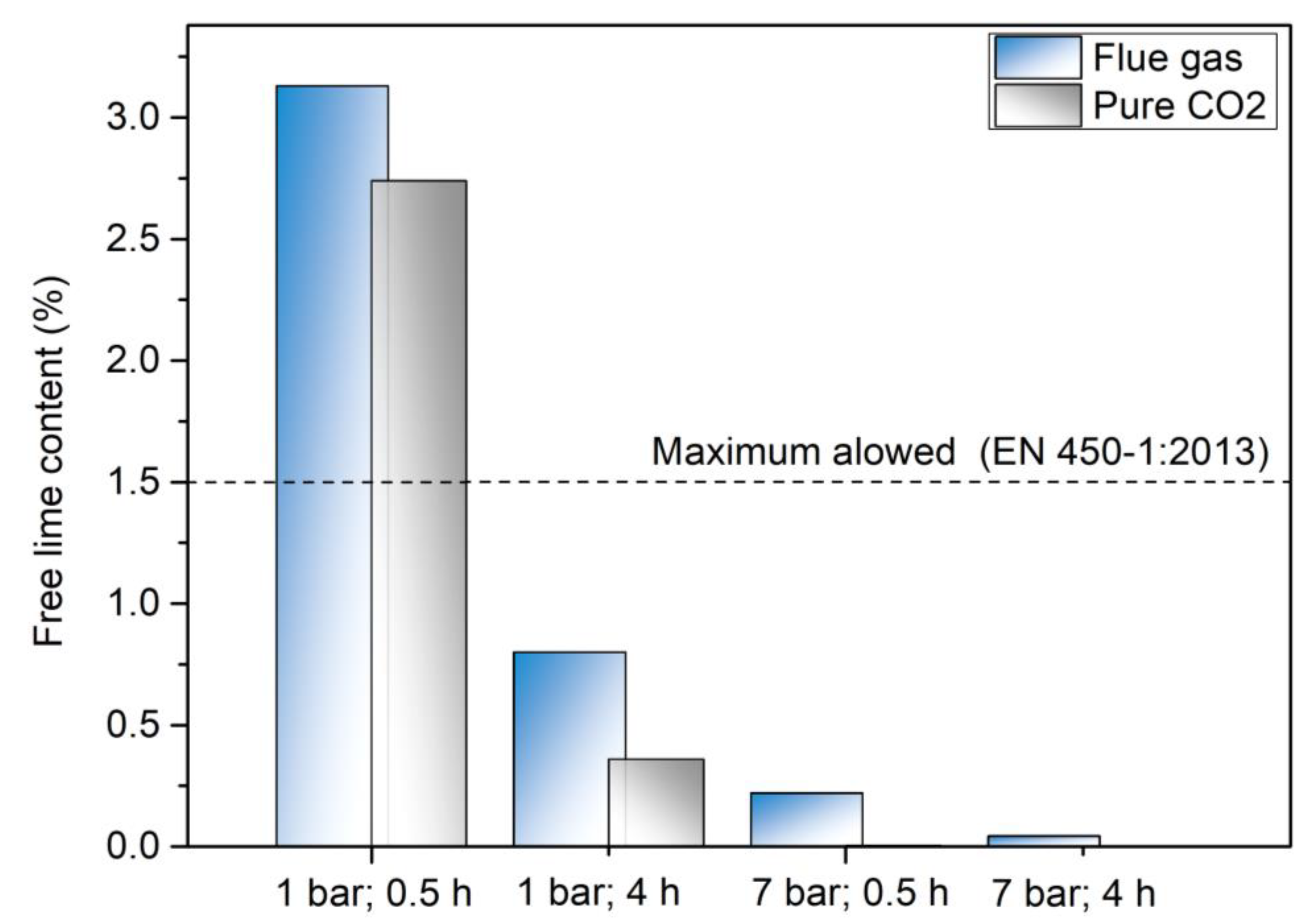
3.3. Free Lime Determination
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- ASTM C618-05, Standard Specification for Coal Fly Ash and Raw or Calcined Natural Pozzolan for Use in Concrete; ASTM International: West Conshohocken, PA, USA, 2005.
- Papayianni, I.; Tsimas, S.; Moutsatsou, A. Standardization aspects concerning high calcium fly ashes. In Proceedings of the 2009 World of Coal Ash (WOCA) Conference, Lexington, KY, USA, 4–7 May 2009. [Google Scholar]
- Fly Ash for Concrete—Part 1: Definition, Specifications and Conformity Criteria 2012; EN 450-1:2013 UNE-EN 450-1:2013; Available online: https://infostore.saiglobal.com/preview/98701122690.pdf?sku=871436_SAIG_NSAI_NSAI_20720611461445 (accessed on 27 October 2021).
- Heidrich, C.; Feuerborn, H.; Weir, A. Coal Combustion Products: A Global Perspective. In Proceedings of the 2013 World of Coal Ash (WOCA) Conference, Lexington, KY, USA, 22–25 April 2013. [Google Scholar]
- Feuerborn, H. Calcareous Ash in Europe—A reflection on technical and legal issues. In Proceedings of the 2nd Hellenic Conference on Utilisation of Industrial By-Products in Construction, Aiani Kozani, Greece, 1–3 June 2009. [Google Scholar]
- Ahmaruzzaman, M. A review on the utilization of fly ash. Prog. Energy Combust. Sci. 2010, 36, 327–363. [Google Scholar] [CrossRef]
- Part 1: Composition, Specifications and Conformity Criteria for Common Cement; EN 197-1:2011 Cement; 2011; Available online: https://www.en-standard.eu/bs-en-197-1-2011-cement-composition-specifications-and-conformity-criteria-for-common-cements/ (accessed on 27 October 2021).
- Feuerborn, H.J.; Müller, B.; Walter, E. Use of Calcareous Fly Ash in Germany. Ash Handl. 2012, 3, 17–29. [Google Scholar]
- Uliasz-Bochenczyk, A. Waste used for CO2 bonding via mineral carbonation. Gospod. Surowcami Miner. 2007, 23, 121–128. [Google Scholar]
- Argiz, C.; Menéndez, E.; Moragues, A.; Sanjuán, M.A. A Fly ash characteristics of Spanish coal-fired power plants. Afinidad 2015, 72, 269–277. [Google Scholar]
- International Energy Agency. Market Report Series Gas 2018; International Energy Agency: Paris, France, 2018. [Google Scholar]
- Papayianni, J. Use of a high-calcium fly ash in blended type cement production. Cem. Concr. Compos. 1993, 15, 231–235. [Google Scholar] [CrossRef]
- Dong, Y.; Jow, J.; Su, J.; Lai, S. Fly Ash Separation Technology and Its Potential Applications. Available online: http://www.mcilvainecompany.com/Decision_Tree/subscriber/Tree/DescriptionTextLinks/Chinese%20flyash%20separation.pdf (accessed on 27 October 2021).
- Naik, T.R.; Singh, S.S. Use of High-Calcium Fly Ash in Cement-Based Construction Materials. In Proceedings of the Fifth CANMET/ACI International Conference on Fly Ash, Silica Fume, Slag and Natural Pozzolans in Concrete; 1994. Available online: https://www.concrete.org/store/productdetail.aspx?ItemID=SP153&Format=DOWNLOAD&Language=English&Units=US_Units (accessed on 27 October 2021).
- Matter, J.M.; Kelemen, P. Permanent storage of carbon dioxide in geological reservoirs by mineral carbonation. Nat. Geosci. 2009, 2, 837–841. [Google Scholar] [CrossRef]
- Lackner, K.S.; Wendt, C.H.; Butt, D.P.; Joyce, E.L.; Sharp, D.H. Carbon dioxide disposal in carbonate minerals. Energy 1995, 20, 1153–1170. [Google Scholar] [CrossRef]
- Li, J.; Hitch, M. Mechanical activation of magnesium silicates for mineral carbonation, a review. Miner. Eng. 2018, 128, 69–83. [Google Scholar] [CrossRef]
- Bobicki, E.R.; Liu, Q.; Xu, Z.; Zeng, H. Carbon capture and storage using alkaline industrial wastes. Prog. Energy Combust. Sci. 2012, 38, 302–320. [Google Scholar] [CrossRef]
- Rahmani, O. CO2 sequestration by indirect mineral carbonation of industrial waste red gypsum. J. CO2 Util. 2018, 27, 374–380. [Google Scholar] [CrossRef]
- Benhelal, E.; Rashid, M.; Holt, C.; Rayson, M.; Brent, G.; Hook, J.; Stockenhuber, M.; Kennedy, E. The utilisation of feed and byproducts of mineral carbonation processes as pozzolanic cement replacements. J. Clean. Prod. 2018, 186, 499–513. [Google Scholar] [CrossRef]
- Fernandezbertos, M.; Simons, S.; Hills, C.; Carey, P. A review of accelerated carbonation technology in the treatment of cement-based materials and sequestration of CO2. J. Hazard. Mater. 2004, 112, 193–205. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, B.; Reddy, K.J.; Argyle, M.D. Field Application of Accelerated Mineral Carbonation. Minerals 2014, 4, 191–207. [Google Scholar] [CrossRef] [Green Version]
- Ebrahimi, A.; Saffari, M.; Milani, D.; Montoya, A.; Valix, M.; Abbas, A. Sustainable transformation of fly ash industrial waste into a construction cement blend via CO2 carbonation. J. Clean. Prod. 2017, 156, 660–669. [Google Scholar] [CrossRef]
- Pei, S.-L.; Pan, S.-Y.; Gao, X.; Fang, Y.-K.; Chiang, P.-C. Efficacy of carbonated petroleum coke fly ash as supplementary cementitious materials in cement mortars. J. Clean. Prod. 2018, 180, 689–697. [Google Scholar] [CrossRef]
- Ji, L.; Yu, H.; Wang, X.; Grigore, M.; French, D.; Gözükara, Y.M.; Yu, J.; Zeng, M. CO2 sequestration by direct mineralisation using fly ash from Chinese Shenfu coal. Fuel Process. Technol. 2017, 156, 429–437. [Google Scholar] [CrossRef]
- Nyambura, M.G.; Mugera, G.W.; Felicia, P.L.; Gathura, N.P. Carbonation of brine impacted fractionated coal fly ash: Implications for CO2 sequestration. J. Environ. Manag. 2011, 92, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Wang, B.; Falzone, G.; La Plante, E.C.; Okoronkwo, M.U.; She, Z.; Oey, T.; Balonis, M.; Neithalath, N.; Pilon, L.; et al. Clinkering-free cementation by fly ash carbonation. J. CO2 Util. 2018, 23, 117–127. [Google Scholar] [CrossRef]
- Ćwik, A.; Casanova, I.; Rausis, K.; Koukouzas, N.; Zarębska, K. Carbonation of high-calcium fly ashes and its potential for carbon dioxide removal in coal fired power plants. J. Clean. Prod. 2018, 202, 1026–1034. [Google Scholar] [CrossRef]
- Ćwik, A.; Casanova, I.; Rausis, K.; Zarębska, K. Utilization of high-calcium fly ashes through mineral carbonation: The cases for Greece, Poland and Spain. J. CO2 Util. 2019, 32, 155–162. [Google Scholar] [CrossRef]
- Aouini, I.; LeDoux, A.; Estel, L.; Mary, S. Pilot Plant Studies for CO2 Capture from Waste Incinerator Flue Gas Using MEA Based Solvent. Oil Gas Sci. Technol. Rev. IFP 2014, 69, 1091–1104. [Google Scholar] [CrossRef] [Green Version]
- Jecht, U. Flue Gas Analysis in Industry—Practical guide for Emission and Process Measurements; Testo: Titisee-Neustadt, Germany, 2004; pp. 1–145. [Google Scholar]
- Rausis, K.; Ćwik, A.; Casanova, I. Phase evolution during accelerated CO2 mineralization of brucite under concentrated CO2 and simulated flue gas conditions. J. CO2 Util. 2019, 37, 122–133. [Google Scholar] [CrossRef]
- Revathy, T.D.R.; Ramachandran, A.; Palanivelu, K. Carbon capture and storage using coal fly ash with flue gas. Clean Technol. Environ. Policy 2021, 1–19. [Google Scholar] [CrossRef]
- Myers, C.A.; Nakagaki, T.; Akutsu, K. Quantification of the CO2 mineralization potential of ironmaking and steelmaking slags under direct gas-solid reactions in flue gas. Int. J. Greenh. Gas Control 2019, 87, 100–111. [Google Scholar] [CrossRef]
- Mouedhen, I.; Kemache, N.; Pasquier, L.-C.; Cecchi, E.; Blais, J.-F.; Mercier, G. Effect of pCO2 on direct flue gas mineral carbonation at pilot scale. J. Environ. Manag. 2017, 198, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Xie, Y.; Zhu, Y.; Lu, X.; Ji, X. Energy Consumption Analysis for CO2 Separation from Gas Mixtures with Liquid Absorbents. Energy Procedia 2014, 61, 2695–2698. [Google Scholar] [CrossRef] [Green Version]
- White, C.M.; Strazisar, B.R.; Granite, E.J.; Hoffman, J.S.; Pennline, H.W. Separation and Capture of CO2 from Large Stationary Sources and Sequestration in Geological Formations—Coalbeds and Deep Saline Aquifers. J. Air Waste Manag. Assoc. 2003, 53, 645–715. [Google Scholar] [CrossRef]
- Galan, I.; Glasser, F.; Baza, D.; Andrade, C. Assessment of the protective effect of carbonation on portlandite crystals. Cem. Concr. Res. 2015, 74, 68–77. [Google Scholar] [CrossRef]
- Kontoyannis, C.G.; Vagenas, N.V. Calcium carbonate phase analysis using XRD and FT-Raman spectroscopy. Analyst 2000, 125, 251–255. [Google Scholar] [CrossRef]
- Mozgawa, W.; Król, M.; Dyczek, J.; Deja, J. Investigation of the coal fly ashes using IR spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 132, 889–894. [Google Scholar] [CrossRef] [PubMed]
- Khouzani, M.F.; Chevrier, D.M.; Güttlein, P.; Hauser, K.; Zhang, P.; Hedin, N.; Gebauer, D. Disordered amorphous calcium carbonate from direct precipitation. CrystEngComm 2015, 17, 4842–4849. [Google Scholar] [CrossRef] [Green Version]
- Vassilev, S.V.; Menendez, R.; Alvarez, D.; Diaz-Somoano, M.; Martinez-Tarazona, M.R. Phase-mineral and chemical composition of coal fly ashes as a basis for their multicomponent utilization. 1. Characterization of feed coals and fly ashes. Fuel 2003, 82, 1793–1811. [Google Scholar] [CrossRef]
- Żyrkowski, M.; Neto, R.C.; Santos, L.F.; Witkowski, K. Characterization of fly-ash cenospheres from coal-fired power plant unit. Fuel 2016, 174, 49–53. [Google Scholar] [CrossRef]
- Rochelle, C.A.; Czernichowski-Lauriol, I.; Milodowski, A. The impact of chemical reactions on CO2 storage in geological formations: A brief review. Geol. Soc. Lond. Spec. Publ. 2004, 233, 87–106. [Google Scholar] [CrossRef]
- Kremer, B.; Kaźmierczak, J.; Stal, L.J. Calcium carbonate precipitation in cyanobacterial mats from sandy tidal flats of the North Sea. Geobiology 2007, 6, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Regnault, O.; Lagneau, V.; Schneider, H. Experimental measurement of portlandite carbonation kinetics with supercritical CO2. Chem. Geol. 2009, 265, 113–121. [Google Scholar] [CrossRef]
- Rodriguez, E.T.; Garbev, K.; Merz, D.; Black, L.; Richardson, I. Thermal stability of C-S-H phases and applicability of Richardson and Groves’ and Richardson C-(A)-S-H(I) models to synthetic C-S-H. Cem. Concr. Res. 2017, 93, 45–56. [Google Scholar] [CrossRef]
- Escardino, A.; García-Ten, J.; Feliu, C.; Saburit, A.; Cantavella, V. Kinetic study of the thermal decomposition process of calcite particles in air and CO2 atmosphere. J. Ind. Eng. Chem. 2012, 19, 886–897. [Google Scholar] [CrossRef] [Green Version]
- Montes-Hernandez, G.; Daval, D.; Chiriac, R.; Renard, F. Growth of Nanosized Calcite through Gas−Solid Carbonation of Nanosized Portlandite under Anisobaric Conditions. Cryst. Growth Des. 2010, 10, 4823–4830. [Google Scholar] [CrossRef]
- Manovic, V.; Anthony, E.J. Carbonation of CaO-based sorbents enhanced by steam addition. Ind. Eng. Chem. Res. 2010, 49, 9105–9110. [Google Scholar] [CrossRef]
- Liu, W.; Su, S.; Xu, K.; Chen, Q.; Xu, J.; Sun, Z.; Wang, Y.; Hu, S.; Wang, X.; Xue, Y.; et al. CO2 sequestration by direct gas–solid carbonation of fly ash with steam addition. J. Clean. Prod. 2018, 178, 98–107. [Google Scholar] [CrossRef]
- Tosun, Y.I. Benefaction from Carbonation of Flue Gas CO2 as Coal Mining Filling. Geomaterials 2014, 4, 64–72. [Google Scholar] [CrossRef] [Green Version]
- Veetil, S.P.; Mercier, G.; Blais, J.F.; Cecchi, E.; Kentish, S. CO2 Sequestration by Direct Dry Gas-Solid Contact of Serpentinite Mining Residues: A Solution for Industrial CO2 Emission. Int. J. Environ. Pollut. Remediat. 2014, 2, 52–58. [Google Scholar] [CrossRef] [Green Version]
- Werner, M.; Hariharan, S.; Bortolan, A.V.; Zingaretti, D.; Baciocchi, R.; Mazzotti, M. Carbonation of Activated Serpentine for Direct Flue Gas Mineralization. Energy Procedia 2013, 37, 5929–5937. [Google Scholar] [CrossRef] [Green Version]
- Kaewmanee, K.; Krammart, P.; Sumranwanich, T.; Choktaweekarn, P.; Tangtermsirikul, S. Effect of free lime content on properties of cement–fly ash mixtures. Constr. Build. Mater. 2013, 38, 829–836. [Google Scholar] [CrossRef]
- Nawaz, A.; Julnipitawong, P.; Krammart, P.; Tangtermsirikul, S. Effect and limitation of free lime content in cement-fly ash mixtures. Constr. Build. Mater. 2016, 102, 515–530. [Google Scholar] [CrossRef]
- Sanna, A.; Uibu, M.; Caramanna, G.; Kuusik, R.; Maroto-Valer, M. A review of mineral carbonation technologies to sequester CO2. Chem. Soc. Rev. 2014, 43, 8049–8080. [Google Scholar] [CrossRef] [Green Version]
- Granite, E.J.; Pennline, H.W.; Connie, S. Mercury Control: For Coal-Derived Gas Streams; John Wiley & Sons: New York, NY, USA, 2015. [Google Scholar]
- Senior, C.; Granite, E.; Linak, W.; Seames, W. Chemistry of Trace Inorganic Elements in Coal Combustion Systems: A Century of Discovery. Energy Fuels 2020, 34, 15141–15168. [Google Scholar] [CrossRef]


| Property of Fly Ash | Unit | Requirement Accord. 450-1:2012 |
|---|---|---|
| LOI | % by mass | 5–9 (depending on class) |
| water requirement | % | ≤95 |
| total phosphate (P2O5) | mg/kg | ≤5 |
| sum SiO2 + Al2O3 + Fe2O3 | % by mass | ≥70 |
| total content of alkalis | % | ≤5 |
| reactive CaO | % | ≤10 |
| sulphate (SO3) | % | ≤3 |
| free CaO | % | if ≥1.5%, fly ash is checked for soundness |
| soundness | mm | ≤10 |
| magnesium oxide MgO | % by mass | ≤4 |
| chloride (Cl−) | % by mass | ≤0.10 |
| Compound | % Content |
|---|---|
| CaO | 35.27 |
| SiO2 | 33.11 |
| Al2O3 | 13.76 |
| MgO | 3.21 |
| Na2O | 1.33 |
| SO3 | 4.98 |
| K2O | 0.95 |
| Fe2O3 | 5.72 |
| TiO2 | 0.67 |
| P2O5 | 0.35 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rausis, K.; Ćwik, A.; Casanova, I.; Zarębska, K. Carbonation of High-Ca Fly Ashes under Flue Gas Conditions: Implications for Their Valorization in the Construction Industry. Crystals 2021, 11, 1314. https://doi.org/10.3390/cryst11111314
Rausis K, Ćwik A, Casanova I, Zarębska K. Carbonation of High-Ca Fly Ashes under Flue Gas Conditions: Implications for Their Valorization in the Construction Industry. Crystals. 2021; 11(11):1314. https://doi.org/10.3390/cryst11111314
Chicago/Turabian StyleRausis, Kwon, Agnieszka Ćwik, Ignasi Casanova, and Katarzyna Zarębska. 2021. "Carbonation of High-Ca Fly Ashes under Flue Gas Conditions: Implications for Their Valorization in the Construction Industry" Crystals 11, no. 11: 1314. https://doi.org/10.3390/cryst11111314
APA StyleRausis, K., Ćwik, A., Casanova, I., & Zarębska, K. (2021). Carbonation of High-Ca Fly Ashes under Flue Gas Conditions: Implications for Their Valorization in the Construction Industry. Crystals, 11(11), 1314. https://doi.org/10.3390/cryst11111314






