Targeted Chemotherapy Delivery via Gold Nanoparticles: A Scoping Review of In Vivo Studies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Literature Search and Study Selection
2.2. Data Analysis
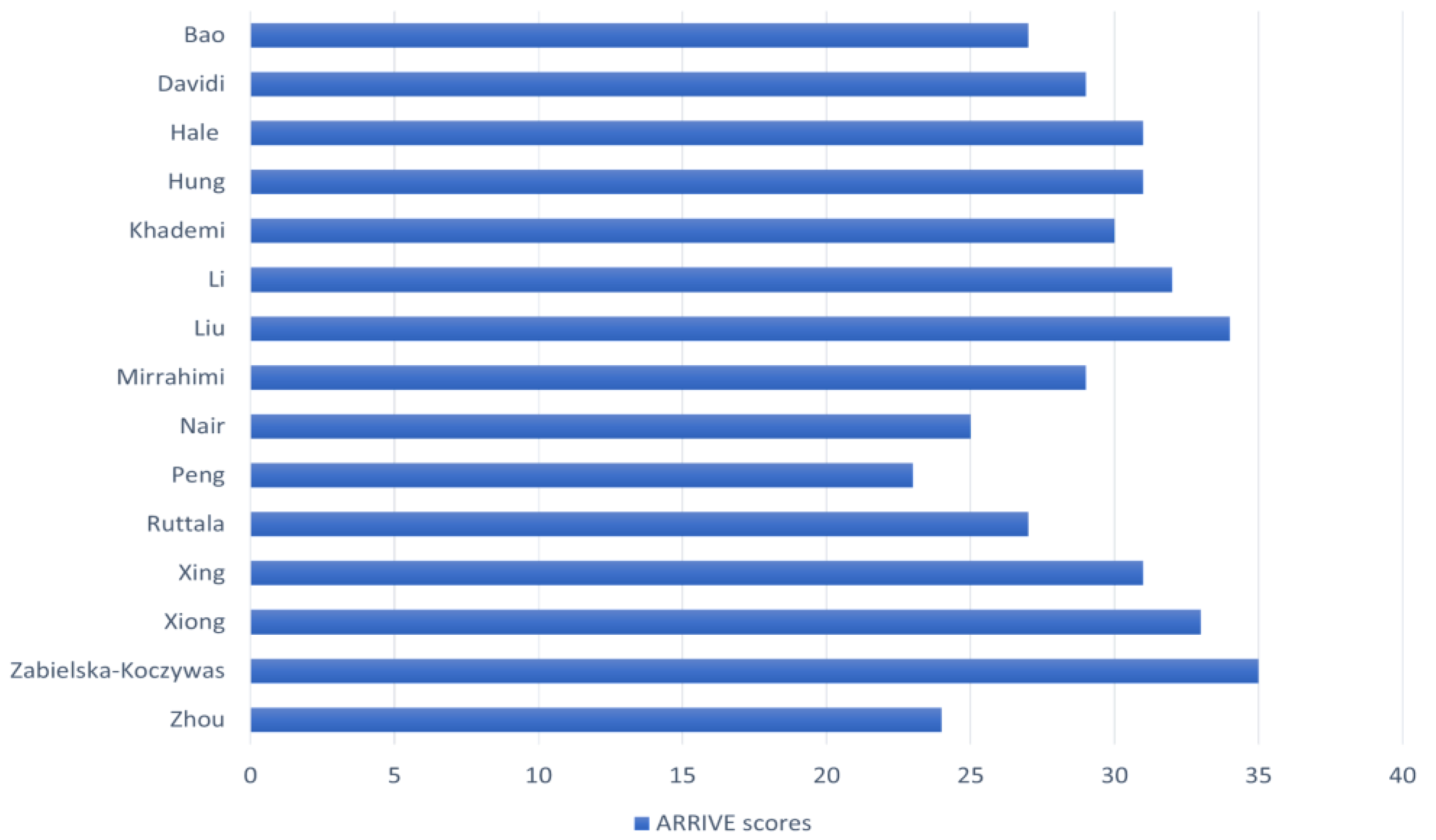
2.3. Quality Assessment
3. Results
3.1. Literature Review and Design of Eligible Studies
3.2. Synthesis and Delivery of AuNPs-Chemotherapy Conjugates (AuNPCC)
3.3. Selection of Chemotherapeutics
3.4. In Vivo Antitumoral Activity of AuNPCC
3.5. Systemic Biodistribution of AuNPCC
4. Discussion
4.1. Literature Overview
4.2. Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Najahi-Missaoui, W.; Arnold, R.D.; Cummings, B.S. Safe nanoparticles: Are we there yet? Int. J. Mol. Sci. 2021, 22, 385. [Google Scholar] [CrossRef]
- Baetke, S.C.; Lammers, T.; Kiessling, F. Applications of nanoparticles for diagnosis and therapy of cancer. Br. J. Radiol. 2015, 88, 20150207. [Google Scholar] [CrossRef]
- Wolfram, J.; Zhu, M.; Yang, Y.; Shen, J.; Gentile, E.; Paolino, D.; Fresta, M.; Nie, G.; Chen, C.; Shen, H.; et al. Safety of nanoparticles in medicine. Curr. Drug Targets 2015, 16, 1671–1681. [Google Scholar] [CrossRef] [Green Version]
- Gustafson, H.H.; Casper, D.H.; Grainger, D.W.; Ghandehari, H. Nanoparticle uptake: The phagocyte problem. Nano today 2015, 10, 487–510. [Google Scholar] [CrossRef] [Green Version]
- Kang, M.S.; Lee, S.Y.; Kim, K.S.; Han, D.W. State of the art biocompatible gold nanoparticles for cancer theragnosis. Pharmaceutics 2020, 12, 701. [Google Scholar] [CrossRef]
- Zhu, L.; Zhou, Z.; Mao, H.; Yang, L. Magnetic nanoparticles for precision oncology: Theranostic magnetic iron oxide nanoparticles for image-guided and targeted cancer therapy. Nanomedicine 2017, 12, 73–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, W.; Xu, Y.; Jin, R.; Wu, C.; Ai, H. MRI tracking of dendritic cells loaded with superparamagnetic iron oxide nanoparticles. Methods Mol. Biol. 2020, 2126, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Exbrayat, J.M.; Moudilou, E.N.; Lapied, E. Harmful effect of nanoparticles on animals. J. Nanotechnol. 2015, 2015, 861092. [Google Scholar] [CrossRef] [Green Version]
- Onaciu, A.; Munteanu, R.; Munteanu, V.C.; Gulei, D.; Raduly, L.; Feder, R.; Pirlog, R.; Atanasov, A.G.; Korban, S.S.; Irimie, A.; et al. Spontaneous and Induced Animal Models for Cancer Research. Diagnostics 2020, 10, 660. [Google Scholar] [CrossRef]
- Hua, S.; De Matos, M.B.; Metselaar, J.M.; Storm, G. Current Trends and Challenges in the Clinical Translation of Nanoparticulate Nanomedicines: Pathways for Translational Development and Commercialization. Front. Pharmacol. 2018, 9, 790. [Google Scholar] [CrossRef]
- Naumenko, V.; Nikitin, A.; Melnikov, A.G.P.; Vodopyanov, S.; Kapitanova, K.; Potashnikova, D.; Vishnevskiy, D.; Alieva, I.; Ilyasov, A.; Eletskaya, B.Z.; et al. Neutrophil-mediated transport is crucial for delivery of short-circulating magnetic nanoparticles to tumors. Acta Biomater. 2020, 104, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Weissleder, R.; Nahrendorf, M.; Pittet, M.J. Imaging macrophages with nanoparticles. Nat. Mater. 2014, 13, 125–138. [Google Scholar] [CrossRef]
- Krenkel, O.; Tacke, F. Liver macrophages in tissue homeostasis and disease. Nat. Rev. Immunol. 2017, 17, 306–321. [Google Scholar] [CrossRef] [PubMed]
- Kapitanova, K.S. Advances and challenges of nanoparticle-based macrophage reprogramming for cancer immunotherapy. Biochemistry 2019, 84, 729–745. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Fathi, P.; Chen, X. Nanoparticle delivery in vivo: A fresh look from intravital imaging. EBioMedicine 2020, 59, 102958. [Google Scholar] [CrossRef]
- Percie du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biol. 2020, 18, e3000410. [Google Scholar] [CrossRef]
- Bao, Q.Y.; Zhang, N.; Geng, D.D.; Xue, J.W.; Merritt, M.; Zhang, C.; Ding, Y. The enhanced longevity and liver targetability of Paclitaxel by hybrid liposomes encapsulating Paclitaxel-conjugated gold nanoparticles. Int. J. Pharm. 2014, 477, 408–415. [Google Scholar] [CrossRef]
- Davidi, E.S.; Sert, N.P.d.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; et al. Cisplatin-conjugated gold nanoparticles as a theranostic agent for head and neck cancer. Head Neck 2018, 40, 70–78. [Google Scholar] [CrossRef]
- Sarah, J.M.; Perrins, R.D.; García, C.E.; Pace, A.; Peral, U.; Patel, K.R.; Robinson, A.; Williams, P.; Ding, Y.; Saito, G.; et al. DM1 Loaded Ultrasmall Gold Nanoparticles Display Significant Efficacy and Improved Tolerability in Murine Models of Hepatocellular Carcinoma. Bioconjug. Chem. 2019, 30, 703–713. [Google Scholar] [CrossRef]
- Hung, W.H.; Zheng, J.H.; Lee, K.C.; Cho, E.C. Doxorubicin conjugated AuNP/biopolymer composites facilitate cell cycle regulation and exhibit superior tumor suppression potential in KRAS mutant colorectal cancer. J. Biotechnol. 2019, 306, 149–158. [Google Scholar] [CrossRef]
- Khademi, Z.; Lavaee, P.; Ramezani, M. Co-delivery of doxorubicin and aptamer against Forkhead box M1 using chitosan-gold nanoparticles coated with nucleolin aptamer for synergistic treatment of cancer cells. Carbohydr. Polym. 2020, 248, 116735. [Google Scholar] [CrossRef]
- Li, Y.; He, D.; Tu, J.; Wang, R.; Zu, C.; Chen, Y.; Yang, W.; Shi, D.; Webster, J.T.; Shen, Y. The comparative effect of wrapping solid gold nanoparticles and hollow gold nanoparticles with doxorubicin-loaded thermosensitive liposomes for cancer thermo-chemotherapy. Nanoscale 2018, 10, 8628–8641. [Google Scholar] [CrossRef]
- Liu, M.; Huang, P.; Wang, W.; Feng, Z.; Zhang, J.; Deng, L.; Dong, A. An injectable nanocomposite hydrogel co-constructed with gold nanorods and paclitaxel-loaded nanoparticles for local chemo-photothermal synergetic cancer therapy. J. Mater. Chem. B 2019, 7, 2667–2677. [Google Scholar] [CrossRef]
- Mirrahimi, M.; Abed, Z.; Beik, J.; Shiri, I.; Dezfuli, A.S.; Mahabadi, V.P.; Kamrava, S.K.; Ghaznavi, H.; Shakeri-Zadeh, A. A thermo-responsive alginate nanogel platform co-loaded with gold nanoparticles and cisplatin for combined cancer chemo-photothermal therapy. Pharmacol. Res. 2019, 143, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Nair, J.B.; Joseph, M.M.; Arya, J.S.; Sreedevi, P.; Sujai, P.T.; Maiti, K.K. Elucidating a Thermoresponsive Multimodal Photo-Chemotherapeutic Nanodelivery Vehicle to Overcome the Barriers of Doxorubicin Therapy. ACS Appl. Mater. Interfaces 2020, 12, 43365–43379. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Xu, J.; Yu, M.; Ning, X.; Huang, Y.; Du, B.; Hernandez, E.; Kapur, P.; Hsieh, J.; Zheng, J. Tuning the In Vivo Transport of Anticancer Drugs Using Renal-Clearable Gold Nanoparticles. Angew. Chem. Int. Ed. Engl. 2019, 58, 8479–8483. [Google Scholar] [CrossRef]
- Ruttala, H.B.; Ramasamy, T.; Poudel, B.K.; Ruttala, R.R.T.; Jin, S.G.; Choi, H.G.; Ku, S.K.; Yong, C.S.; Kim, J.O. Multi-responsive albumin-lonidamine conjugated hybridized gold nanoparticle as a combined photothermal-chemotherapy for synergistic tumor ablation. Acta Biomater. 2020, 101, 531–543. [Google Scholar] [CrossRef]
- Xing, S.; Zhang, X.; Luo, L.; Cao, W.; Li, L.; He, Y.; An, J.; Gao, D. Doxorubicin/gold nanoparticles coated with liposomes for chemo-photothermal synergetic antitumor therapy. Nanotechnology 2018, 29, 405101. [Google Scholar] [CrossRef]
- Xiong, X.; Arvizo, R.R.; Saha, S.; Robertson, D.J.; McMeekin, S.; Bhattacharya, R.; Mukherjee, P. Sensitization of ovarian cancer cells to cisplatin by gold nanoparticles. Oncotarget 2014, 5, 6453–6465. [Google Scholar] [CrossRef]
- Zabielska-Koczywąs, K.; Wojtalewicz, A.; Użarowska, E.; Klejman, A.; Wojtkowska, A.; Dolka, I.; Wojnicki, M.; Sobczak, K.; Wójcik, M.; Shen, H.; et al. Distribution of Glutathione-Stabilized Gold Nanoparticles in Feline Fibrosarcomas and Their Role as a Drug Delivery System for Doxorubicin-Preclinical Studies in a Murine Model. Int. J. Mol. Sci. 2018, 19, 1021. [Google Scholar] [CrossRef] [Green Version]
- Zhou, F.; Feng, B.; Yu, H.; Wang, D.; Wang, T.; Liu, J.; Meng, Q.; Wang, S.; Zhang, P.; Zhang, Z.; et al. Cisplatin Prodrug-Conjugated Gold Nanocluster for Fluorescence Imaging and Targeted Therapy of the Breast Cancer. Theranostics 2016, 6, 679–687. [Google Scholar] [CrossRef]
- ud Din, F.; Aman, W.; Ullah, I.; Qureshi, O.S.; Mustapha, O.; Shafique, S.; Zeb, A. Effective use of nanocarriers as drug delivery systems for the treatment of selected tumors. Int. J. Nanomed. 2017, 12, 7291–7309. [Google Scholar] [CrossRef] [Green Version]
- Yingchoncharoen, P.; Kalinowski, D.S.; Richardson, D.R. Lipid-Based Drug Delivery Systems in Cancer Therapy: What Is Available and What Is Yet to Come. Pharmacol. Rev. 2016, 68, 701–787. [Google Scholar] [CrossRef] [Green Version]
- Prados, J.; Melguizo, C.; Ortiz, R.; Velez, C.; Alvarez, P.J.; Arias, J.L.; Ruiz, M.A.; Gallardo, V.; Aranega, A. Doxorubicin-loaded nanoparticles: New advances in breast cancer therapy. Anticancer Agents Med. Chem. 2012, 12, 1058–1070. [Google Scholar] [CrossRef] [PubMed]
- Norouzi, M.; Yathindranath, V.; Thliveris, J.A.; Kopec, B.M.; Siahaan, T.J.; Miller, D.W. Doxorubicin-loaded iron oxide nanoparticles for glioblastoma therapy: A combinational approach for enhanced delivery of nanoparticles. Sci. Rep. 2020, 10, 11292. [Google Scholar] [CrossRef]
- Songbo, M.; Lang, H.; Xinyong, C.; Bin, X.; Ping, Z.; Liang, S. Oxidative stress injury in doxorubicin-induced cardiotoxicity. Toxicol. Lett. 2019, 307, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Koleini, N.; Nickel, B.E.; Edel, A.L.; Fandrich, R.R.; Ravandi, A.; Kardami, E. Oxidized phospholipids in Doxorubicin-induced cardiotoxicity. Chem. Biol. Interact. 2019, 303, 35–39. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Yi, M.; Huang, Y.P. Oxymatrine Ameliorates Doxorubicin-Induced Cardiotoxicity in Rats. Cell Physiol. Biochem. 2017, 43, 626–635. [Google Scholar] [CrossRef] [PubMed]
- Uyar, T.B.; Wu, K.; He, M.; Khan, I.; Royzen, M.; Yigit, M.V. Switchable Fluorescence of Doxorubicin for Label-Free Imaging of Bioorthogonal Drug Release. Chem. Med. Chem. 2020, 15, 988–994. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Yang, A.; Bai, Y.; Kong, D.; Lv, F. Dual fluorescence imaging-guided programmed delivery of doxorubicin and CpG nanoparticles to modulate tumor microenvironment for effective chemo-immunotherapy. Biomaterials 2020, 230, 119659. [Google Scholar] [CrossRef] [PubMed]
- Charbgoo, F.; Nejabat, M.; Abnous, K.; Soltani, F.; Taghdisi, S.M.; Alibolandi, M.; Shier, W.T.; Steele, T.W.J.; Ramezani, M. Gold nanoparticle should understand protein corona for being a clinical nanomaterial. J. Control. Release 2018, 272, 39–53. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Wang, J.; Mu, Q.; Su, G. In vivo Protein Corona Formation: Characterizations, Effects on Engineered Nanoparticles’ Biobehaviors, and Applications. Front. Bioeng. Biotechnol. 2021, 9, 263. [Google Scholar] [CrossRef]
- Chenthamara, D.; Subramaniam, S.; Ramakrishnan, S.G.; Krishnaswamy, S.; Essa, M.M.; Lin, F.H.; Qoronfleh, M.W. Therapeutic efficacy of nanoparticles and routes of administration. Biomater. Res. 2019, 23, 20. [Google Scholar] [CrossRef] [PubMed]
- Amina, S.J.; Guo, B. A review on the synthesis and functionalization of gold nanoparticles as a drug delivery vehicle. Int. J. Nanomed. 2020, 7, 9823–9857. [Google Scholar] [CrossRef] [PubMed]
- Jennings, T.; Strouse, G. Past, present and future of gold nanoparticles. Adv. Exp. Med. Biol. 2007, 620, 34. [Google Scholar] [CrossRef] [PubMed]
- Bracamonte, M.V.; Bollo, S.; Labbe, P.; Rivas, G.A.; Ferreyra, N.F. Quaternized chitosan as support for the assembly of gold nanoparticles and glucose oxidase. Physiochemical characterization of the platform and evaluation of its biocatalytic activity. Electrochim. Acta 2011, 56, 1316–1322. [Google Scholar] [CrossRef]
- Aillon, K.L.; Xie, Y.; El-Gendy, N.; Berkland, C.J.; Forrest, M.L. Nanomaterials physiochemical properties on in vivo toxicity. Adv. Drug. Deliv. Rev. 2009, 61, 457–466. [Google Scholar] [CrossRef] [Green Version]
- Iravani, S.; Korbekandi, H.; Mirmohammadi, S.V.; Zolfaghari, B. Synthesis of silver nanoparticles: Chemical, physical and biological methods. Res. Pharm. Sci. 2014, 9, 385–406. [Google Scholar]
- Turkevich, J.; Stevenson, P.C.; Hillier, J. Nucleation and growth process in the synthesis of colloidal gold. Discuss Faraday Soc. 1951, 11, 55–75. [Google Scholar] [CrossRef]
- Perala, S.R.; Kumar, S. On the mechanism of metal nanoparticle synthesis in the Brust-Schiffrin method. Langmuir 2013, 29, 9863–9873. [Google Scholar] [CrossRef]
- Uehara, A.; Booth, S.G.; Chang, S.Y.; Schroeder, S.L.; Imai, T.; Hashimoto, T.; Mosselmans, J.F.; Dryfe, R.A. Electrochemical Insight into the Brust-Schiffrin Synthesis of Au Nanoparticles. J. Am. Chem. Soc. 2015, 137, 15135–15144. [Google Scholar] [CrossRef]
- Zhao, P.; Li, N.; Astruc, D. State of the art in gold nanoparticle synthesis. Coord. Chem. Rev. 2013, 257, 638–665. [Google Scholar] [CrossRef]
- Sani, A.; Cao, C.; Cui, D. Toxicity of gold nanoparticles (AuNPs): A review. Biochem. Biophys. Rep. 2021, 26, 100991. [Google Scholar] [CrossRef]
- Elahi, N.; Kamali, M.; Baghersad, M.H. Recent biomedical applications of gold nanoparticles: A review. Talanta 2018, 184, 537–556. [Google Scholar] [CrossRef]
- Yeh, Y.C.; Creran, B.; Rotello, V.M. Gold nanoparticles: Preparation, properties, and applications in bionanotechnology. Nanoscale 2012, 4, 1871–1880. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, Y.; Ding, T.; Liu, J.; Zhao, H. Multifunctional gold nanoparticles: A novel nanomaterial for various medical applications and biological activities. Front. Bioeng. Biotechnol. 2020, 8, 990. [Google Scholar] [CrossRef] [PubMed]
- Musielak, M.; Potoczny, J.; Boś-Liedke, A.; Kozak, M. The Combination of Liposomes and Metallic Nanoparticles as Multifunctional Nanostructures in the Therapy and Medical Imaging-A Review. Int. J. Mol. Sci. 2021, 22, 6229. [Google Scholar] [CrossRef] [PubMed]
- Chithrani, D.B.; Dunne, M.; Stewart, J.; Allen, C.; Jaffray, D.A. Cellular uptake and transport of gold nanoparticles incorporated in a liposomal carrier. Nanomedicine 2010, 6, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Li, M.; Dey, R.; Chen, Y. Nanomaterials for cancer therapy: Current progress and perspectives. J. Hematol. Oncol. 2021, 14, 85. [Google Scholar] [CrossRef]

| First Author | Novelty |
|---|---|
| Bao [17] | Using hybrid liposome with AuNPs to investigate the therapeutic outcome |
| Davidi [18] | AuNPs acting as drug carrier, radiosensitizer and contrast agent |
| Hale [19] | Using 2nm AuNPs conjugated with maytansine analogue for the treatment of hepatocellular carcinoma |
| Hung [20] | Significant inhibition of mutated KRAS gene in colorectal cancer cells using AuNPs with Doxurubicin (DXR) |
| Khademi [21] | Efficient usage of Chitosan coated-AuNPs with aptamers and DXR on cancer cell lines |
| Li [22] | Reducing toxicity and improving tumoral tissue destruction with heated-hallow AuNPs with thermosensitive liposomal DXR carriers |
| Liu [23] | Using chemo-photothermal synergic cancer therapy through AuNPs-Paclitaxel (PTX) and a hydrogel with gold nanorods with phototermal properties |
| Mirrahimi [24] | Using a novel nanocomplex comprising alginate nanogel co-loaded with Cisplatin (CIS) and AuNPs for chemo-phothermal therapy |
| Nair [25] | Enhanced anti-tumor effect and diminished system toxicity through chemo-photothermal therapy with hallow AuNPs and docetaxel |
| Peng [26] | The use of renal-clearable AuNPs with DXR |
| Ruttala [27] | Usage of a nanoplatform created from AuNPs-Lonidamine-Albumin-Aptamer for targeted tumoral destruction |
| Xing [28] | Using AuNPs-DXRcomplex co-encapsulated withing a liposome for targeted chemo-photothermal therapy |
| Xiong [29] | Prevention of tumoral CIS-induced chemoresistence throught AuNPs pretreatment |
| Zabielska-Koczywas [30] | The biodistribution and the effect of Glutathione-stabilized AuNPs with DXR in Feline Injection Site Sarcoma |
| Zhou [31] | The diagnostics and therapeutic effect of fluorescent gold nanoclusters conjugated with CIS and folic acid |
| First Author | Year of Publication | Type of AuNPCC | Chemotherapeutic | Type of Tumor | Route of Administration |
|---|---|---|---|---|---|
| Bao [17] | 2014 | loaded in liposomes | PTX | Hepatic carcinoma | intravascular |
| Davidi [18] | 2017 | simple conjugates | CIS | A431 (squamous carcinoma) | intravascular |
| Hale [19] | 2018 | maytansine conjugated AuNPs (Brust–Schiffrin synthesis) | Maytansine | HCC | intravascular |
| Hung [20] | 2019 | biopolymer composite | DXR | DLD-1 (colorectal adenocarcinoma) | intraperitoneal |
| Khademi [21] | 2020 | biopolymer composite | DXR | 4T1 (breast carcinoma) | intravascular |
| Li [22] | 2018 | loaded in liposomes | DXR | MCF-7 (breast carcinoma) | intravascular |
| Liu [23] | 2019 | biopolymer composite | PTX | 4T1 (breast carcinoma) | subcutaneous |
| Mirrahimi [24] | 2019 | nanogel construct | CIS | CT26 (colon adenocarcinoma) | intraperitoneal |
| Nair [25] | 2020 | folate-calix construct | DXR | HeLa (cervical cancer); A549 (lung adenocarcinoma) | intraperitoneal |
| Peng [26] | 2019 | simple conjugates | DXR | 4T1 (breast carcinoma) | intravascular |
| Ruttala [27] | 2020 | simple conjugates | Ionidamine | DU-145 (prostate cancer) | intravascular |
| Xing [28] | 2018 | loaded in liposomes | DXR | U14 (cervical carcinoma) | intravascular |
| Xiong [29] | 2014 | simple conjugates | CIS | SK-OV-3 (ovarian carcinoma) | intraperitoneal |
| Zabielska-Koczywas [30] | 2018 | glutathione conjugates | DXR | FFS1 (feline fibrosarcoma) | intravascular |
| Zhou [31] | 2016 | folate-calix construct | CIS | 4T1 (breast carcinoma) | intravascular |
| First Author | Type of Surface Ligand | Type of Carrier |
|---|---|---|
| Bao [17] | PEG | liposomes |
| Davidi [18] | PEG7 | not used |
| Hale [19] | direct surface bond | not used |
| Hung [20] | PEG; PEI | not used |
| Khademi [21] | chitosan | not used |
| Li [22] | direct surface bond | liposomes |
| Liu [23] | direct surface bond | nanogel |
| Mirrahimi [24] | direct surface bond | nanogel |
| Nair [25] | Folic Acid | not used |
| Peng [26] | PEG; MBA | not used |
| Ruttala [27] | aptamer AS1411 | not used |
| Xing [28] | direct surface bond | liposomes |
| Xiong [29] | direct surface bond | not used |
| Zabielska-Koczywas [30] | glutathione | not used |
| Zhou [31] | Folic Acid | not used |
| First Author | Route of Administration | Ex Vivo/In Vivo Analysis | Imaging Evaluation |
|---|---|---|---|
| Bao [17] | intravascular | ex vivo | chromatography |
| Davidi [18] | intravascular | ex vivo | atomic absorption spectroscopy |
| Khademi [21] | intravascular | ex vivo | NIR fluorescence |
| Li [22] | intravascular | in vivo | NIR fluorescence |
| Nair [25] | intraperitoneal | ex vivo | ICP-MS |
| Ruttala [27] | intravascular | in vivo | FOBI fluorescence imaging |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morărașu, Ș.; Iacob, Ș.; Tudorancea, I.; Luncă, S.; Dimofte, M.-G. Targeted Chemotherapy Delivery via Gold Nanoparticles: A Scoping Review of In Vivo Studies. Crystals 2021, 11, 1169. https://doi.org/10.3390/cryst11101169
Morărașu Ș, Iacob Ș, Tudorancea I, Luncă S, Dimofte M-G. Targeted Chemotherapy Delivery via Gold Nanoparticles: A Scoping Review of In Vivo Studies. Crystals. 2021; 11(10):1169. https://doi.org/10.3390/cryst11101169
Chicago/Turabian StyleMorărașu, Ștefan, Ștefan Iacob, Ionuț Tudorancea, Sorinel Luncă, and Mihail-Gabriel Dimofte. 2021. "Targeted Chemotherapy Delivery via Gold Nanoparticles: A Scoping Review of In Vivo Studies" Crystals 11, no. 10: 1169. https://doi.org/10.3390/cryst11101169
APA StyleMorărașu, Ș., Iacob, Ș., Tudorancea, I., Luncă, S., & Dimofte, M.-G. (2021). Targeted Chemotherapy Delivery via Gold Nanoparticles: A Scoping Review of In Vivo Studies. Crystals, 11(10), 1169. https://doi.org/10.3390/cryst11101169







