Ionic Liquids as Protein Crystallization Additives
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
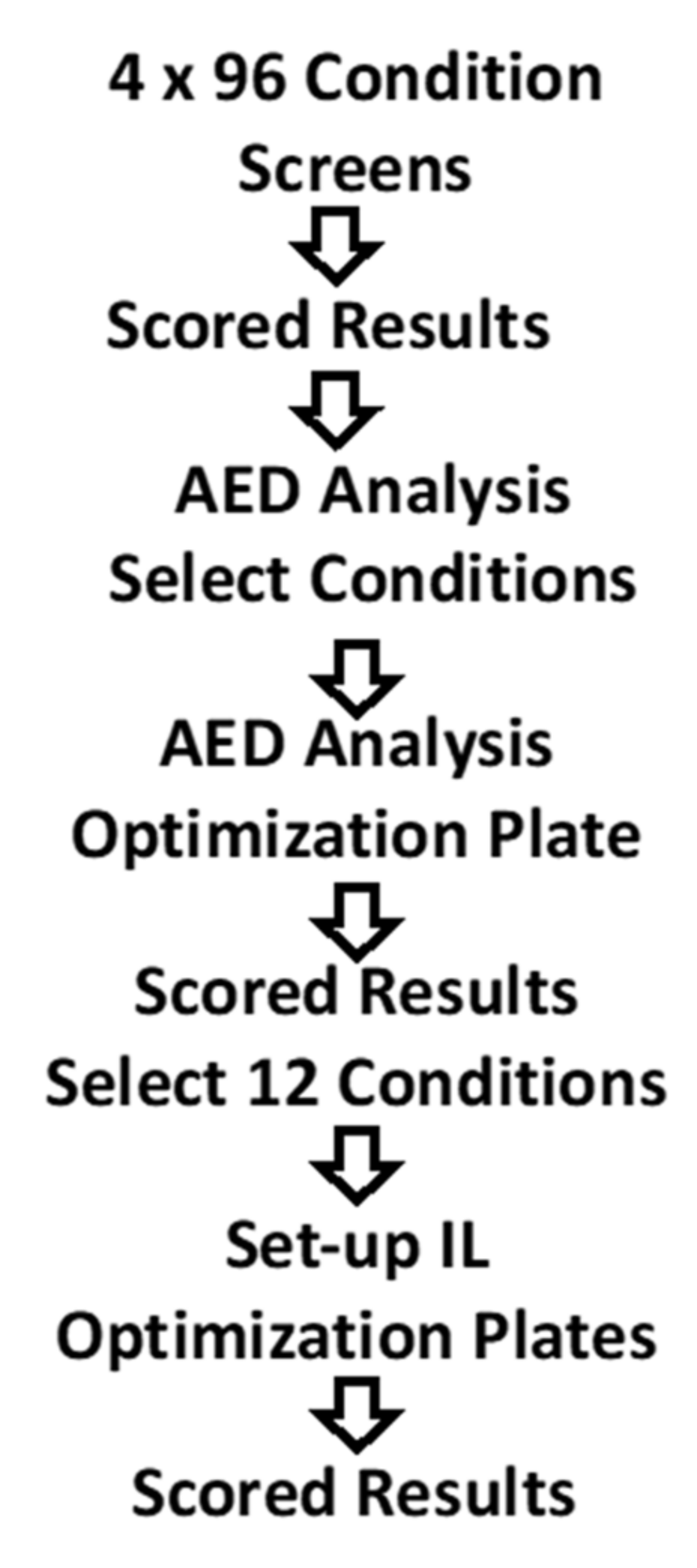
2.2. Methods
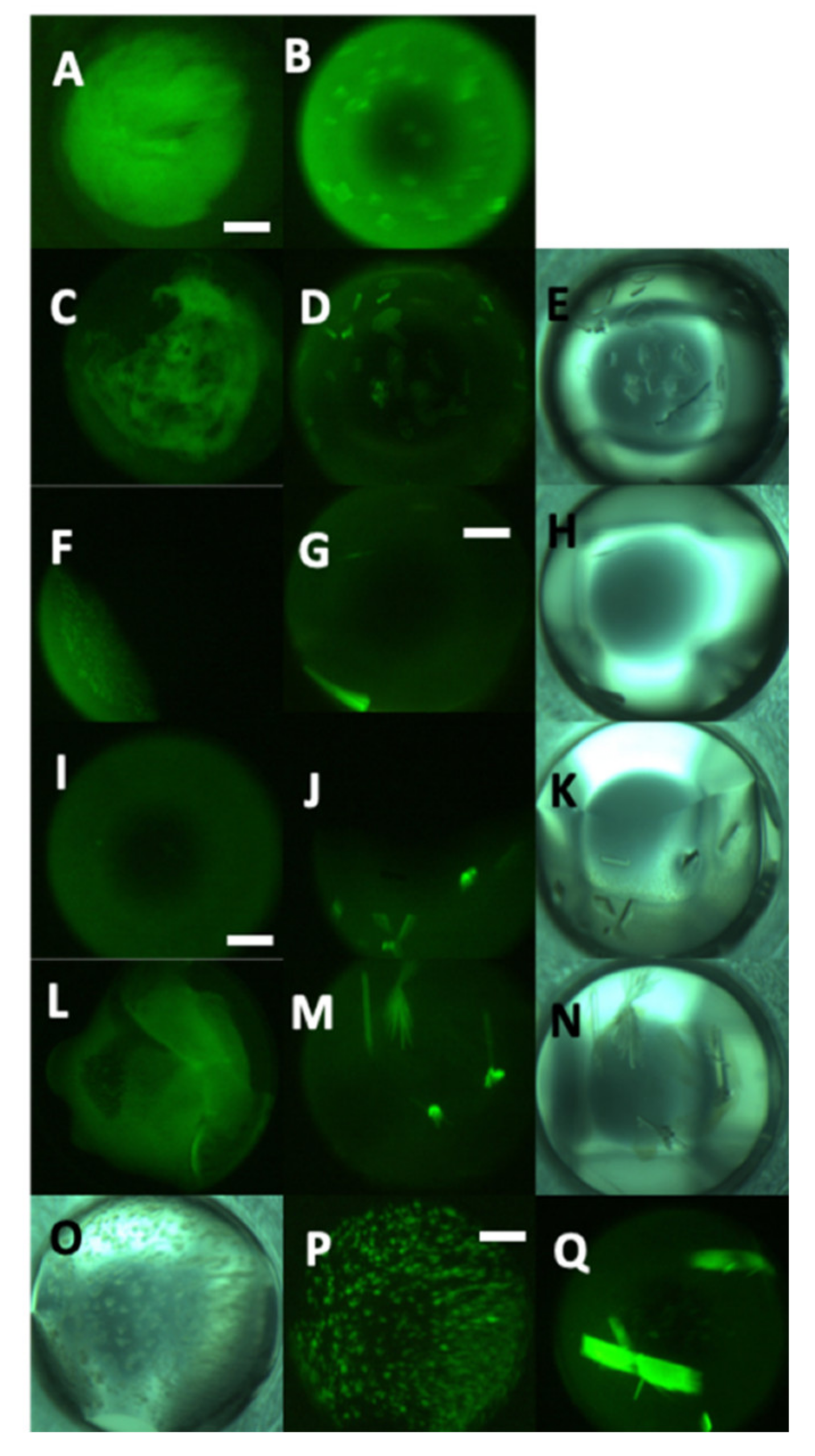
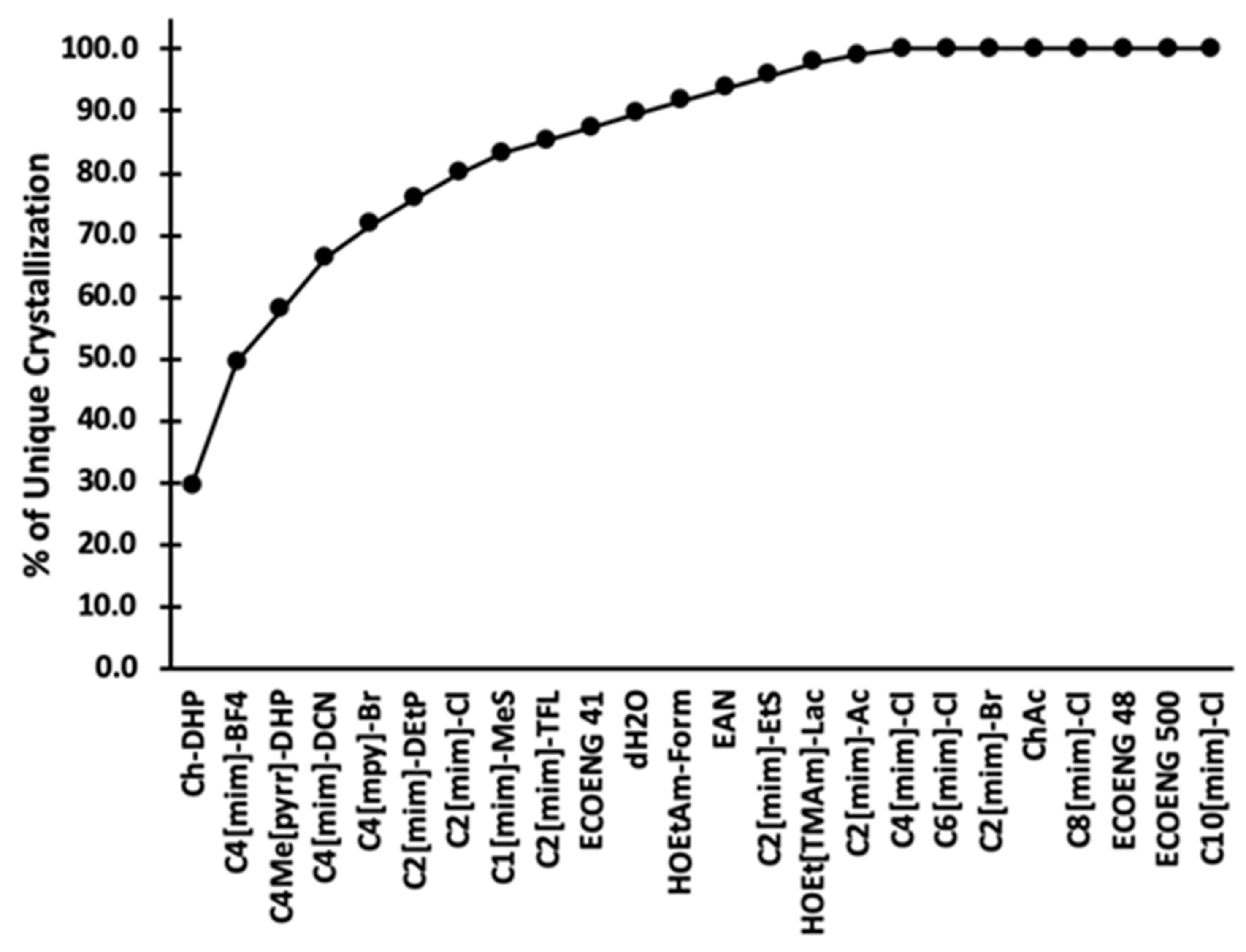
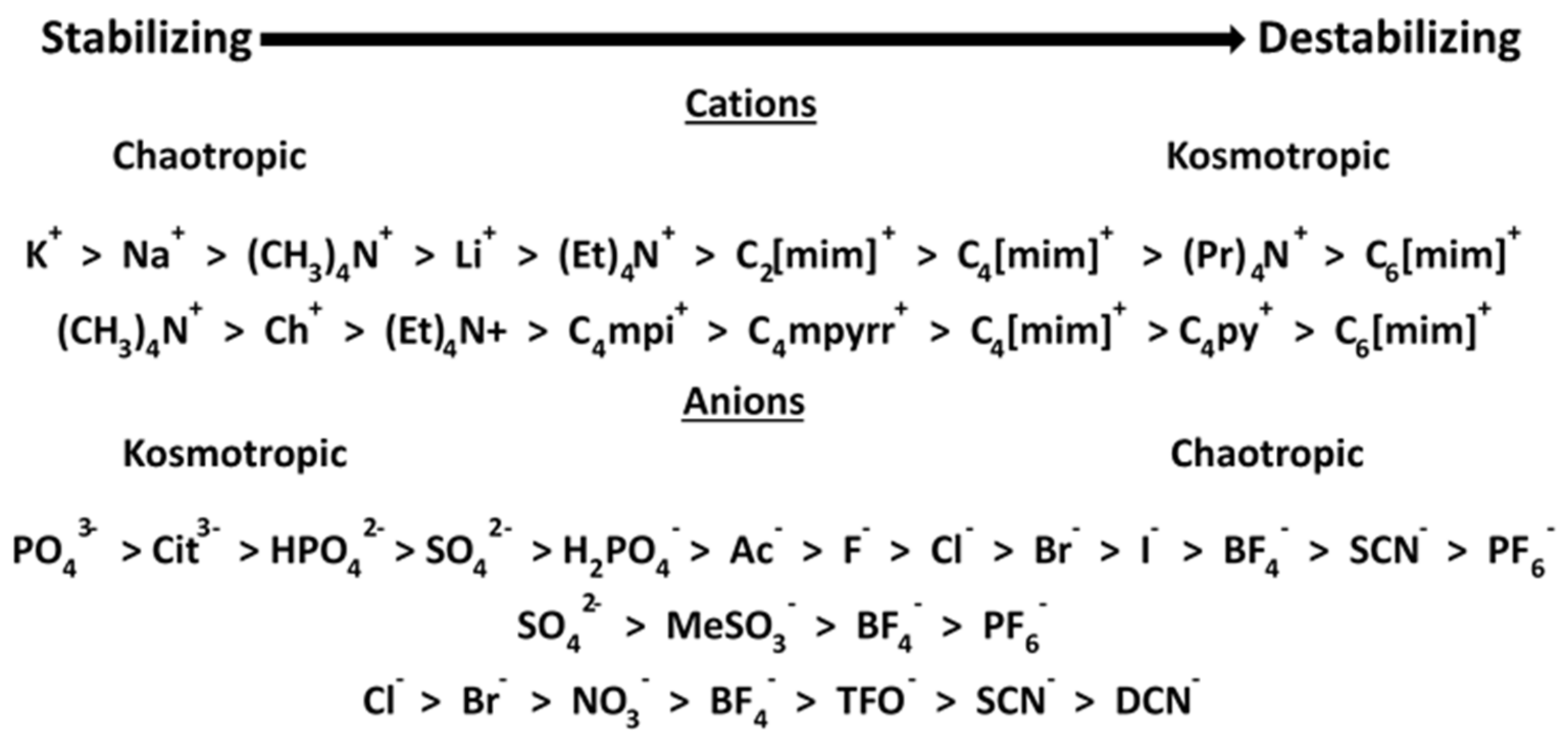
3. Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Garlitz, J.A.; Summers, C.A.; Flowers, R.A.; Borgstahl, G.E.O. Ethylammonium nitrate: A protein crystallization reagent. Acta Cryst. D 1999, 55, 2037–2038. [Google Scholar] [CrossRef] [PubMed]
- Sey, M.L.; Paley, M.S.; Turner, M.B.; Rogers, R.D. Protein Crystallization Using Room Temperature Ionic Liquids. Cryst. Growth Des. 2007, 7, 787–793. [Google Scholar]
- Judge, R.A.; Takahashi, S.; Longenecker, K.L.; Fry, E.H.; Abad-Zapatero, C.; Chiu, M. The Effect of Ionic Liquids on Protein Crystallization and X-ray Diffraction Resolution. Cryst. Growth Des. 2009, 9, 3463–3469. [Google Scholar] [CrossRef]
- Kennedy, D.F.; Drummond, C.J.; Peat, T.S.; Newman, J. Evaluating Protic Ionic Liquids as Protein Crystallization Additives. Cryst. Growth Des. 2011, 11, 1777–1785. [Google Scholar] [CrossRef]
- Hebel, D.; Ürdingen, M.; Hekmat, D.; Weuster-Botz, D. Development and Scale up of High-Yield Crystallization Processes of Lysozyme and Lipase Using Additives. Cryst. Growth Des. 2013, 13, 2499–2506. [Google Scholar] [CrossRef]
- Li, X.X.; Xu, X.D.; Dan, Y.Y.; Zhang, M.L. The crystallization of lysozyme and thaumatin with ionic liquid. Crystallogr. Rep. 2009, 54, 1285–1288. [Google Scholar] [CrossRef]
- Mann, J.P.; McCluskey, A.; Atkin, R. Activity and thermal stability of lysozyme in alkylammonium formate ionic liquids—influence of cation modification. Green Chem. 2009, 11, 785–792. [Google Scholar] [CrossRef]
- Buchfink, R.; Tischer, A.; Patil, G.; Rudolph, R.; Lange, C. Ionic liquids as refolding additives: Variation of the anion. J. Biotechnol. 2010, 150, 64–72. [Google Scholar] [CrossRef]
- Yamamoto, E.; Yamaguchi, S.; Nagamune, T. Protein Refolding by N-Alkylpyridinium and N-Alkyl-N-methylpyrrolidinium Ionic Liquids. Appl. Biochem. Biotechnol. 2011, 164, 957–967. [Google Scholar] [CrossRef]
- Reinhard, L.; Mayerhofer, H.; Geerlof, A.; Mueller-Dieckmann, J.; Weiss, M.S. Optimization of protein buffer cocktails using Thermofluor. Acta Cryst. F 2013, 69, 209–214. [Google Scholar] [CrossRef]
- Ericsson, U.B.; Hallberg, B.M.; DeTitta, G.T.; Dekker, N.; Nordlund, P. Thermofluor-based high-throughput stability optimization of proteins for structural studies. Anal. Biochem. 2006, 357, 289–298. [Google Scholar] [CrossRef]
- Wang, Z.; Dang, L.; Han, Y.; Jiang, P.; Wei, H. Crystallization Control of Thermal Stability and Morphology of Hen Egg White Lysozyme Crystals by Ionic Liquids. J. Agric. Food Chem. 2010, 58, 5444–5448. [Google Scholar] [CrossRef]
- Wang, Z.; Xiao, H.; Han, Y.; Jiang, P.; Zhou, Z. The Effect of Four Imidazolium Ionic Liquids on Hen Egg White Lysozyme Solubility. J. Chem. Eng. Data 2011, 56, 1700–1703. [Google Scholar] [CrossRef]
- Turner, M.B.; Holbrey, J.D.; Spear, S.K.; Pusey Marc, L.; Rogers, R.D. The Effect of Oxygen-Containing Functional Groups on Protein Stability in Ionic Liquid Solutions. In Ionic Liquids IIIB: Fundamentals, Progress, Challenges, and Opportunities; ACS Symposium Series; Rogers, R.D., Seddon, K.R., Eds.; ACS Publication: Washington, DC, USA, 2004; Volume 902, pp. 233–243. [Google Scholar]
- Amygina, V.; Moiseev, V.; Rodina, E.; Vorobyeva, N.; Popov, A.; Kurilova, S.; Nazarova, T.; Avaeva, S.; Bartunik, H. Reversible Inhibition of Escherichia coli Inorganic Pyrophosphatase by Fluoride: Trapped Catalytic Intermediates in Cryo-crystallographic Studies. J. Mol. Biol. 2007, 366, 1305–1317. [Google Scholar] [CrossRef] [PubMed]
- Kajander, T.; Kellosalo, J.; Goldman, A. Inorganic pyrophosphatases: One substrate, three mechanisms. FEBS Lett. 2013, 587, 1863–1869. [Google Scholar] [CrossRef] [PubMed]
- Halonen, P.; Tammenkoski, M.; Niiranen, L.; Parfenyev, A.N.; Goldman, A.; Baykov, A.; Lahti, R. Effects of active site mutations on the metal binding affinity, catalytic competence, and stability of the family II pyrophosphatase from Bacillus subtilis. Biochemistry 2005, 44, 4004–4010. [Google Scholar] [CrossRef]
- Kuhn, N.J.; Ward, S. Purification, properties, and multiple forms of a manganese-activated inorganic pyrophosphatase from Bacillus subtilis. Arch. Biochem. Biophys. 1998, 354, 47–56. [Google Scholar] [CrossRef]
- Lee, H.S.; Cho, Y.; Kim, Y.-J.; Lho, T.-O.; Cha, S.-S.; Lee, J.-H.; Kang, S.G. A novel inorganic pyrophosphatase in Thermococcus onnurineus NAFEMS. Microb. Lett. 2009, 300, 68–74. [Google Scholar] [CrossRef][Green Version]
- Hughes, R.C.; Ng, J. Can small laboratories do structural genomics? Cryst. Growth Des. 2007, 7, 2226–2238. [Google Scholar] [CrossRef]
- Marsic, D.; Hughes, R.; Byrne-Steele, M.; Ng, J. PCR-based gene synthesis to produce recombinant proteins for crystallization. BMC Biotechnol. 2008, 8, 44. [Google Scholar] [CrossRef][Green Version]
- Orsythe, E.; Achari, A.; Pusey, M.L. Trace fluorescent labeling for high-throughput crystallography. Acta Cryst. D 2006, 62, 339–346. [Google Scholar] [CrossRef]
- Pusey, M.; Barcena, J.; Morris, M.; Singhal, A.; Yuan, Q.; Ng, J. Trace fluorescent labeling for protein crystallization. Acta Cryst. F 2015, 71, 806–814. [Google Scholar] [CrossRef] [PubMed]
- Dinç, I.; Pusey, M.; Aygün, R. Optimizing associative experimental design for protein crystallization screening. IEEE Trans. NanoBiosci. 2016, 15, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Brodersen, D.E.; Andersen, G.R.; Andersen, C.B.F. MIMER: An Automated Spreadsheet-Based Crystallization System. Acta Cryst. F 2013, 69, 815–820. [Google Scholar] [CrossRef] [PubMed]
- D’Arcy, A.; Villard, F.; Marsh, M. An automated microseed matrix-screening method for protein crystallization. Acta Cryst. D 2007, 63, 550–554. [Google Scholar] [CrossRef] [PubMed]
- Luft, J.R.; DeTitta, G.T. A method to produce microseed stock for use in the crystallization of biological macromolecules. Acta Cryst. D 1999, 55, 988–993. [Google Scholar] [CrossRef]
- Sievers, F.; Wilm, A.; Dineen, D.; Gibson, T.J.; Karplus, K.; Li, W.; López, R.; McWilliam, H.; Remmert, M.; Söding, J.; et al. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol. Syst. Biol. 2011, 7, 539. [Google Scholar] [CrossRef]
- Sievers, F.; Higgins, D.G. Clustal Omega for making accurate alignments of many protein sequences. Protein Sci. 2017, 27, 135–145. [Google Scholar] [CrossRef]
- Sievers, F.; Barton, G.J.; Higgins, D.G. Multiple sequence alignment. In Bioinformatics; Baxevanis, A.D., Bader, G.D., Wishart, D.S., Eds.; Wiley: Hoboken, NJ, USA, 2020; Volume 227, pp. 227–250. [Google Scholar]
- Constantinescu, D.; Weingärtner, H.; Herrmann, C. Protein denaturation by ionic liquids and the Hofmeister series: A case study of aqueous solutions of ribonuclease Angw. Chem. Int. Ed. 2007, 46, 8887–8889. [Google Scholar] [CrossRef]
- Jenkins, H.D.B.; Marcus, Y. Viscosity ß-coefficients of ions in solution. Chem. Rev. 1995, 95, 2695–2794. [Google Scholar] [CrossRef]
- Lu, L.; Hu, Y.; Huang, X.; Qu, Y. A Bioelectrochemical Method for the Quantitative Description of the Hofmeister Effect of Ionic Liquids in Aqueous Solution. J. Phys. Chem. B 2012, 116, 11075–11080. [Google Scholar] [CrossRef]
- Zhao, H.; Olubajo, O.; Song, Z.; Sims, A.L.; Person, T.E.; Lawal, R.A.; Holley, L.A. Effect of kosmotropicity of ionic liquids on the enzyme stability in aqueous solutions. Bioorganic Chem. 2006, 34, 15–25. [Google Scholar] [CrossRef]
- Nostro, P.L.; Ninham, B.W. Hofmeister Phenomena: An Update on Ion Specificity in Biology. Chem. Rev. 2012, 112, 2286–2322. [Google Scholar] [CrossRef]
- Lee, L.L.Y.; Lee, J.C. Thermal stability of proteins in the presence of poly(ethylene glycols). Biochemistry 1987, 26, 7813–7819. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Sharma, V.K.; Kalonia, D.S. Effect of polyols on polyethylene glycol (PEG)-induced precipitation of proteins: Impact on solubility, stability and conformation. Int. J. Pharm. 2009, 366, 38–43. [Google Scholar] [CrossRef]
- De Lencastre Novaes, L.C.; Mazzola, P.G.; Pessoa, A., Jr.; Penna, T.C.V. Effect of polyethylene glycol on the thermal stability of green fluorescent protein. Biotechnol. Prog. 2010, 26, 252–256. [Google Scholar] [CrossRef] [PubMed]
- Samanta, N.; Das Mahanta, D.; Hazra, S.; Kumar, G.S.; Mitra, R.K. Short chain polyethylene glycols unusually assist thermal unfolding of human serum albumin. Biochimie 2014, 104, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z. Hofmeister effects: An explanation for the impact of ionic liquids on biocatalysis. J. Biotechnol. 2009, 144, 12–22. [Google Scholar] [CrossRef]
- Fujita, K.; MacFarlane, D.R.; Forsyth, M.; Yoshizawa-Fujita, M.; Murata, K.; Nakamura, N.; Ohno, H. Solubility and Stability of Cytochrome c in Hydrated Ionic Liquids: Effect of Oxo Acid Residues and Kosmotropicity. Biomacromolecules 2007, 8, 2080–2086. [Google Scholar] [CrossRef]
- Akdogan, Y.; Junk, M.J.N.; Hinderberger, D. Effect of Ionic Liquids on the Solution Structure of Human Serum Albumin. Biomacromolecules 2011, 12, 1072–1079. [Google Scholar] [CrossRef] [PubMed]
- Fiebig, O.C.; Mancini, E.; Caputo, G.; Vaden, T.D. Quantitative Evaluation of Myoglobin Unfolding in the Presence of Guanidinium Hydrochloride and Ionic Liquids in Solution. J. Phys. Chem. B 2013, 118, 406–412. [Google Scholar] [CrossRef] [PubMed]
- Constatinescu, D.; Herrmann, C.; Weingärtner, H. Patterns of protein unfolding and protein aggregation in ionic liquids. Phys. Chem. Chem. Phys. 2010, 12, 1756–1763. [Google Scholar] [CrossRef]
- Shaposhnikova, A.; Kuty, M.; Chaloupkova, R.; Damborsky, J.; Smatanova, I.K.; Minofar, B.; Prudnikova, T. Stabilization of Haloalkane Dehalogenase Structure by Interfacial Interaction with Ionic Liquids. Crystals 2021, 11, 1052. [Google Scholar] [CrossRef]


| IL | Abbreviation | Source |
|---|---|---|
| 1-Ethyl-3-methylimidazolium chloride | C2[mim]-Cl | Fluka |
| 1-Butyl-3-methylimidazolium chloride | C4[mim]-Cl | Sigma |
| 1-Hexyl-3-methylimidazolium chloride | C6[mim]-Cl | Solvent Innovations |
| 1-Octyl-3-methylimidazolium chloride | C8[mim]-Cl | Iolitec |
| 1-Decyl-3-methylimidazolium chloride | C10[mim]-Cl | Sigma |
| 1-Butyl-3-methylimidazolium diethyleneglycol monomethylether sulfate | ECOENG 41 | Solvent Innovations |
| 1-Octyl-3-methylimidazolium diethyleneglycol monomethylether sulfate | ECOENG 48 | Solvent Innovations |
| 1-Methyl-3-methyl-midazolium methyl sulfate | C1[mim]-MeS | Solvent Innovations |
| 1-Ethyl-3-methylimidazolium diethyl phosphate | C2[mim]-DEtP | Iolitec |
| 1-Ethyl-3-methylimidazolium triflate | C2[mim]-TFL | Iolitec |
| 1-Ethyl-3-methylimidazolium acetate | C2[mim]-Ac | Iolitec |
| 1-Ethyl-3-methylimidazolium ethylsulfate | C2[mim]-EtS | Iolitec |
| 1-Ethyl-3-methylimidazolium bromide | C2[mim]-Br | Fluka |
| 1-Butyl-3-methylimidazolium tetrafluoroborate | C4[mim]-BF4 | Sigma |
| 1-Butyl-3-methylimidazolium dicyanamide | C4[mim]-DCN | Iolitec |
| Choline dihydrogen phosphate | Ch-DHP | Iolitec |
| N-Butyl, N-methylpyrrolidinium dihydrogen phosphate | C4Me[pyrr]-DHP | [2] |
| N-Methyl-N-butyl-pyridinium bromide | C4[mpy]-Br | Solvent Innovations |
| Ethyl ammonium nitrate | EAN | Iolitec |
| 2-Hydroxyethyl-trimethylammonium L-(+)-lactate | HOEt[TMAm]-Lac | Sigma |
| Choline acetate | Ch-Ac | Sigma |
| 2-Hydroxyethylammonium formate | HOEtAm-Form | Iolitec |
| Cocosalkyl pentaethoxy methylammonium Methylsulfate | ECOENG 500 | Solvent Innovations |
| E. coli | S. typhimurium | F. tularensis | C. jejuni | K. pneumoniae | H. influenzae | A. baumannii | S. pyrogenes | S. pneumoniae | |
|---|---|---|---|---|---|---|---|---|---|
| E. coli | 100.00 | 94.32 | 63.01 | 51.16 | 42.86 | 33.33 | 67.43 | 18.67 | 19.28 |
| S. typhimurium | 94.32 | 100.00 | 61.27 | 50.00 | 41.71 | 33.33 | 65.14 | 18.67 | 19.28 |
| F. tularensis | 63.01 | 61.27 | 100.00 | 52.05 | 42.44 | 35.09 | 56.07 | 16.46 | 17.68 |
| C. jejuni | 51.16 | 50.00 | 52.05 | 100.00 | 40.70 | 31.58 | 49.42 | 21.47 | 19.63 |
| K. pneumoniae | 42.86 | 41.71 | 42.44 | 40.70 | 100.00 | 32.00 | 40.23 | 14.12 | 16.47 |
| H. influenzae | 33.33 | 33.33 | 35.09 | 31.58 | 32.00 | 100.00 | 31.79 | 16.27 | 15.06 |
| A. baumannii | 67.43 | 65.14 | 56.07 | 49.42 | 40.23 | 31.79 | 100.00 | 21.21 | 21.21 |
| S. pyrogenes | 18.67 | 18.67 | 16.46 | 21.47 | 14.12 | 16.27 | 21.21 | 100.00 | 82.64 |
| S. pneumoniae | 19.28 | 19.28 | 17.68 | 19.63 | 16.47 | 15.06 | 21.21 | 82.64 | 100.00 |
| IL | Pcpt→Xtl | Non-Faceted→Xtl | All |
|---|---|---|---|
| Ch-DHP | 28 | 5 | 49 |
| C4[mim]-BF4 | 22 | 4 | 43 |
| C4Me[pyrr]-DHP | 14 | 1 | 26 |
| C4[mim]-DCN | 11 | 0 | 22 |
| C1[mim]-MeS | 11 | 0 | 18 |
| C2[mim]-DEtP | 10 | 4 | 28 |
| C2[mim]-TFL | 9 | 3 | 25 |
| C4[mpy]-Br | 9 | 2 | 21 |
| ECOENG 41 | 9 | 1 | 14 |
| C2[mim]-Cl | 9 | 0 | 25 |
| C2[mim]-Ac | 8 | 4 | 19 |
| dH2O | 8 | 3 | 29 |
| HOEtAm-Form | 8 | 3 | 20 |
| EAN | 8 | 2 | 23 |
| C2[mim]-EtS | 8 | 1 | 24 |
| HOEt[TMAm]-Lac | 6 | 4 | 17 |
| C4[mim]-Cl | 6 | 3 | 30 |
| C6[mim]-Cl | 6 | 2 | 14 |
| C2[mim]-Br | 5 | 2 | 23 |
| ChAc | 5 | 1 | 20 |
| C8[mim]-Cl | 5 | 1 | 10 |
| ECOENG 48 | 3 | 0 | 5 |
| ECOENG 500 | 2 | 0 | 8 |
| C10[mim]-Cl | 0 | 0 | 6 |
| IL | Xtl→Xtl | 5,6→Xtl | 0–4→Xtl |
|---|---|---|---|
| C4[mim]-BF4 | 6 | 1 | 13 |
| Ch-DHP | 3 | 6 | 8 |
| C1[mim]-MeS | 2 | 0 | 8 |
| C4Me[pyrr]-DHP | 2 | 0 | 7 |
| C2[mim]-TFL | 4 | 2 | 5 |
| C2[mim]-Ac | 3 | 2 | 5 |
| C2[mim]-DEtP | 4 | 2 | 5 |
| ECOENG 41 | 0 | 0 | 5 |
| HOEt[TMAm]-Lac | 3 | 3 | 4 |
| HOEtAm-Form | 2 | 2 | 4 |
| ChAc | 5 | 1 | 4 |
| C2[mim]-Cl | 7 | 0 | 4 |
| C4[mim]-DCN | 5 | 0 | 4 |
| C6[mim]-Cl | 2 | 2 | 3 |
| EAN | 3 | 2 | 3 |
| C2[mim]-Br | 5 | 1 | 3 |
| C2[mim]-EtS | 7 | 1 | 3 |
| C4[mpy]-Br | 2 | 0 | 3 |
| ECOENG 500 | 1 | 0 | 3 |
| dH2O | 5 | 2 | 2 |
| C4[mim]-Cl | 7 | 1 | 2 |
| C8[mim]-Cl | 1 | 1 | 2 |
| ECOENG 48 | 0 | 0 | 1 |
| C10[mim]-Cl | 3 | 0 | 0 |
| Ch-DHP | C4[mim]-BF4 | C4Me[pyrr]-DHP | C4[mim]-DCN | |
|---|---|---|---|---|
| Total Instances | 49 | 42 | 26 | 22 |
| Ch-DHP | 100% | 21% (9) | 15% (4) | 23% (5) |
| C4[mim]-BF4 | 100% | 27% (7) | 15% (3) | |
| C4Me[pyrr]-DHP | 100% | 5% (1) | ||
| C4[mim]-DCN | 100% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tarver, C.L.; Yuan, Q.; Pusey, M.L. Ionic Liquids as Protein Crystallization Additives. Crystals 2021, 11, 1166. https://doi.org/10.3390/cryst11101166
Tarver CL, Yuan Q, Pusey ML. Ionic Liquids as Protein Crystallization Additives. Crystals. 2021; 11(10):1166. https://doi.org/10.3390/cryst11101166
Chicago/Turabian StyleTarver, Crissy L., Qunying Yuan, and Marc L. Pusey. 2021. "Ionic Liquids as Protein Crystallization Additives" Crystals 11, no. 10: 1166. https://doi.org/10.3390/cryst11101166
APA StyleTarver, C. L., Yuan, Q., & Pusey, M. L. (2021). Ionic Liquids as Protein Crystallization Additives. Crystals, 11(10), 1166. https://doi.org/10.3390/cryst11101166






