Effect of KyAl4(Si8-y) O20(OH)4 Calcined Based-Clay on the Microstructure and Mechanical Performances of High-Performance Concrete
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. HPC Production
2.2.1. Mix Proportions
2.2.2. Batching Procedure and Curing Conditions
2.3. Test Methods
2.3.1. Workability
2.3.2. Setting Times Test
2.3.3. Mechanical Properties
2.3.4. Non-Destructive Tests on HPC Mixtures
3. Results and Discussions
3.1. Material Characterisation
3.1.1. Physical Properties
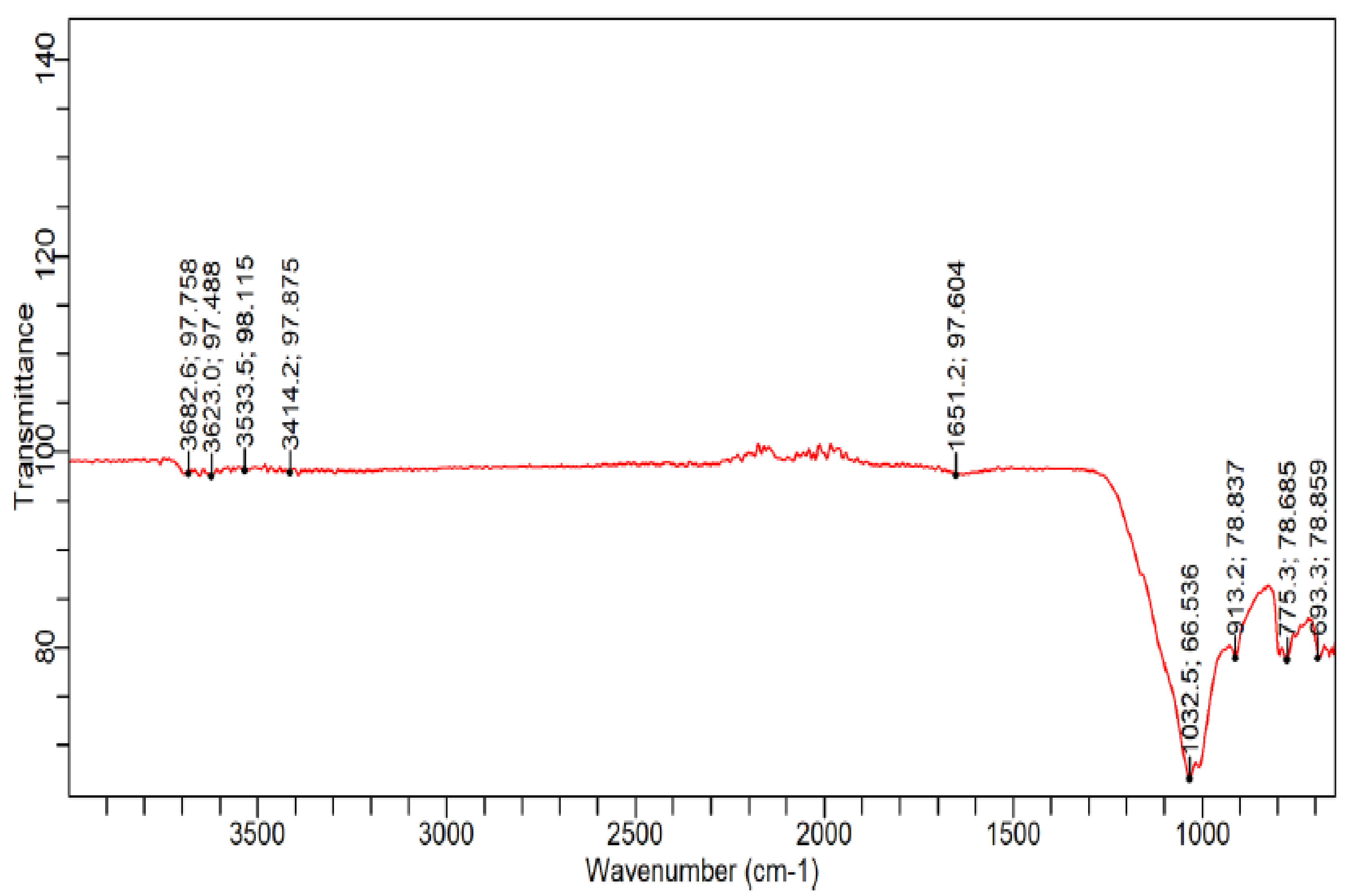
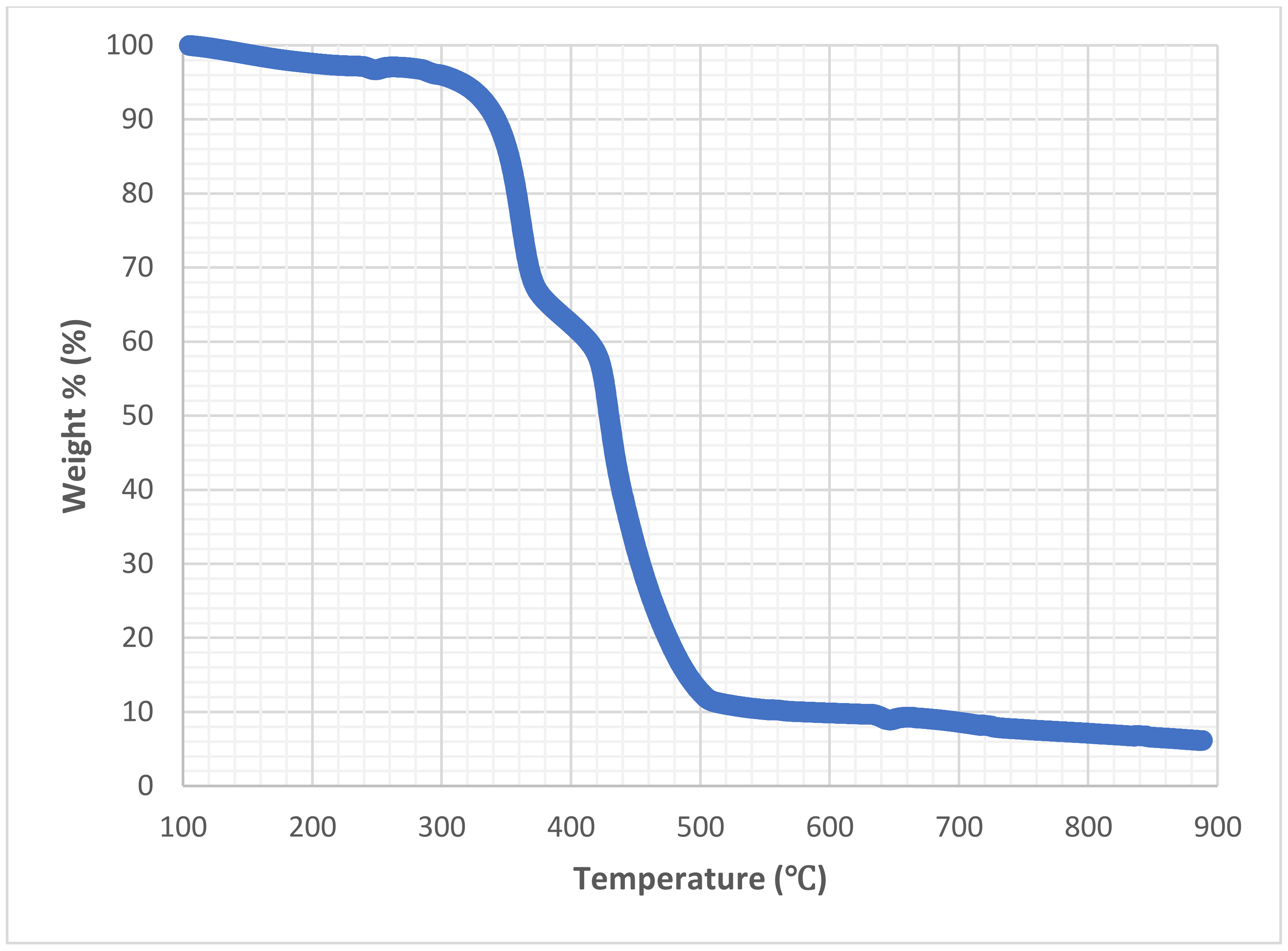
3.1.2. Chemical and Microstructure Analyses of Binders
3.2. Fresh Properties
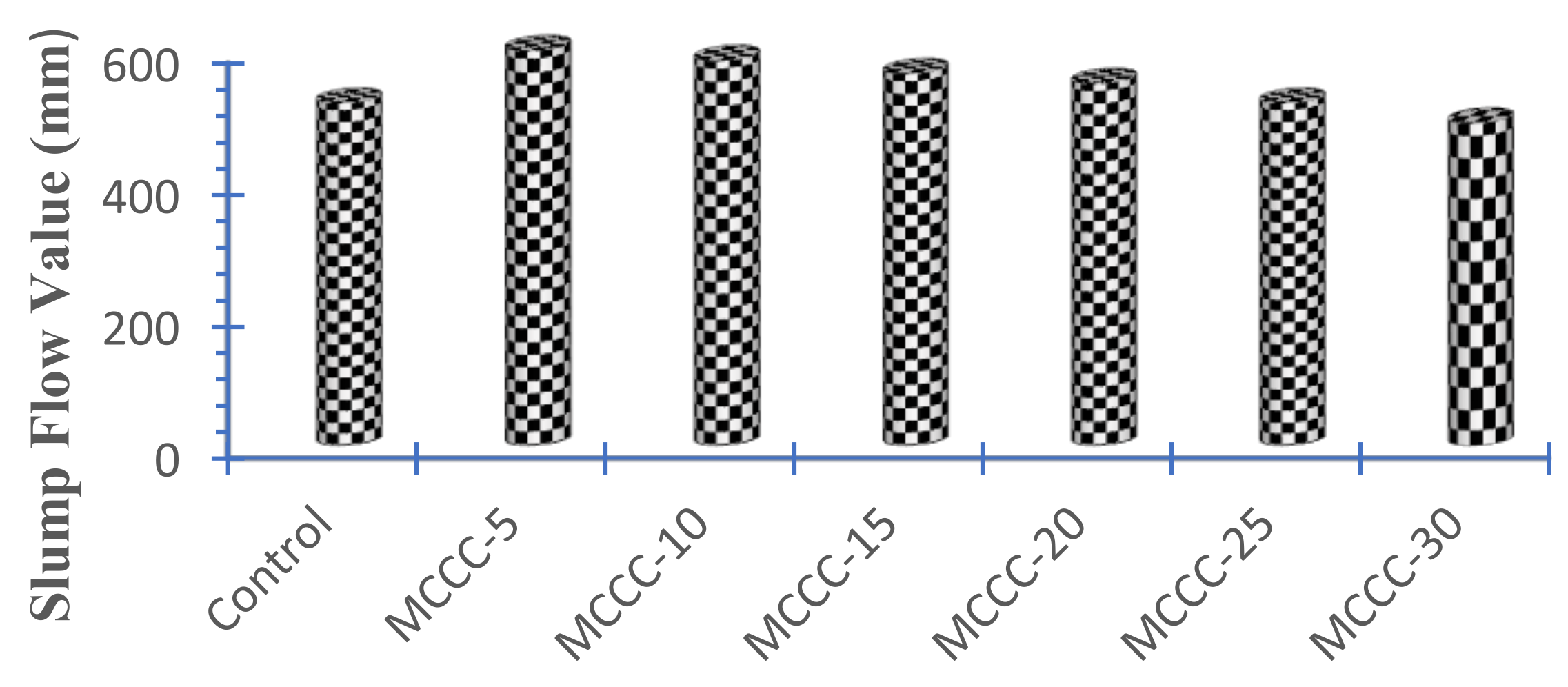
3.2.1. Slump Flow Test
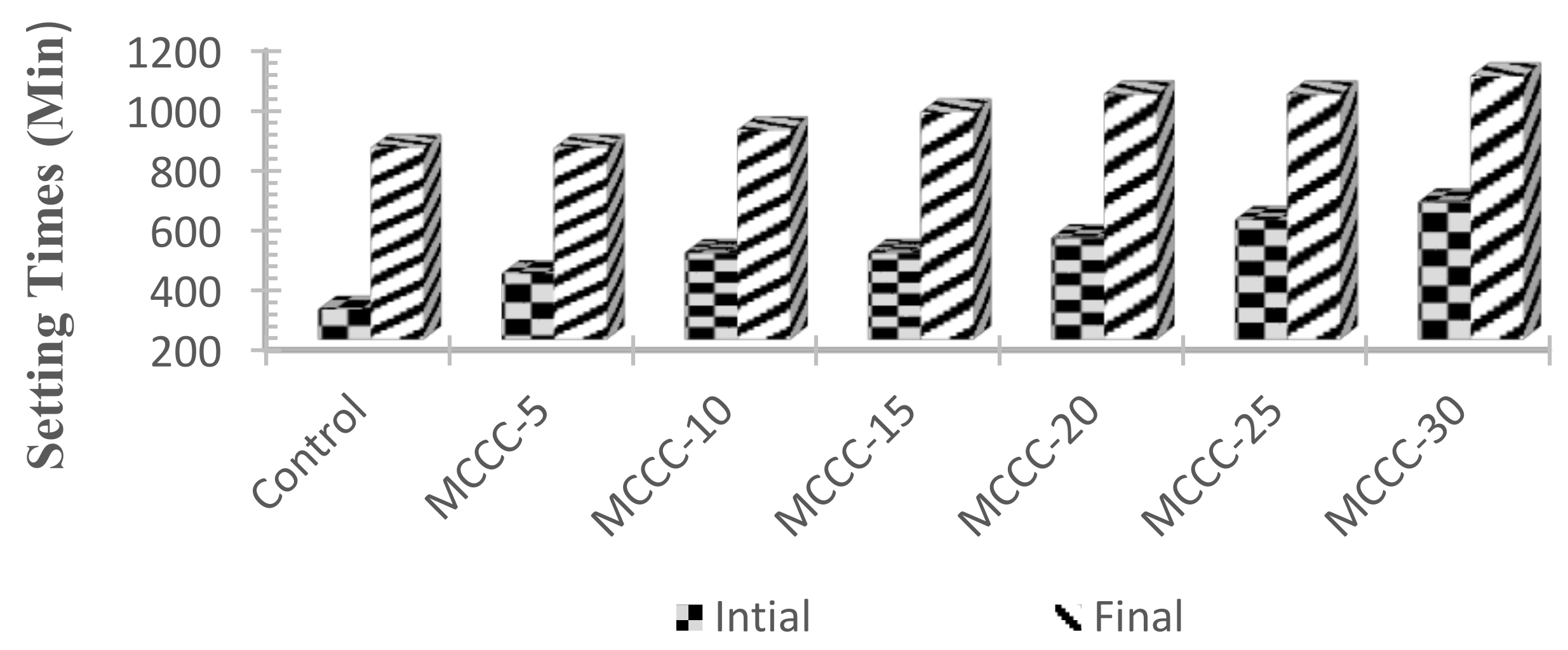
3.2.2. Initial and Final Setting Times
3.3. Mechanical Properties
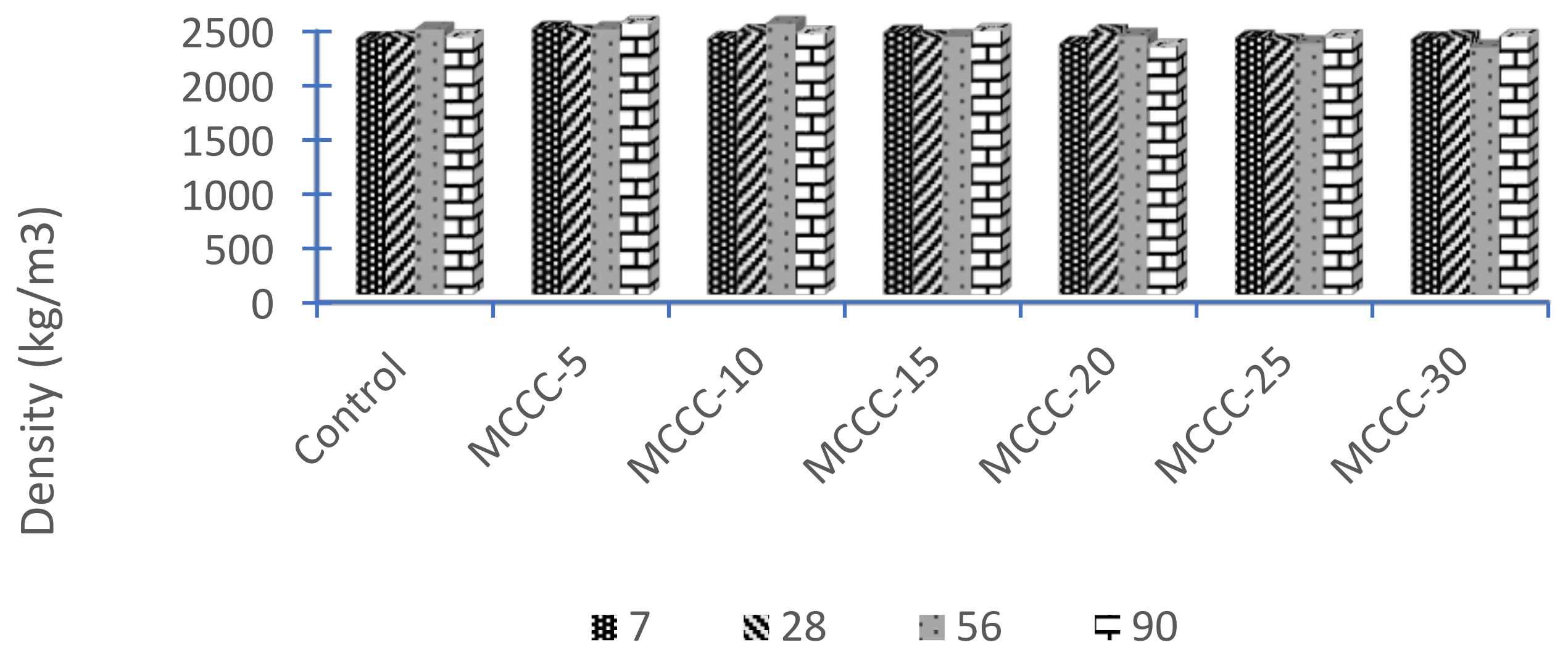
3.3.1. Density of HPC
3.3.2. Compressive Strength of HPC with MCC
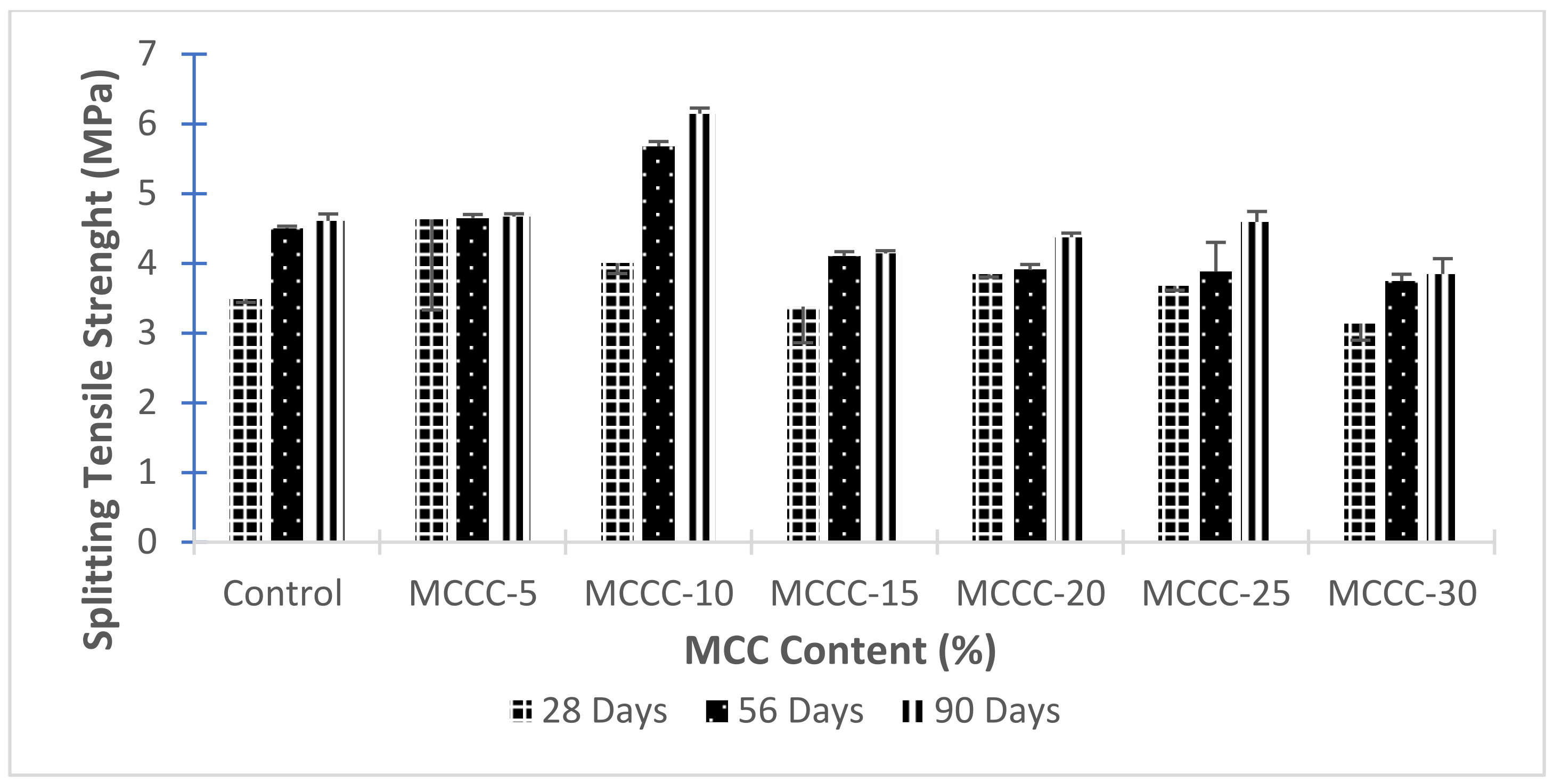
3.3.3. Splitting Tensile Strength of HPCs with MCC
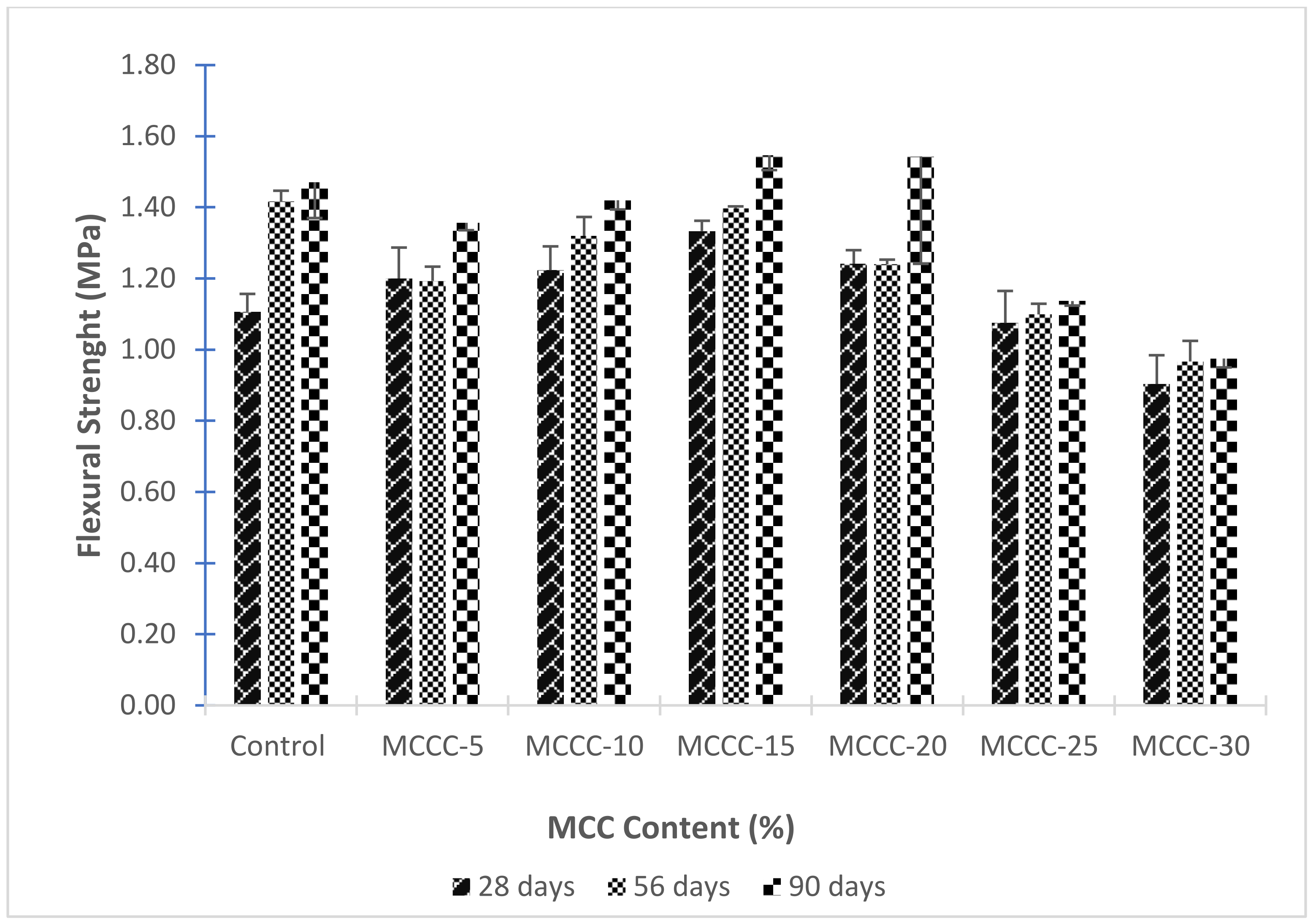
3.3.4. Flexural Strength of HPCs with MCC
3.4. Non-destructive Tests on Hardened HPC Mixtures
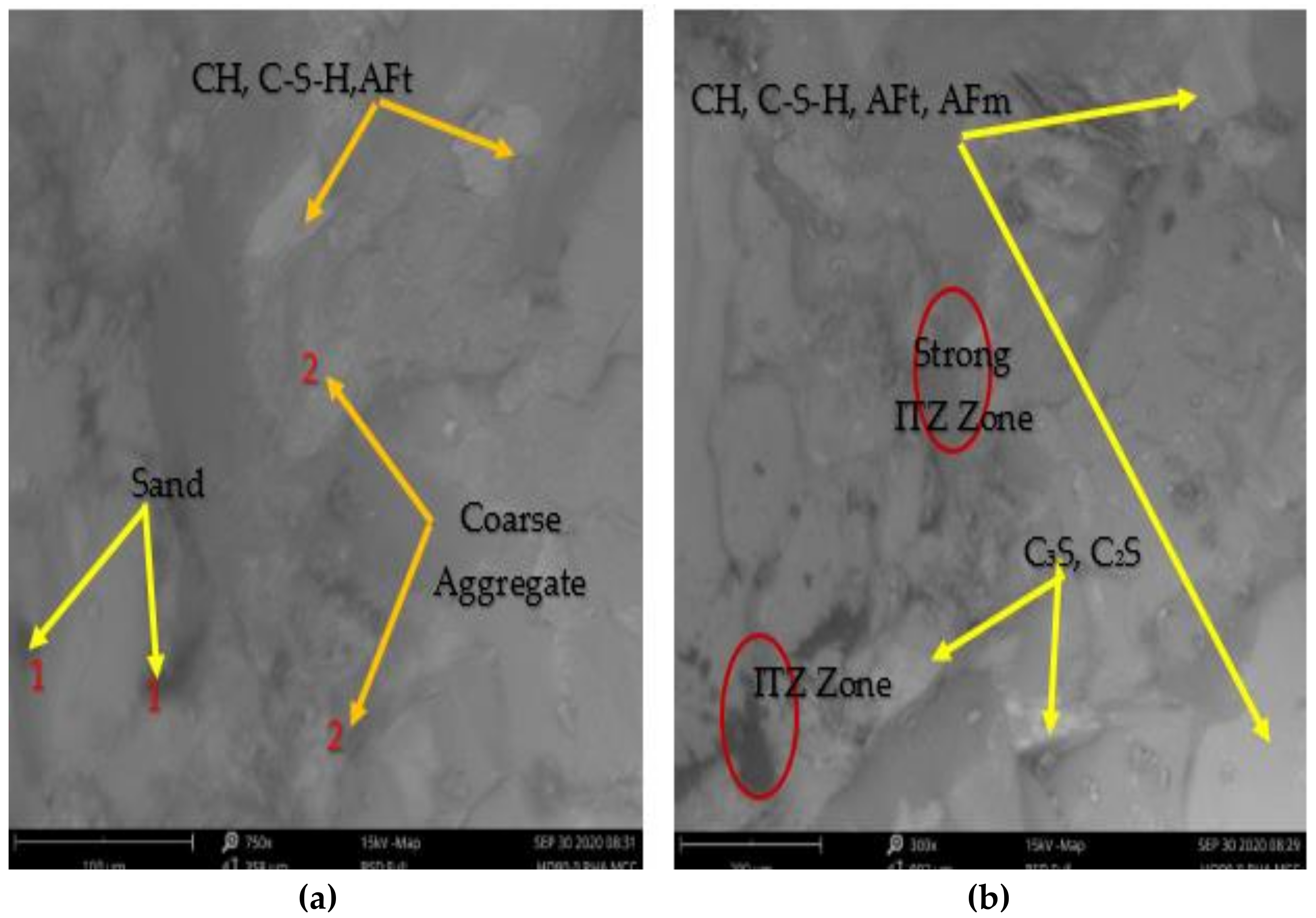
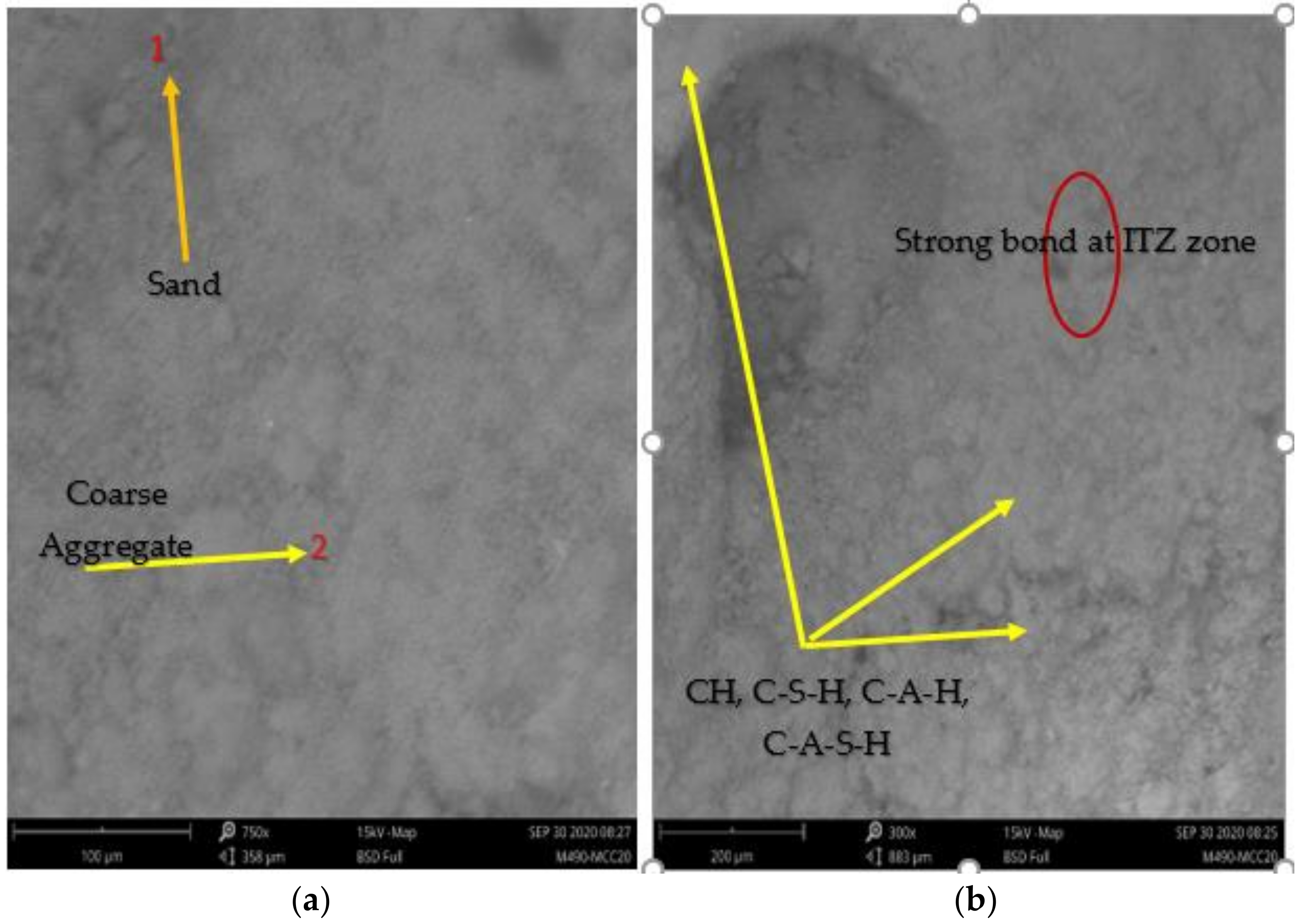
3.4.1. SEM/EDX Analysis
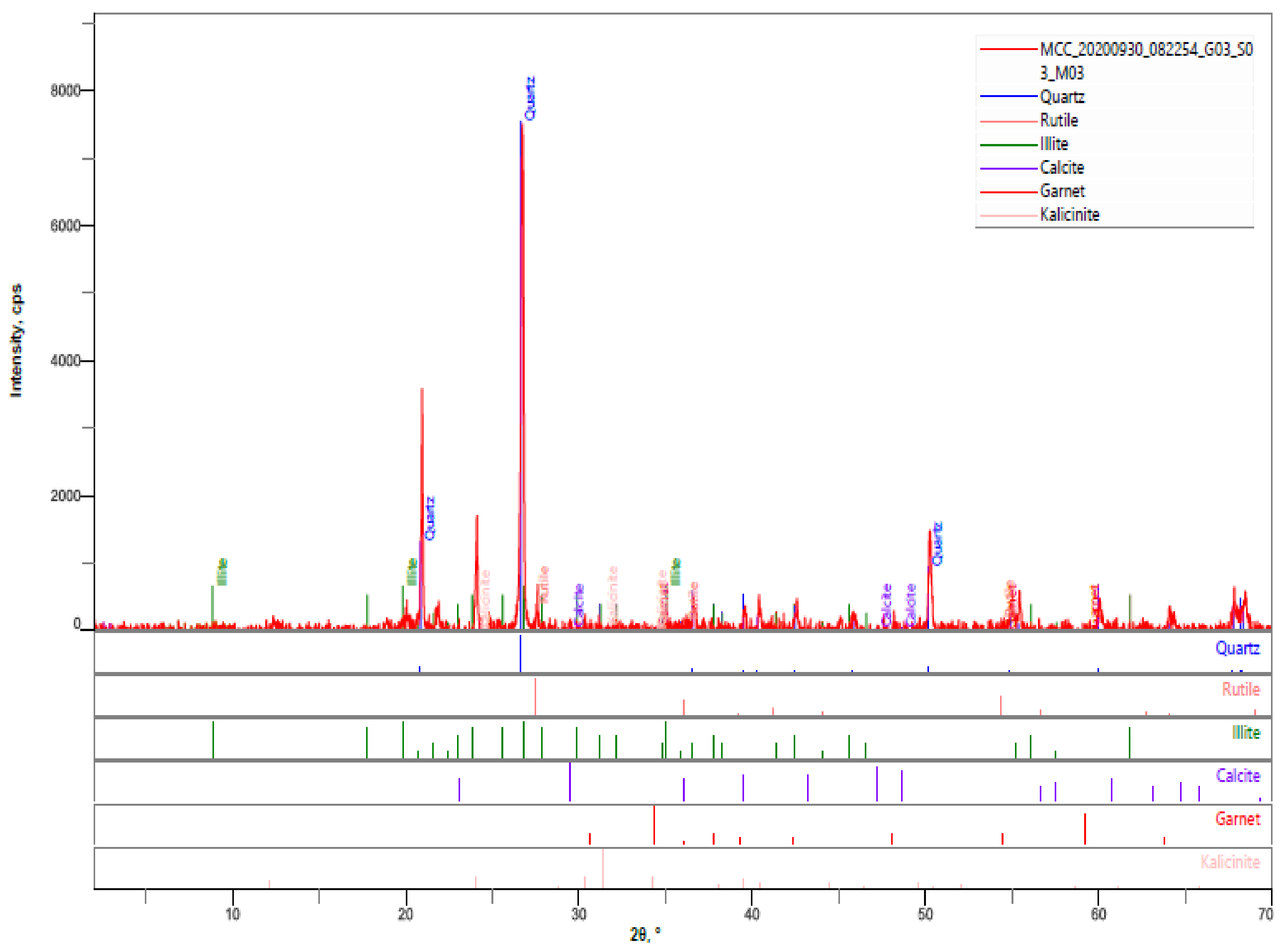
3.4.2. XRD Analysis
4. Conclusions
- The MCCC slump flow is consistent with HPC mixtures having a slump flow range of 450 mm to 600 mm;
- MCC based HPC mixtures showed a gradual increase in the initial and final setting time;
- The average density of the activated HPC samples containing MCC and the control varies from 2390 kg/m3 to 2387 kg/m3 for all ages, conforming to the minimum density of normal weight concrete of 2400 kg/m3;
- The blended CEM II with MCC HPCs could not produce the target designed strength of Class 1 HPC (50–75 MPa) at 28 days, while later age curing showed a promising result. HPC specimen type MCCC-10 had the highest compressive strength at 56- and 90-days curing ages;
- The addition of MCC has a moderate effect on the splitting tensile strength of the HPC, especially at later hydration days. The 10% cement replacement with MCC (MCCC-10) is sufficient to accelerate 28, 56 and 90 days splitting tensile strength of the HPCs;
- The MCC-based cement paste matrix highlights the light grey background fragment spread over the surface, assigned hydration products (C3S, CH, C-S-H, AFm and AFt). EDX result of MCCC-10 and MCCC-20 mixtures showed the dominance of calcium, silicon and oxygen in the elemental atomic weight% compositions, respectively;
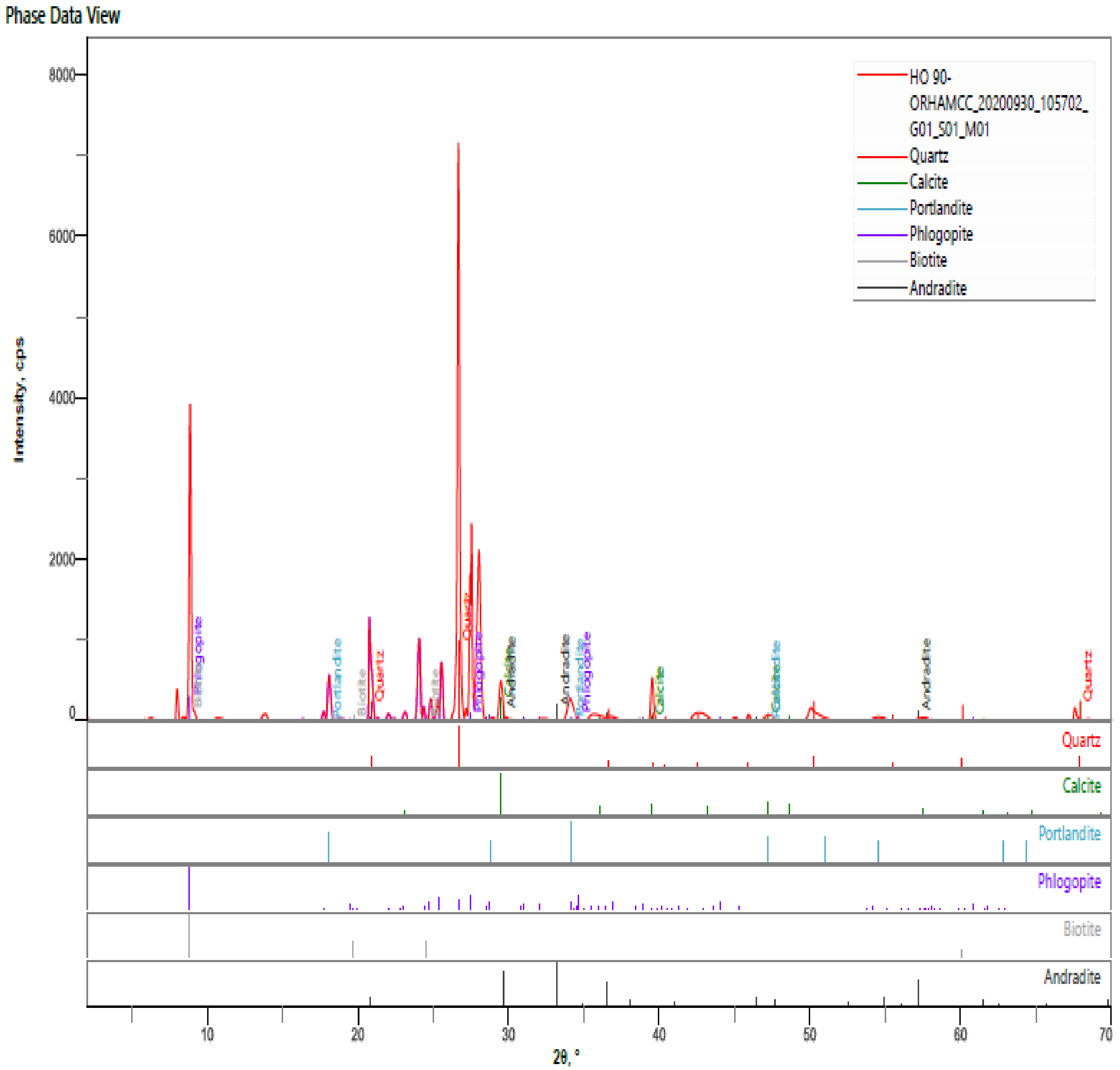
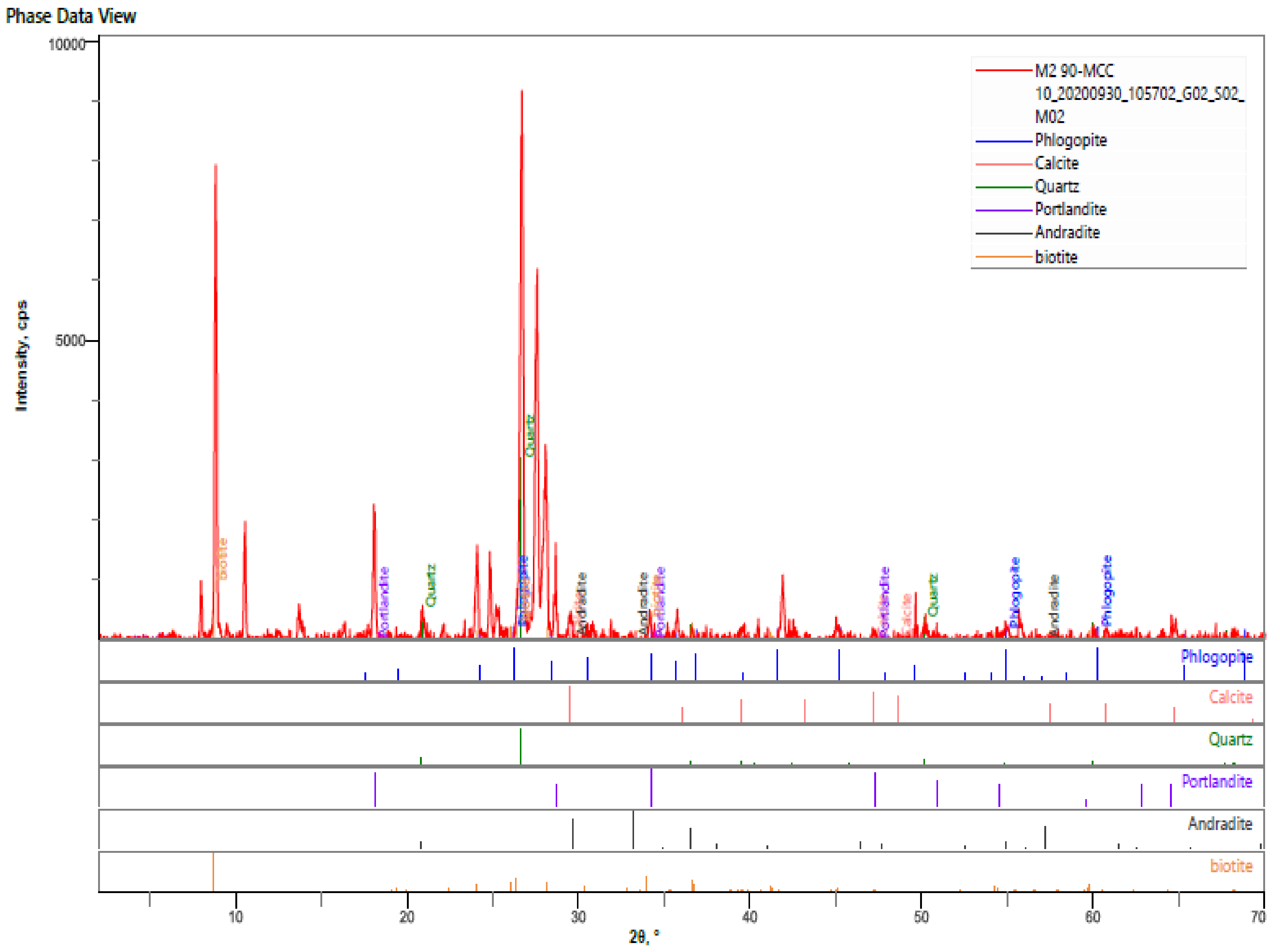
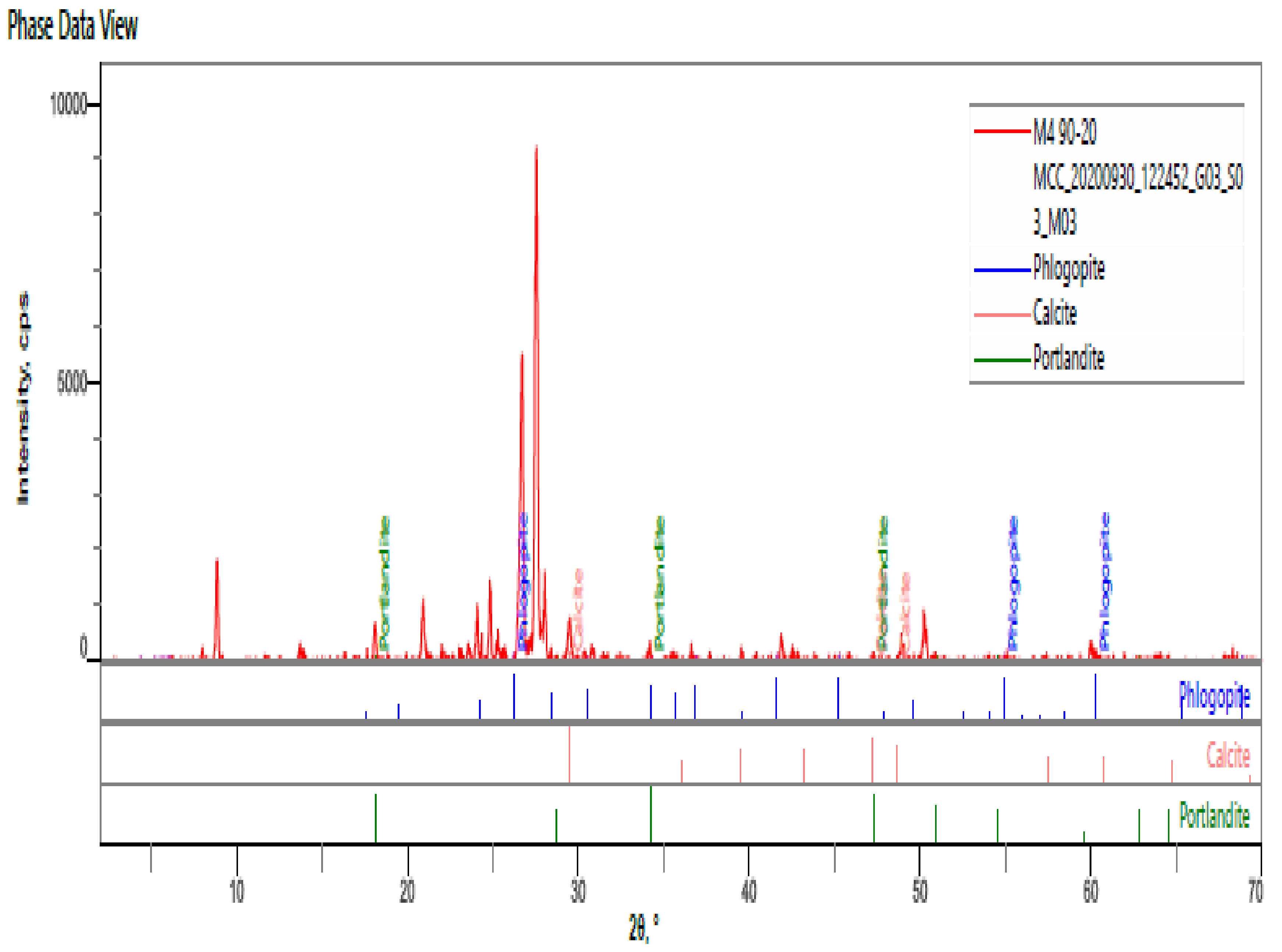
- The hardened HPC control sample at 90 days revealed quartz, calcite, portlandite, phlogopite, biotite, andradite as the major mineral phases of the hardened paste. The percentages intensities of portlandite peaks reduced to 3% for both MCCC-10 and MCCC-20 mixtures, indicating depletion of portlandite in the MCC blended cement with the control sample.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AEC | Architecture, engineering, and construction |
| AFm | Monosulfate |
| AFt | Ettringite |
| BET | Brunauer-Emmett-Teller |
| C2S | Dicalcium silicate |
| C3S | Tricalcium silicate |
| Cc | Coefficient of curvature |
| CEM II | Portland-limestone cement |
| CH | Calcium hydroxide |
| C-S-H | Calcium silicate hydrate |
| Cu | Coefficient of uniformity |
| FM | Fineness modulus |
| FTIR-ATR | Fourier transform infrared-Attenuated total reflection |
| HPC | High-performance concrete |
| ITZ | Interfacial transition zone |
| KyAl4(Si8-y) O20(OH)4 | Illite clay-based mineral |
| MCC | Meta-illite calcined clay |
| NBRRI | Nigeria Building and Road Research Institute |
| PC | Portland cement |
| PCE | Polycarboxylic ether |
| PSD | Particle size distribution |
| SAP | Superabsorbent polymers |
| SCM | Supplementary cementitious material |
| SEM-EDX | Scanning electron microscopy-energy dispersive X-ray |
| SG | Specific gravity |
| SSA | Specific surface area |
| TGA | Thermogravimetric analysis |
| W/B | Water-to-binder ratio |
| XRD | X-ray diffraction |
| XRF | X-ray fluorescence |
References
- Han, C.; Shen, W.; Ji, X.; Wang, Z.; Ding, Q.; Xu, G.; Lv, Z.; Tang, X. Behavior of high performance concrete pastes with different mineral admixtures in simulated seawater environment. Constr. Build. Mater. 2018, 187, 426–438. [Google Scholar] [CrossRef]
- Nduka, D.O.; Ameh, J.O.; Joshua, O.; Ojelabi, R. Awareness and Benefits of Self-Curing Concrete in Construction Projects: Builders and Civil Engineers Perceptions. Buildings 2018, 8, 109. [Google Scholar] [CrossRef] [Green Version]
- Di Bella, C.; Griffa, M.; Ulrich, T.; Lura, P. Early-age elastic properties of cement-based materials as a function of decreasing moisture content. Cem. Concr. Res. 2016, 89, 87–96. [Google Scholar] [CrossRef]
- Aïtcin, P.C. High-Performance Concrete; Taylor & Francis e-Library: New York, NY, USA, 2004. [Google Scholar]
- Abbas, S.; Nehdi, M.L.; Saleem, M.A. Ultra-high-performance concrete: Mechanical performance, durability, sustainability and implementation challenges. Int. J. Concr. Struct. Mater. 2016, 10, 271–295. [Google Scholar] [CrossRef] [Green Version]
- Zhou, D.; Wang, R.; Tyrer, M.; Wong, H.; Cheeseman, C. Sustainable infrastructure development through use of calcined excavated waste clay as a supplementary cementitious material. J. Clean. Prod. 2017, 168, 1180–1192. [Google Scholar] [CrossRef]
- Nwankwo, C.O.; Bamigboye, G.O.; Davies, I.E.; Michaels, T.A. High volume Portland cement replacement: A review. Constr. Build. Mater. 2020, 260, 120445. [Google Scholar] [CrossRef]
- Fapohunda, C.; Akinbile, B.; Shittu, A. Structure and properties of mortar and concrete with rice husk ash as partial replacement of ordinary Portland cement—A review. Int. J. Sustain. Built Environ. 2017, 6, 675–692. [Google Scholar] [CrossRef]
- Chen, Y.; Rodriguez, C.R.; Li, Z.; Chen, B.; Çopuroğlu, O.; Schlangen, E. Effect of different grade levels of calcined clays on fresh and hardened properties of ternary-blended cementitious materials for 3D printing. Cem. Concr. Compos. 2020, 114, 103708. [Google Scholar] [CrossRef]
- Marchetti, G.; Rahhal, V.; Pavlík, Z.; Pavlíková, M.; Irassar, E.F. Assessment of packing, flowability, hydration kinetics, and strength of blended cements with illitic calcined shale. Constr. Build. Mater. 2020, 254, 119042. [Google Scholar] [CrossRef]
- Song, T.; Ren, Z.; Li, H.; Sun, X.; Xue, M.; Yan, S. Modification of illite with calcium pimelate and its in-fluence on the crystallisation and mechanical property of isotactic polypropylene. Compos. Part A Appl. Sci. Manuf. 2019, 123, 200–207. [Google Scholar] [CrossRef]
- Avet, F.; Snellings, R.; Diaz, A.A.; Ben Haha, M.; Scrivener, K. Development of a new rapid, relevant and reliable (R3) test method to evaluate the pozzolanic reactivity of calcined kaolinitic clays. Cem. Concr. Res. 2016, 85, 1–11. [Google Scholar] [CrossRef]
- Trümer, A.; Ludwig, H.-M.; Schellhorn, M.; Diedel, R. Effect of a calcined Westerwald bentonite as supplementary cementitious material on the long-term performance of concrete. Appl. Clay Sci. 2019, 168, 36–42. [Google Scholar] [CrossRef]
- Ferreiro, S.; Herfort, D.; Damtoft, J. Effect of raw clay type, fineness, water-to-cement ratio and fly ash addition on workability and strength performance of calcined clay–Limestone Portland cements. Cem. Concr. Res. 2017, 101, 1–12. [Google Scholar] [CrossRef]
- Irassar, E.F.; Bonavetti, V.L.; Castellano, C.C.; Trezza, M.A.; Rahhal, V.F.; Cordoba, G.; Lemma, R. Calcined illite-chlorite shale as supplementary cementing material: Thermal treatment, grinding, color and pozzolanic activity. Appl. Clay Sci. 2019, 179, 105143. [Google Scholar] [CrossRef]
- Laidani, Z.E.-A.; Benabed, B.; Abousnina, R.; Gueddouda, M.K.; Kadri, E.-H. Experimental investigation on effects of calcined bentonite on fresh, strength and durability properties of sustainable self-compacting concrete. Constr. Build. Mater. 2020, 230, 117062. [Google Scholar] [CrossRef]
- European Committee for Standardization. 197-1: 2011. Cement, Composition, Specifications and Conformity Criteria for Common Cements; British Standard Institution (BSI): London, UK, 2011. [Google Scholar]
- Nigeria Industrial Standard [NIS] 444-1. Composition, Specification and Conformity Criteria for Common Cements; Standards Organization of Nigeria: Abuja, Nigeria, 2004.
- Olawuyi, B.J.; Boshoff, W. Influence of SAP content and curing age on air void distribution of high performance concrete using 3D volume analysis. Constr. Build. Mater. 2017, 135, 580–589. [Google Scholar] [CrossRef]
- European Committee for Standardization. 1008, 2002, Methods of Test for Water for Making Concrete (Including Notes on the Suitability of the Water); British Standards Institutions: London, UK, 2002. [Google Scholar]
- Aïtcin, P.C. High Performance Concrete; CRC Press: Boca Raton, FL, USA, 1998. [Google Scholar]
- Beushausen, H.; Dehn, F. High-Performance Concrete. Fulton’s Concrete Technology, 9th ed.; Cement and Concrete Institute: Midrand, South Africa, 2009; pp. 297–304. [Google Scholar]
- Neville, A.M. Properties of Concrete, 5th ed.; Pearson Educational Limited: London, UK, 2012. [Google Scholar]
- Olawuyi, B.J.; Boshoff, W.P. Influence of superabsorbent polymer on the splitting tensile strength and fracture energy of high-performance concrete. In Proceedings of the MATEC Web of Conferences; EDP Sciences: Paris, France, 2018; Volume 199, p. 11004. [Google Scholar]
- Olawuyi, B.J. The Mechanical Behaviour of High-Performance Concrete with Superabsorbent Polymers (SAP). Ph.D. Thesis, University of Stellenbosch, Stellenbosch, South Africa, 2016. [Google Scholar]
- Mermerdaş, K.; Gesoğlu, M.; Güneyisi, E.; Özturan, T. Strength development of concretes incorporated with metakaolin and different types of calcined kaolins. Constr. Build. Mater. 2012, 37, 766–774. [Google Scholar] [CrossRef]
- Shafiq, N.; Nuruddin, M.F.; Khan, S.U.; Ayub, T. Calcined kaolin as cement replacing material and its use in high strength concrete. Constr. Build. Mater. 2015, 81, 313–323. [Google Scholar] [CrossRef]
- Rodriguez, C.; Tobon, J.I. Influence of calcined clay/limestone, sulfate and clinker proportions on cement performance. Constr. Build. Mater. 2020, 251, 119050. [Google Scholar] [CrossRef]
- Du, H.; Dai Pang, S. High-performance concrete incorporating calcined kaolin clay and limestone as cement substitute. Constr. Build. Mater. 2020, 264, 120152. [Google Scholar] [CrossRef]
- Cordoba, G.; Irassar, E.F. Sulfate performance of calcined illitic shales. Constr. Build. Mater. 2021, 291, 123215. [Google Scholar] [CrossRef]
- BSI. BS EN 12390-3, 5 & 6: Testing Hardened Concrete. Making and Curing Specimens for Strength Tests; BSI: London, UK, 2009. [Google Scholar]
- European Committee for Standardization. 12350-5. 2009. Testing Fresh Concrete-Part 5: Flow Table Test; European Committee for Standardization: Brussels, Belgium, 2009. [Google Scholar]
- ASTM. ASTM C 403: Standard Test Method for Time of Setting of Concrete Mixtures by Penetration Resistance; ASTM; BSI: West Conshohocken, PA, USA, 2008. [Google Scholar]
- BS EN 12390-3: 2019: Testing Hardened Concrete. Compressive Strength of Test Specimens; BSI: London, UK, 2019.
- International Union of Testing and Research Laboratories for Materials and Structures (RILEM). CPC4—Compressive Strength of Concrete 1975, TC14-CPC, RILEM Technical Recommendations for the Testing and Use of Construction Materials; E & FN, Spon: London, UK, 1994; pp. 17–18. [Google Scholar]
- BS EN 12390-5: 2019: Testing Hardened Concrete. Flexural Strength of Test Specimens; BSI: London, UK, 2019.
- Hollanders, S.; Adriaens, R.; Skibsted, J.; Cizer, Ö.; Elsen, J. Pozzolanic reactivity of pure calcined clays. Appl. Clay Sci. 2016, 132, 552–560. [Google Scholar] [CrossRef]
- Ihekweme, G.O.; Shondo, J.N.; Orisekeh, K.I.; Kalu-Uka, G.M.; Nwuzor, I.C.; Onwualu, A.P. Characterisation of certain Nigerian clay minerals for water purification and other industrial applications. Heliyon 2020, 6, e03783. [Google Scholar] [CrossRef]
- Zhou, D. Developing Supplementary Cementitious Materials from Waste London Clay. Ph.D. Thesis, Imperial College of London, London, UK, 2016. [Google Scholar]
- Garg, N.; Skibsted, J. Pozzolanic reactivity of a calcined interstratified illite/smectite (70/30) clay. Cem. Concr. Res. 2016, 79, 101–111. [Google Scholar] [CrossRef]
- Mehta, P.K.; Monteiro, P.J. Concrete: Microstructure, Properties, and Materials; McGraw-Hill Education: New York, NY, USA, 2014. [Google Scholar]
- Elgamouz, A.; Tijani, N.; Shehadi, I.; Hasan, K.; Kawam MA, F. Characterisation of the firing behaviour of an illite-kaolinite clay mineral and its potential use as membrane support. Heliyon 2019, 5, e02281. [Google Scholar]
- Ma, Y.; Shi, C.; Lei, L.; Sha, S.; Zhou, B.; Liu, Y.; Xiao, Y. Research progress on polycarboxylate based superplasticisers with tolerance to clays—A review. Constr. Build. Mater. 2020, 255, 119386. [Google Scholar] [CrossRef]
- Cai, R.; He, Z.; Tang, S.; Wu, T.; Chen, E. The early hydration of metakaolin blended cements by non-contact impedance measurement. Cem. Concr. Compos. 2018, 92, 70–81. [Google Scholar] [CrossRef]
- Schulze, S.E.; Rickert, J. Suitability of natural calcined clays as supplementary cementitious material. Cem. Concr. Compos. 2019, 95, 92–97. [Google Scholar] [CrossRef]
- Vejmelková, E.; Koňáková, D.; Doleželová, M.; Scheinherrová, L.; Svora, P.; Keppert, M.; Černý, R. Effect of cal-cined Czech claystone on the properties of high performance concrete: Microstructure, strength and durability. Constr. Build. Mater. 2018, 168, 966–974. [Google Scholar] [CrossRef]
- Mindess, S.; Young, F.J.; Darwin, D. Concrete, 2nd ed.; Prentile Hall: Prentile Hall, NJ, USA, 2003; p. 644. [Google Scholar]



| Constituents | Mix Blends (kg/m3) | ||||||
|---|---|---|---|---|---|---|---|
| Control | MCCC-5 | MCCC-10 | MCCC-15 | MCCC-20 | MCCC-25 | MCCC-30 | |
| Water | 156 | 156 | 156 | 156 | 156 | 156 | 156 |
| Cement (CEM II) | 540 | 513 | 486 | 459 | 432 | 405 | 378 |
| MCC | 0 | 27 | 54 | 81 | 108 | 135 | 162 |
| Coarse aggregate | 1050 | 1050 | 1050 | 1050 | 1050 | 1050 | 1050 |
| Sand (≥300 µm) | 700 | 700 | 700 | 700 | 700 | 700 | 700 |
| SAP (0.3% bwob) | 1.62 | 1.62 | 1.62 | 1.62 | 1.62 | 1.62 | 1.62 |
| Superplastiser (1.5% bwob) | 8.10 | 8.10 | 8.10 | 8.10 | 8.10 | 8.10 | 8.10 |
| Water/binder (W/B) * | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 |
| Additional water | 20.30 | 20.30 | 20.30 | 20.30 | 20.30 | 20.30 | 20.30 |
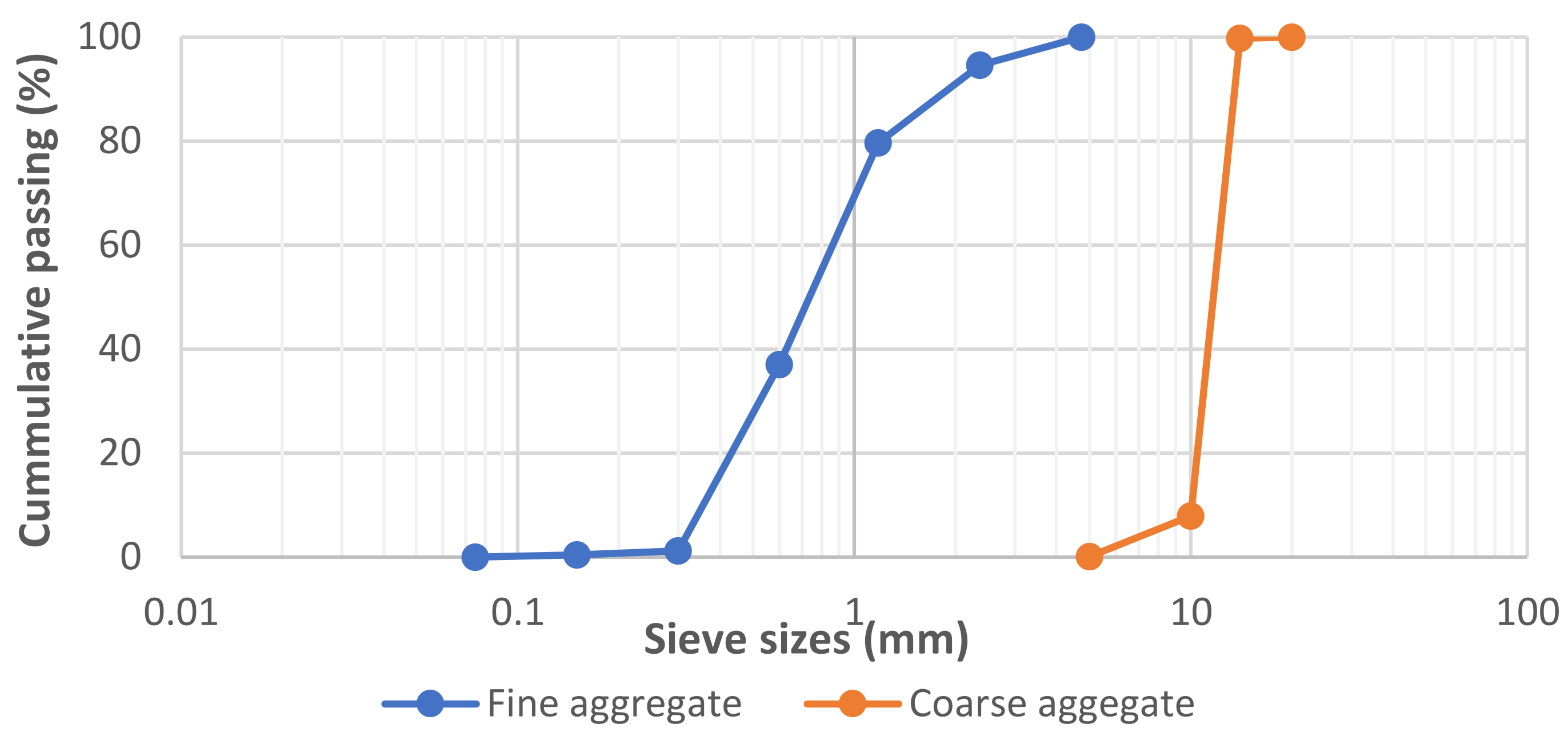
| Properties | Sand | Granite |
|---|---|---|
| Fineness modulus | 2.87 | - |
| Specific gravity | 2.65 | 2.7 |
| Water absorption, % | 1.44 | 1.26 |
| Aggregate crushing value, % | - | 28 |
| Aggregate impact value, % | - | 11 |
| D10 | 360 | 10,000 |
| D30 | 540 | 11,000 |
| D60 | 860 | 13,000 |
| Cu | 2.39 | 1.30 |
| Cc | 0.94 | 0.93 |
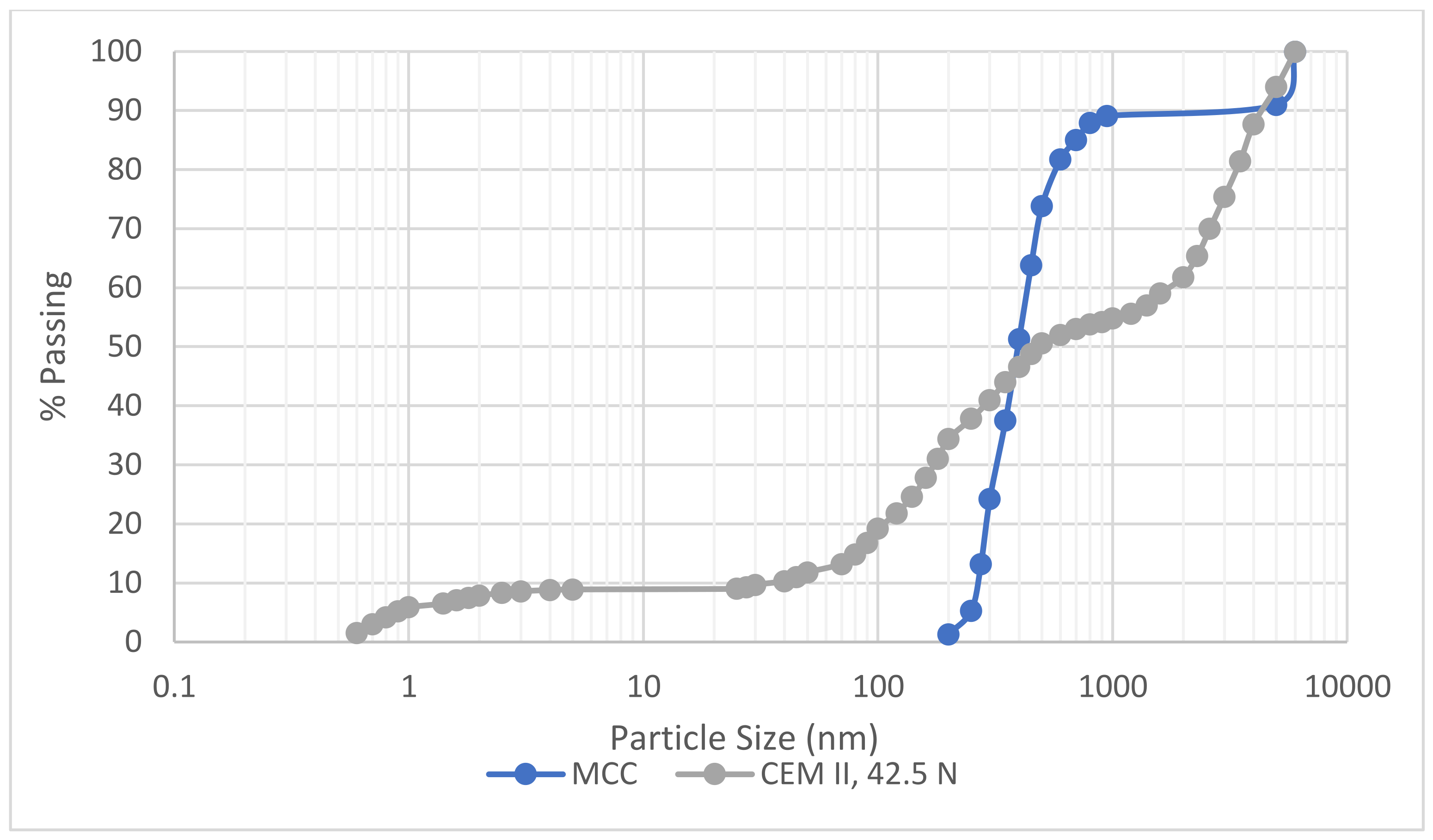
| Binders | Properties | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| BET SSA (m2/g) | Specific Gravity | Initial and Final Setting Time (min) | Soundness (%) | D90 | D50 | D10 | Pore Diameter Mode—DA (nm) | ||
| SinglePoint | MultiPoint | ||||||||
| CEM II | 5.590 × 102 | 8.182 × 102 | 3.12 | 90 and 205 | 0.75 | 4000 | 48.8 | 4.88 | 2.92 |
| MCC | 3.026 × 102 | 4.649 × 102 | 2.81 | 950 | 450 | 275 | 2.88 | ||
| Oxides | MCC (%) | CEM II (%) |
|---|---|---|
| SiO2 | 60.92 | 15.38 |
| Al2O3 | 20.92 | 4.14 |
| Fe2O3 | 3.43 | 3.19 |
| CaO | 1.38 | 56.92 |
| MgO | 0.24 | 2.44 |
| SO3 | 0.00 | 1.59 |
| K2O | 0.35 | 0.21 |
| Na2O | 0.00 | 0.04 |
| M2O5 | 0.12 | 0.04 |
| P2O5 | 0.18 | 0.28 |
| TiO2 | 3.24 | 0.21 |
| LOI | 7.68 | 15.59 |
| SiO2 + Al2O3 + Fe2O3 | 85.27 | 22.71 |
| Total | 98.46 | 100.00 |
| Element Symbol | Element Name | Atomic Concentration | ||
|---|---|---|---|---|
| Control | MCCC-10 | MCCC-20 | ||
| Si | Silicon | 34.35 | 28.72 | 5.08 |
| Ca | Calcium | 18.78 | 38.99 | 70.90 |
| O | Oxygen | 22.24 | 22.92 | 15.37 |
| Fe | Iron | 5.11 | 2.69 | 1.20 |
| Al | Aluminium | 10.20 | 1.43 | 1.18 |
| K | Potassium | 2.94 | 1.30 | 1.19 |
| Na | Sodium | 2.59 | 0.00 | 0.37 |
| Ti | Titanium | 1.13 | 0.00 | 0.51 |
| Ag | Silver | 0.50 | 0.58 | 0.46 |
| S | Sulfur | 0.51 | 0.95 | 0.60 |
| P | Phosphorus | 0.52 | 0.93 | 0.60 |
| Mg | Magnesium | 0.62 | 0.25 | 0.59 |
| ‘C | Carbon | 0.53 | 0.33 | 0.65 |
| Cl | Chlorine | 0.00 | 0.90 | 0.76 |
| Zn | Zinc | 0.00 | 0.00 | 0.54 |
| Ti | Titanium | 0.00 | 0.00 | 0.51 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nduka, D.O.; Olawuyi, B.J.; Fagbenle, O.I.; Fonteboa, B.G. Effect of KyAl4(Si8-y) O20(OH)4 Calcined Based-Clay on the Microstructure and Mechanical Performances of High-Performance Concrete. Crystals 2021, 11, 1152. https://doi.org/10.3390/cryst11101152
Nduka DO, Olawuyi BJ, Fagbenle OI, Fonteboa BG. Effect of KyAl4(Si8-y) O20(OH)4 Calcined Based-Clay on the Microstructure and Mechanical Performances of High-Performance Concrete. Crystals. 2021; 11(10):1152. https://doi.org/10.3390/cryst11101152
Chicago/Turabian StyleNduka, David O., Babatunde J. Olawuyi, Olabosipo I. Fagbenle, and Belén G. Fonteboa. 2021. "Effect of KyAl4(Si8-y) O20(OH)4 Calcined Based-Clay on the Microstructure and Mechanical Performances of High-Performance Concrete" Crystals 11, no. 10: 1152. https://doi.org/10.3390/cryst11101152
APA StyleNduka, D. O., Olawuyi, B. J., Fagbenle, O. I., & Fonteboa, B. G. (2021). Effect of KyAl4(Si8-y) O20(OH)4 Calcined Based-Clay on the Microstructure and Mechanical Performances of High-Performance Concrete. Crystals, 11(10), 1152. https://doi.org/10.3390/cryst11101152






