The Crystallization Process of Vaterite Microdisc Mesocrystals via Proto-Vaterite Amorphous Calcium Carbonate Characterized by Cryo-X-ray Absorption Spectroscopy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Synthesis
2.3. X-ray Absorption Spectroscopy (XAS) Measurement
3. Results and Discussion
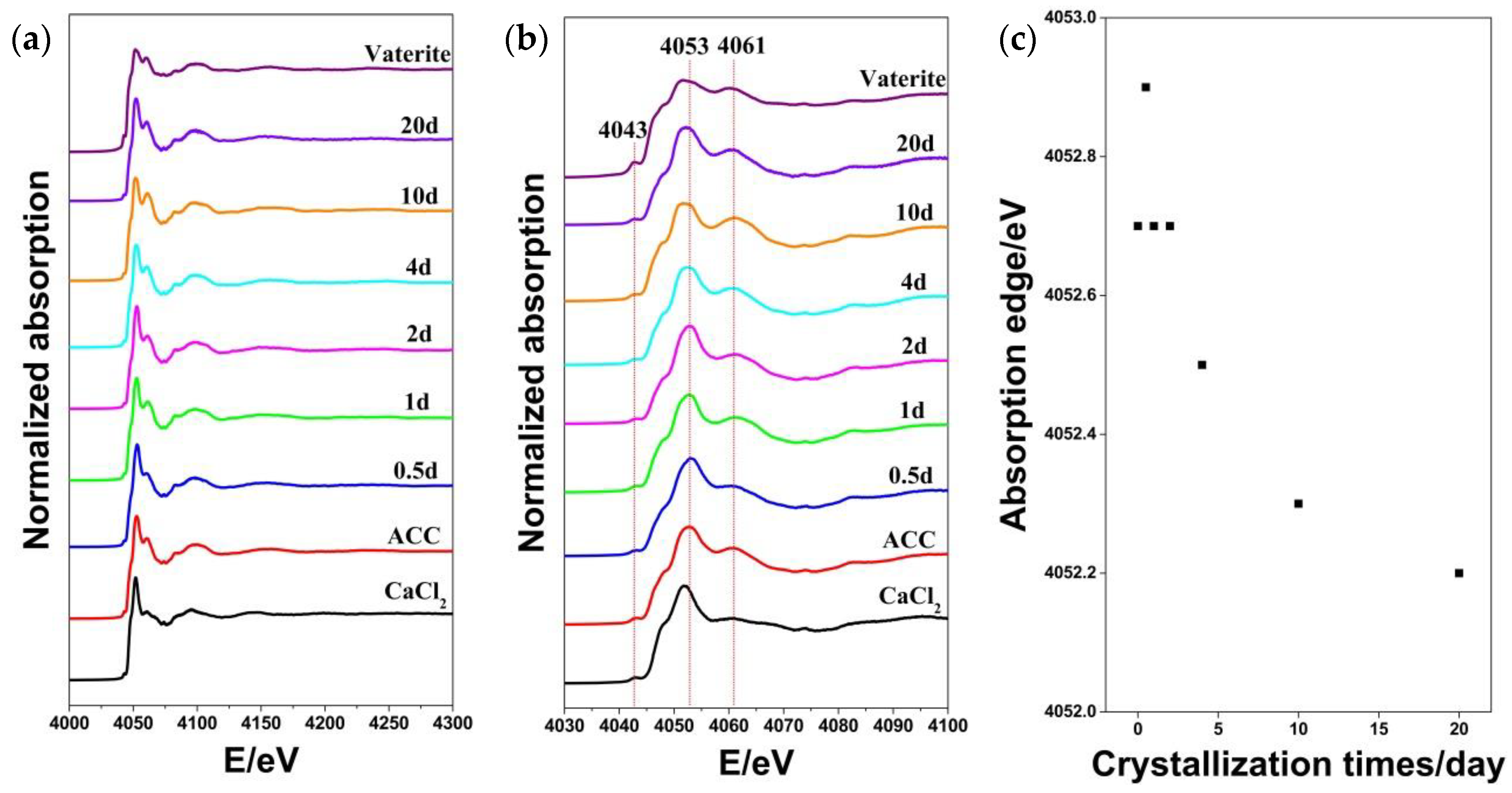
3.1. Ca K-Edge X-ray Absorption Near Edge Structure (XANES) Spectra
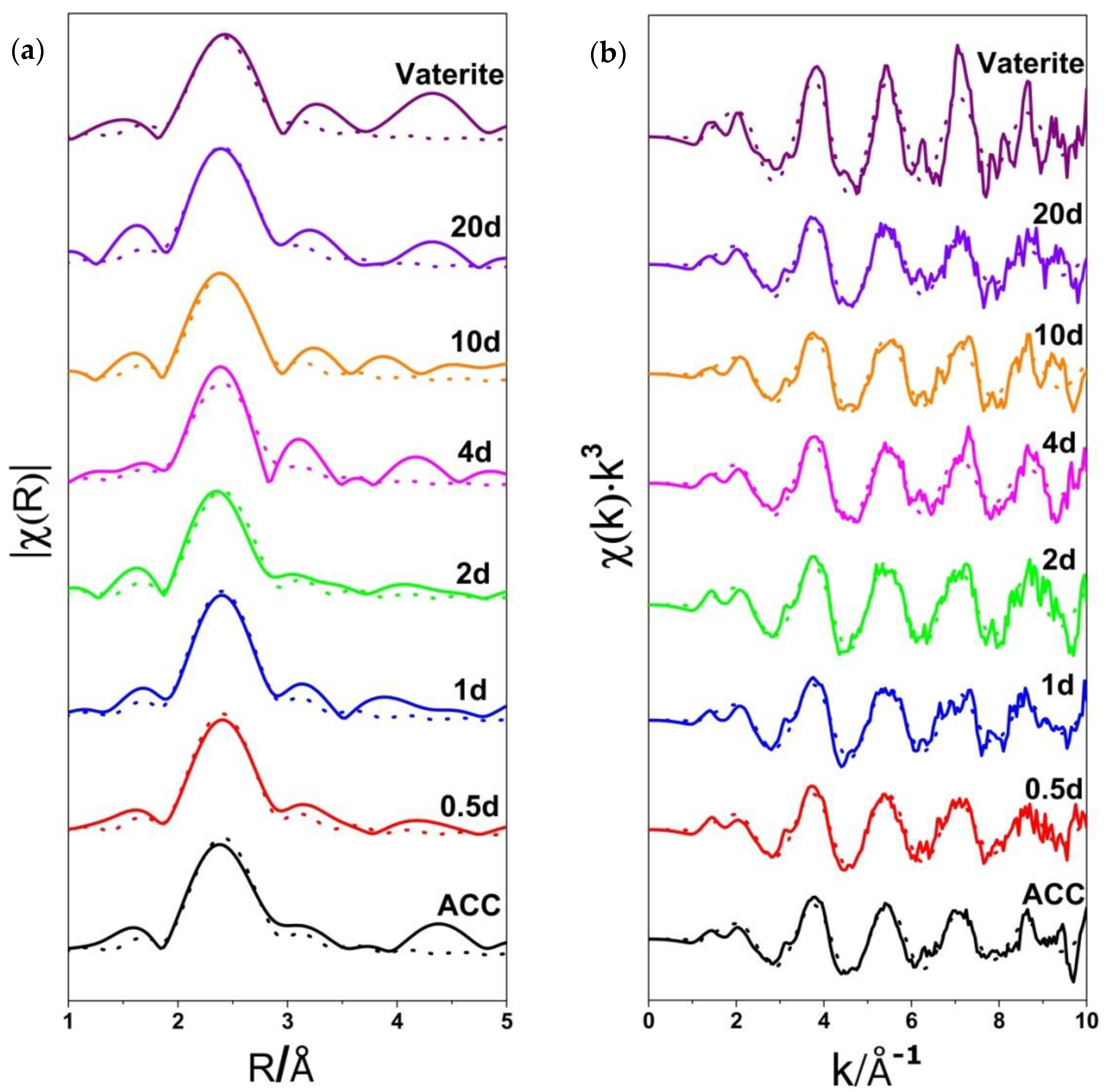
3.2. Ca K-Edge Extended X-ray Absorption Fine Structure (EXAFS) Spectra
3.3. Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A

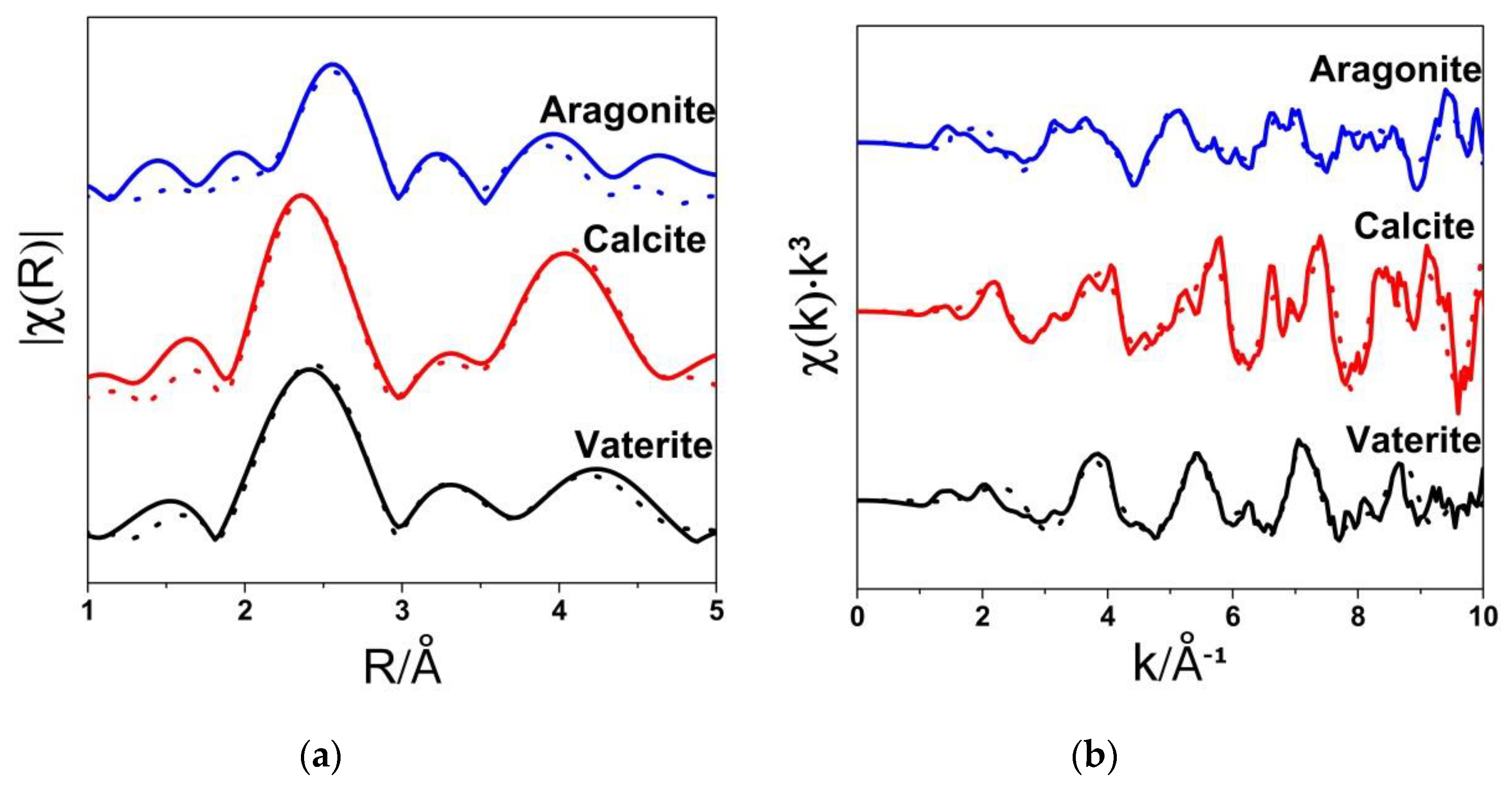
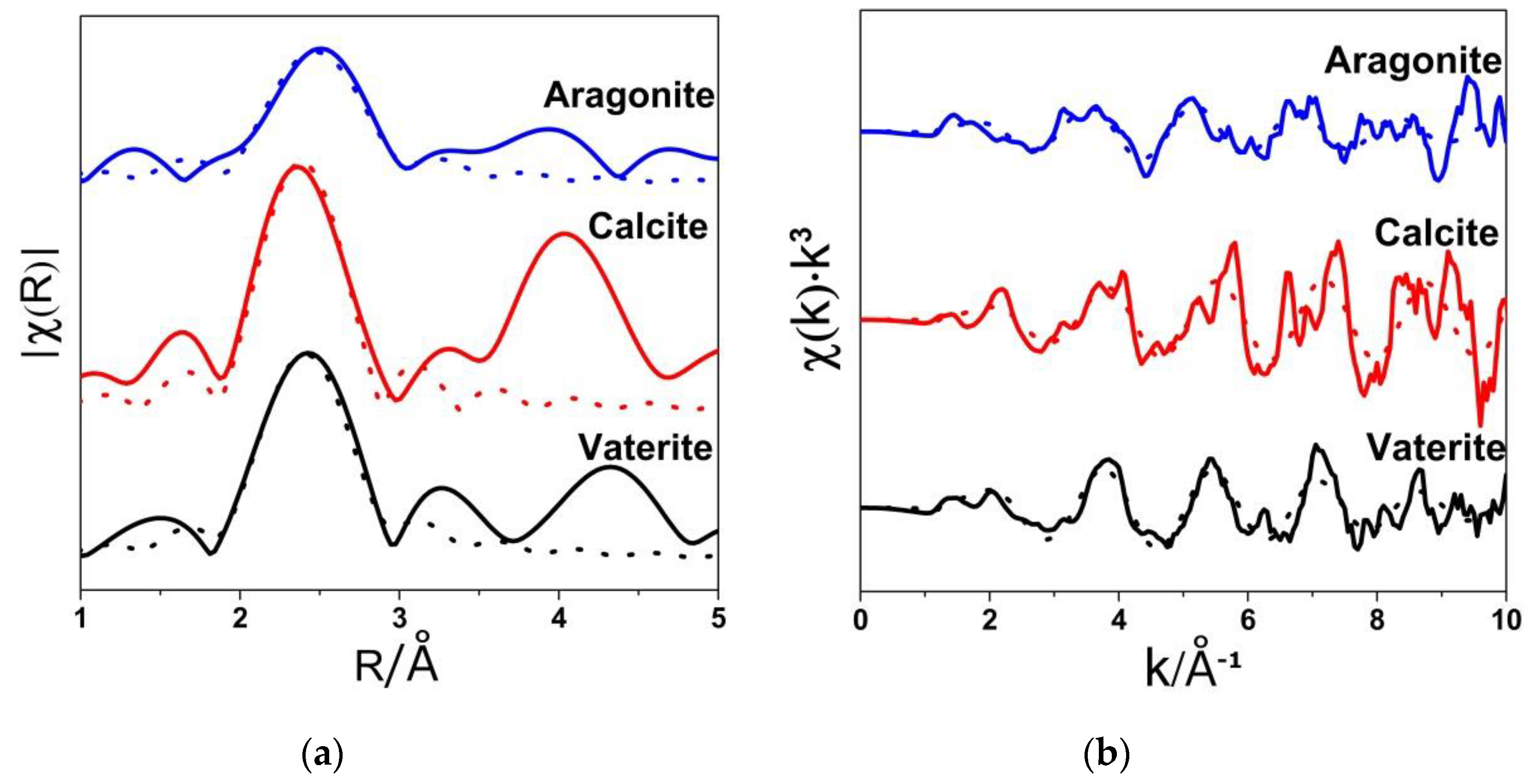
| Sample | N | R(Å) | 103σ2(Å2) |
|---|---|---|---|
| Vaterite | 6 | 2.38 | 6 |
| Calcite | 6 | 2.35 | 0.3 |
| Aragonite | 9 | 2.47 | 0.9 |
References
- Sommerdijk, N.A.J.M.; de With, G. Biomimetic CaCO3 Mineralization using Designer Molecules and Interfaces. Chem. Rev. 2008, 108, 4499–4550. [Google Scholar] [CrossRef] [PubMed]
- Koishi, A.; Fernandez-Martinez, A.; Van Driessche, A.E.S.; Michot, L.J.; Pina, C.M.; Pimentel, C.; Lee, B.; Montes-Hernandez, G. Surface Wetting Controls Calcium Carbonate Crystallization Kinetics. Chem. Mater. 2019, 31, 3340–3348. [Google Scholar] [CrossRef]
- Falini, G.; Albeck, S.; Weiner, S.; Addadi, L. Control of aragonite or calcite polymorphism by mollusk shell macromolecules. Science 1996, 271, 67–69. [Google Scholar] [CrossRef]
- Nemeth, P.; Mugnaioli, E.; Gemmi, M.; Czuppon, G.; Demeny, A.; Spotl, C. A nanocrystalline monoclinic CaCO3 precursor of metastable aragonite. Sci. Adv. 2018, 4, 6178–6190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kabalah-Amitai, L.; Mayzel, B.; Kauffmann, Y.; Fitch, A.N.; Bloch, L.; Gilbert, P.U.P.A.; Pokroy, B. Vaterite Crystals Contain Two Interspersed Crystal Structures. Science 2013, 340, 454–457. [Google Scholar] [CrossRef] [Green Version]
- Zou, Z.Y.; Habraken, W.J.E.M.; Matveeva, G.; Jensen, A.C.S.; Bertinetti, L.; Hood, M.A.; Sun, C.Y.; Gilbert, P.U.P.A.; Polishchuk, I.; Pokroy, B.; et al. A hydrated crystalline calcium carbonate phase: Calcium carbonate hemihydrate. Science 2019, 363, 396–400. [Google Scholar] [CrossRef]
- Gebauer, D.; Gunawidjaja, P.N.; Ko, J.Y.; Bacsik, Z.; Aziz, B.; Liu, L.; Hu, Y.; Bergstrom, L.; Tai, C.W.; Sham, T.K.; et al. Proto-calcite and proto-vaterite in amorphous calcium carbonates. Angew. Chem. Int. Ed. 2010, 49, 8889–8891. [Google Scholar] [CrossRef]
- Du, H.; Steinacher, M.; Borca, C.; Huthwelker, T.; Murello, A.; Stellacci, F.; Amstad, E. Amorphous CaCO3: Influence of the Formation Time on Its Degree of Hydration and Stability. J. Am. Chem. Soc. 2018, 140, 14289–14299. [Google Scholar] [CrossRef] [Green Version]
- DeVol, R.T.; Sun, C.Y.; Marcus, M.A.; Coppersmith, S.N.; Myneni, S.C.B.; Gilbert, P.U.P.A. Nanoscale Transforming Mineral Phases in Fresh Nacre. J. Am. Chem. Soc. 2015, 137, 13325–13333. [Google Scholar] [CrossRef]
- Farhadi-Khouzani, M.; Chevrier, D.M.; Zhang, P.; Hedin, N.; Gebauer, D. Water as the Key to Proto-Aragonite Amorphous CaCO3. Angew. Chem. Int. Ed. 2016, 55, 8117–8120. [Google Scholar] [CrossRef]
- Walker, J.M.; Marzec, B.; Nudelman, F. Solid-State Transformation of Amorphous Calcium Carbonate to Aragonite Captured by CryoTEM. Angew. Chem. Int. Ed. 2017, 56, 11740–11743. [Google Scholar] [CrossRef] [PubMed]
- Levi-Kalisman, Y.; Raz, S.; Weiner, S.; Addadi, L.; Sagi, I. Structural differences between biogenic amorphous calcium carbonate phases using X-ray absorption spectroscopy. Adv. Funct. Mater. 2002, 12, 43–48. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Y.; Xie, H.; Su, B.L.; Yao, B.; Yin, Y.X.; Li, S.P.; Chen, F.; Fu, Z.Y. Calcium Carbonate Nanoplate Assemblies with Directed High-Energy Facets: Additive-Free Synthesis, High Drug Loading, and Sustainable Releasing. ACS Appl. Mater. Interfaces 2015, 7, 15686–15691. [Google Scholar] [CrossRef] [PubMed]
- Schenk, A.S.; Cantaert, B.; Kim, Y.Y.; Li, Y.T.; Read, E.S.; Semsarilar, M.; Armes, S.P.; Meldrum, F.C. Systematic Study of the Effects of Polyamines on Calcium Carbonate Precipitation. Chem. Mater. 2014, 26, 2703–2711. [Google Scholar] [CrossRef]
- Li, X.Q.; Zeng, H.C. Calcium Carbonate Nanotablets: Bridging Artificial to Natural Nacre. Adv. Mater. 2012, 24, 6277–6282. [Google Scholar] [CrossRef]
- Imai, H.; Tochimoto, N.; Nishino, Y.; Takezawa, Y.; Oaki, Y. Oriented Nanocrystal Mosaic in Monodispersed CaCO3 Microspheres with Functional Organic Molecules. Cryst. Growth Des. 2012, 12, 876–882. [Google Scholar] [CrossRef]
- Sand, K.K.; Rodriguez-Blanco, J.D.; Makovicky, E.; Benning, L.G.; Stipp, S.L.S. Crystallization of CaCO3 in Water-Alcohol Mixtures: Spherulitic Growth, Polymorph Stabilization, and Morphology Change. Cryst. Growth Des. 2012, 12, 842–853. [Google Scholar] [CrossRef]
- Zhang, J.; Yao, B.; Ping, H.; Fu, Z.Y.; Li, Y.; Wang, W.M.; Wang, H.; Wang, Y.C.; Zhang, J.Y.; Zhang, F. Template-free synthesis of hierarchical porous calcium carbonate microspheres for efficient water treatment. RSC Adv. 2016, 6, 472–480. [Google Scholar] [CrossRef]
- Sun, R.; Willhammar, T.; Grape, E.S.; Stromme, M.; Cheung, O. Mesoscale Transformation of Amorphous Calcium Carbonate to Porous Vaterite Microparticles with Morphology Control. Cryst. Growth Des. 2019, 19, 5075–5087. [Google Scholar] [CrossRef]
- Magnabosco, G.; Polishchuk, I.; Pokroy, B.; Rosenberg, R.; Cölfen, H.; Falini, G. Synthesis of calcium carbonate in trace water environments. Chem. Commun. 2017, 53, 4811–4814. [Google Scholar] [CrossRef] [Green Version]
- Rugabirwa, B.; Murindababisha, D.; Wang, H.T.; Li, J. A High-Pressure Gas Solid Carbonation Route to Produce Vaterite. Cryst. Growth Des. 2019, 19, 242–248. [Google Scholar] [CrossRef]
- Lu, H.; Lutz, H.; Roeters, S.J.; Hood, M.A.; Schafer, A.; Munoz-Espi, R.; Berger, R.; Bonn, M.; Weidner, T. Calcium-Induced Molecular Rearrangement of Peptide Folds Enables Biomineralization of Vaterite Calcium Carbonate. J. Am. Chem. Soc. 2018, 140, 2793–2796. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Pacella, M.S.; Athanasiadou, D.; Nelea, V.; Vali, H.; Hazen, R.M.; Gray, J.J.; McKee, M.D. Chiral acidic amino acids induce chiral hierarchical structure in calcium carbonate. Nat. Commun. 2017, 8, 15066. [Google Scholar] [CrossRef] [PubMed]
- Svenskaya, Y.I.; Fattah, H.; Inozemtseva, O.A.; Ivanova, A.G.; Shtykov, S.N.; Gorin, D.A.; Parakhonskiy, B.V. Key Parameters for Size- and Shape-Controlled Synthesis of Vaterite Particles. Cryst. Growth Des. 2018, 18, 331–337. [Google Scholar] [CrossRef]
- Niederberger, M.; Cölfen, H. Oriented attachment and mesocrystals: Non-classical crystallization mechanisms based on nanoparticle assembly. Phys. Chem. Chem. Phys. 2006, 8, 3271–3287. [Google Scholar] [CrossRef]
- Jehannin, M.; Rao, A.; Cölfen, H. New Horizons of Nonclassical Crystallization. J. Am. Chem. Soc. 2019, 141, 10120–10136. [Google Scholar] [CrossRef]
- Xto, J.; Wetter, R.; Borca, C.N.; Frieh, C.; van Bokhoven, J.A.; Huthwelker, T. Droplet-based in situ X-ray absorption spectroscopy cell for studying crystallization processes at the tender X-ray energy range. RSC Adv. 2019, 9, 34004–34010. [Google Scholar] [CrossRef] [Green Version]
- Pouget, E.M.; Bomans, P.H.H.; Dey, A.; Frederik, P.M.; de With, G.; Sommerdijk, N.A.J.M. The Development of Morphology and Structure in Hexagonal Vaterite. J. Am. Chem. Soc. 2010, 132, 11560–11565. [Google Scholar] [CrossRef]
- Rodriguez-Blanco, J.D.; Shaw, S.; Benning, L.G. The kinetics and mechanisms of amorphous calcium carbonate (ACC) crystallization to calcite, via vaterite. Nanoscale 2011, 3, 265–271. [Google Scholar] [CrossRef]
- Avaro, J.; Moon, E.M.; Rose, J.; Rose, A.L. Calcium coordination environment in precursor species to calcium carbonate mineral formation. Geochim. Cosmochim. Acta 2019, 259, 344–357. [Google Scholar] [CrossRef]
- Koningsberger, D.C.; Prins, R. (Eds.) X-ray Absorption: Principles, Applications and Techniques of EXAFS, SEXAFS and XANES; Wiley: New York, NY, USA, 1988; Volume 1. [Google Scholar]
- Becker, A.; Bismayer, U.; Epple, M.; Fabritius, H.; Hasse, B.; Shi, J.; Ziegler, A. Structural characterisation of X-ray amorphous calcium carbonate (ACC) in sternal deposits of the crustacea Porcellio scaber. Dalton Trans. 2003, 551–555. [Google Scholar] [CrossRef]
- Zhang, Y.Z.; Qiao, L.; Yan, H.J.; Zizak, I.; Zaslansky, P.; Li, Y.F.; Qi, L.M.; Ma, Y.R. Vaterite microdisc mesocrystals exposing the (001) facet formed via transformation from proto-vaterite amorphous calcium carbonate. Cryst. Growth Des. 2020, 20, 3482–3492. [Google Scholar] [CrossRef]
- Görlin, M.; Chernev, P.; Ferreira de Araújo, J.; Reier, T.; Dresp, S.; Paul, B.; Krähnert, R.; Dau, H.; Strasser, P. Oxygen Evolution Reaction Dynamics, Faradaic Charge Efficiency, and the Active Metal Redox States of Ni–Fe Oxide Water Splitting Electrocatalysts. J. Am. Chem. Soc. 2016, 138, 5603–5614. [Google Scholar] [CrossRef] [PubMed]
- Newville, M. IFEFFIT: Interactive XAFS analysis and FEFF fitting. J. Synchrotron Rad. 2001, 8, 322–324. [Google Scholar] [CrossRef] [PubMed]
- Rehr, J.J.; Deleon, J.M.; Zabinsky, S.I.; Albers, R.C. Theoretical X-Ray Absorption Fine-Structure Standards. J. Am. Chem. Soc. 1991, 113, 5135–5140. [Google Scholar] [CrossRef]
- Christy, A.G. A Review of the Structures of Vaterite: The Impossible, the Possible, and the Likely. Cryst. Growth Des. 2017, 17, 3567–3578. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.W.; Zhang, F.X.; Zhang, J.M.; Ewing, R.C.; Becker, U.; Cai, Z.H. Carbonate orientational order and superlattice structure in vaterite. J. Cryst. Growth 2014, 407, 78–86. [Google Scholar] [CrossRef] [Green Version]
- Calvin, S. XAFS in a Nutshell. In XAFS for Everyone; CRC Press: Boca Raton, FL, USA, 2012; pp. 20–21. [Google Scholar]
- Sun, S.; Chevrier, D.M.; Zhang, P.; Gebauer, D.; Cölfen, H. Distinct Short-Range Order Is Inherent to Small Amorphous Calcium Carbonate Clusters (<2 nm). Angew. Chem. Int. Ed. 2016, 55, 12206–12209. [Google Scholar]
- Gebauer, D.; Cölfen, H. Prenucleation clusters and non-classical nucleation. Nano Today 2011, 6, 564–584. [Google Scholar] [CrossRef] [Green Version]
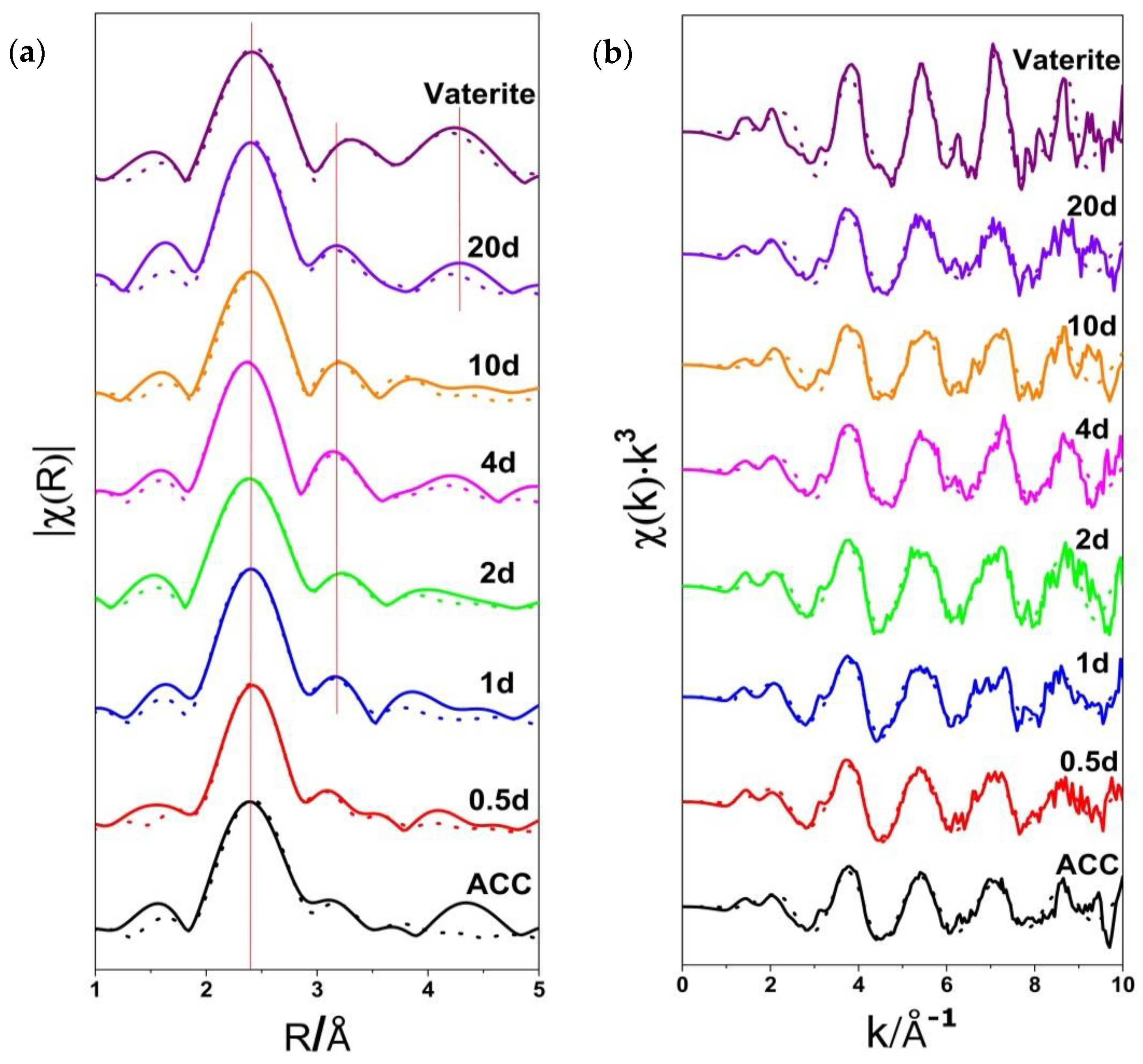
| Sample | N | R(Å) | 103σ2(Å2) |
|---|---|---|---|
| proto-vaterite ACC | 3.5 (±0.8) | 2.38 (±0.007) | 6 (± 3) |
| vaterite-0.5d | 3.4 (±1.1) | 5 (±4) | |
| vaterite-1d | 3.5 (±0.9) | 6 (±3) | |
| vaterite-2d | 3.7 (±1.1) | 3 (±4) | |
| vaterite-4d | 4.3 (±1.1) | 8 (±3) | |
| vaterite-10d | 3.7 (±0.9) | 6 (±3) | |
| vaterite-20d | 4.3 (±1.4) | 8(±5) | |
| vaterite reference | 6 (±0) | 6(±3) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiao, L.; Zizak, I.; Zaslansky, P.; Ma, Y. The Crystallization Process of Vaterite Microdisc Mesocrystals via Proto-Vaterite Amorphous Calcium Carbonate Characterized by Cryo-X-ray Absorption Spectroscopy. Crystals 2020, 10, 750. https://doi.org/10.3390/cryst10090750
Qiao L, Zizak I, Zaslansky P, Ma Y. The Crystallization Process of Vaterite Microdisc Mesocrystals via Proto-Vaterite Amorphous Calcium Carbonate Characterized by Cryo-X-ray Absorption Spectroscopy. Crystals. 2020; 10(9):750. https://doi.org/10.3390/cryst10090750
Chicago/Turabian StyleQiao, Li, Ivo Zizak, Paul Zaslansky, and Yurong Ma. 2020. "The Crystallization Process of Vaterite Microdisc Mesocrystals via Proto-Vaterite Amorphous Calcium Carbonate Characterized by Cryo-X-ray Absorption Spectroscopy" Crystals 10, no. 9: 750. https://doi.org/10.3390/cryst10090750
APA StyleQiao, L., Zizak, I., Zaslansky, P., & Ma, Y. (2020). The Crystallization Process of Vaterite Microdisc Mesocrystals via Proto-Vaterite Amorphous Calcium Carbonate Characterized by Cryo-X-ray Absorption Spectroscopy. Crystals, 10(9), 750. https://doi.org/10.3390/cryst10090750





