Quantitative Structure–Property Relationships from Experiments for CH4 Storage and Delivery by Metal–Organic Frameworks
Abstract
1. Introduction
2. Materials and Methods
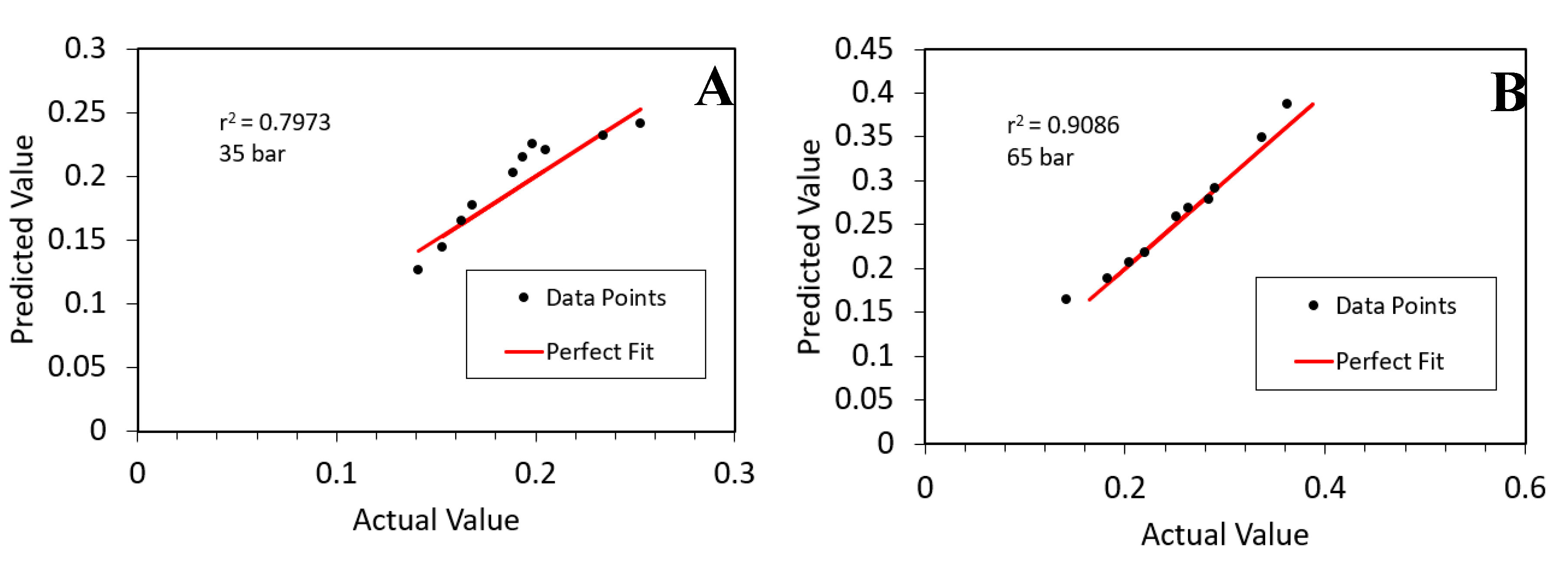
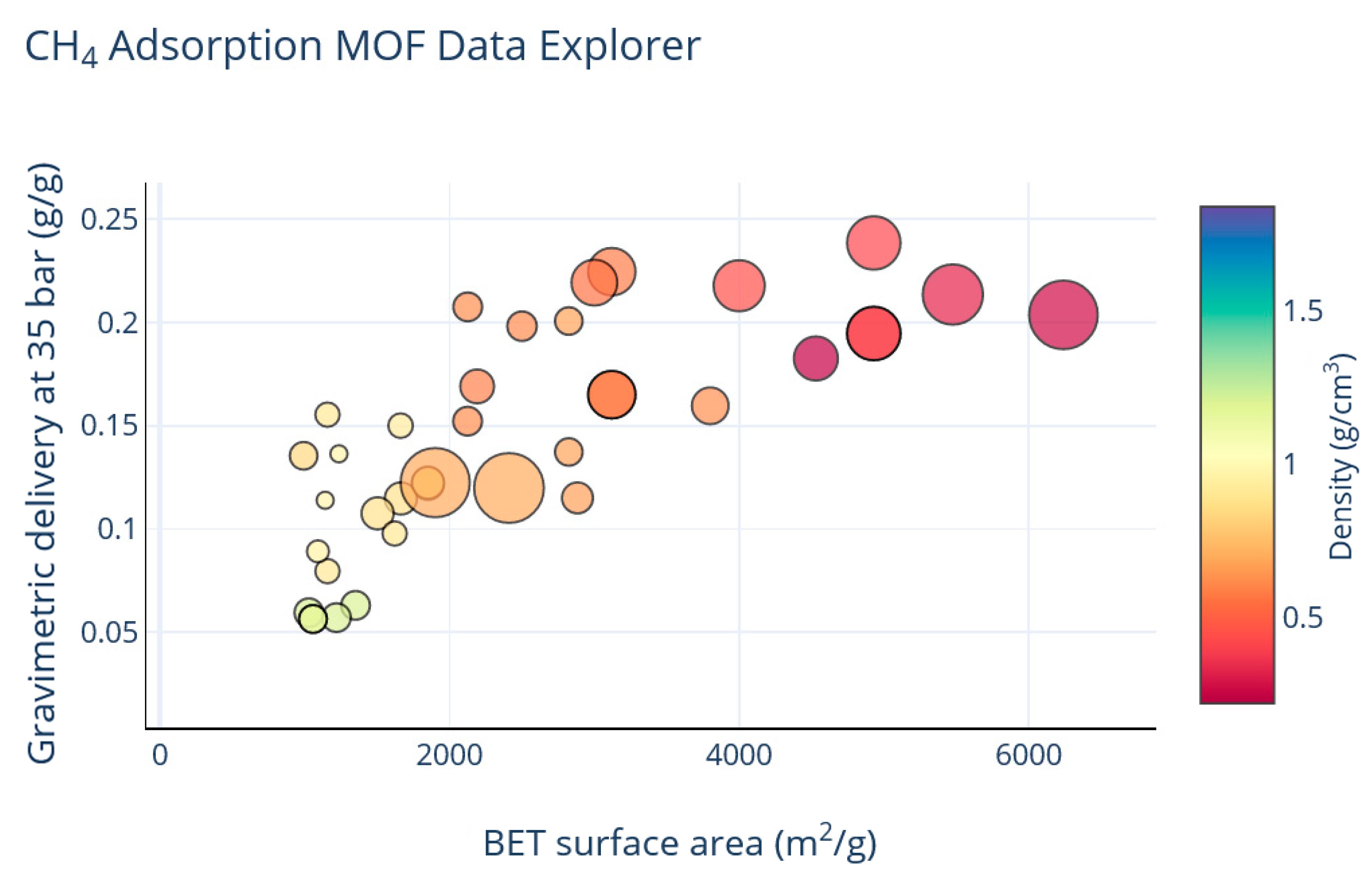
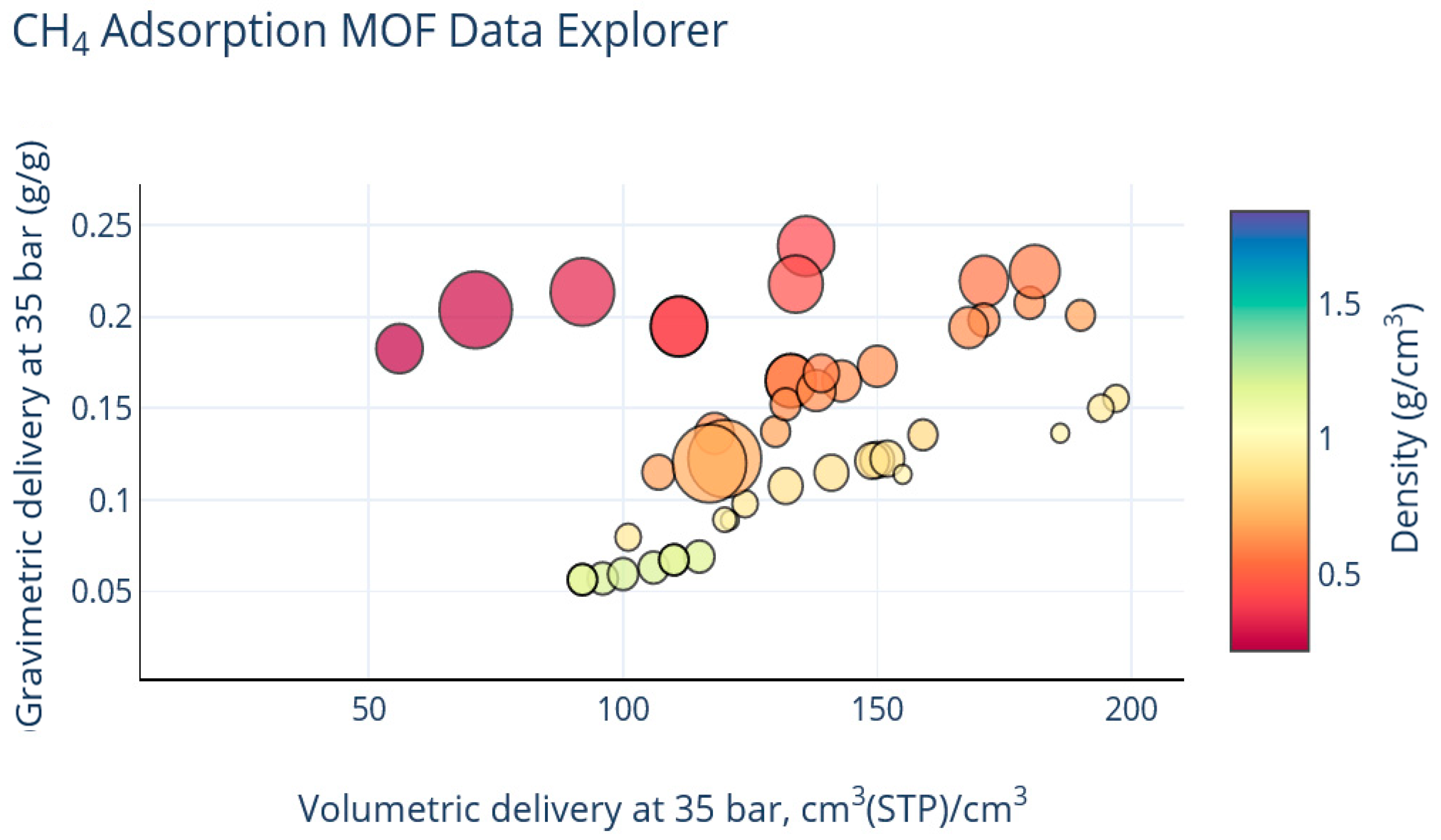
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Funding
Acknowledgments
Conflicts of Interest
References
- Zhou, H.C.; Long, J.R.; Yaghi, O.M. Introduction to metal-organic frameworks. Chem. Rev. 2012, 112, 673–674. [Google Scholar] [CrossRef]
- Safaei, M.; Foroughi, M.M.; Ebrahimpoor, N.; Jahani, S.; Omidi, A.; Khatami, M. A review on metal-organic frameworks: Synthesis and applications. TrAC Trends Anal. Chem. 2019, 118, 401–425. [Google Scholar] [CrossRef]
- Metal–Organic Frameworks (MOFs). Available online: https://pubs.rsc.org/en/content/articlehtml/2014/cs/c4cs90059f?page=search (accessed on 13 August 2020).
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The Chemistry and Applications of Metal-Organic Frameworks. Science 2013, 341, 1230444-1-1230444-13. [Google Scholar] [CrossRef]
- Omar, M.Y.; Markus, J.K.; Christian, S.D. Emergence of Metal-Organic Frameworks. Available online: https://application.wiley-vch.de/books/sample/3527345027_c01.pdf (accessed on 13 August 2020).
- Ji, Z.; Wang, H.; Canossa, S.; Wuttke, S.; Yaghi, O.M. Pore Chemistry of Metal–Organic Frameworks. Adv. Funct. Mater. 2020, 2000238, 1–24. [Google Scholar]
- Chui, S.S.Y.; Lo, S.M.F.; Charmant, J.P.H.; Orpen, A.G.; Williams, I.D. A chemically functionalizable nanoporous material [Cu3(TMA)2 (H2O)3](n). Science 1999, 283, 1148–1150. [Google Scholar] [CrossRef]
- Spanopoulos, I.; Tsangarakis, C.; Klontzas, E.; Tylianakis, E.; Froudakis, G.; Adil, K.; Belmabkhout, Y.; Eddaoudi, M.; Trikalitis, P.N. Reticular Synthesis of HKUST-like tbo-MOFs with Enhanced CH4 Storage. J. Am. Chem. Soc. 2016, 138, 1568–1574. [Google Scholar] [CrossRef]
- Peng, Y.; Krungleviciute, V.; Eryazici, I.; Hupp, J.T.; Farha, O.K.; Yildirim, T. Methane storage in metal-organic frameworks: Current records, surprise findings, and challenges. J. Am. Chem. Soc. 2013, 135, 11887–11894. [Google Scholar] [CrossRef] [PubMed]
- Getzschmann, J.; Senkovska, I.; Wallacher, D.; Tovar, M.; Fairen-Jimenez, D.; Düren, T.; Van Baten, J.M.; Krishna, R.; Kaskel, S. Methane storage mechanism in the metal-organic framework Cu 3(btc)2: An in situ neutron diffraction study. Microporous Mesoporous Mater. 2010, 136, 50–58. [Google Scholar] [CrossRef]
- Vikrant, K.; Kumar, V.; Kim, K.H.; Kukkar, D. Metal-organic frameworks (MOFs): Potential and challenges for capture and abatement of ammonia. J. Mater. Chem. A 2017, 5, 22877–22896. [Google Scholar] [CrossRef]
- Todaro, M.; Buscarino, G.; Sciortino, L.; Alessi, A.; Messina, F.; Taddei, M.; Ranocchiari, M.; Cannas, M.; Gelardi, F.M. Decomposition Process of Carboxylate MOF HKUST-1 Unveiled at the Atomic Scale Level. J. Phys. Chem. C 2016, 120, 12879–12889. [Google Scholar] [CrossRef]
- Li, H.; Eddaoudi, M.; O’Keeffe, M.; Yaghi, O.M. Design and synthesis of an exceptionally stable and highly porous metal-organic framework. Nature 1999, 402, 276–279. [Google Scholar] [CrossRef]
- Varun Mehra ARPA-E is Here to Stay. Available online: https://scienceprogress.org/wp-content/uploads/2013/01/ARPA-Ebrief.pdf (accessed on 12 August 2020).
- Jiang, J.; Furukawa, H.; Zhang, Y.B.; Yaghi, O.M. High Methane Storage Working Capacity in Metal-Organic Frameworks with Acrylate Links. J. Am. Chem. Soc. 2016, 138, 10244–10251. [Google Scholar] [CrossRef] [PubMed]
- Mason, J.A.; Veenstra, M.; Long, J.R. Evaluating metal-organic frameworks for natural gas storage. Chem. Sci. 2014, 5, 32–51. [Google Scholar] [CrossRef]
- Hechinger, M.; Leonhard, K.; Marquardt, W. What is Wrong with Quantitative Structure–Property Relations Models Based on Three-Dimensional Descriptors? J. Chem. Inf. Model. 2012, 52, 1984–1993. [Google Scholar] [CrossRef]
- Sezginel, K.B.; Uzun, A.; Keskin, S. Multivariable linear models of structural parameters to predict methane uptake in metal–organic frameworks. Chem. Eng. Sci. 2015, 124, 125–134. [Google Scholar] [CrossRef]
- Liu, L.; Konstas, K.; Hill, M.R.; Telfer, S.G. Programmed pore architectures in modular quaternary metal-organic frameworks. J. Am. Chem. Soc. 2013, 135, 17731–17734. [Google Scholar] [CrossRef]
- Guo, Z.; Wu, H.; Srinivas, G.; Zhou, Y.; Xiang, S.; Chen, Z.; Yang, Y.; Zhou, W.; O’Keeffe, M.; Chen, B. A metal-organic framework with optimized open metal sites and pore spaces for high methane storage at room temperature. Angew. Chemie Int. Ed. 2011, 50, 3178–3181. [Google Scholar] [CrossRef]
- Kalidindi, S.B.; Nayak, S.; Briggs, M.E.; Jansat, S.; Katsoulidis, A.P.; Miller, G.J.; Warren, J.E.; Antypov, D.; Corà, F.; Slater, B.; et al. Chemical and structural stability of zirconium-based metal-organic frameworks with large three-dimensional pores by linker engineering. Angew. Chemie Int. Ed. 2015, 54, 221–226. [Google Scholar] [CrossRef]
- Karagiaridi, O.; Bury, W.; Mondloch, J.E.; Hupp, J.T.; Farha, O.K. Solvent-assisted linker exchange: An alternative to the de novo synthesis of unattainable metal-organic frameworks. Angew. Chemie Int. Ed. 2014, 53, 4530–4540. [Google Scholar] [CrossRef]
- Stock, N.; Biswas, S. Synthesis of metal-organic frameworks (MOFs): Routes to various MOF topologies, morphologies, and composites. Chem. Rev. 2012, 112, 933–969. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Doonan, C.J.; Furukawa, H.; Ferreira, R.B.; Towne, J.; Knobler, C.B.; Wang, B.; Yaghi, O.M. Multiple functional groups of varying ratios in metal-organic frameworks. Science 2010, 327, 846–850. [Google Scholar] [CrossRef] [PubMed]
- Moghadam, P.Z.; Fairen-Jimenez, D.; Snurr, R.Q. Efficient identification of hydrophobic MOFs: Application in the capture of toxic industrial chemicals. J. Mater. Chem. A 2015, 4, 529–536. [Google Scholar] [CrossRef]
- Casco, M.E.; Rey, F.; Jordá, J.L.; Rudić, S.; Fauth, F.; Martínez-Escandell, M.; Rodríguez-Reinoso, F.; Ramos-Fernández, E.V.; Silvestre-Albero, J. Paving the way for methane hydrate formation on metal-organic frameworks (MOFs). Chem. Sci. 2016, 7, 3658–3666. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.G.; Camp, J.; Haranczyk, M.; Sikora, B.J.; Bury, W.; Krungleviciute, V.; Yildirim, T.; Farha, O.K.; Sholl, D.S.; Snurr, R.Q. Computation-ready, experimental metal-organic frameworks: A tool to enable high-throughput screening of nanoporous crystals. Chem. Mater. 2014, 26, 6185–6192. [Google Scholar] [CrossRef]
- Ohno, H.; Mukae, Y. Machine Learning Approach for Prediction and Search: Application to Methane Storage in a Metal–Organic Framework. J. Phys. Chem. C 2016, 120, 23963–23968. [Google Scholar] [CrossRef]
- Mahmoud, E.; Ali, L.; El Sayah, A.; Alkhatib, A.S.; Abdulsalam, H.; Juma, M.; Al-Muhtaseb, H.A. Implementing Metal-Organic Frameworks for Natural Gas Storage. Crystals 2019, 9, 406. [Google Scholar] [CrossRef]
- Moellmer, J.; Moeller, A.; Dreisbach, F.; Glaeser, R.; Staudt, R. High pressure adsorption of hydrogen, nitrogen, carbon dioxide and methane on the metal-organic framework HKUST-1. Microporous Mesoporous Mater. 2011, 138, 140–148. [Google Scholar] [CrossRef]
- Deng, H.; Grunder, S.; Cordova, K.E.; Valente, C.; Furukawa, H.; Hmadeh, M.; Gándara, F.; Whalley, A.C.; Liu, Z.; Asahina, S.; et al. Large-pore apertures in a series of metal-organic frameworks. Science 2012, 336, 1018–1023. [Google Scholar] [CrossRef]
- Gómez-Gualdrón, D.A.; Wilmer, C.E.; Farha, O.K.; Hupp, J.T.; Snurr, R.Q. Exploring the limits of methane storage and delivery in nanoporous materials. J. Phys. Chem. C 2014, 118, 6941–6951. [Google Scholar] [CrossRef]
- Wilmer, C.E.; Farha, O.K.; Yildirim, T.; Eryazici, I.; Krungleviciute, V.; Sarjeant, A.A.; Snurr, R.Q.; Hupp, J.T. Gram-scale, high-yield synthesis of a robust metal–organic framework for storing methane and other gases. Energy Environ. Sci. 2013, 6, 1158–1163. [Google Scholar] [CrossRef]
- Loiseau, T.; Serre, C.; Huguenard, C.; Fink, G.; Taulelle, F.; Henry, M.; Bataille, T.; Férey, G. A Rationale for the Large Breathing of the Porous Aluminum Terephthalate (MIL-53) Upon Hydration. Chem. A Eur. J. 2004, 10, 1373–1382. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Srinivas, G.; Wilmer, C.E.; Eryazici, I.; Snurr, R.Q.; Hupp, J.T.; Yildirim, T.; Farha, O.K. Simultaneously high gravimetric and volumetric methane uptake characteristics of the metal-organic framework NU-111. Chem. Commun. 2013, 49, 2992–2994. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Zhou, W.; Yildirim, T.; Chen, B. A series of metal-organic frameworks with high methane uptake and an empirical equation for predicting methane storage capacity. Energy Environ. Sci. 2013, 6, 2735–2744. [Google Scholar] [CrossRef]
- Prajwal, B.P.; Ayappa, K.G. Evaluating methane storage targets: From powder samples to onboard storage systems. Adsorption 2014, 20, 769–776. [Google Scholar] [CrossRef]
- Wen, H.-M.M.; Li, B.; Yuan, D.; Wang, H.; Yildirim, T.; Zhou, W.; Chen, B. A porous metal-organic framework with an elongated anthracene derivative exhibiting a high working capacity for the storage of methane. J. Mater. Chem. A 2014, 2, 11516–11522. [Google Scholar] [CrossRef]
- Li, L.; Tang, S.; Wang, C.; Lv, X.; Jiang, M.; Wu, H.; Zhao, X. High gas storage capacities and stepwise adsorption in a UiO type metal-organic framework incorporating Lewis basic bipyridyl sites. Chem. Commun. 2014, 50, 2304–2307. [Google Scholar] [CrossRef]
- Chen, Z.; Li, P.; Anderson, R.; Wang, X.; Zhang, X.; Robison, L.; Redfern, L.R.; Moribe, S.; Islamoglu, T.; Gómez-Gualdrón, D.A.; et al. Balancing volumetric and gravimetric uptake in highly porous materials for clean energy. Science 2020, 368, 297–303. [Google Scholar] [CrossRef]
- Fernandez, M.; Woo, T.K.; Wilmer, C.E.; Snurr, R.Q. Large-Scale Quantitative Structure–Property Relationship (QSPR) Analysis of Methane Storage in Metal–Organic Frameworks. J. Phys. Chem. C 2013, 117, 7681–7689. [Google Scholar] [CrossRef]
- Farha, O.K.; Özgür Yazaydın, A.; Eryazici, I.; Malliakas, C.D.; Hauser, B.G.; Kanatzidis, M.G.; Nguyen, S.T.; Snurr, R.Q.; Hupp, J.T. De novo synthesis of a metal–organic framework material featuring ultrahigh surface area and gas storage capacities. Nat. Chem. 2010, 2, 944–948. [Google Scholar] [CrossRef]
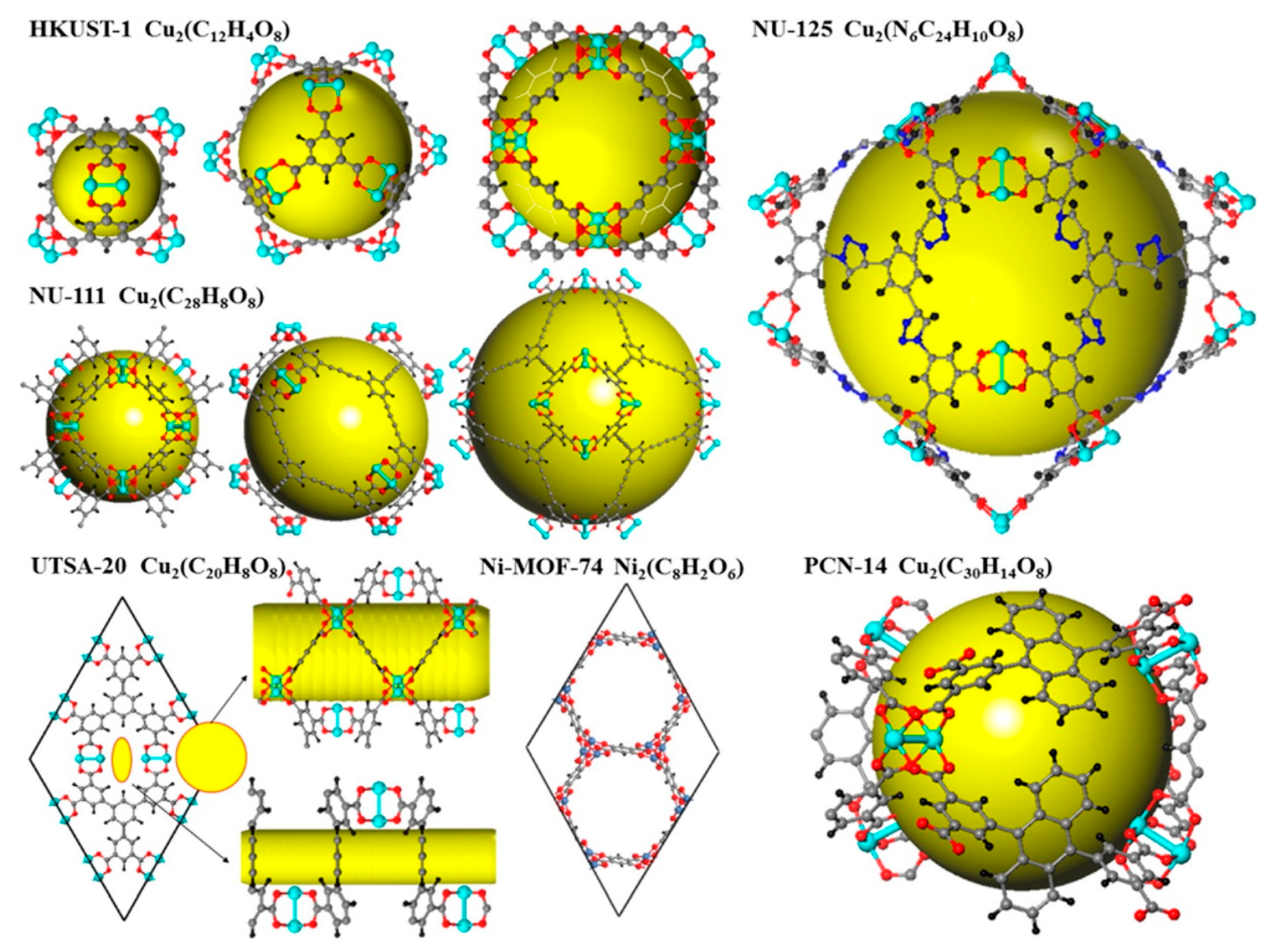

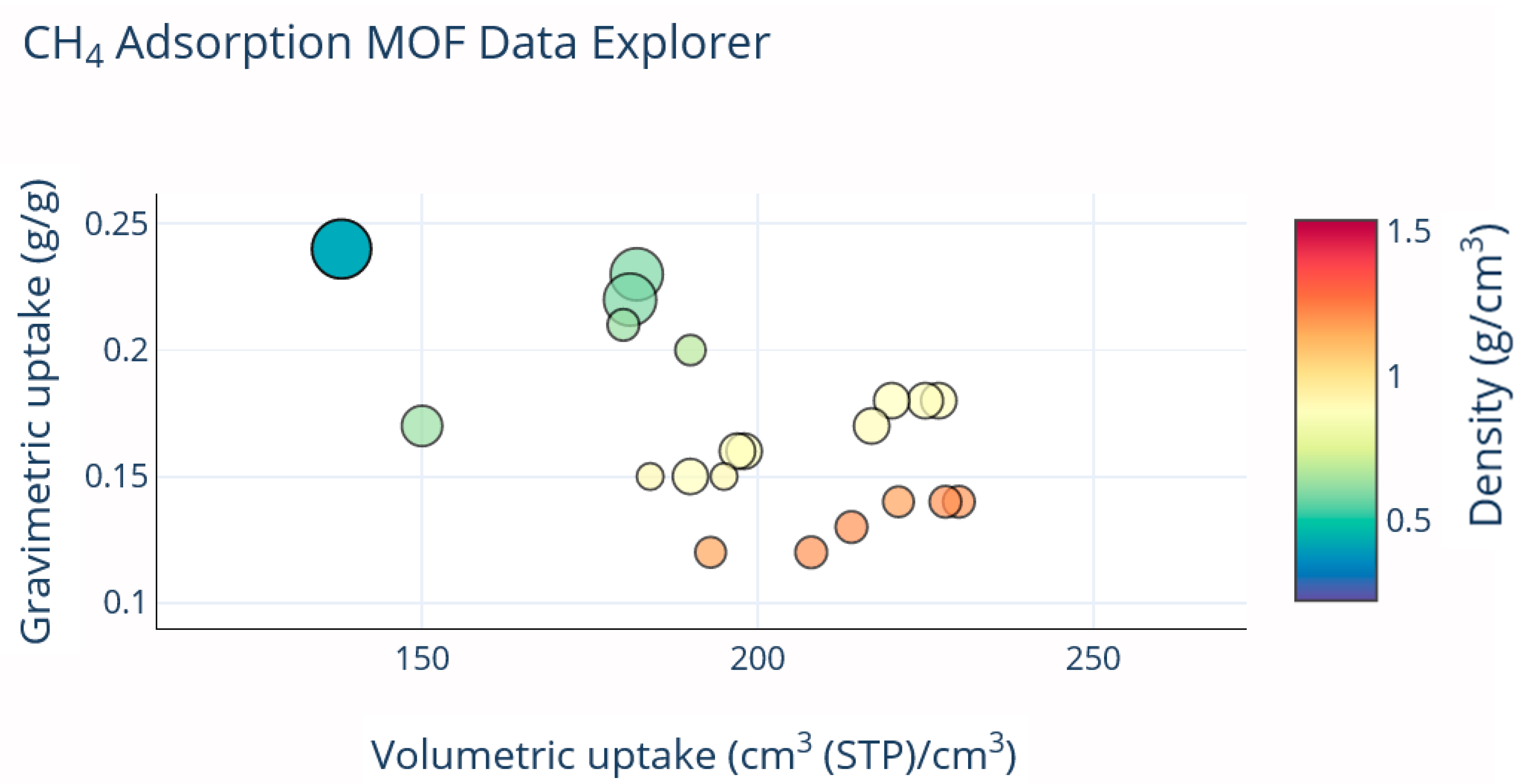
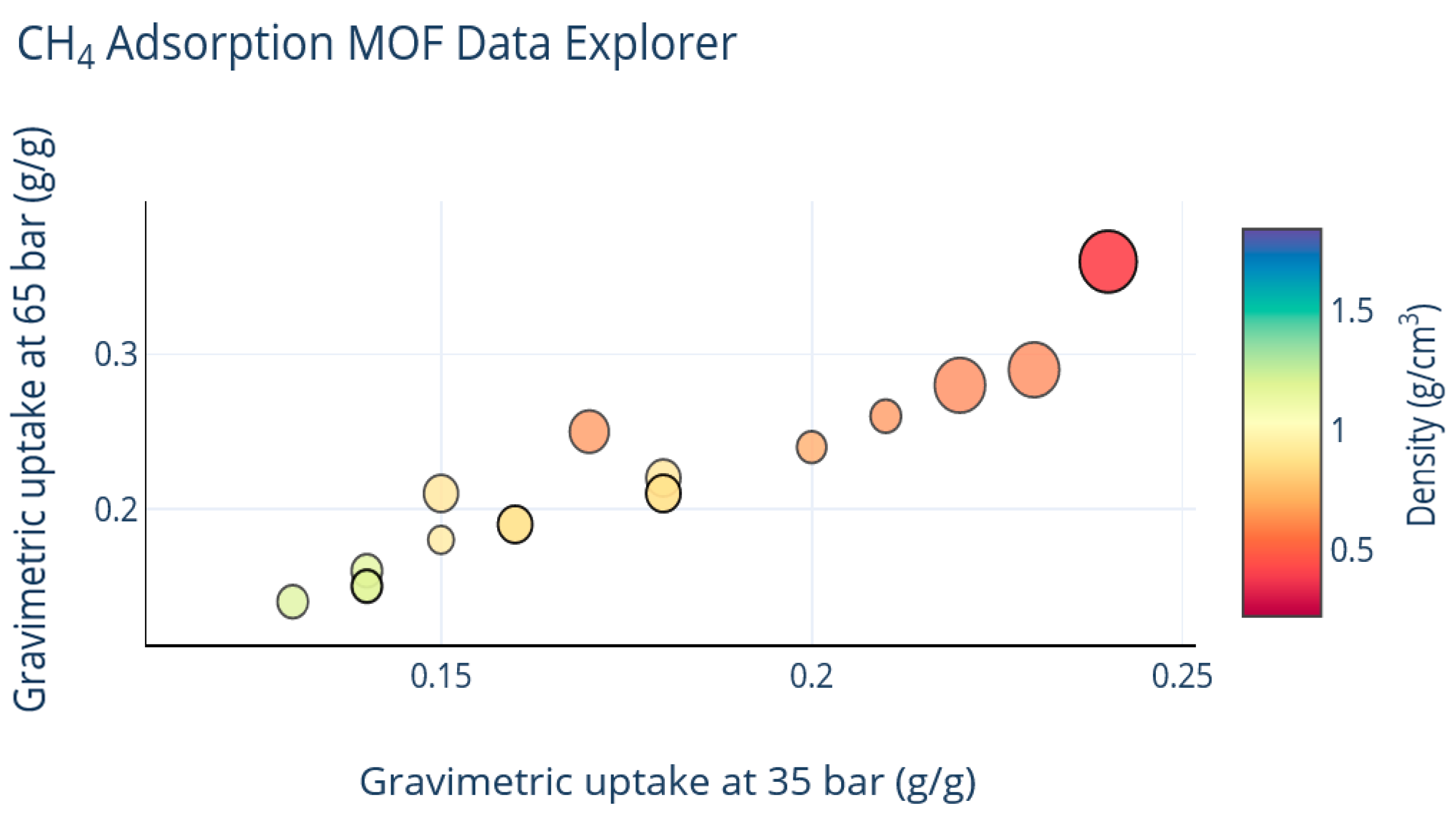


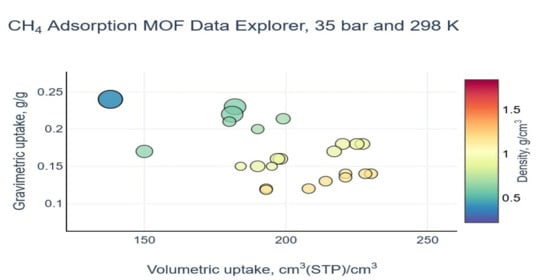
| MOF | VP (cm3g−1) a | BET (m2g−1) | Uptake b (cm3cm−3) | Delivery c (cm3cm−3) | T (K) | P (bar) | Uptake b (cm3cm−3) | Delivery c (cm3cm−3) | T (K) | P (bar) | Qst kJ mol−1 | REF |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PCN-61 | 1.36 | 3000 | 171 | 127 | 298 | 35 | 219 | 174 | 298 | 65 | - | [4] |
| HKUST-1 | 0.71 | 1555 | 190 | - | 303 | 35 | 254 | - | 303 | 65 | 20.7 | [30] |
| MgMOF-74 | 0.69 | - | 200 | 113 | 298 | 35 | 230 | 142 | 298 | 65 | 18.5 | [16] |
| MOF-5 | 1.4 | - | 150 | 118 | 298 | 35 | 214 | 182 | 298 | 65 | 12.3 | [16] |
| Cu-TDPAT | 0.93 | 1938 | 181 | 122 | 298 | 35 | 222 | 163 | 298 | 65 | - | [31] |
| PCN-14 | 0.83 | 1984 | 202 | 125 | 298 | 35 | 239 | 160 | 298 | 65 | 17.6 | [16] |
| CoMOF-74 | 0.51 | - | 221 | 110 | 298 | 35 | 249 | 136 | 298 | 65 | 19.5 | [16] |
| PCN-61 | 1.36 | 3000 | 171 | 127 | 298 | 35 | 219 | 174 | 298 | 65 | - | [24] |
| MOF-210 | 3.60 | 6240 | 83 | 71 | 298 | 35 | 143 | 131 | 298 | 65 | - | [4] |
| PCN-14 | 0.85 | 2000 | 195 | 122 | 298 | 35 | 230 | 157 | 298 | 65 | 18.7 | [9] |
| NU-111 | 2.09 | 4930 | 138 | 111 | 298 | 35 | 206 | 179 | 298 | 65 | 14.2 | [9] |
| NU-140 | 1.97 | 4300 | 138 | 108 | 298 | 35 | 200 | 170 | 298 | 65 | 14 | [32] |
| NU-125 | 1.29 | 3120 | 181 | 133 | 298 | 35 | 228 | 180 | 298 | 58 | 15.5 | [33] |
| NiMOF-74 | 0.47 | 1218 | 214 | 94 | 298 | 35 | 236 | 116 | 298 | 65 | - | [34] |
| NU-111 | 2.09 | 4930 | 138 | 111 | 298 | 35 | 206 | 179 | 298 | 65 | 15.2 | [35] |
| NOTT-109 | 0.850 | 2110 | 196 | 125 | 300 | 35 | 242 | 170 | 300 | 65 | 17.1 | [36] |
| ZJU-5 | 1.074 | 2823 | 190 | 130 | 300 | 35 | 228 | 168 | 300 | 65 | 15.3 | [37] |
| ZJU-25 | 1.183 | 2124 | 180 | 132 | 300 | 35 | 229 | 181 | 300 | 63 | 15.1 | [38] |
| NU-135 | 1.02 | 2530 | 187 | 127 | 298 | 35 | 230 | 170 | 298 | 65 | 16.6 | [39] |
| NOTT-100 | 0.677 | 1661 | 195 | 104 | 300 | 35 | 230 | 139 | 300 | 65 | 18.1 | [36] |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahmoud, E. Quantitative Structure–Property Relationships from Experiments for CH4 Storage and Delivery by Metal–Organic Frameworks. Crystals 2020, 10, 700. https://doi.org/10.3390/cryst10080700
Mahmoud E. Quantitative Structure–Property Relationships from Experiments for CH4 Storage and Delivery by Metal–Organic Frameworks. Crystals. 2020; 10(8):700. https://doi.org/10.3390/cryst10080700
Chicago/Turabian StyleMahmoud, Eyas. 2020. "Quantitative Structure–Property Relationships from Experiments for CH4 Storage and Delivery by Metal–Organic Frameworks" Crystals 10, no. 8: 700. https://doi.org/10.3390/cryst10080700
APA StyleMahmoud, E. (2020). Quantitative Structure–Property Relationships from Experiments for CH4 Storage and Delivery by Metal–Organic Frameworks. Crystals, 10(8), 700. https://doi.org/10.3390/cryst10080700