Synthesis, Crystal Structure and Solid State Transformation of 1,2-Bis[(1-methyl-1H-imidazole-2-yl)thio]ethane
Abstract
1. Introduction
2. Results and Discussion
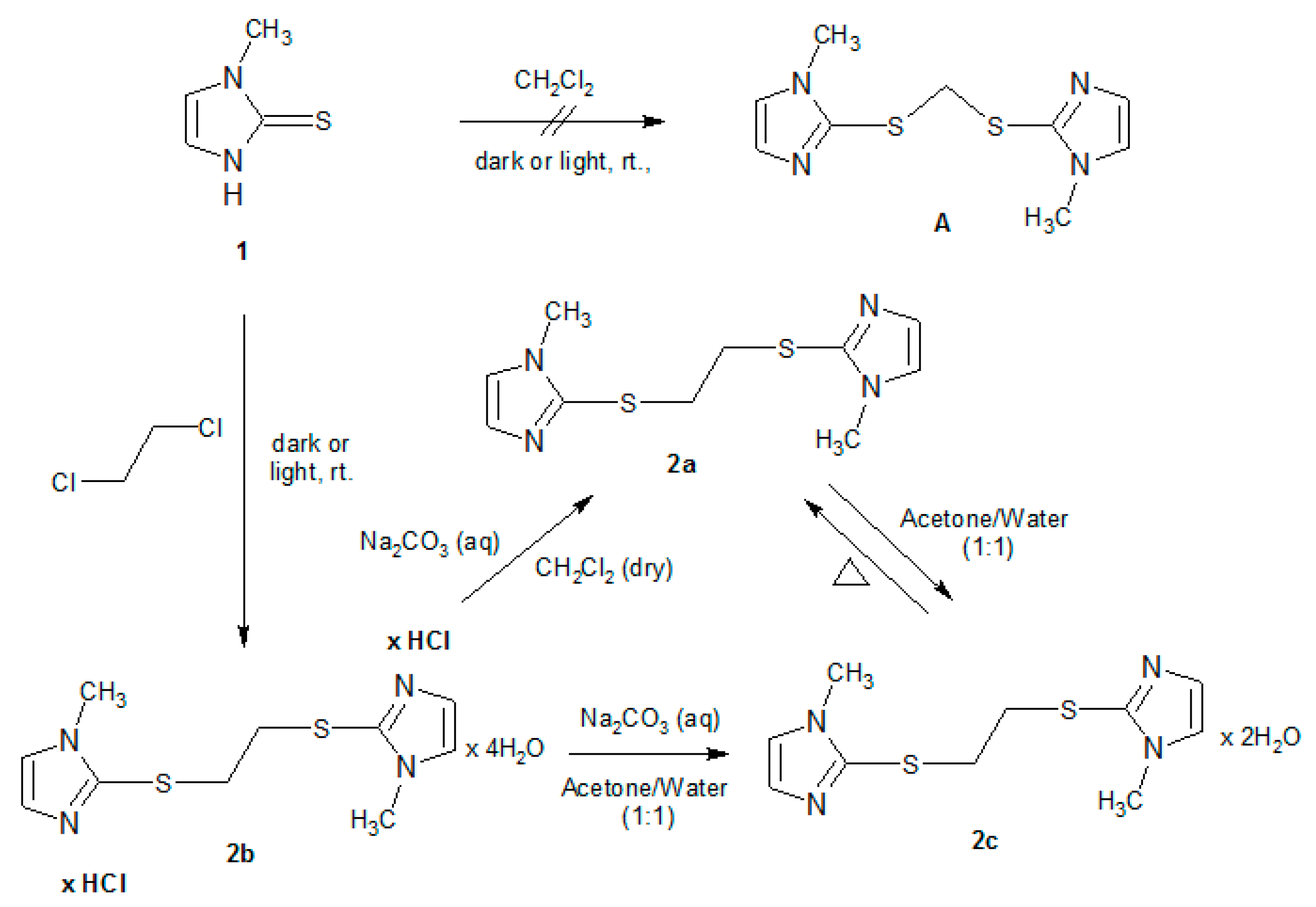
2.1. Synthesis of Bis Derivative 2
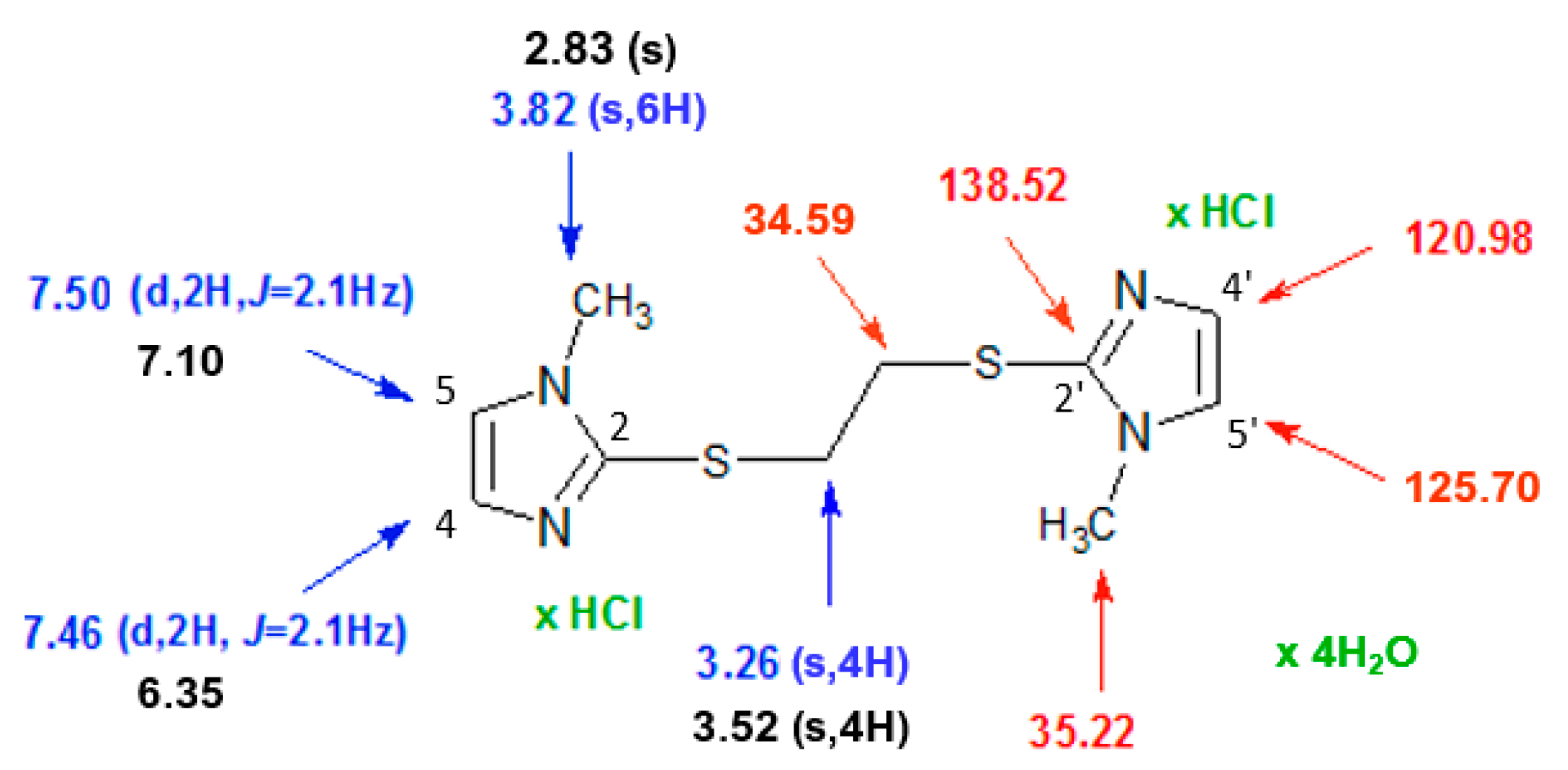
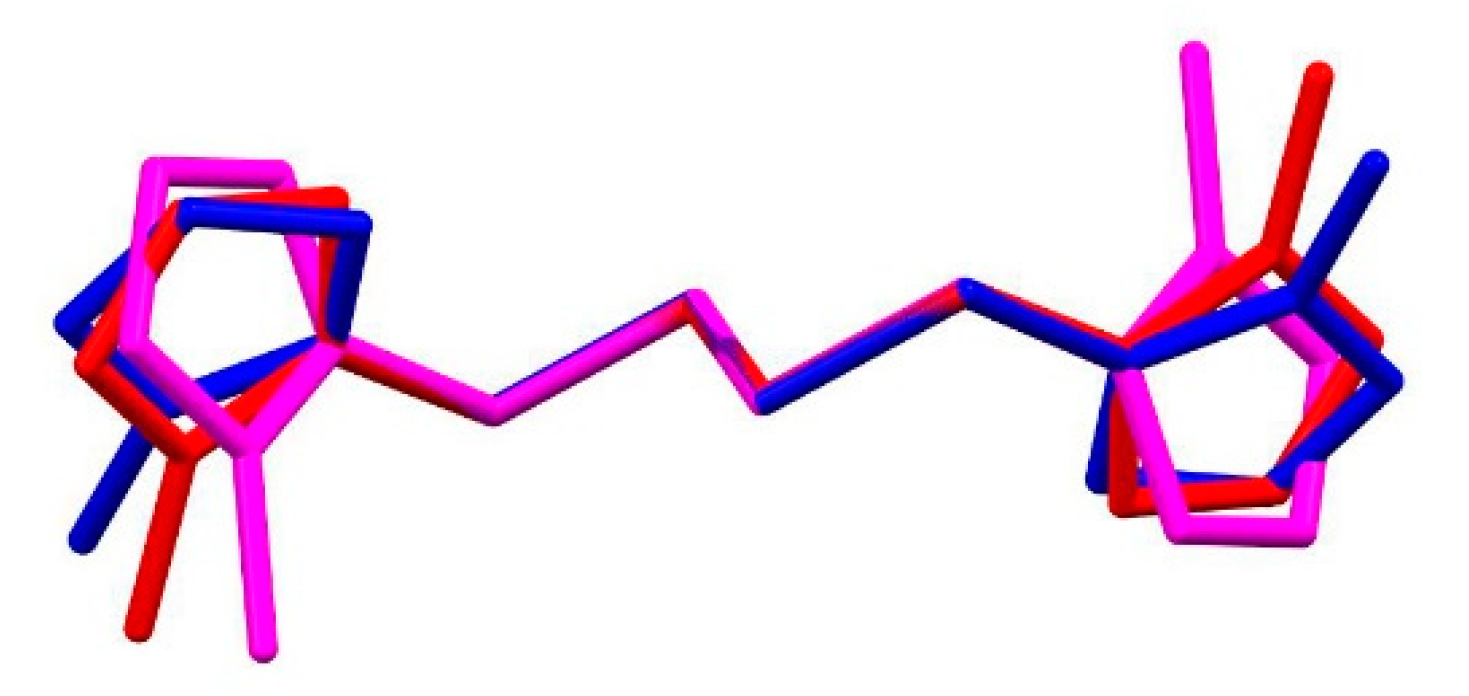
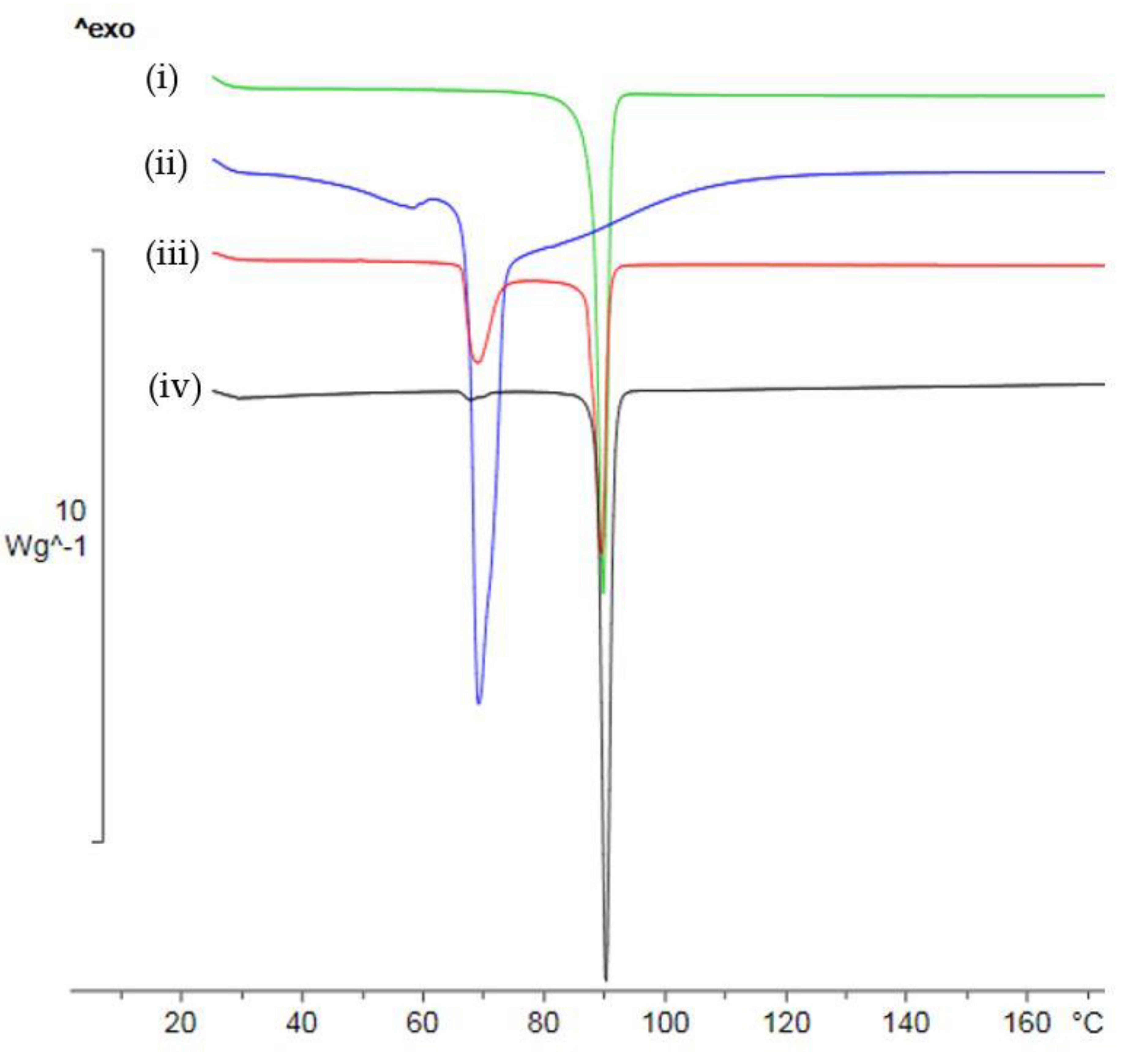
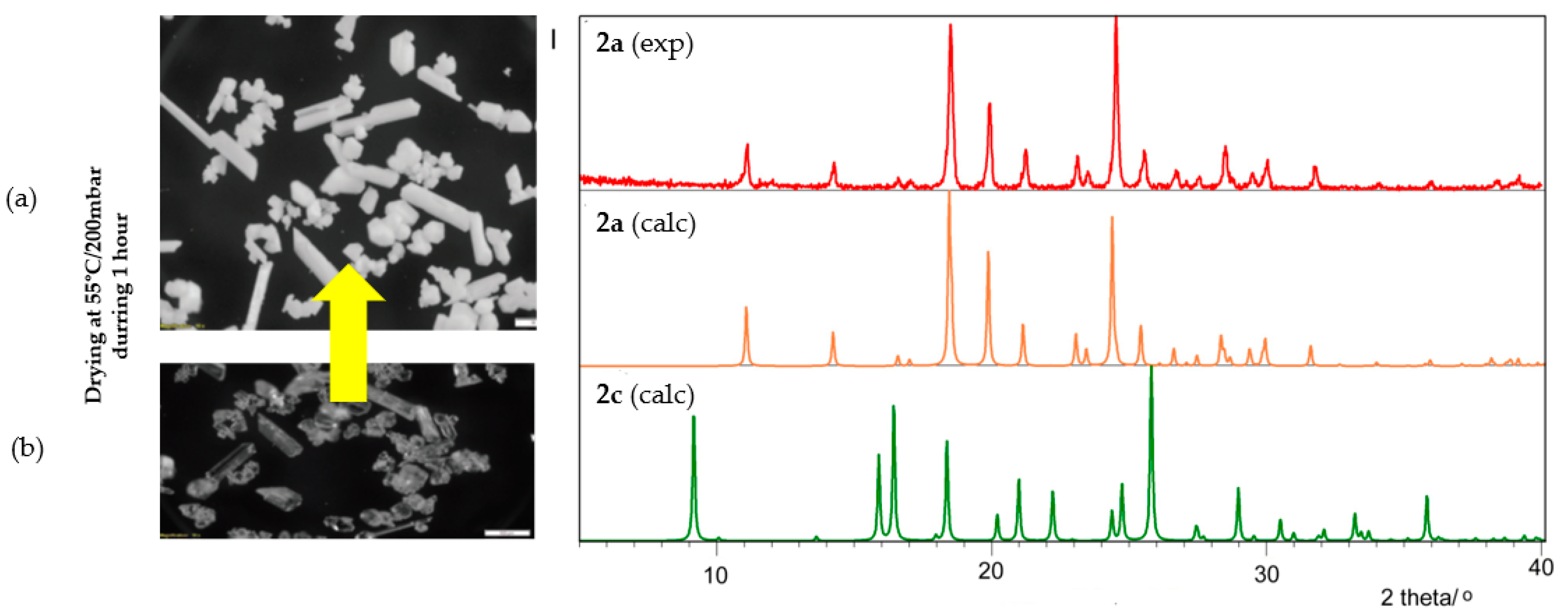
2.2. Characterization of 1,2-Bis[(1-methyl-1H-imidazole-2-yl)thio]ethane Forms 2a–2c
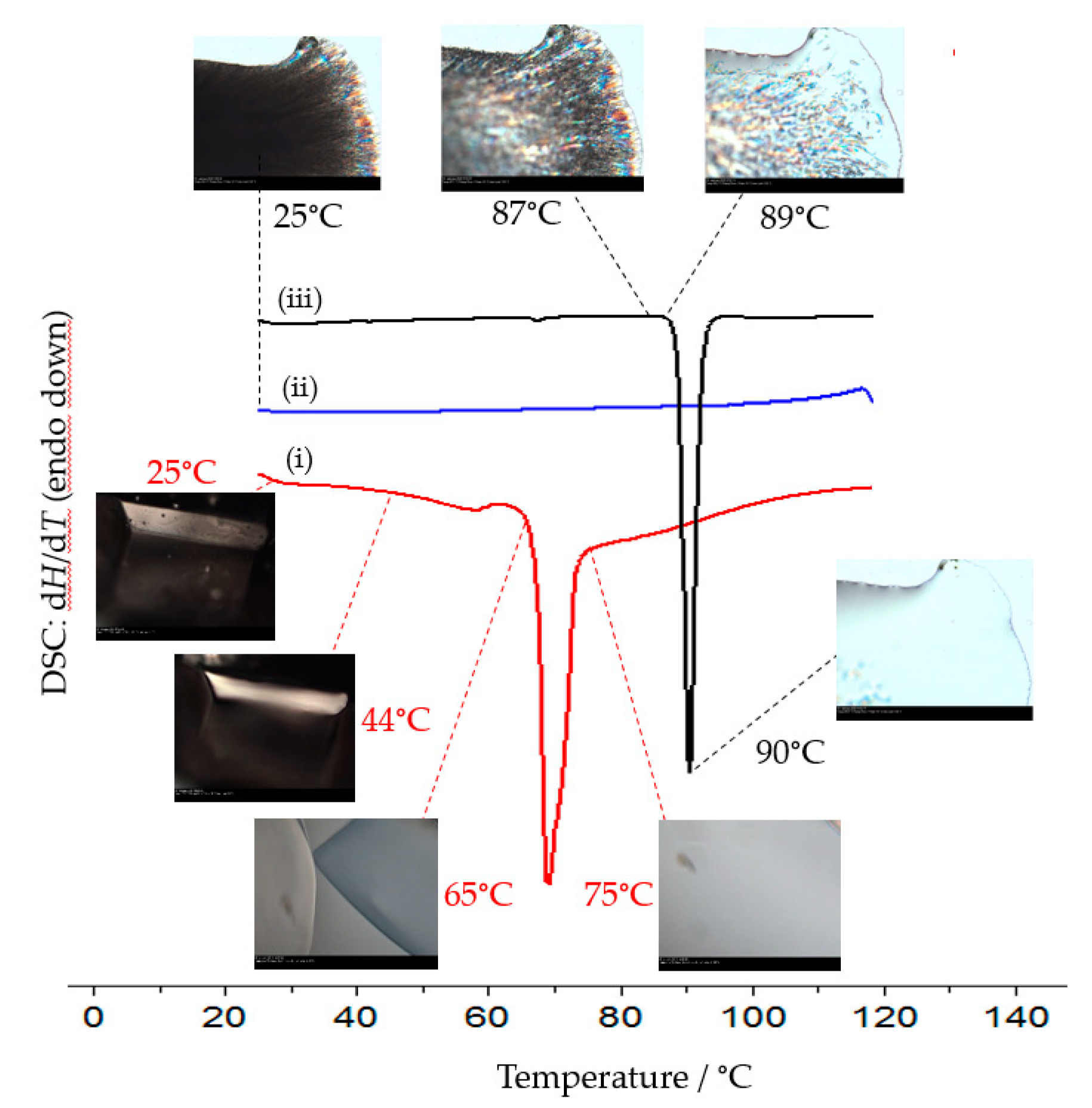
2.3. Dehydration Behaviour of Dihydrate 2c and Its Transformation to the Anhydrous Form 2a
3. Materials and Methods
3.1. General
3.2. Synthesis of 1,2-Bis[(1-methyl-1H-imidazole-2-yl)thio]ethane Dihydrochloride Tetrahydrate (2b)
3.3. Synthesis of Anhydrous 1,2-Bis[(1-methyl-1H-imidazole-2-yl)thio]ethane (2a)
3.4. Synthesis of 1,2-Bis[(1-methyl-1H-imidazole-2-yl)thio]ethane Dihydrate (2c)
3.5. Powder X-ray Diffraction
3.6. Single Crystal X-ray Diffraction Analysis and Structure Determination
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Aboul-Enein, H.Y.; Al-Badr, A.A. Analytical Profile of Methimazole. In Analytical Profiles of Drug Substances; Florey, K., Ed.; Academic Press: New York, NY, USA, 1979; Volume 8, pp. 351–370. [Google Scholar]
- Qingjian, L.; Mingli, S. Synthesis of noncyclic crown ethers with methimazole heterocycle as a terminal group. Youji Huaxue 1992, 12, 509–513. [Google Scholar]
- Qingjian, L.; Mingli, S.; Chongqiu, J.; Fengling, L. Syntheses and coordination properties of bridged bis(methimazole) compounds. Gaodeng Xuexiao Huaxue Xuebao 1992, 13, 328–331. [Google Scholar]
- Silva, R.M.; Smith, M.D.; Gardinier, J.R. Unexpected New Chemistry of the Bis(thioimidazolyl)methanes. J. Org. Chem. 2005, 70, 8755–8763. [Google Scholar] [CrossRef] [PubMed]
- Hassanaly, P.; Dou, H.J.M.; Metzger, J.; Assef, G.; Kister, J.S. Alkylation of 2-Thioxo-2,3-dihydroimidazole and its 1-Methyl Derivative under Phase-Transfer Conditions. Synthesis 1997, 4, 253–254. [Google Scholar]
- Pilaniya, K.; Chandrawanshi, H.K.; Pilaniya, U.; Manchandani, P.; Jain, P.; Singh, N. Recent trends in the impurity profile of pharmaceuticals. J. Adv. Pharm. Technol. Res. 2010, 1, 302–310. [Google Scholar]
- Infantes, L.; Motherwell, S. Water clusters in organic molecular crystals. Cryst. Eng. Comm. 2002, 4, 454–461. [Google Scholar] [CrossRef]
- Aaltonen, J.; Allesø, M.; Mirza, S.; Koradia, V.; Gordon, K.C.; Rantanen, J. Solid form screening—A review. Eur. J. Pharm. Biopharm. 2009, 71, 23–37. [Google Scholar] [CrossRef]
- Larsen, A.S.; Ruggiero, M.T.; Johansson, K.E.; Zeitler, J.A.; Rantanen, J. Tracking Dehydration Mechanisms in Crystalline Hydrates with Molecular Dynamics Simulations. Cryst. Growth Des. 2017, 17, 5017–5022. [Google Scholar] [CrossRef]
- Galwey, A.K. Structure and order in thermal dehydration of crystalline solids. Thermochim. Acta 2000, 355, 181–238. [Google Scholar] [CrossRef]
- Petit, S.; Coquerel, G. Mechanism of Several Solid−Solid Transformations between Dihydrated and Anhydrous Copper (II) 8-Hydroxyquinolinates. Proposition for a Unified Model for the Dehydration of Molecular Crystals. Chem. Mater. 1996, 2247–2258. [Google Scholar] [CrossRef]
- Morris, K.R. Structural Aspects of Hydrates and Solvates. In Polymorphism in Pharmaceutical Solids; Brittain, H.G., Ed.; Marcel Dekker Inc.: New York, NY, USA, 1999; pp. 125–182. [Google Scholar]
- Mimura, H.; Kitamura, S.; Kitagawa, T.; Kohda, S. Characterization of the non-stoichiometric and isomorphic hydration and solvation in FK041 clathrate. Colloids Surf. B: Biointerfaces 2002, 26, 397–406. [Google Scholar] [CrossRef]
- Dumić, M.; Vinković, M.; Orešić, M.; Meštrović, E.; Danilovski, A.; Dumbović, A.; Knežević, Z.; Lazarevski, G.; Filić, D.; Cinčić, D.; et al. Isostructural Pseudopolymorphs of 9-Deoxo-9a-aza-9a-methyl-9a-homoerithromycin A. U.S. 7,569,549 B2, 4 August 2009. [Google Scholar]
- Dumić, M.; Vinković, M.; Orešić, M.; Meštrović, E.; Danilovski, A.; Dumbović, A.; Knežević, Z.; Lazarevski, G.; Filić, D.; Cinčić, D.; et al. Novel Amorphous 9-Deoxo-9a-aza-9a-methyl-9a-homoerithromycin A, Process for Preparing the Same, and Uses Thereof. U.S. 6,936,591 B2, 30 August 2005. [Google Scholar]
- Fujii, K.; Uekusa, H.; Itoda, N.; Yonemochi, E.; Terada, K. Mechanism of Dehydration–Hydration Processes of Lisinopril Dihydrate Investigated by ab Initio Powder X-ray Diffraction Analysis. Cryst. Growth Des. 2012, 12, 6165–6172. [Google Scholar] [CrossRef]
- Mizoguchi, R.; Uekusa, H. Elucidating the Dehydration Mechanism of Ondansetron Hydrochloride Dihydrate with a Crystal Structure. Cryst. Growth Des. 2018, 18, 6142–6149. [Google Scholar] [CrossRef]
- Byrn, S.R.; Pfeiffer, R.R.; Stowell, J.G. Solid-State Chemistry of Drugs, 2nd ed.; SSCI Inc.: West Lafayette, IN, USA, 1999; p. 292. [Google Scholar]
- CrysAlisPro Software System, version 1.171.39.46; Rigaku Oxford Diffraction: Oxford, UK, 2018.
- Farrugia, L.J. WinGX and ORTEP for Windows: An update. J. Appl. Cryst. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, 71, 3–8. [Google Scholar]
- Spek, A.L. Structure validation in chemical crystallography. Acta Cryst. 2009, 65, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Macrae, C.F.; Bruno, I.J.; Chisholm, J.A.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Rodriguez-Monge, L.; Taylor, R.; Van De Streek, J.; Wood, P.A. Mercury CSD 2.0—New features for the visualization and investigation of crystal structures. J. Appl. Cryst. 2008, 41, 466–470. [Google Scholar] [CrossRef]
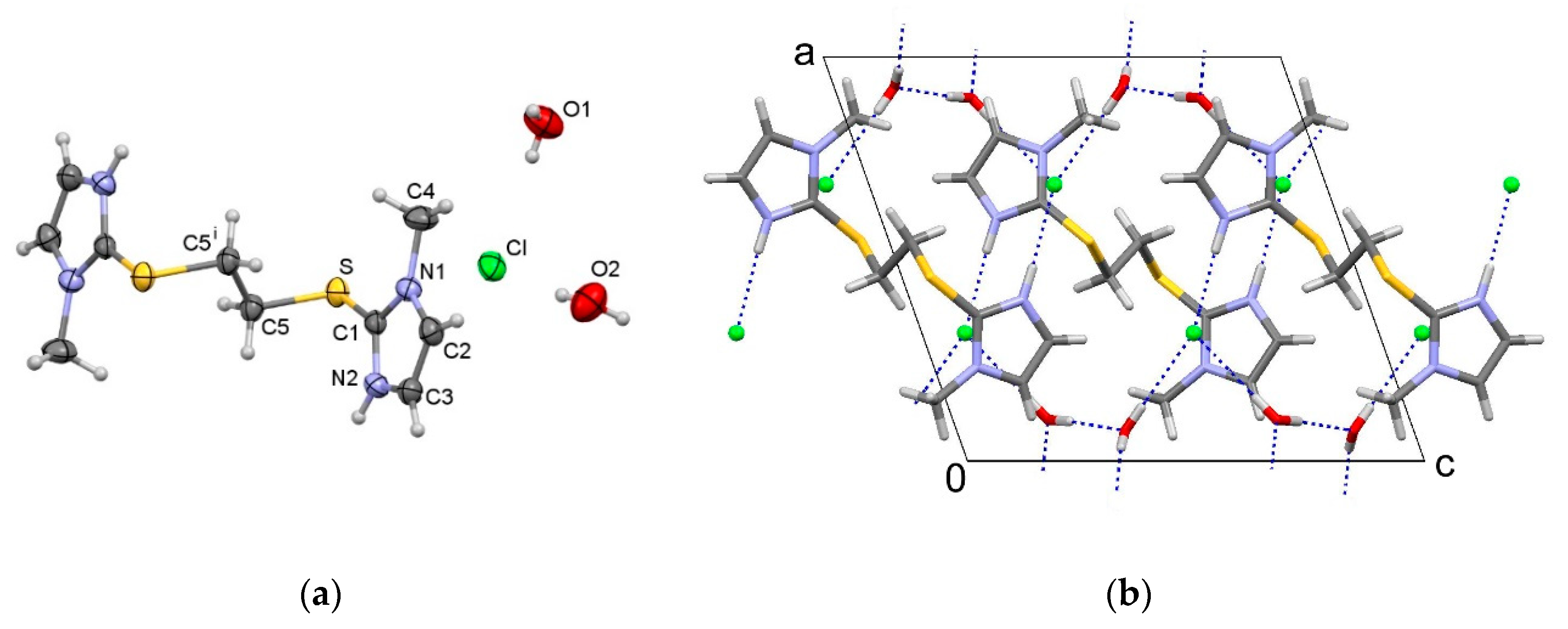
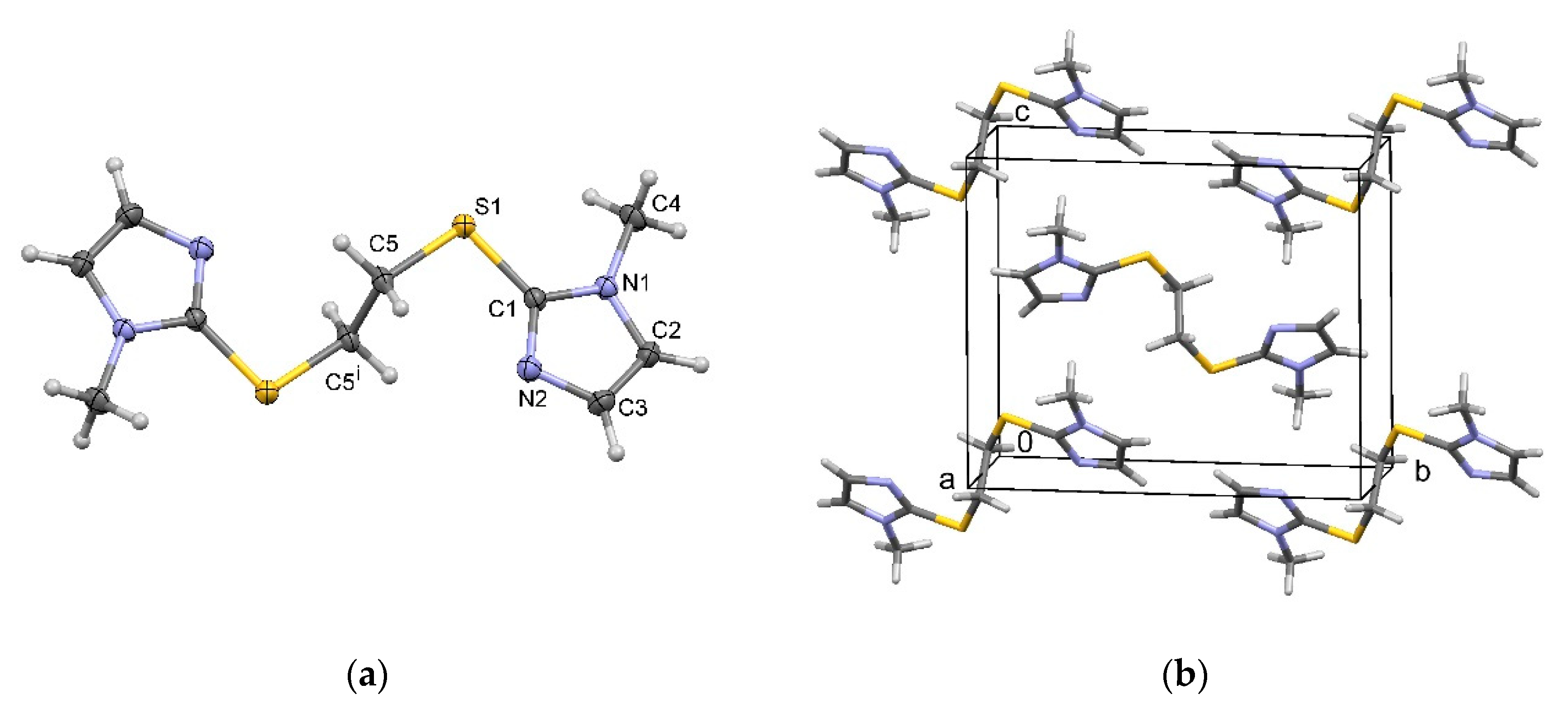


| Form | Donor-H···Acceptor | d(D-H)/Å | d(H-A)/Å | d(D···A)/Å | <d(D-H···A)/° |
|---|---|---|---|---|---|
| N2-H2···Cl a | 0.86 | 2.26 | 3.112 (2) | 172 | |
| O1-H11···O2 b | 0.78 (4) | 1.98 (4) | 2.749 (5) | 169 (5) | |
| 2b | O1-H12···Cl c | 0.75 (5) | 2.44 (5) | 3.190 (4) | 178 (6) |
| O2 -H21···Cl | 0.78 (6) | 2.42 (6) | 3.198 (4) | 174 (5) | |
| O2 -H22···O1 | 0.83 (3) | 1.93 (3) | 2.756 (5) | 172 (3) | |
| O1-H1···N2 d | 0.81 (3) | 2.03 (3) | 2.827 (3) | 167 (4) | |
| 2c | O1-H12···O1 e | 0.87 (4) | 1.92 (4) | 2.787 (4) | 176 (4) |
| C4-4B···O1 f | 0.96 | 2.58 | 3.489 (4) | 157 |
| Compound | 2a | 2b | 2c |
|---|---|---|---|
| Chemical formula | C10H14N4S2 | [C10H16N4S2]Cl2·4(H2O) | C10H14N4S2·2(H2O) |
| Formula weight | 254.37 | 399.35 | 290.40 |
| Crystal system | Monoclinic | Monoclinic | Trigonal |
| Space group | P 21/c | P 21/c | R–3 |
| a/Å | 4.7035 (7) | 11.1667 (10) | 19.3142 (13) |
| b/Å | 12.3772 (14) | 7.7629 (7) | 19.3142 (13) |
| c/Å | 10.3316 (11) | 11.8970 (13) | 10.3283 (5) |
| α/° | 90 | 90 | 90 |
| β/° | 92.772 (14) | 109.637 (11) | 90 |
| γ/° | 90 | 90 | 180 |
| Z | 2 | 2 | 9 |
| F (000) | 268 | 420 | 1386 |
| T/K | 150 | 292 | 292 |
| V/ų | 600.76 (13) | 971.32 (18) | 3336.75 (5) |
| Dx /g.cm3 | 1.406 | 1.365 | 1.301 |
| S | 0.933 | 0.900 | 1.047 |
| R | 0.0493 | 0.049 | 0.0398 |
| wR (F2) | 0.1063 | 0.1272 | 0.0994 |
| No. of reflections | 972 | 2329 | 1448 |
| CCDC | 2013658 | 2013659 | 2013660 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Štefan, L.; Matković-Čalogović, D.; Filić, D.; Dumić, M. Synthesis, Crystal Structure and Solid State Transformation of 1,2-Bis[(1-methyl-1H-imidazole-2-yl)thio]ethane. Crystals 2020, 10, 667. https://doi.org/10.3390/cryst10080667
Štefan L, Matković-Čalogović D, Filić D, Dumić M. Synthesis, Crystal Structure and Solid State Transformation of 1,2-Bis[(1-methyl-1H-imidazole-2-yl)thio]ethane. Crystals. 2020; 10(8):667. https://doi.org/10.3390/cryst10080667
Chicago/Turabian StyleŠtefan, Leo, Dubravka Matković-Čalogović, Darko Filić, and Miljenko Dumić. 2020. "Synthesis, Crystal Structure and Solid State Transformation of 1,2-Bis[(1-methyl-1H-imidazole-2-yl)thio]ethane" Crystals 10, no. 8: 667. https://doi.org/10.3390/cryst10080667
APA StyleŠtefan, L., Matković-Čalogović, D., Filić, D., & Dumić, M. (2020). Synthesis, Crystal Structure and Solid State Transformation of 1,2-Bis[(1-methyl-1H-imidazole-2-yl)thio]ethane. Crystals, 10(8), 667. https://doi.org/10.3390/cryst10080667




