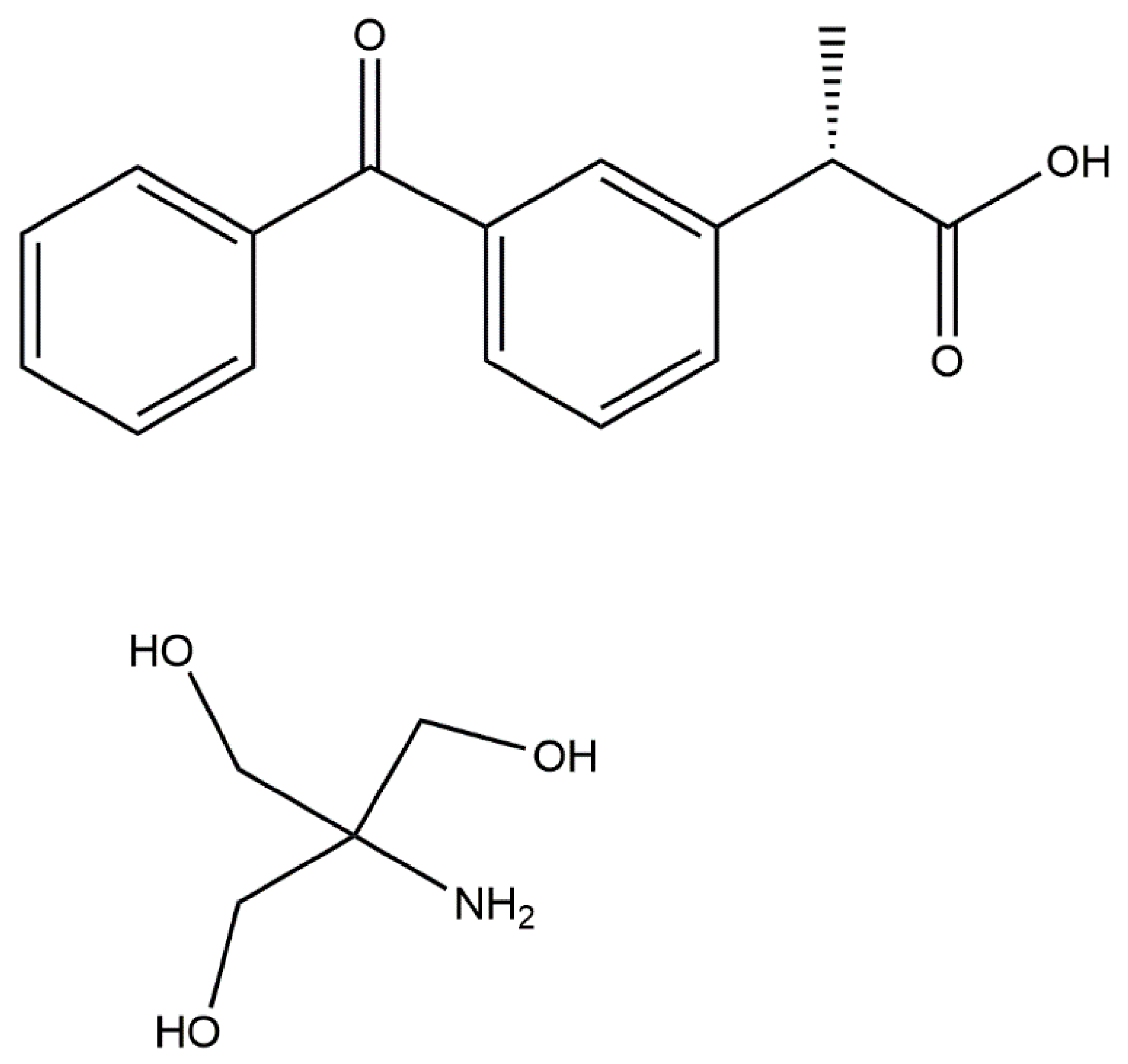
A Combined Crystallographic and Computational Study on Dexketoprofen Trometamol Dihydrate Salt
Abstract
1. Introduction
2. Materials and Methods
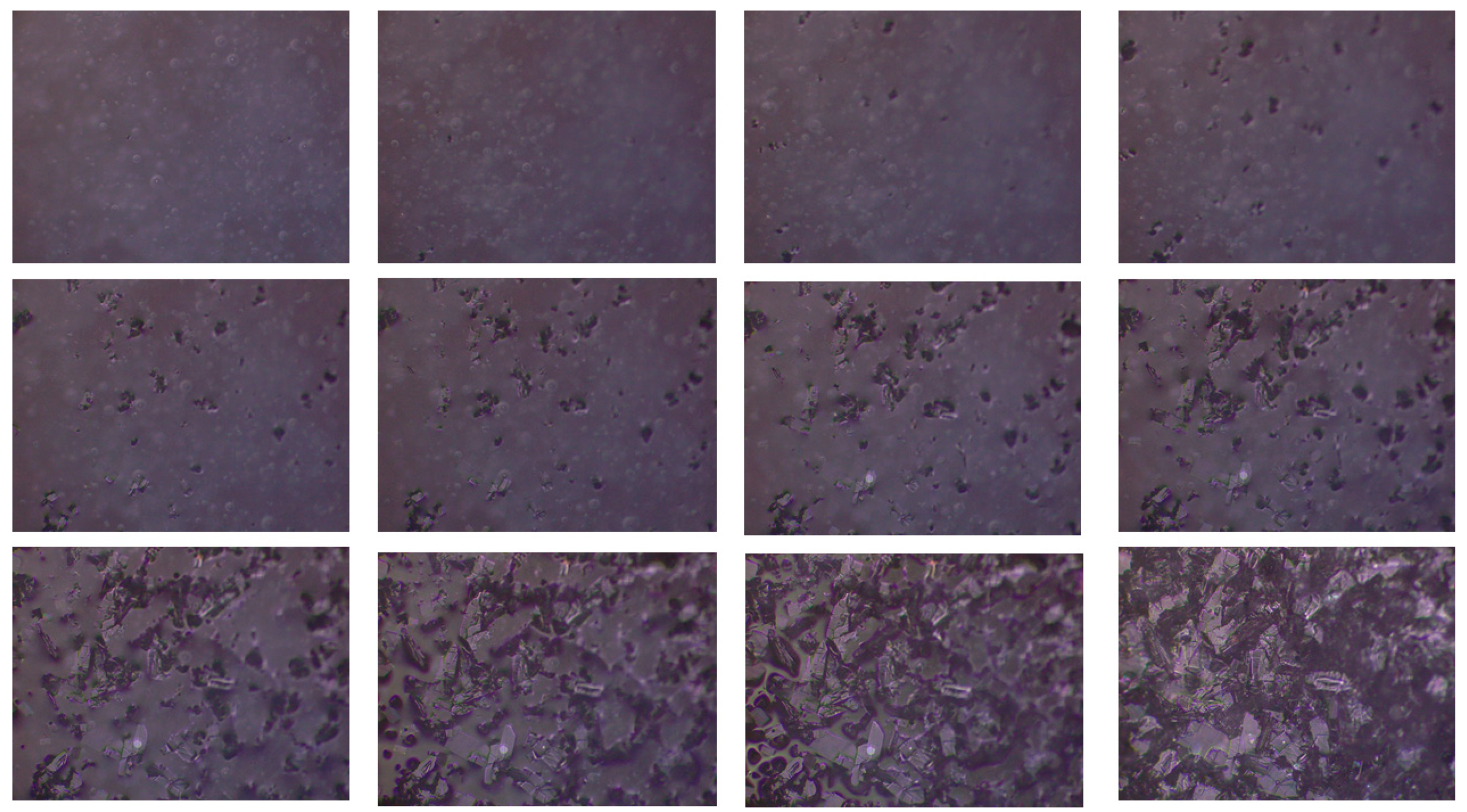
2.1. Procedures of DK-T_A Hydration and DK-T_2H2O Crystallization
2.2. X-ray Powder Diffraction (XRPD)
2.3. Single Crystal X-Ray Data Collection and Structure Solution
2.4. Computational Methods
3. Results and Discussion
3.1. Procedures of DK-T_A Hydration and DK-T_2H2O Crystallization
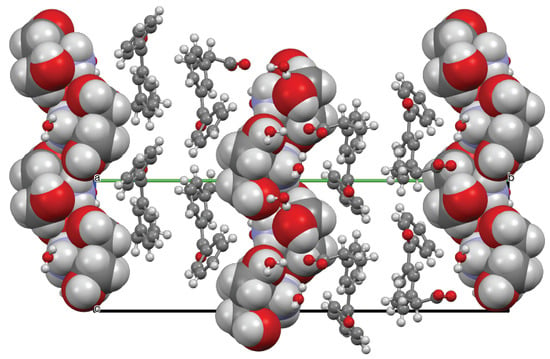
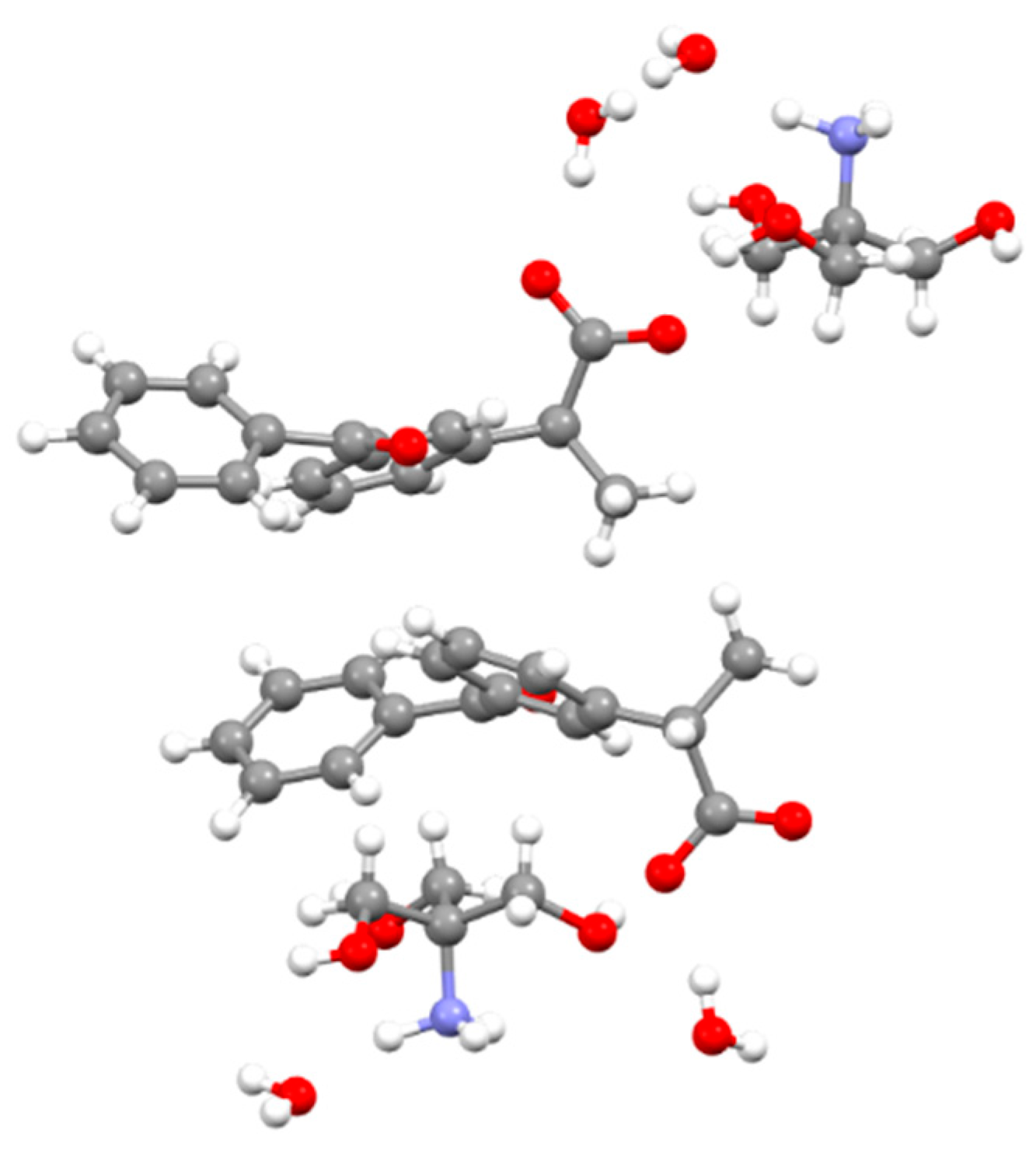
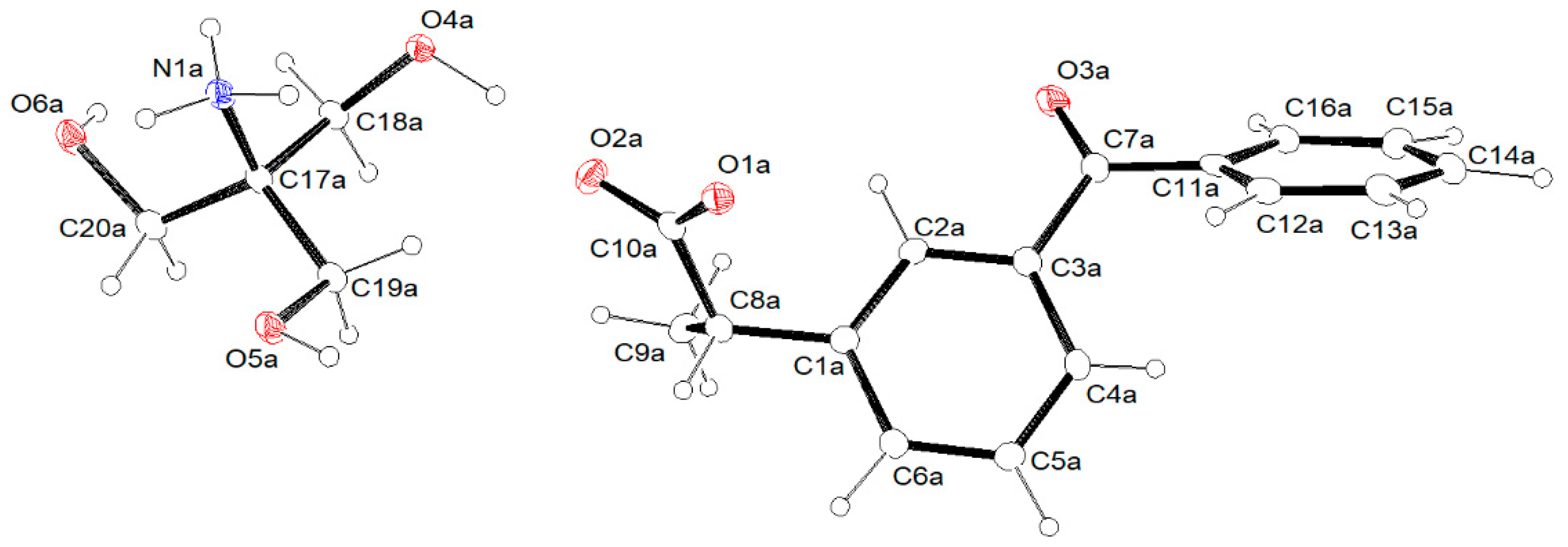
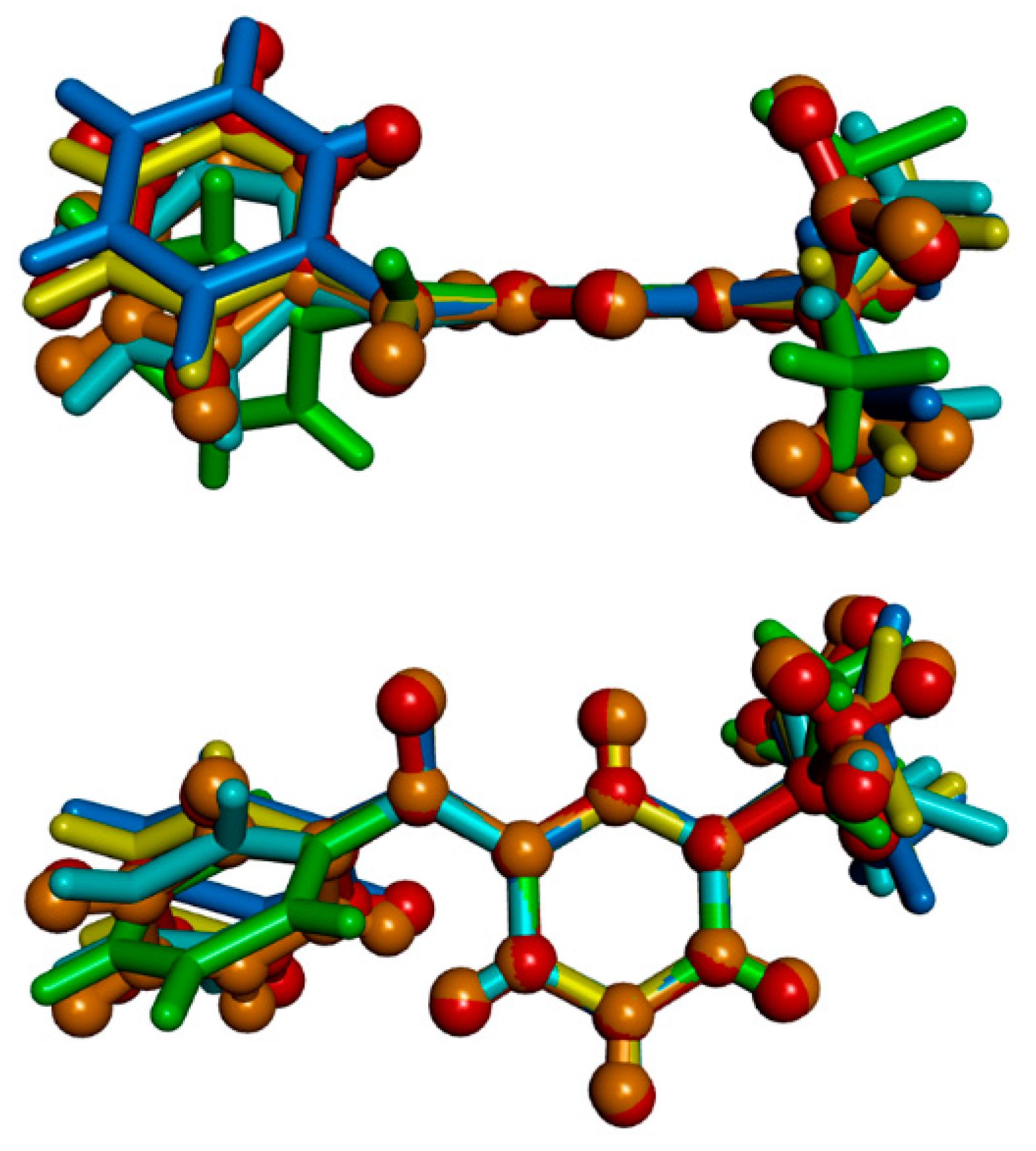
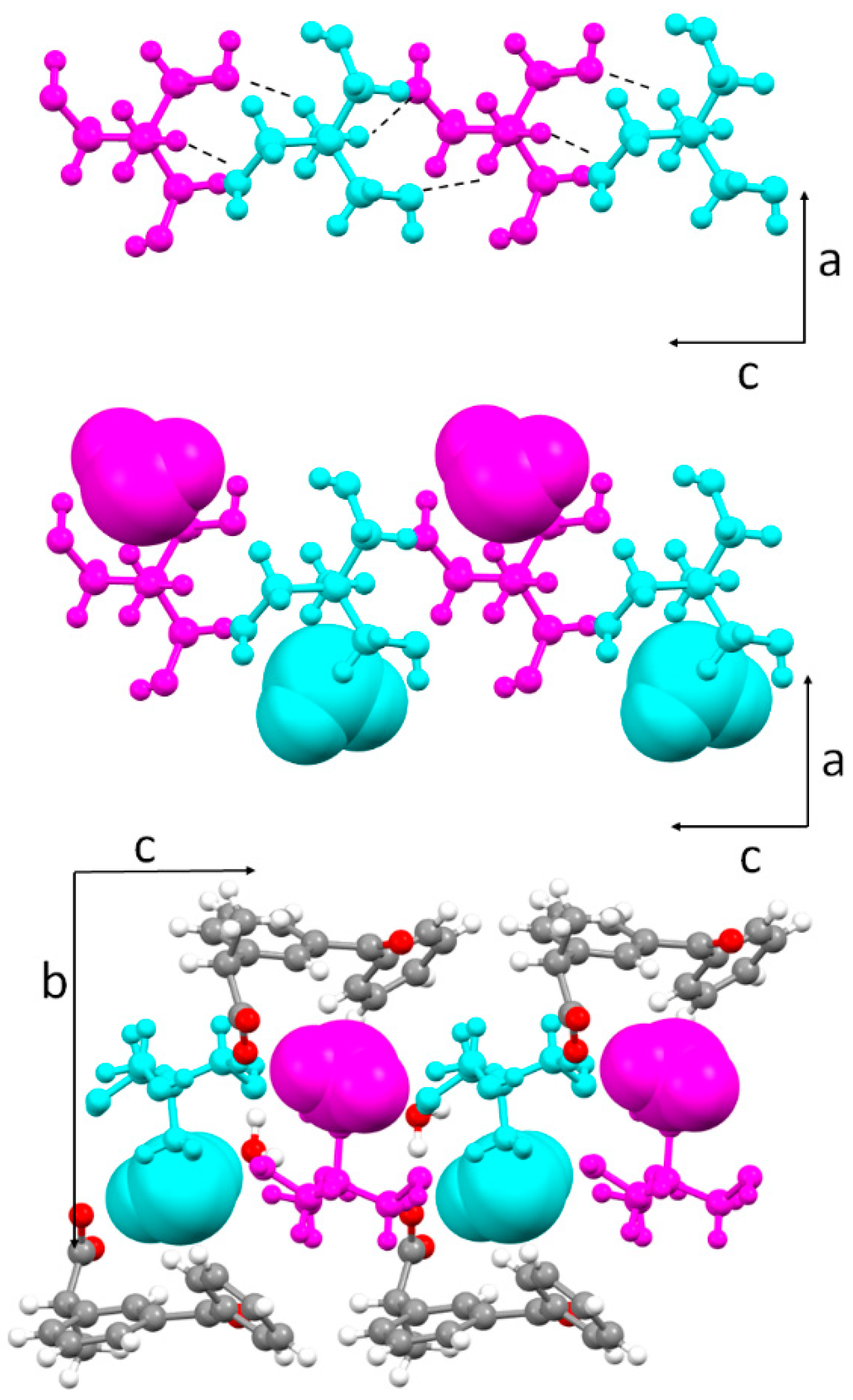
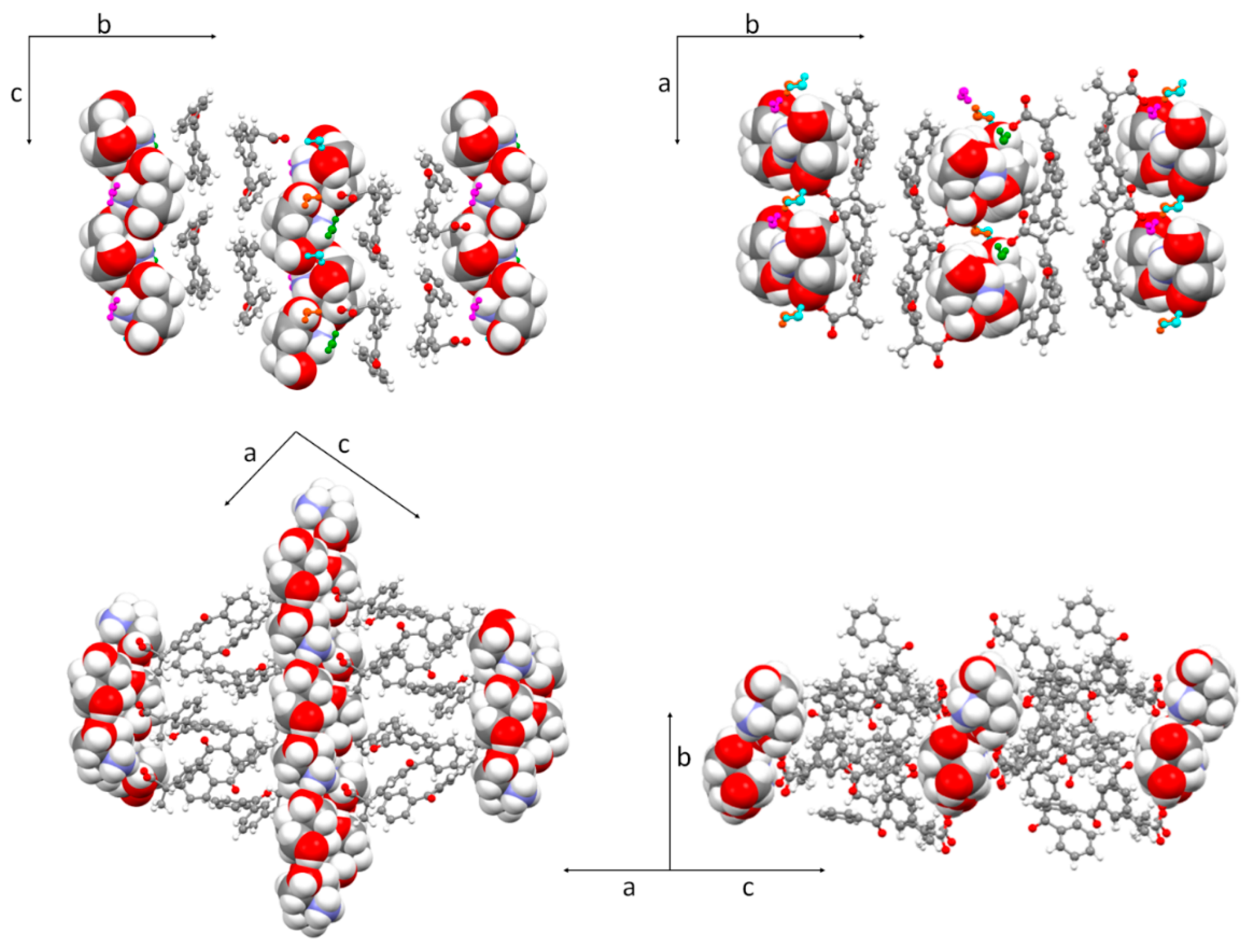
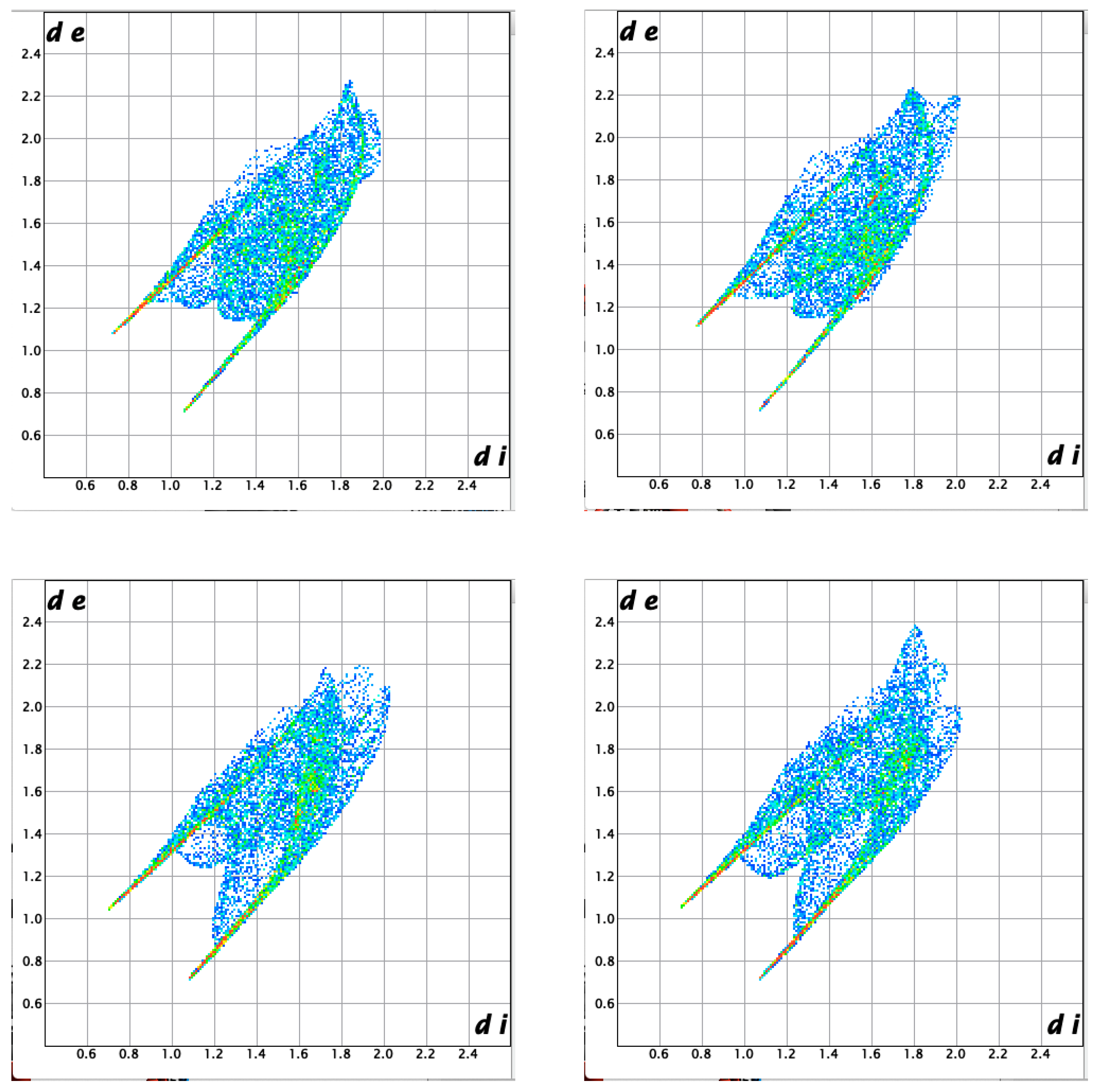
3.2. Molecular and Crystal Structure from Single-Crystal X-Ray Diffraction
3.3. Crystal Structure from Computational Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Byrn, S.R.; Zografi, G.; Chen, X.S. Solid-State Properties of Pharmaceutical Materials, 1st ed.; John Wiley & Sons: New York, NY, USA, 2017; pp. 38–39. [Google Scholar]
- Nangia, A. Pseudopolymorph: Retain This Widely Accepted Term. Cryst. Growth Des. 2006, 6, 2–4. [Google Scholar] [CrossRef]
- Brittain, H.G. Theory and principles of polymorphic systems. In Polymorphism in Pharmaceutical Solids; Brittain, H.G., Ed.; Marcel Dekker Inc.: New York, NY, USA, 1999; pp. 1–33. [Google Scholar]
- Aaltonen, J.; Allesø, M.; Mirza, S.; Koradia, V.; Gordon, K.C.; Rantanen, J. Solid form screening–a review. Eur. J. Pharm. Biopharm. 2009, 71, 23–37. [Google Scholar] [CrossRef] [PubMed]
- Morris K., R.; Rodríguez-Hornedo, N. Encyclopaedia of Pharmaceutical Technology, 3rd ed.; Swarbrick, J., Boylan, J., Eds.; Marcel Dekker: New York, NY, USA, 1993; pp. 393–440. [Google Scholar]
- Tiana, F.; Qua, Q.; Zimmermann, A.; Munka, T.; Jørgensend, A.C.; Rantanena, Y. Factors affecting crystallization of hydrates. J. Pharm. Pharmacol. 2010, 62, 1534–1546. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Jiang, L.; Huang, Y.; Wang, J.R.; Mei, X. Solid-State Characterization and Transformation of Various Creatine Phosphate Sodium Hydrates. J. Pharm. Sci. 2014, 103, 3688–3695. [Google Scholar] [CrossRef]
- Hickey, M.B.; Peterson, M.L.; Manas, E.S.; Alvarez, J.; Haeffner, F.; Almarsson, O. Hydrates and Solid-State Reactivity: A Survey of β-Lactam Antibiotics. J. Pharm. Sci. 2007, 96, 1090–1099. [Google Scholar] [CrossRef]
- Martins Santos, O.M.; Dias Reis, M.E.; Tavares Jacon, J.; Esselin de Sousa Lino, M.; Savioli Simões, J.; Doriguetto, A.C. Polymorphism: An evaluation of the potential risk to the quality of drug products from the Farmácia Popular Rede Própria. Braz. J. Pharm. Sci. 2014, 50, 1–24. [Google Scholar] [CrossRef][Green Version]
- Aitipamula, S.; Vangala, V.R. X-Ray Crystallography and its Role in Understanding the Physicochemical Properties of Pharmaceutical Cocrystals. Indian Inst. Sci. 2017, 97, 227–243. [Google Scholar] [CrossRef]
- Beloborodova, A.A.; Minkov, V.S.; Rychkov, D.A.; Rybalova, T.V.; Boldyreva, E.V. First Evidence of Polymorphism in Furosemide Solvates. Growth Des. 2014, 17, 2333–2341. [Google Scholar] [CrossRef]
- Maestrelli, F.; Rossi, P.; Paoli, P.; De Luca, E.; Mura, P. The role of solid state properties of flufenamic acid. J. Pharm. Biom. Anal. 2020, 14, 113058–113066. [Google Scholar] [CrossRef]
- Rossi, P.; Paoli, P.; Ienco, A.; Biagi, D.; Valleri, M.; Conti, L. A new crystal form of the NSAID dexketoprofen. Acta Cryst. Sect. C 2019, 75, 783–792. [Google Scholar] [CrossRef]
- Paoli, P.; Rossi, P.; Chelazzi, L.; Altamura, M.; Fedi, V.; Giannotti, D. Solid State Investigation and Characterization of a Nepadutant Precursor: Polymorphic and Pseudopolymorphic Forms of MEN11282. Cryst. Growth Des. 2016, 16, 5294–5304. [Google Scholar] [CrossRef]
- Drebushchak, T.N.; Mikhailenko, M.A.; Brezgunova, M.E.; Shakhtshneider, T.P.; Kuznetsova, S.A. Crystal Structure Of Betulin Ethanol Solvate. J. Struct.Chem. 2010, 51, 798–801. [Google Scholar] [CrossRef]
- Drebushchak, V.A.; McGregor, L.; Rychkov, D.A. Cooling rate ‘‘window’’ in the crystallizationof metacetamol form II. J. Therm. Anal. Calorim. 2017, 127, 1807–1814. [Google Scholar] [CrossRef]
- Paoli, P.; Rossi, P.; Macedi, E.; Ienco, A.; Chelazzi, L.; Bartolucci, G.L.; Bruni, B. Similar but Different: The Case of Metoprolol Tartrate and Succinate Salts. Cryst. Growth Des. 2016, 16, 789–799. [Google Scholar] [CrossRef]
- Rossi, P.; Paoli, P.; Chelazzi, L.; Conti, L.; Bencini, A. Metroprolol Fumarate: Crystal Structure from Powder X-ray Diffraction Data and Comparison with the Tartrate and Succinate Salts. Cryst. Growth Des. 2018, 18, 7015–7026. [Google Scholar] [CrossRef]
- Rossi, P.; Macedi, E.; Paoli, P.; Bernazzani, L.; Carignani, E.; Borsacchi, S.; Geppi, M. Solid-Solid Transition between Hydrated Racemic Compound and Anhydrous Conglomerate in Na-Ibuprofen: A Combined X-ray Diffraction, Solid-State NMR, Calorimetric, and Computational Study. Cryst. Growth Des. 2014, 14, 2441–2452. [Google Scholar] [CrossRef]
- McGregor, L.; Rychkov, D.A.; Coster, P.L.; Day, S.; Drebushchak, V.A.; Achkasov, A.F.; Nichol, G.S.; Pulham, C.R.; Boldyreva, E.V. A new polymorph of metacetamol. CrystEngComm 2015, 17, 6183–6192. [Google Scholar] [CrossRef]
- Yang, P.; Qin, C.; Du, S.; Jia, L.; Qin, Y.; Gong, J.; Wu, S. Crystal Structure, Stability and Desolvation of the Solvates of Sorafenib Tosylate. Crystals 2019, 9, 367. [Google Scholar] [CrossRef]
- Tarakanova, E.G.; Voloshenko, G.I.; Kislina, I.S.; Mayorov, V.D.; Yukhnevich, G.V.; Lyashchenko, A.K. Composition and Structure of Hydrates Formed in Aqueous Solutions of Formic Acid. J. Struct.Chem. 2019, 60, 255–267. [Google Scholar] [CrossRef]
- Rossi, P.; Paoli, P.; Milazzo, S.; Chelazzi, L.; Ienco, A.; Conti, L. Investigating Differences and Similarities between Betaxolol Polymorphs. Crystals 2019, 9, 509. [Google Scholar] [CrossRef]
- Paoli, P.; Milazzo, S.; Rossi, P.; Ienco, A. Rationalization of Lattice Thermal Expansion for Beta-Blocker Organic Crystals. Crystals 2020, 10, 350. [Google Scholar] [CrossRef]
- Rossi, P.; Paoli, P.; Chelazzi, L.; Conti, L.; Bencini, A. The solid-state structure of the β-blocker metoprolol: A combined experimental and in silico investigation. Acta Cryst. 2019, C75, 87–96. [Google Scholar] [CrossRef]
- Rychkov, D.A. A Short Review of Current Computational Concepts for High-Pressure Phase Transition Studies in Molecular Crystals. Crystals 2020, 10, 81. [Google Scholar] [CrossRef]
- Rychkov, D.A.; Stare, J.; Boldyreva, E.V. Pressure-driven phase transition mechanisms revealed by quantum-chemistry: L-serine polymorphs. Phys. Chem. Chem. Phys. 2017, 19, 6671–6676. [Google Scholar] [CrossRef] [PubMed]
- Giovannoni, M.P.; Schepetkin, I.; Quinn, M.T.; Cantini, N.; Crocetti, L.; Guerrini, G.; Iacovone, A.; Paoli, P.; Rossi, P.; Bartolucci, G.; et al. Synthesis, biological evaluation, and molecular modelling studies of potent human neutrophil elastase (HNE) inhibitors. J. Enz. Inhib. Med. Chem. 2018, 33, 1108–1124. [Google Scholar] [CrossRef] [PubMed]
- Giovannoni, M.P.; Crocetti, L.; Cantini, N.; Guerrini, G.; Vergelli, C.; Iacovone, A.; Teodori, E.; Schepetkin, I.; Quinn, M.T.; Ciattini, S.; et al. New 3-unsubstituted isoxazolones as potent human neutrophil elastase inhibitors: Synthesis and molecular dynamic simulation. Drug Develop. Res. 2020, 81, 338–349. [Google Scholar] [CrossRef] [PubMed]
- Hanna, M.; Moon, J.Y. A review of dexketoprofen trometamol in acute pain. Curr. Med. Res. Opin. 2019, 35, 189–202. [Google Scholar] [CrossRef]
- Bosch, M.; Mannucci, S.; Torras, E.; Falorni, R.; Gonzales, J.M. Polymorphic forms of dexketoprofen trometamol, preparation and pharmaceutical compositions thereof. European Patent EP1739072 A1, March 2007. [Google Scholar]
- Farshi, F.; Soylemez, S.; Koc, F.; Durmus, S. A process for preparing dexketoprofen trometamol form A and form B crystals. International Patent WO2011/001213 A1, June 2009. [Google Scholar]
- Rossi, P.; Paoli, P.; Chelazzi, L.; Milazzo, S.; Biagi, D.; Valleri, M.; Ienco, A.; Valtancoli, B.; Conti, L. Relationships between Anhydrous and Solvated Species of Dexketoprofen Trometamol: A Solid-State Point of View. Cryst. Growth Des. 2020, 20, 226–336. [Google Scholar] [CrossRef]
- Bruker. BrukerAPEX2; Bruker AXS Inc.: Madison, WI, USA, 2012. [Google Scholar]
- Bruker. Bruker SAINT; Bruker AXS Inc.: Madison, WI, USA, 2012. [Google Scholar]
- Burla, M.C.; Caliandro, R.; Camalli, M.; Carrozzini, B.; Cascarano, G.L.; Da Caro, L.; Giacovazzo, C.; Polidori, G.; Spagna, R. An improved tool for crystal structure determination and refinement. J. Appl. Crystallogr. 2005, 38, 381–388. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Nardelli, M. PARST95 - an update to PARST: A system of Fortran routines for calculating molecular structure parameters from the results of crystal structure analyses. J. Appl. Crystallogr. 1995, 28, 659. [Google Scholar] [CrossRef]
- Farrugia, L. WinGX and ORTEP for Windows: An update. J. Appl. Cryst. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Macrae, C.F.; Bruno, I.J.; Chisholm, J.A.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Rodriguez-Monge, E.; Taylor, R.; van de Streek, J.; Wood, P.A.; et al. Mercury CSD 2.0—New Features for the Visualization and Investigation of Crystal Structures. J. Appl. Crystallogr. 2008, 41, 466–470. [Google Scholar] [CrossRef]
- Discovery Studio 2019 Client; BIOVIA: San Diego, CA, USA, 2019.
- Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Spackman, P.R.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer17 (2017); University of Western Australia: Perth, Australia, 2017. [Google Scholar]
- Dovesi, R.; Erba, A.; Orlando, R.; Zicovich-Wilson, C.M.; Civalleri, B.; Maschio, L.; Rérat, M.; Casassa, S.; Baima, J.; Salustro, S.; et al. Quantum-mechanical condensed matter simulations with CRYSTAL. WIREs Comput. Mol. Sci. 2018, 8, e1360. [Google Scholar] [CrossRef]
- Sure, R.; Grimme, S. Corrected small basis set Hartree-Fock method for large systems. J. Comput. Chem. 2013, 34, 1672–1685. [Google Scholar] [CrossRef]
- Cutini, M.; Civalleri, B.; Corno, M.; Orlando, R.; Brandenburg, J.G.; Maschio, L.; Ugliengo, P. Assessment of different quantum mechanical methods for the prediction of structure and cohesive energy of molecular crystals. J. Chem. Theory Comput. 2016, 12, 3340–3352. [Google Scholar] [CrossRef]
- IUPAC. Compendium of Chemical Terminology, 2nd ed.; the "Gold Book"; McNaught, A.D., Wilkinson, A., Eds.; Blackwell Scientific Publications: Oxford, UK, 1997. [Google Scholar]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Cryst. Sect. B 2016, 72, 171–179. [Google Scholar]
- Etter, M.C.; MacDonald, J.C.; Bernstein, J. Graph-set analysis of hydrogen-bond patterns in organic crystals. Acta Crystallogr., Sect. B Struct. Sci. 1990, 46, 256–262. [Google Scholar] [CrossRef]
- Kitaigorodskii, A.I. Organic Chemical Crystallography; Consultants Bureau: New York, NY, USA, 1961; pp. 106–110. [Google Scholar]
- Werner, J.E.; Swift, J.A. Data mining the Cambridge Structural Database for hydrate–anhydrate pairs with SMILES strings. CrystEngComm 2020. [Google Scholar] [CrossRef]
- Morris, K.R. Structural Aspects of Hydrates and Solvates. In Polymorphism in Pharmaceutical Solids; Brittain, H.G., Ed.; Marcel Dekker Inc.: New York, NY, USA, 1999; pp. 126–179. [Google Scholar]
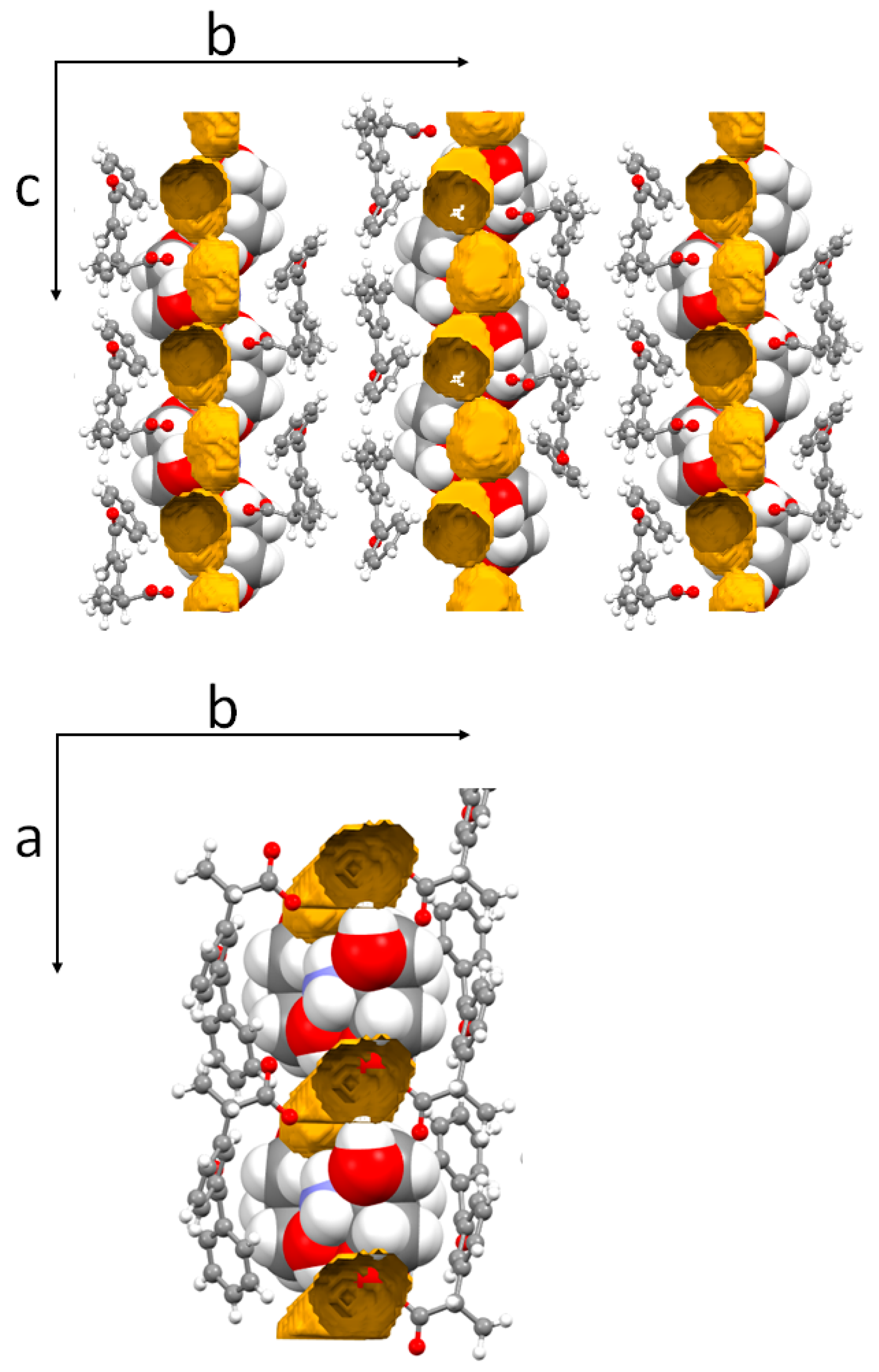
| DK-T_2H2O | |
|---|---|
| Formula | [C4H12NO3]+[C16H13O3]− .2H2O |
| MW | 411.44 |
| T (K) | 100 |
| λ (Å) | 1.54178 |
| Crystal system, space group | Monoclinic, P21 |
| Unit cell dimensions (Å, °) | a = 8.480(4) b = 27.7440(10); β = 90.479(3) c = 8.7770(4) |
| Volume (Å3) | 2064.89(15) |
| Z, Dc (mg/cm3) | 4, 1.323 |
| μ (mm−1) | 0.857 |
| R1 [I>2σ(I)] | 0.0639 |
| wR2 (all) | 0.1672 |
| GOFs (Goodness of fit) | 1.041 |
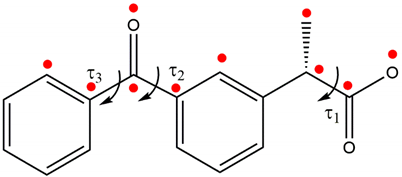 | ||
|---|---|---|
| DK | ||
| a | b | |
| τ1 = C9-C8-C10-O1 | −163.0(5) | −158.7(5) |
| τ2 = C2-C3-C7-O3 | −22.1 (9) | −30.3(9) |
| τ3 = O3-C7-C11-C12 | −40.0(9) | −35.4(9) |
| X…Y (Å), H…Y (Å), X-H…Y (°) | X…Y (Å), H…Y (Å), X-H…Y (°) | ||
|---|---|---|---|
| Strongest donor | |||
| N1A-H1N1…O1WA | 2.784(7)/2.020(4) /140.6(3) | ||
| N1A-H1N2…O5B1 | 2.749(6)/1.933(4)/148.3(3) | ||
| N1A-H1N3…O6B | 2.883(6)/ 2.095(4)/144.3(3) | ||
| Water…Water | |||
| N1B-H1N6…O1WB | 2.787(7)/2.034(4)/139.2(3) | O1WA-H1W2…O2WA2 | 2.801(6)/1.93(6)/171(5) |
| N1B-H1N5…O4A | 2.757(6)/1.953(4)/146.4(3) | O1WB-H1W3…O2WB3 | 2.825(6)/1.98(6)/163(5) |
| N1B-H1N4…O5A3 | 2.880(6)/2.096(4) /143.6(3) | ||
| Strongest acceptor | Water (acceptor) …trometamol (donor) | ||
| O1A…H6OA-O6A4 | 2.711(6)/2.04(8)/161(9) | O2WA…H6OB-O6B | 2.786(6)/1.94(8)/177(8) |
| O2A…H4OA-O4A5 | 2.586(6)/1.65(7) /153(6) | O2WB…H5OA-O5A2 | 2.779(6)/1.91(8)/169(8) |
| O1A…H1W4-O1WB4 | 2.903(6)/2.01(5) /174(4) | ||
| O1A…H2W1-O2WA4 | 2.717(6)/1.85(4) /170(3) | ||
| Water (donor) …trometamol (acceptor) | |||
| O1B…H4OB-O4B3 | 2.708(6)/2.01(8)/167(9) | O2WA-H2W2…O4B7 | 2.817(6) /1.96(6)/175(6) |
| O2B…H5OB-O5B6 | 2.588(6)/1.76(8) /174(8) | O2WB-H2W4…O6A | 2.811(6) /1.94(6)/172(5) |
| O1B…H1W1-O1WA | 2.884(6)/2.00(5) /173(4) | ||
| O1B…H2W3-O2WB7 | 2.728(6)/1.86(4) /164(3) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rossi, P.; Paoli, P.; Milazzo, S.; Chelazzi, L.; Giovannoni, M.P.; Guerrini, G.; Ienco, A.; Valleri, M.; Conti, L. A Combined Crystallographic and Computational Study on Dexketoprofen Trometamol Dihydrate Salt. Crystals 2020, 10, 659. https://doi.org/10.3390/cryst10080659
Rossi P, Paoli P, Milazzo S, Chelazzi L, Giovannoni MP, Guerrini G, Ienco A, Valleri M, Conti L. A Combined Crystallographic and Computational Study on Dexketoprofen Trometamol Dihydrate Salt. Crystals. 2020; 10(8):659. https://doi.org/10.3390/cryst10080659
Chicago/Turabian StyleRossi, Patrizia, Paola Paoli, Stella Milazzo, Laura Chelazzi, Maria Paola Giovannoni, Gabriella Guerrini, Andrea Ienco, Maurizio Valleri, and Luca Conti. 2020. "A Combined Crystallographic and Computational Study on Dexketoprofen Trometamol Dihydrate Salt" Crystals 10, no. 8: 659. https://doi.org/10.3390/cryst10080659
APA StyleRossi, P., Paoli, P., Milazzo, S., Chelazzi, L., Giovannoni, M. P., Guerrini, G., Ienco, A., Valleri, M., & Conti, L. (2020). A Combined Crystallographic and Computational Study on Dexketoprofen Trometamol Dihydrate Salt. Crystals, 10(8), 659. https://doi.org/10.3390/cryst10080659