Zeolite-Like Boron Imidazolate Frameworks (BIFs): Synthesis and Application
Abstract
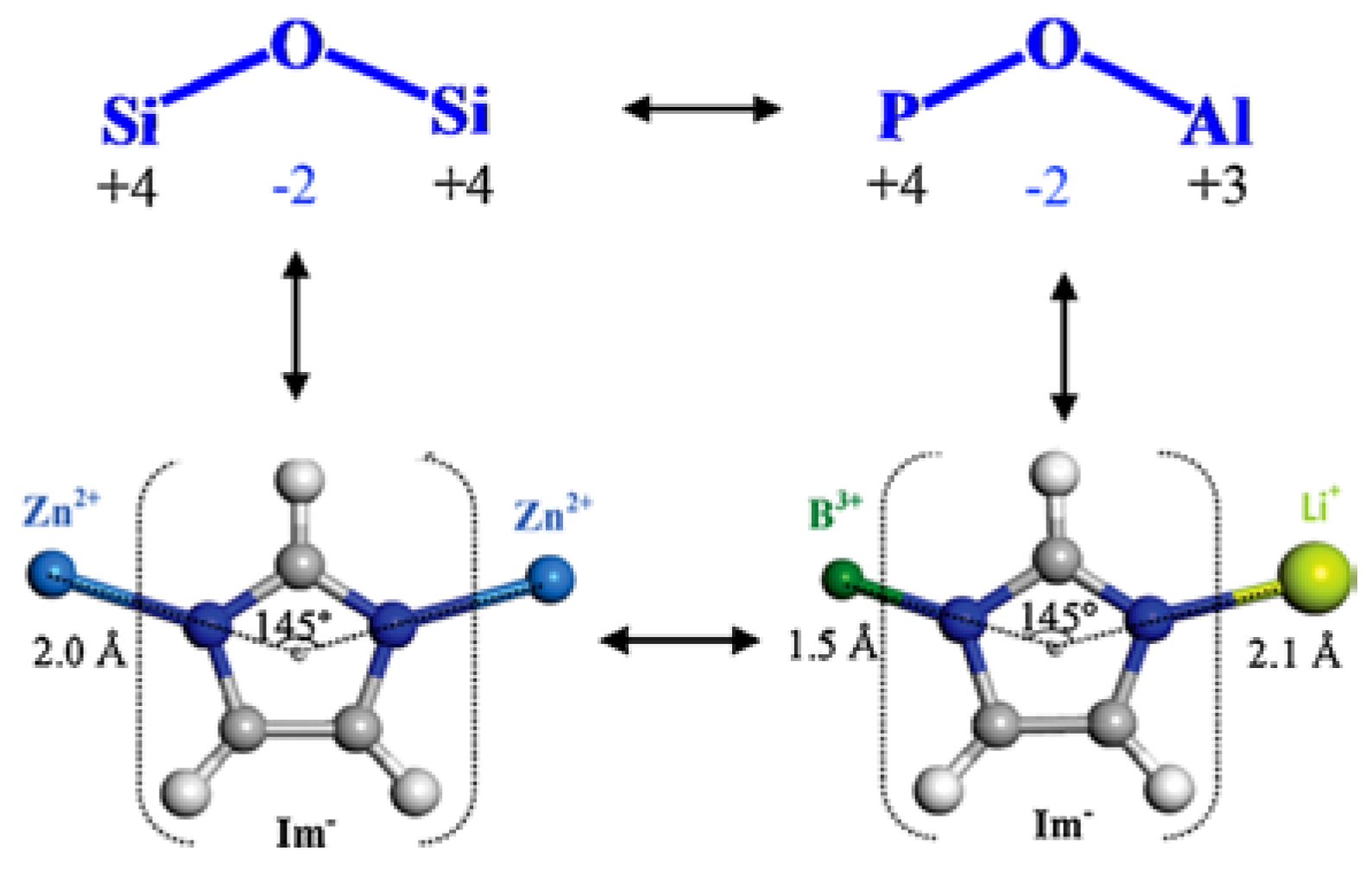
1. Introduction
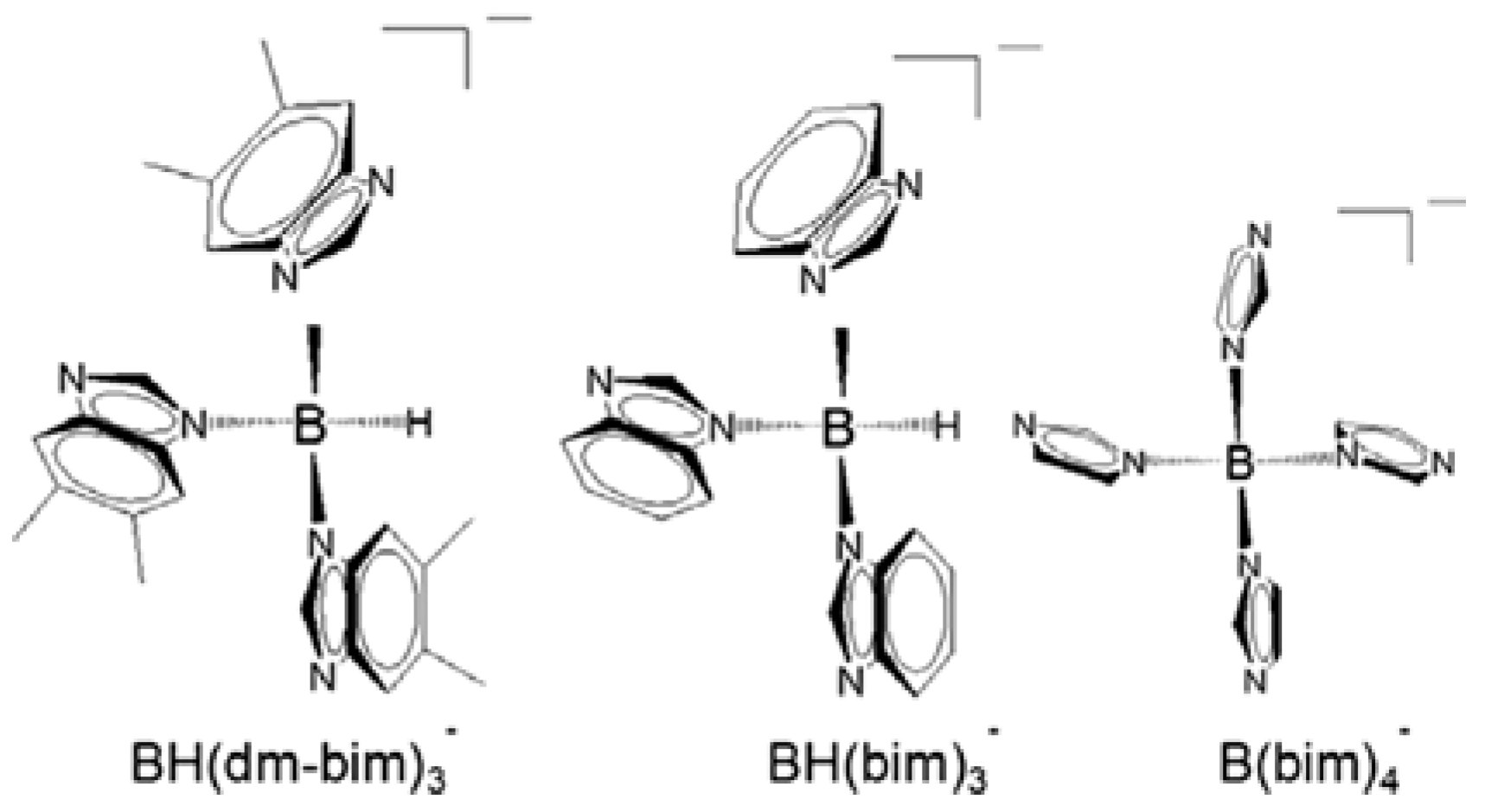
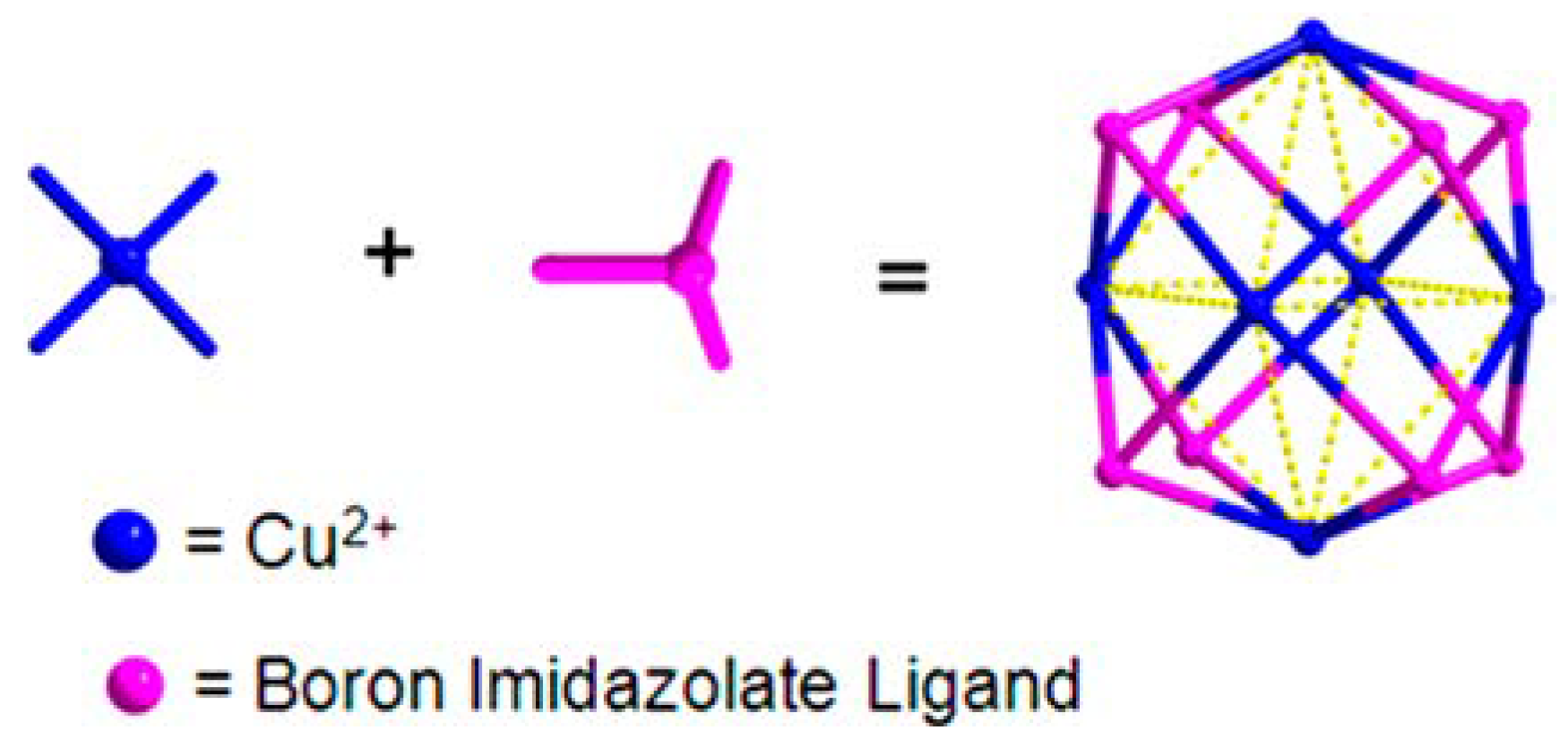
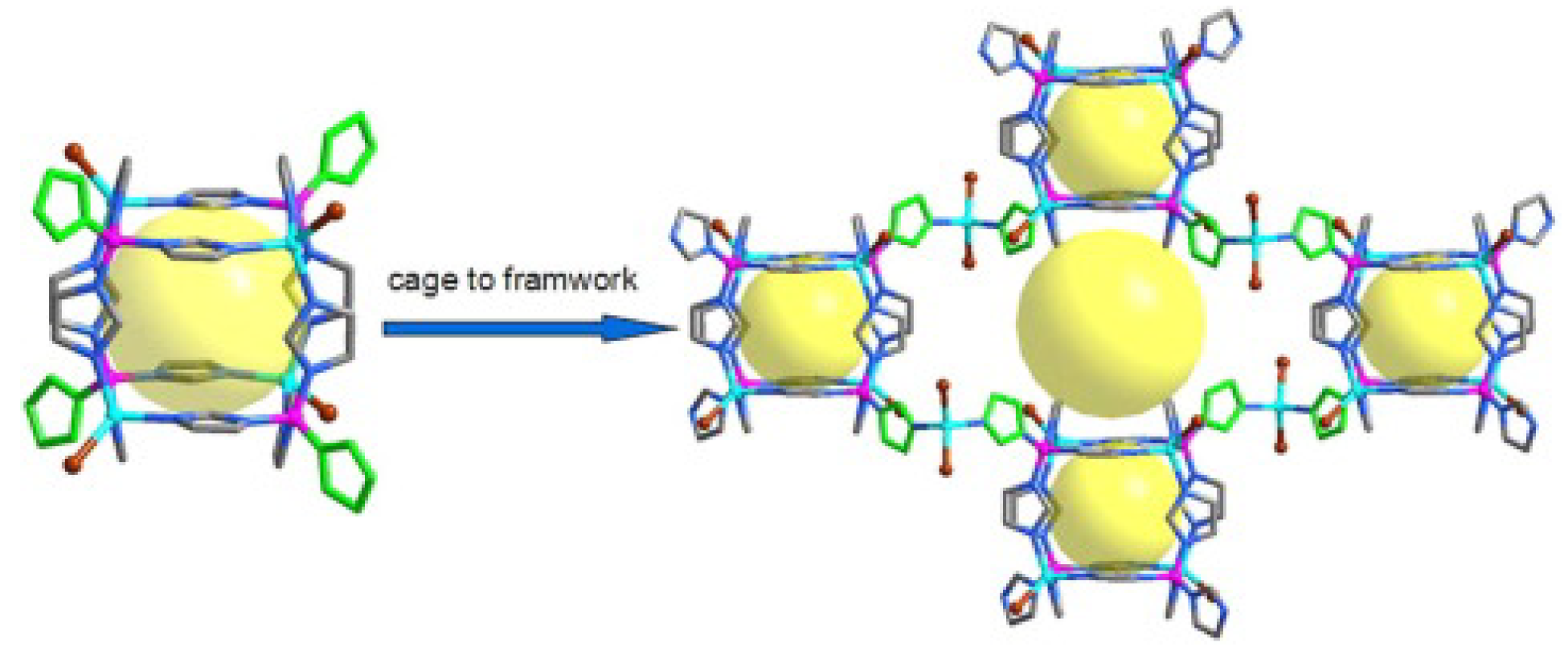
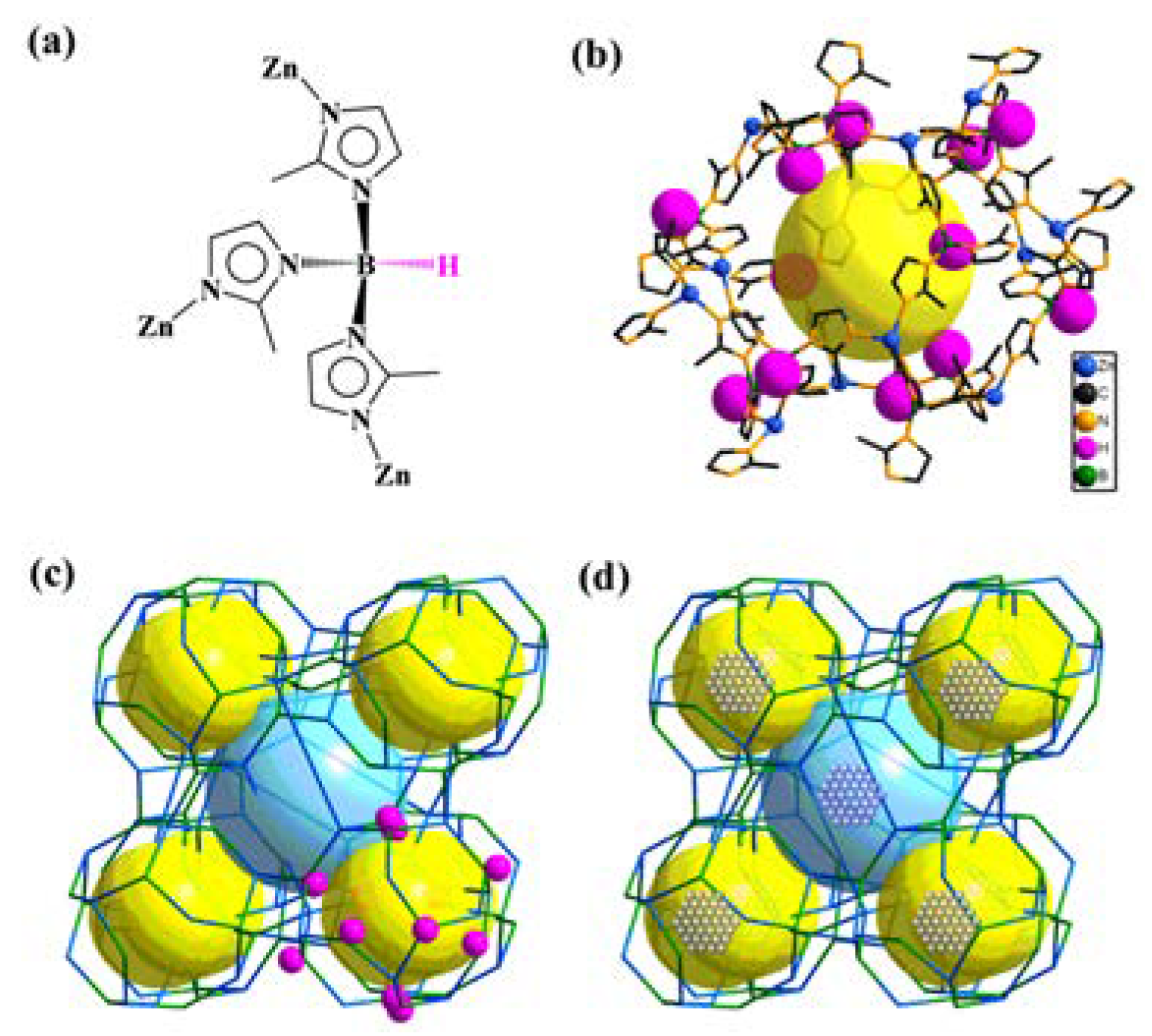
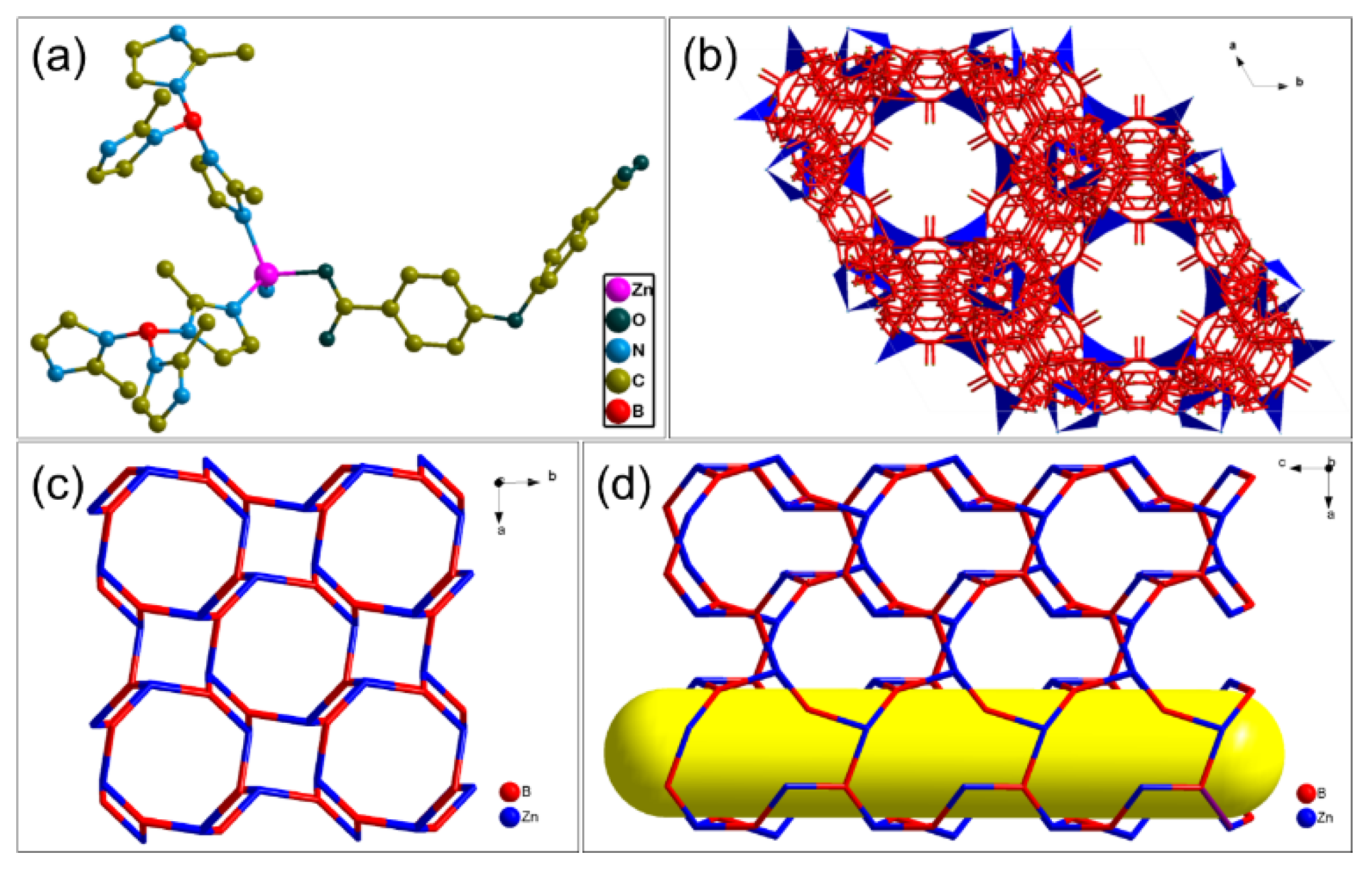
2. Rational Construction of Boron Imidazolate Frameworks
3. Potential Applications for the BIF Materials
3.1. Gas Adsorption and Separation
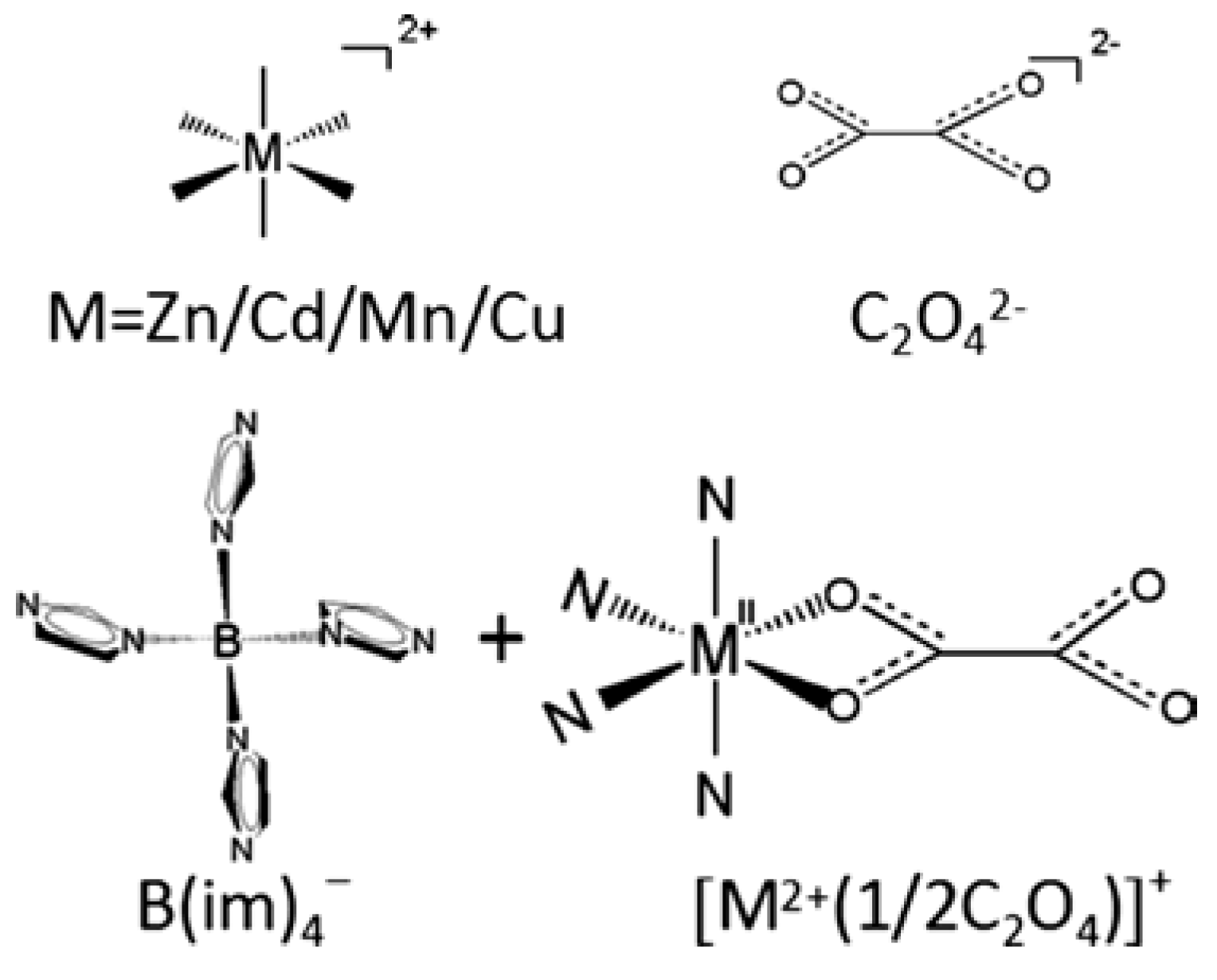
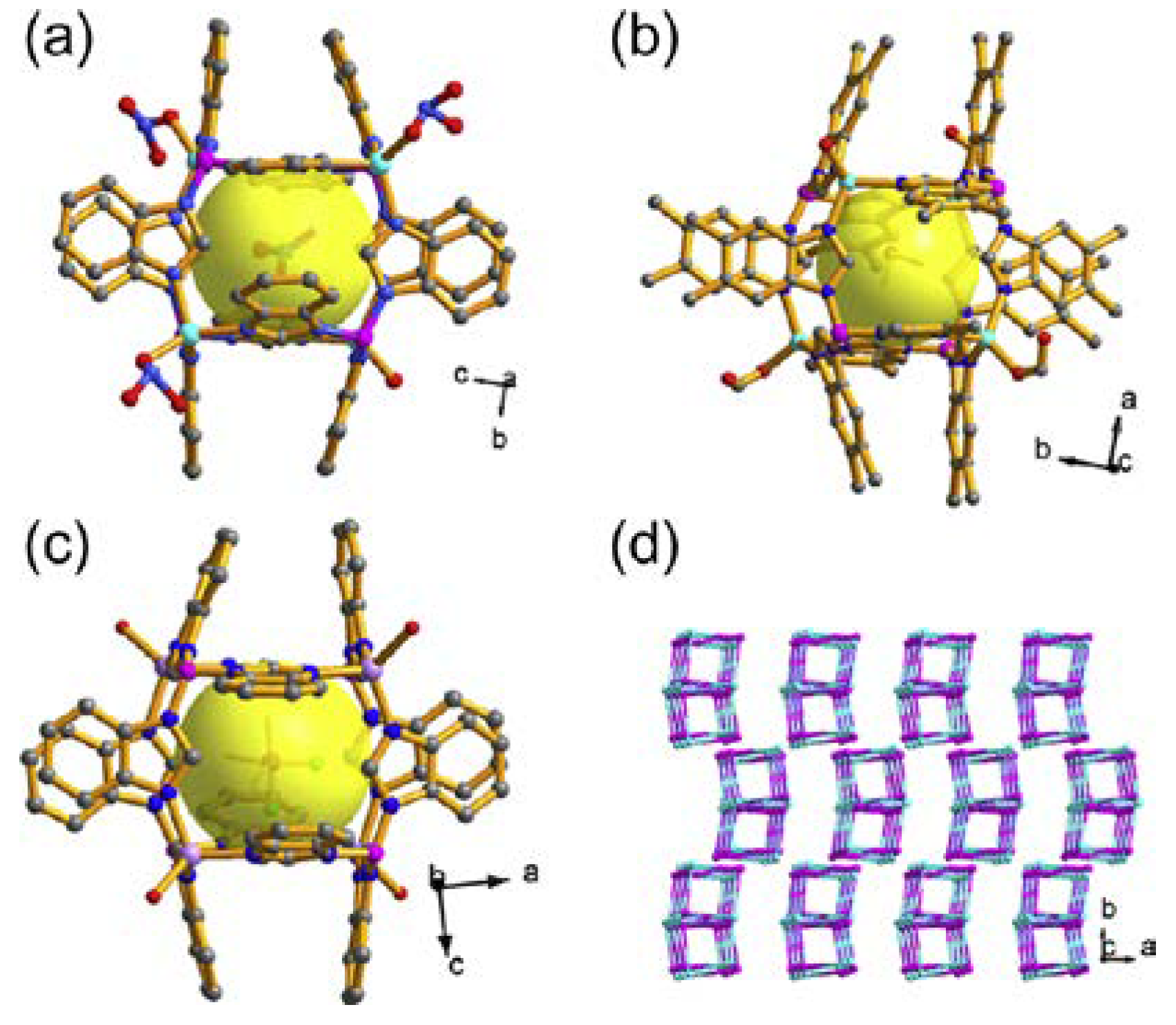
3.2. Reductive Catalysis
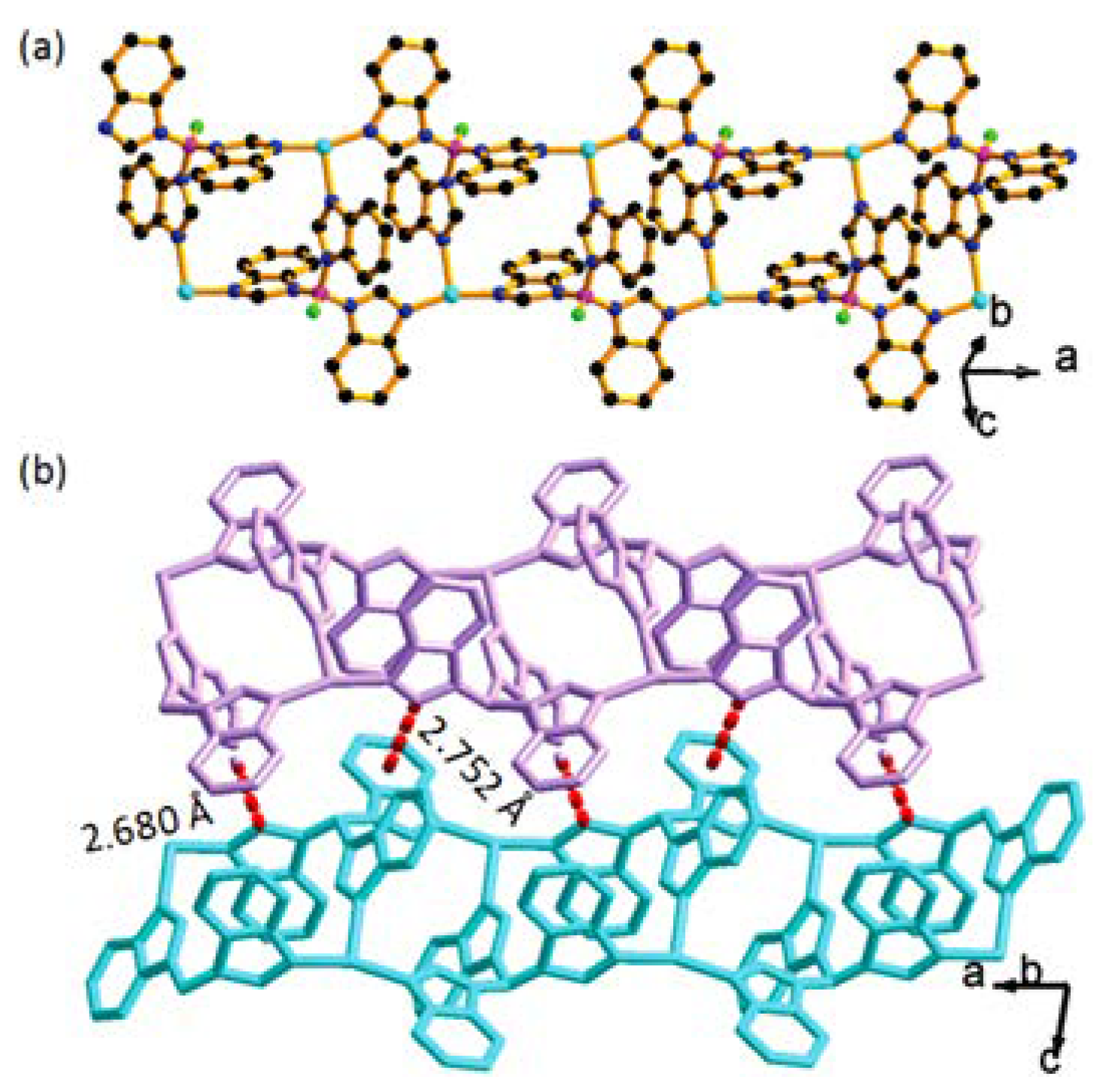
3.3. Solid-State Photoluminescent and Mechanxochromic Properties of BIF Matrices
3.4. Photocatalysis
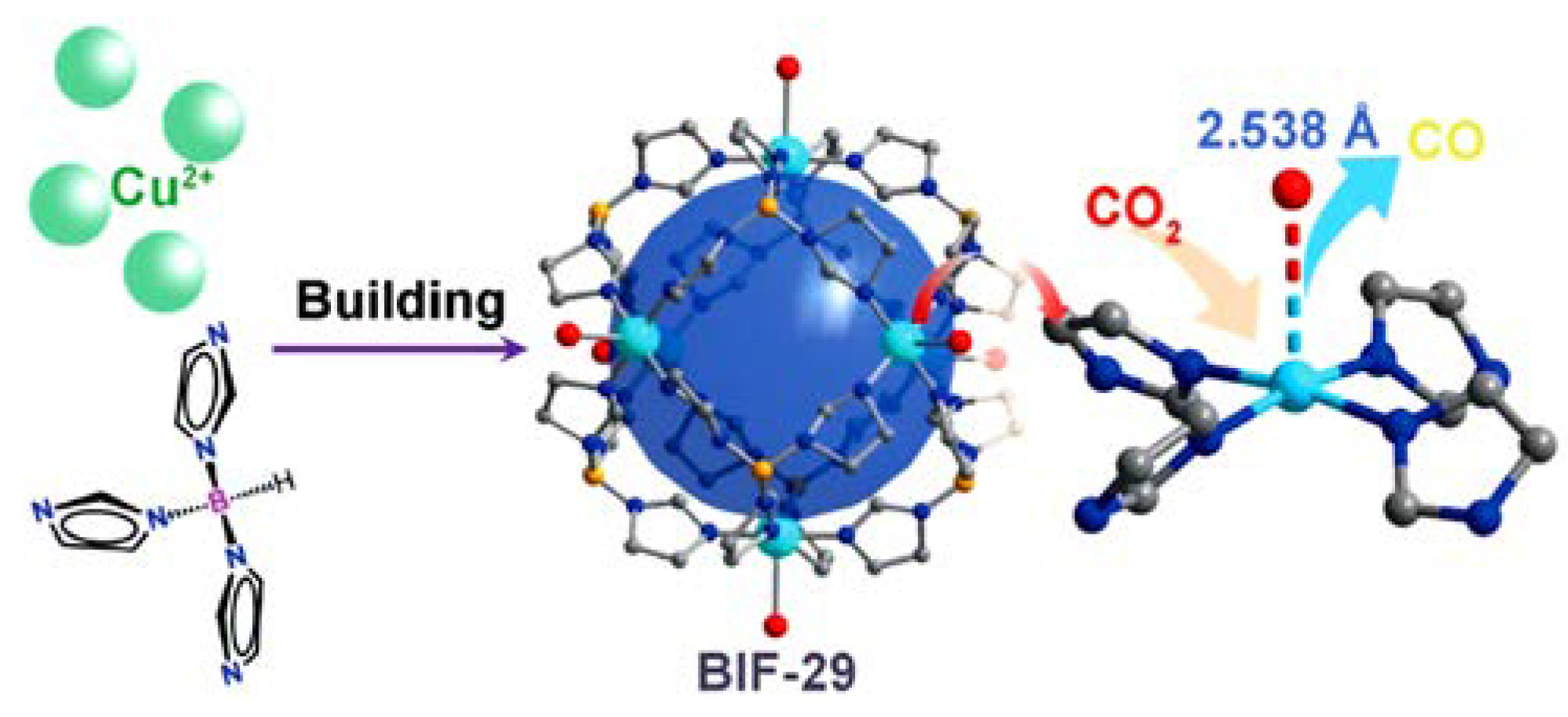
3.5. CO2 Photocatalytic Reduction
3.6. Electrocatalysis
4. BIF Nanohybrids with Metal Nanoparticles
5. BIF-Based Composites with Graphitic Material
6. BIFs-derived Materials
6.1. 2D BIFs-Derived Nanosheet Materials
6.2. BIFs-Derived Graphitic Materials
7. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Schuth, F.; Schmidt, W. Microporous and mesoporous materials. Adv. Mater. 2002, 14, 629–638. [Google Scholar] [CrossRef]
- Li, J.; Corma, A.; Yu, J. Synthesis of new zeolite structures. Chem. Soc. Rev. 2015, 44, 7112–7127. [Google Scholar] [CrossRef]
- Seoane, B.; Castellanos, S.; Dikhtiarenko, A.; Kapteijn, F.; Gascon, J. Multi-Scale Crystal Engineering of Metal Organic Frameworks. Coord. Chem. Rev. 2016, 307, 147–187. [Google Scholar] [CrossRef]
- Eddaoudi, M.; Sava, D.F.; Eubank, J.F.; Adila, K.; Guillerma, V. Zeolite-like metal-organic frameworks (ZMOFs): Design, synthesis, and properties. Chem. Soc. Rev. 2015, 44, 228–249. [Google Scholar] [CrossRef] [PubMed]
- Cheetham, K.; Ferey, G.; Loiseau, T. Angew. Chem. Int. Ed. 1999, 38, 3268–3292. [CrossRef]
- Chen, B.; Yang, Z.; Zhu, Y.; Xia, Y. Zeolitic Imidazolate Framework Materials: Recent Progress in Synthesis and Applications. J. Mater. Chem. A 2014, 2, 16811–16831. [Google Scholar] [CrossRef]
- Li, J.; Yu, J.; Xu, R. Progress in heteroatom-containing aluminophosphate molecular sieves. Proc. R. Soc. A 2012, 468, 1955–1967. [Google Scholar] [CrossRef]
- Wilson, S.T.; Lok, B.M.; Messina, C.A.; Cannan, T.R.; Flanigen, E.M. Aluminophosphate molecular sieves: A new class of microporous crystalline inorganic solids. J. Am. Chem. Soc. 1982, 104, 1146–1147. [Google Scholar] [CrossRef]
- Cora, F.; Alfredsson, M.; Barker, C.M.; Bell, R.G.; Foster, M.D.; Saadoune, I.; Simperler, A.; Catlow, C.R.A. Modeling the framework stability and catalytic activity of pure and transition metal-doped zeotypes. J. Solid State Chem. 2003, 176, 496–529. [Google Scholar] [CrossRef]
- Hartmann, M.; Elangovan, S.P. Catalysis with Microporous Aluminophosphates and Silicoaluminophosphates Containing Transition Metals. In Advances in Nanoporous Materials; Ernst, S., Ed.; Elsevier: Amsterdam, The Netherlands, 2010; Volume 1, pp. 1–336. [Google Scholar]
- Rowsell, J.L.C.; Yaghi, O.M. Metal-organic frameworks: A new class of porous materials. Microporous Mesoporous Mater. 2004, 73, 3–14. [Google Scholar] [CrossRef]
- Férey, G. Hybrid porous solids: Past, present, future. Chem. Soc. Rev. 2008, 37, 191–214. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.-C.; Long, G.; Yaghi, O.M. Introduction to Metal-Organic Frameworks. Chem. Rev. 2012, 112, 673–674. [Google Scholar] [CrossRef] [PubMed]
- Diercks, K.S.; Kalmutzki, M.G.; Diercks, N.G.; Yaghi, O.M. Conceptual Advances from Werner Complexes to Metal-Organic Frameworks. ACS Cent. Sci. 2018, 4, 1457–1464. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.X.; Liu, M.; Wen, T.; Zhang, J. Synthetic design of functional boron imidazolate frameworks. Coord. Chem. Rev. 2016, 307, 255–266. [Google Scholar] [CrossRef]
- Khay, I.; Chaplais, G.; Nouali, H.; Ortiz, G.; Marichal, C.; Patarin, J. Assessment of the Energetic Performances of Various ZIFs with SOD or RHO Topology Using High Pressure Water Intrusion-Extrusion Experiments. Dalton Trans. 2016, 45, 4392–4400. [Google Scholar] [CrossRef]
- Tan, Y.-X.; Wang, F.; Zhang, J. Design and synthesis of multifunctional metal-organic zeolites. Chem. Soc. Rev. 2018, 47, 2130–2144. [Google Scholar] [CrossRef]
- Corma, A. State of the art and future challenges of zeolites as catalysts. J. Catal. 2003, 216, 298–312. [Google Scholar] [CrossRef]
- O’Keeffe, M.; Peskov, M.A.; Ramsden, S.J.; Yaghi, O.M. The Reticular Chemistry Structure Resource (RCSR) Database of, and Symbols for, Crystal Nets. Acc. Chem. Res. 2008, 41, 1782–1789. [Google Scholar] [CrossRef]
- Bavykina, A.; Kolobov, N.; Khan, I.S.; Bau, J.A.; Ramirez, A.; Gascon, J. Metal-Organic Frameworks in Heterogeneous Catalysis: Recent Progress, New Trends, and Future Perspectives. Chem. Rev. 2020. [Google Scholar] [CrossRef]
- Petit, C. Present and future of MOF research in the field of adsorption and molecular separation. Curr. Opin. Chem. Eng. 2018, 20, 132–142. [Google Scholar] [CrossRef]
- Dhaka, S.; Kumar, R.; Deep, A.; Kurade, M.B.; Ji, S.W.; Jeon, B.H. Metal-organic frameworks (MOFs) for the removal of emerging contaminants from aquatic environments. Coord. Chem. Rev. 2019, 380, 330–352. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, Y.-W. Metal-Organic frameworks for biomedical applications. Small 2020. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-S. Metal-organic frameworks for biosensing and bioimaging applications. Coord. Chem. Rev. 2017, 349, 139–155. [Google Scholar] [CrossRef]
- Tian, Y.; Cai, C.; Ji, Y.; You, X.; Peng, S.; Lee, G. [Co5(Im)10_2MB]∞: A Metal-Organic Open-Framework with Zeolite-Like Topology. Angew. Chem. Int. Ed. 2002, 41, 1384–1386. [Google Scholar] [CrossRef]
- Noh, K.; Lee, J.; Kim, J. Compositions and Structures of Zeolitic Imidazolate Frameworks. Isr. J. Chem. 2018, 58, 1075–1088. [Google Scholar] [CrossRef]
- Wu, T.; Bu, X.; Zhang, J.; Feng, P. New Zeolitic Imidazolate Frameworks: From Unprecedented Assembly of Cubic Clusters to Ordered Cooperative Organization of Complementary Ligands. Chem. Mater. 2008, 20, 7377–7382. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, T.; Zhou, C.; Chen, S.; Feng, P.; Bu, X. Zeolitic Boron Imidazolate Frameworks. Angew. Chem. Int. Ed. 2009, 48, 2542–2545. [Google Scholar] [CrossRef]
- Brekalo, I.; Deliz, D.E.; Kane, C.M.; Frišcic, T.; Holman, K.T. Templates in the Mechanochemical Synthesis of RHO Topology Zeolitic Imidazolate Frameworks (ZIFs). Molecules 2020, 25, 633. [Google Scholar] [CrossRef]
- Moosavi, S.M.; Boyd, P.G.; Sarkisov, L.; Smit, B. Improving the Mechanical Stability of Metal-Organic Frameworks Using Chemical Caryatids. ACS Cent. Sci. 2018, 4, 832–839. [Google Scholar] [CrossRef]
- Phan, A.; Doonan, C.J.; Uribe-Romo, F.J.; Knobler, C.B.; O’Keeffe, M.; Yaghi, O.M. Synthesis, Structure, and Carbon Dioxide Capture Properties of Zeolitic Imidazolate Frameworks. Acc. Chem. Res. 2010, 43, 58–67. [Google Scholar] [CrossRef]
- Hayashi, H.; Cote, A.P.; Furukawa, H.; O’Keeffe, M.; Yaghi, O.M. Zeolite a imidazolate framework. Nat. Mater. 2007, 6, 501–506. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, R.; Phan, A.; Wang, B.; Knobler, C.; Furukawa, H.; O’Keeffe, M.; Yaghi, O.M. High-throughput synthesis of zeolitic imidazolate frameworks and application to CO2 capture. Science 2008, 319, 939–943. [Google Scholar] [CrossRef] [PubMed]
- Kaneti, V.V.; Dutta, S.; Hossain, M.S.A.; Shiddiky, M.J.A.; Tung, K.-L.; Shieh, F.-K.; Tsung, C.-K.; Wu, K.C.-W.; Yamauchi, Y. Strategies for Improving the Functionality of Zeolitic Imidazolate Frameworks: Tailoring Nanoarchitectures for Functional Applications. Adv. Mater. 2017, 29, 1700213. [Google Scholar] [CrossRef]
- Zhang, H.-X.; Fu, H.-R.; Li, H.-Y.; Zhang, J.; Bu, X. Porous ctn-Type Boron Imidazolate Framework for Gas Storage and Separation. Chem. Eur. J. 2013, 19, 11527–11530. [Google Scholar] [CrossRef]
- Baburin, I.A.; Assfour, B.; Seifert, G.; Leoni, S. Polymorphs of lithium-boron imidazolates: Energy landscape and hydrogen storage properties. Dalton Trans. 2011, 40, 3796–3798. [Google Scholar] [CrossRef]
- Wang, F.; Shu, Y.-B.; Bu, X.; Zhang, J. Zeolitic Boron Imidazolate Frameworks with 4-Connected Octahedral Metal Centers. Chem. Eur. J. 2012, 18, 11876–11879. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Furukawa, H.; Gandara, F.; Nguyen, H.T.; Cordova, K.E.; Yaghi, O.M. Selective Capture of Carbon Dioxide under Humid Conditions by Hydrophobic Chabazite-Type Zeolitic Imidazolate Frameworks. Angew. Chem. Int. Ed. 2014, 53, 10645–10648. [Google Scholar] [CrossRef]
- Jiang, J.; Zhao, Y.; Yaghi, O.M. Covalent Chemistry beyond Molecules. J. Am. Chem. Soc. 2016, 138, 3255–3265. [Google Scholar] [CrossRef]
- Wen, T.; Zheng, Y.; Zhang, J.; Davey, K.; Qiao, S.-Z. Co (II) Boron Imidazolate Framework with Rigid Auxiliary Linkers for Stable Electrocatalytic Oxygen Evolution Reaction. Adv. Sci. 2019, 6, 1801920. [Google Scholar] [CrossRef]
- Zhang, H.-X.; Wang, F.; Yang, H.; Tan, Y.X.; Zhang, J.; Bu, X. Interrupted Zeolite LTA and ATN-Type Boron Imidazolate Frameworks. J. Am. Chem. Soc. 2011, 133, 11884–11887. [Google Scholar] [CrossRef]
- Zhang, H.-X.; Liu, M.; Xu, G.; Liu, L.; Zhang, J. Selectivity of CO2 via Pore Space Partition in Zeolitic Boron Imidazolate Frameworks. Chem. Commun. 2016, 52, 3552–3555. [Google Scholar] [CrossRef]
- Zhang, D.-X.; Zhang, H.-X.; Wen, T.; Li, D.-S.; Zhang, J. Mechanochromic Cu(i) Boron Imidazolate Frameworks with Low-dimensional Structures and Reducing Function. Inorg. Chem. Front. 2016, 3, 263–267. [Google Scholar] [CrossRef]
- Wen, T.; Zhang, J. Rational design of metal boron imidazolate cages to frameworks. Inorg. Chim. Acta 2017, 460, 89–92. [Google Scholar] [CrossRef]
- Zhang, H.-X.; Hong, Q.-L.; Li, J.; Wang, F.; Huang, X.; Chen, S.; Tu, W.; Yu, D.; Xu, R.; Zhou, T.; et al. Isolated Square-Planar Copper Center in Boron Imidazolate Nanocages for Photocatalytic Reduction of CO2 to CO. Angew. Chem. Int. Ed. 2019, 58, 11752–11756. [Google Scholar] [CrossRef]
- Wen, T.; Zhang, D.-X.; Zhang, H.-X.; Zhang, H.-B.; Zhang, J.; Li, D.-S. Redox-active Cu(i) boron imidazolate framework for mechanochromic and catalytic applications. Chem. Commun. 2014, 50, 8754–8756. [Google Scholar] [CrossRef] [PubMed]
- Wen, T.; Chen, E.-X.; Zhang, D.-X.; Zhang, J. Synthesis of borocarbonitride from a multifunctional Cu(I) boron imidazolate framework. Dalton Trans. 2016, 45, 5223–5228. [Google Scholar] [CrossRef]
- Wen, T.; Zhang, D.X.; Liu, J.; Zhang, H.X.; Zhang, J. Facile synthesis of bimetal Au-Ag nanoparticles in a Cu(i) boron imidazolate framework with mechanochromic properties. Chem. Commun. 2015, 51, 1353–1355. [Google Scholar] [CrossRef]
- Zhang, D.-X.; Liu, J.; Zhang, H.-X.; Wen, T.; Zhang, J. A Rational Strategy to Construct a Neutral Boron Imidazolate Framework with Encapsulated Small-Size Au−Pd Nanoparticles for Catalysis. Inorg. Chem. 2015, 54, 6069–6071. [Google Scholar] [CrossRef]
- Liu, L.; Wen, T.; Chen, S.; Zhang, J. A zeolitic Cd (II) boron imidazolate framework with sensing and catalytic properties. J. Solid State Chem. 2015, 231, 185–189. [Google Scholar] [CrossRef]
- Wen, T.; Liu, M.; Chen, S.; Li, Q.; Du, Y.; Zhou, T.; Ritchie, C.; Zhang, J. 2D Boron Imidazolate Framework Nanosheets with Electrocatalytic Applications for Oxygen Evolution and Carbon Dioxide Reduction Reaction. Small 2020, 1907669. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, D.-X.; Chen, S.; Wen, T. Loading Ag nanoparticles on Cd II boron imidazolate framework for photocatalysis. J. Solid State Chem. 2016, 237, 32–35. [Google Scholar] [CrossRef]
- Liu, M.; Chen, S.; Wen, T.; Zhang, J. Encapsulation of Ln (III) ions/Ag nanoparticles within Cd (II) boron imidazolate frameworks for tuning luminescence emission. Chem. Commun. 2016, 52, 8577–8580. [Google Scholar] [CrossRef] [PubMed]
- Wen, T.; Zheng, Y.; Xu, C.C.; Zhang, J.; Jaroniec, M.; Qiao, S.Z. A boron imidazolate framework with mechanochromic and electrocatalytic properties. Mater. Horiz. 2018, 5, 1151–1155. [Google Scholar] [CrossRef]
- Hong, Q.-L.; Zhang, H.-X.; Zhang, J. Synthesis of boron imidazolate frameworks with cobalt clusters for efficient visible-light driven CO2 reduction. J. Mater. Chem. A 2019, 7, 17272–17276. [Google Scholar] [CrossRef]
- Qia, Y.; Yea, J.; Ren, S.; Lva, J.; Zhang, S.; Che, Y.; Ning, G. In-situ synthesis of metal nanoparticles@metal-organic frameworks: Highly effective catalytic performance and synergistic antimicrobial activity. J. Hazard. Mater. 2020, 387, 121687. [Google Scholar] [CrossRef] [PubMed]
- De Azevedo, D.C.S.; Cardoso, D.; Fraga, M.A.; Pastore, H.O. Zeolites for a Sustainable World. Microporous Mesoporous Mater. 2017, 254, 1–2. [Google Scholar] [CrossRef]
- Newsam, J.M. The Zeolite Cage Structure. Science 1986, 231, 1093–1099. [Google Scholar] [CrossRef]
- Hong, Q.-L.; Zhang, H.-X.; Wen, Y.-H.; Zhang, J. One unique neutral boron imidazolate framework with fluorescent property. Inorg. Chem. Commun. 2018, 95, 130–133. [Google Scholar] [CrossRef]
- Lu, Z.; Knobler, C.B.; Furukawa, H.; Wang, B.; Liu, G.; Yaghi, O.M. Synthesis and Structure of Chemically Stable Metal−Organic Polyhedra. J. Am. Chem. Soc. 2009, 131, 12532–12533. [Google Scholar] [CrossRef]
- Furukawa, H.; Kim, J.; Plass, K.E.; Yaghi, O.M. Crystal Structure, Dissolution, and Deposition of a 5 nm Functionalized Metal−Organic Great Rhombicuboctahedron. J. Am. Chem. Soc. 2006, 128, 8398–8399. [Google Scholar] [CrossRef]
- Zhang, G.; Mastalerz, M. Organic cage compounds—from shape-persistency to function. Chem. Soc. Rev. 2014, 43, 1934–1947. [Google Scholar] [CrossRef] [PubMed]
- Rostamnia, S.; Karimi, Z. Preparation and catalytically study of metal–organic frameworks of amine/MIL-53 (Al) as a powerful option in the rapid N-formylation condensation in neat conditions. Inorg. Chim. Acta 2015, 428, 133. [Google Scholar] [CrossRef]
- Rostamnia, S.; Alamgholiloo, H.; Liu, X. Pd-grafted open metal site copper-benzene-1,4-dicarboxylate metal organic frameworks (Cu-BDC MOF’s) as promising interfacial catalysts for sustainable Suzuki coupling. J. Colloid Interface Sci. 2016, 46, 310–317. [Google Scholar] [CrossRef]
- Lee, J.; Kwak, H.J.; Choe, W. Evolution of form in metal-organic frameworks. Nat. Commun. 2017, 8, 14070. [Google Scholar] [CrossRef]
- Zhang, D.-X.; Zhang, H.-X.; Li, H.-Y.; Wen, T.; Zhang, J. Targeted design of a cubic boron imidazolate cage with sensing and reducing functions. Dalton Trans. 2015, 44, 9367–9369. [Google Scholar] [CrossRef]
- Zhang, D.X.; Zhang, H.X.; Li, H.Y.; Wen, T.; Zhang, J. Self-Assembly of Metal Boron Imidazolate Cages. Cryst. Growth Des. 2015, 15, 2433–2436. [Google Scholar] [CrossRef]
- Ramirez, J.R.; Yang, H.; Kane, C.M.; Ley, A.N.; Holman, K.T. Reproducible Synthesis and High Porosity of Mer-Zn(Im)2 (ZIF-10): Exploitation of an Apparent Double-Eight Ring Template. J. Am. Chem. Soc. 2016, 138, 12017–12020. [Google Scholar] [CrossRef] [PubMed]
- Frišˇcic, T.; Halasz, I.; Beldon, P.J.; Belenguer, A.M.; Adams, F.; Kimber, S.A.J.; Honkimäki, V.; Dinnebier, R.E. Real-Time and in Situ Monitoring of Mechanochemical Milling Reactions. Nat. Chem. 2013, 5, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Jeazet, H.B.T.; Staudt, C.; Janiak, C. Metal-organic frameworks in mixed-matrix membranes for gas separation. Dalton Trans. 2012, 41, 14003–14027. [Google Scholar] [CrossRef]
- Gangu, K.K.; Maddila, S.; Mukkamala, S.B.; Jonnalagadda, S.B. A review on contemporary Metal-Organic Framework materials. Inorg. Chim. Acta 2016, 446, 61–74. [Google Scholar] [CrossRef]
- Isaeva, V.I.; Barkova, M.I.; Kustov, L.M.; Syrtsova, D.A.; Efimova, E.A.; Teplyakov, V.V. In Situ synthesis of novel ZIF-8 membranes on polymeric and inorganic supports. J. Mater. Chem. A. 2015, 3, 7469–7476. [Google Scholar] [CrossRef]
- Hillman, F.; Heong, H.-K. Linker-Doped Zeolitic Imidazolate Frameworks (ZIFs) and Their Ultrathin Membranes for Tunable Gas Separations. ACS Appl. Mater. Interfaces. 2019, 11, 18377–18385. [Google Scholar] [CrossRef] [PubMed]
- Noguera-Díaz, A.; Villarroel-Rocha, J.; Ting, V.P.; Bimbo, N.; Sapag, K.; Mays, T.J. Flexible ZIFs: Probing guest-induced flexibility with CO2, N2 and Ar adsorption. J. Chem. Technol. Biotechnol. 2019, 94, 3787–3792. [Google Scholar] [CrossRef]
- Stepanov, A.V.; Mel’nik, C.E.; Isaeva, V.I.; Kapustin, G.I.; Chernyshev, V.V.; Veselovsky, V.V. The Henry reaction catalyzed by zeolitic imidazolate framework ZIF-8. Mend. Commun. 2018, 28, 88–90. [Google Scholar] [CrossRef]
- Carraro, F.; Velásquez-Hernández, M.D.J.; Astria, E.; Liang, W.; Twight, L.; Parise, C.; Ge, M.; Huang, Z.; Ricco, R.; Zou, X.; et al. Phase dependent encapsulation and release profile of ZIF-based biocomposites. Chem. Sci. 2020, 11, 3397–3404. [Google Scholar] [CrossRef]
- Zhang, H.-X.; Liu, M.; Bu, X.; Zhang, J. Zeolitic BIF Crystal Directly Producing Noble-Metal Nanoparticles in Its Pores for Catalysis. Sci. Rep. 2014, 4, 3923. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.; Forrest, K.A.; Furukawa, H.; Eckert, J.; Space, B. Hydrogen Adsorption in a Zeolitic Imidazolate Framework with lta Topology. J. Phys. Chem. C 2018, 122, 15435–15445. [Google Scholar] [CrossRef]
- Tang, Y.-H.; Wang, F.; Liu, J.-X.; Zhang, J. Diverse tetrahedral tetrazolate frameworks with N-rich surface. Chem. Commun. 2016, 52, 5625–5628. [Google Scholar] [CrossRef]
- D’Alessandro, D.M.; Smit, B.; Long, J.R. Carbon Dioxide Capture: Prospects for New Materials. Angew. Chem. Int. Ed. 2010, 49, 6058–6082. [Google Scholar] [CrossRef]
- Xu, G.; Zhang, H.; Wei, J.; Zhang, H.-X.; Wu, X.; Li, Y.; Li, C.; Zhang, J.; Ye, J. Integrating the g-C3N4 Nanosheet with B-H Bonding Decorated Metal−Organic Framework for CO2 Activation and Photoreduction. ACS Nano 2018, 12, 5333–5340. [Google Scholar] [CrossRef]
- He, Y.; Zhang, Z.; Xiang, S.; Fronczek, F.R.; Krishna, R.; Chen, B. A robust doubly interpenetrated metal-organic framework constructed from a novel aromatic tricarboxylate for highly selective separation of small hydrocarbons. Chem. Commun. 2012, 48, 6493–6495. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Morris, C.G.; Lu, Z.; Yan, Y.; Godfrey, H.G.W.; Murray, C.; Tang, C.C.; Thomas, S.; Yang, K.M.; Schröder, M. Selective Hysteretic Sorption of Light Hydrocarbons in a Flexible Metal-Organic Framework Material. Chem. Mater. 2016, 28, 2331–2340. [Google Scholar] [CrossRef]
- Tan, L.-L.; Li, H.; Qiu, Y.-C.; Chen, D.-X.; Wang, X.; Pan, R.-Y.; Wang, Y.; Zhang, S.X.-A.; Wang, B.; Yang, Y.-W. Stimuli-responsive metal–organic frameworks gated by pillar [5] arene supramolecular switches. Chem. Sci. 2015, 6, 1640–1644. [Google Scholar] [CrossRef] [PubMed]
- Knebel, A.; Zhou, C.; Huang, A.; Zhang, J.; Kustov, L.; Caro, J. Smart Metal-Organic Frameworks (MOFs): Switching Gas Permeation through MOF Membranes by External Stimuli Smart Metal-Organic Frameworks (MOFs): Switching Gas Permeation through MOF Membranes by External Stimuli. Chem. Eng. Technol. 2018, 41, 224–234. [Google Scholar] [CrossRef]
- Zheng, G.; de Marchi, S.; Llpez-Puente, V.; Sentosun, K.; Polavarapu, L.; Perez-Juste, I.; Hill, S.; Bals, E.H.; Liz-Marzan, L.M.; Pastoriza-Santos, I.; et al. Encapsulation of Single Plasmonic Nanoparticles within ZIF-8 and SERS Analysis of the MOF Flexibility. Small 2016, 12, 3935–3943. [Google Scholar] [CrossRef]
- Hu, P.; Morabito, J.V.; Tsung, C.-K. Core-Shell Catalysts of Metal Nanoparticle Core and Metal-Organic Framework. Shell ACS Catal. 2014, 4, 4409–4419. [Google Scholar] [CrossRef]
- Zhu, Q.L.; Shen, C.J.; Tan, C.H.; Sheng, T.L.; Hu, S.M.; Wu, X.T. Bright blue emissions with temperature-dependent quantum yields from microporous metal–organic frameworks. Chem. Commun. 2012, 48, 531–533. [Google Scholar] [CrossRef]
- Rocha, J.; Carlos, L.D.; Paz, F.A.A.; Ananias, D. Luminescent multifunctional lanthanides-based metal-organic frameworks. Chem. Soc. Rev. 2011, 40, 926–940. [Google Scholar] [CrossRef]
- Vikrant, K.; Giri, B.S.; Raza, N.; Roy, K.; Kim, K.H.; Rai, B.N.; Singh, R.S. Recent advancements in bioremediation of dye: Current status and challenges. Bioresour. Technol. 2018, 253, 355–367. [Google Scholar] [CrossRef]
- Bedia, J.; Muelas-Ramos, V.; Peñas-Garzón, M.; Gómez-Avilés, A.; Rodríguez, J.J.; Belver, C. A Review of Photocatalytic Water Purification with Metal Organic Frameworks. Catalysts 2019, 19, 52. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Li, Z.; Garcia, H. Catalysis and photocatalysis by metal organic frameworks. Chem. Soc. Rev. 2018, 47, 8134–8172. [Google Scholar] [CrossRef] [PubMed]
- Alkhati, I.I.; Garlisi, C.; Pagliaro, M.; Al-Ali, K.; Palmisano, G. Metal-organic frameworks for photocatalytic CO2 reduction under visible radiation: A review of strategies and applications. Catal. Today 2020, 340, 209–224. [Google Scholar] [CrossRef]
- Sibo, W.; Wangshu, Y.; Jinliang, L.; Zhengxin, D.; Xinchen, W. Cobalt imidazolate metal–organic frameworks photosplit CO2 under mild reaction conditions. Angew. Chem. Int. Ed. 2014, 53, 1034–1038. [Google Scholar]
- Wang, S.; Lin, J.; Wang, X. Semiconductor-redox catalysis promoted by metal-organic frameworks for CO2 reduction. J. Chem. Soc. Faraday Trans. 2014, 16, 14656–14660. [Google Scholar] [CrossRef]
- Martínez-Vargas, D.X.; Sandoval-Rangel, L.; Campuzano-Calderon, O.; Romero-Flores, M.; Lozano, F.J.; Nigam, K.D.P.; Mendoza, A. Montesinos-Castellanos. Recent Advances in Bifunctional Catalysts for the Fischer–Tropsch Process: One-Stage Production of Liquid Hydrocarbons from Syngas A. Ind. Eng. Chem. Res. 2019, 58, 15872–15901. [Google Scholar] [CrossRef]
- Zhuang, L.; Ge, L.; Yang, Y.; Li, M.; Jia, Y.; Yao, X.; Zhu, Z. Ultrathin Iron-Cobalt Oxide Nanosheets with Abundant Oxygen Vacancies for the Oxygen Evolution Reaction. Adv. Mater. 2017, 29, 1606793. [Google Scholar] [CrossRef]
- Dou, S.; Li, X.Y.; Tao, L.; Huo, J.; Wang, S.Y. Cobalt nanoparticle-embedded carbon nanotube/porous carbon hybrid derived from MOF-encapsulated Co3O4 for oxygen electrocatalysis. Chem. Commun. 2016, 52, 9727–9730. [Google Scholar] [CrossRef]
- Song, F.; Hu, X.L. Exfoliation of layered double hydroxides for enhanced oxygen evolution catalysis. Nat. Commun. 2014, 5, 4477. [Google Scholar] [CrossRef]
- Rincon, R.A.; Ventosa, E.; Tietz, F.; Masa, J.; Seisel, S.; Kuznetsov, V.; Schuhmann, W. Evaluation of Perovskites as Electrocatalysts for the Oxygen Evolution Reaction. ChemPhysChem 2014, 15, 2810–2816. [Google Scholar] [CrossRef]
- Ding, M.; Cai, X.; Jiang, H.-L. Improving MOF stability: Approaches and applications. Chem. Sci. 2019, 10, 10209–10230. [Google Scholar] [CrossRef]
- Wu, H.B.; Lou, X.W. Metal-organic frameworks and their derived materials for electrochemical energy storage and conversion: Promises and challenges. Sci. Adv. 2017, 3, eaap9252. [Google Scholar] [CrossRef] [PubMed]
- Li, F.L.; Shao, Q.; Huang, X.; Lang, J.P. Nanoscale Trimetallic Metal-Organic Frameworks Enable Efficient Oxygen Evolution Electrocatalysis. Angew. Chem. Int. Ed. 2018, 57, 1888–1892. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Liu, J.; Sun, Y.; Wang, X.; Geng, Z.; Shi, F.; Wang, X.; Zhang, W.; Feng, S.; Wang, Y.; et al. Optimized Co2+(Td)–O–Fe3+(Oh) electronic states in a spinel electrocatalyst for highly efficient oxygen evolution reaction performance. Inorg. Chem. Front. 2019, 6, 3295–3301. [Google Scholar] [CrossRef]
- Grosu, Y.; Gomes, S.; Renaudin, G.; Grolier, J.P.E.; Eroshenko, V.; Nedelec, J.M. Stability of zeolitic imidazolate frameworks: Effect of forced water intrusion and framework flexibility dynamics. RSC Adv. 2015, 5, 89498–89502. [Google Scholar] [CrossRef]
- Chen, L.; Xu, Q. Metal-Organic Framework Composites for Catalysis. Matter 2019, 1, 57–89. [Google Scholar] [CrossRef]
- Falcaro, P.; Ricco, R.; Yazdi, A.; Imaz, I.; Furukawa, S.; Maspoch, D.; Ameloot, R.; Evans, J.D.; Doonan, C.J. Application of Metal and Metal Oxide Nanoparticles@MOFs. Coord. Chem. Rev. 2016, 307, 237–254. [Google Scholar] [CrossRef]
- Moon, H.R.; Limb, D.-W.; Suh, M.P. Fabrication of metal nanoparticles in metal-organic frameworks. Chem. Soc. Rev. 2013, 42, 1807–1824. [Google Scholar] [CrossRef]
- Yu, J.; Mu, C.; Yan, B.; Qin, X.; Shen, C.; Xue, H.; Pang, H. Nanoparticle/MOF composites: Preparations and applications. Mater. Horiz. 2017, 4, 557–569. [Google Scholar] [CrossRef]
- Rösler, C.; Fischer, R.A. Metal-organic frameworks as hosts for nanoparticles. CrystEngComm 2015, 17, 199–217. [Google Scholar] [CrossRef]
- Isaeva, V.I.; Eliseev, O.L.; Chernyshev, V.V.; Bondarenko, T.N.; Vergun, V.V.; Kapustin, G.I.; Lapidus, A.L.; Kustov, L.M. Palladium nanoparticles embedded in MOF matrices: Catalytic activity and structural stability in iodobenzene methoxycarbonylation. Polyhedron 2019, 158, 55–64. [Google Scholar] [CrossRef]
- Isaeva, V.I.; Eliseev, O.L.; Kazantsev, R.V.; Chernyshev, V.V.; Tarasov, A.L.; Davydov, P.E.; Lapidus, A.L.; Kustov, L.M. Effect of the support morphology on the performance of Co nanoparticles deposited on metal-organic framework MIL-53(Al) in Fischer-Tropsch synthesis. Polyhedron 2019, 157, 389–395. [Google Scholar] [CrossRef]
- Stakheev, A.Y.; Isaeva, V.I.; Markov, P.V.; Turova, O.V.; Mashkovskii, I.S.; Kapustin, G.I.; Saifutdinov, B.R.; Kustov, L.M. Novel catalysts for selective hydrogenation of CC triple bond based on Pd nanoparticles immobilized in phenylenecarboxylate frameworks (NH2)MIL-53(Al). Russ. Chem. Bull. Int. Ed. 2015, 64, 284–290. [Google Scholar] [CrossRef]
- Isaeva, V.I.; Prokudina, N.I.; Kozlova, L.M.; Kustov, L.M.; Glukhov, L.M.; Tarasov, A.L.; Beletskaya, I.P. Hydroamination of phenylacetylene in the presence of gold-containing catalytic systems supported on carriers modified by ionic liquids. Russ. Chem. Bull. Int. Ed. 2015, 64, 2811–2815. [Google Scholar] [CrossRef]
- Belyaeva, E.V.; Isaeva, V.I.; Said-Galiev, E.E.; Tkachenko, O.P.; Savilov, S.V.; Egorov, A.V.; Kozlova, L.M.; Sharf, V.Z.; Kustov, L.M. New method for catalyst preparation based on metal-organic framework MOF-5 for the partial hydrogenation of phenylacetylene. Russ. Chem. Bull. Int. Ed. 2014, 63, 396–403. [Google Scholar] [CrossRef]
- Cheon, Y.E.; Suh, M.P. Enhanced hydrogen storage by palladium nanoparticles fabricated in a redox-active metal-organic framework. Angew. Chem. Int. Ed. 2009, 48, 2899–2903. [Google Scholar] [CrossRef]
- Xiong, Y.Y.; Li, J.Q.; Yan, C.S.; Gao, H.Y.; Zhou, J.P.; Gong, L.L.; Luo, M.B.; Zhang, L.; Meng, P.P.; Luo, F. MOF catalysis of Fe-II-to-Fe-III reaction for an ultrafast and one-step generation of the Fe2O3@MOF composite and uranium(VI) reduction by iron (II) under ambient conditions. Chem. Commun. 2016, 52, 9538–9541. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Cai, Y.; Sun, Z.; Liu, J.; Liu, C.; Wei, J.; Li, W.; Liu, C.; Wang, Y.; Zhao, D. Multifunctional mesoporous composite microspheres with well designed nanostructure: A highly integrated catalyst system. J. Am. Chem. Soc. 2010, 132, 8466–8473. [Google Scholar] [CrossRef] [PubMed]
- Duan, M.; Jiang, L.; Zeng, G.; Wang, D.; Tang, W.; Liang, J.; Wang, H.; He, D.; Liu, Z.; Tang, L. Bimetallic nanoparticles/metal-organic frameworks: Synthesis, applications and challenges. Appl. Mater. Today 2020, 19, 100564. [Google Scholar] [CrossRef]
- Tate, G.; Kenvin, A.; Diao, W.; Monnier, J.R. Preparation of Pt-containing bimetallic and trimetallic catalysts using continuous electroless deposition methods. Catal. Today 2019, 334, 113–121. [Google Scholar] [CrossRef]
- Liu, X.-W.; Sun, T.-J.; Hu, J.-L.; Wang, S.-D. Composites of metal–organic frameworks and carbon-based materials: Preparations, functionalities and applications. J. Mater. Chem. A 2016, 4, 3584–3616. [Google Scholar] [CrossRef]
- Zhu, L.; Meng, L.; Shi, J. Metal-organic frameworks/carbon-based materials for environmental remediation: State-of-the-art mini-review. J. Environ. Manag. 2019, 232, 964–977. [Google Scholar] [CrossRef]
- Abdol, A.M.; Sadeghazadeh, S.; Jalaly, M.; Khatibi, M.M. Constructing a three-dimensional graphene structure via bonding layers by ion beam irradiation. Sci. Rep. 2019, 9, 8127. [Google Scholar] [CrossRef] [PubMed]
- Paszkiewicz, S.; Szymczyk, A.; Sui, X.; Wagner, H.; Linares, A.; Ezquerra, T.A.; Rosłaniec, Z. Synergetic effect of single-walled carbon nanotubes (SWCNT) and graphene nanoplatelets (GNP) in electrically conductive PTT-block-PTMO hybrid nanocomposites prepared by in situ polymerization. Compos. Sci. Technol. 2015, 118, 72–77. [Google Scholar] [CrossRef]
- Prolongo, S.; Redondo, O.; Campo, M.; Ureña, A. Heat dissipation on electrical conductor composites by combination of carbon nanotubes and graphene nanoplatelets. J. Coat. Technol. Res. 2019, 16, 491–498. [Google Scholar] [CrossRef]
- Kidanemariam, A.; Lee, J.; Park, J. Recent Innovation of Metal-Organic Frameworks for Carbon Dioxide Photocatalytic Reduction. Polymers 2019, 11, 2090. [Google Scholar] [CrossRef]
- Wang, X.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J.M.; Domen, K.; Antonietti, M. A Metal-free Polymeric Photocatalyst for Hydrogen Production from Water under Visible Light. Nat. Mater. 2009, 8, 76–80. [Google Scholar] [CrossRef]
- Ingaki, M.; Tsumura, T.; Kinumoto, T.; Toyoda, M. Graphitic carbon nitrides (g-C3N4) with comparative discussion to carbon materials. Carbon 2019, 141, 580–607. [Google Scholar] [CrossRef]
- Shi, L.; Wang, T.; Zhang, H.; Chang, K.; Ye, J. Electrostatic Self-Assembly of Nanosized Carbon Nitride Nanosheet onto a Zirconium Metal-Organic Framework for Enhanced Photocatalytic CO2 Reduction. Adv. Funct. Mater. 2015, 25, 5360–5367. [Google Scholar] [CrossRef]
- Wang, R.; Gu, L.N.; Zhou, J.J.; Liu, X.L.; Teng, F.; Li, C.H.; Shen, Y.H.; Yuan, Y.P. Quasi-Polymeric Metal-Organic Framework UiO-66/g-C3N4, Heterojunctions for Enhanced Photocatalytic Hydrogen Evolution under Visible Light Irradiation. Adv. Mater. Interfaces 2015, 2, 1500037. [Google Scholar] [CrossRef]
- Wang, H.; Yuan, X.; Wu, Y.; Zeng, G.; Chen, X.; Leng, L.; Li, H. Synthesis and Applications of Novel Graphitic Carbon Nitride/Metal-Organic Frameworks Mesoporous Photocatalyst for Dyes Removal. Appl. Catal. B 2015, 174, 445–454. [Google Scholar] [CrossRef]
- Su, P.; Iwase, K.; Nakanishi, S.; Hashimoto, K.; Kamiya, K. Nickel-Nitrogen-Modified Graphene: An Efficient Electrocatalyst for the Reduction of Carbon Dioxide to Carbon Monoxide. Small 2016, 12, 6083–6089. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, Z.; Zhao, X.; Yao, T.; Chen, W.; You, R.; Zhao, C.; Wu, G.; Wang, J.; Huang, W.; et al. Regulation of Coordination Number Over Single Co Sites: Triggering the Efficient Electroreduction of CO2. Angew. Chem. Int. Ed. 2018, 57, 1944–1948. [Google Scholar] [CrossRef]
- Zhao, M.; Huang, Y.; Peng, Y.; Huang, Z.; Ma, Q.; Zhang, H. Two-dimensional metal–organic framework nanosheets: Synthesis and applications. Chem. Soc. Rev. 2018, 47, 6267–6295. [Google Scholar] [CrossRef]
- Zhao, S.; Wang, Y.; Dong, J.; He, C.-T.; Yin, H.; An, P.; Zhao, K.; Zhang, X.; Gao, C.; Zhang, L.; et al. Ultrathin metal-organic framework nanosheets for electrocatalytic oxygen evolution. Nat. Energy 2016, 1, 16184. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Liu, Z.; Xie, C.; Feng, S.; Liu, D.; Shao, M.; Wang, S. Layered Double Hydroxide Nanosheets with Multiple Vacancies Obtained by Dry Exfoliation as Highly Efficient Oxygen Evolution Electrocatalysts. Angew. Chem. Int. Ed. 2017, 56, 5867–5871. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhao, M.; Lin, Y.S. Stability of ZIF-8 in water under ambient conditions. Microporous Mesoporous Mater. 2019, 279, 201–210. [Google Scholar] [CrossRef]



| BIF Structure | Composition | Topology | Pore Diameter, nm | Application | Reference |
|---|---|---|---|---|---|
| BIF-6 | CuBH(im)3]n | Stacked ladder-like chain | Mechanochromic properties | [43] | |
| BIF-20 | Zn2(BH(mim)3)2(obb) | 3-Connected; interrupted LTA | 1.2, 1.6, 0.31 | Gas adsorption; reduction catalysis with Ag and Au-NP | [41] |
| BIF-24 | {Zn3[HB (mim)3]4} (NO3)2 | (3,4)-Connected; ctn | H2 storage, CO2 adsorption, light hydrocarbon separation | [35] | |
| BIF-26 | Zn4[BH(bim)3]4NO3)4 | M4B4 cage-based | [44] | ||
| BIF-27 | Zn4[BH(dm-bim)3]4.(CO2)4 | M4B4 cage-based | [44] | ||
| BIF-32 | Zn4[B(im)4]4Cl4 | M4B4 cage-based | 1.07 × 1.88 | [44] | |
| BIF-33 | Zn/Cu6[BH(im)3]8]I4· 8H2O | M6B8 cage-based | CO2 photocatalytic reduction | [44,45] | |
| BIF-34 | [CuBH(dm-bim)3]n_(solvent)x | 2D Stacked ladder-like chain | Mechanochromic properties | [46] | |
| BIF-36 | [CuBH(dm-bim)3] | 2D Stacked ladder-like chain | Reduction catalysis with Au–Ag–Pd-NP; Precursor for borocarbonitride—high temperature conductor; adsorbent for 4-nitrophenol | [47] | |
| BIF-38 | (CuHB(im)3 | 2-Fold interpene trating; ths | mechanochromic properties | [48] | |
| BIF-39(M) | MB(im)4·0.5C2O4 (M = Zn, Mn, Cu, Cd) | (4,4)-Connected layers | Reduction catalysis with Au–Pd-NP | [49] | |
| BIF-40 | [CuBH(bim)3]n | 2D Stacked ladder-like chain | Mechanochromic properties | [43] | |
| BIF-41 | [CoB(im)4(ad)0.5] 3.5H2O | (4,5)-Connected; GIS derivative | 0.7; 0.34 × 0.45—windows; 0.5 × 0.5—1D channels | Gas adsorption | [42] |
| BIF-42 | CdB(im)4(ndc)0.5] DMF | ABW derivative; tcj/hc | 0.4 × 0.5—windows; | [42] | |
| BIF-51 | [Cd(ac)B(im)4]∙DMF | ABW | Luminescent emission and sensor of nitrobenzene | [50] | |
| BIF-51 | [Cd(ac)B(im)4]∙DMF | ABW | 0.45 × 1.16 channels | Ag-NP@BIF-51 Catalytic reduction | [50] |
| BIF-58 | Co4[BH(bim)3]4. (OH)3.BF4 | M4B4 cage- based | Photocatalyst to degrade organic dyes | [44] | |
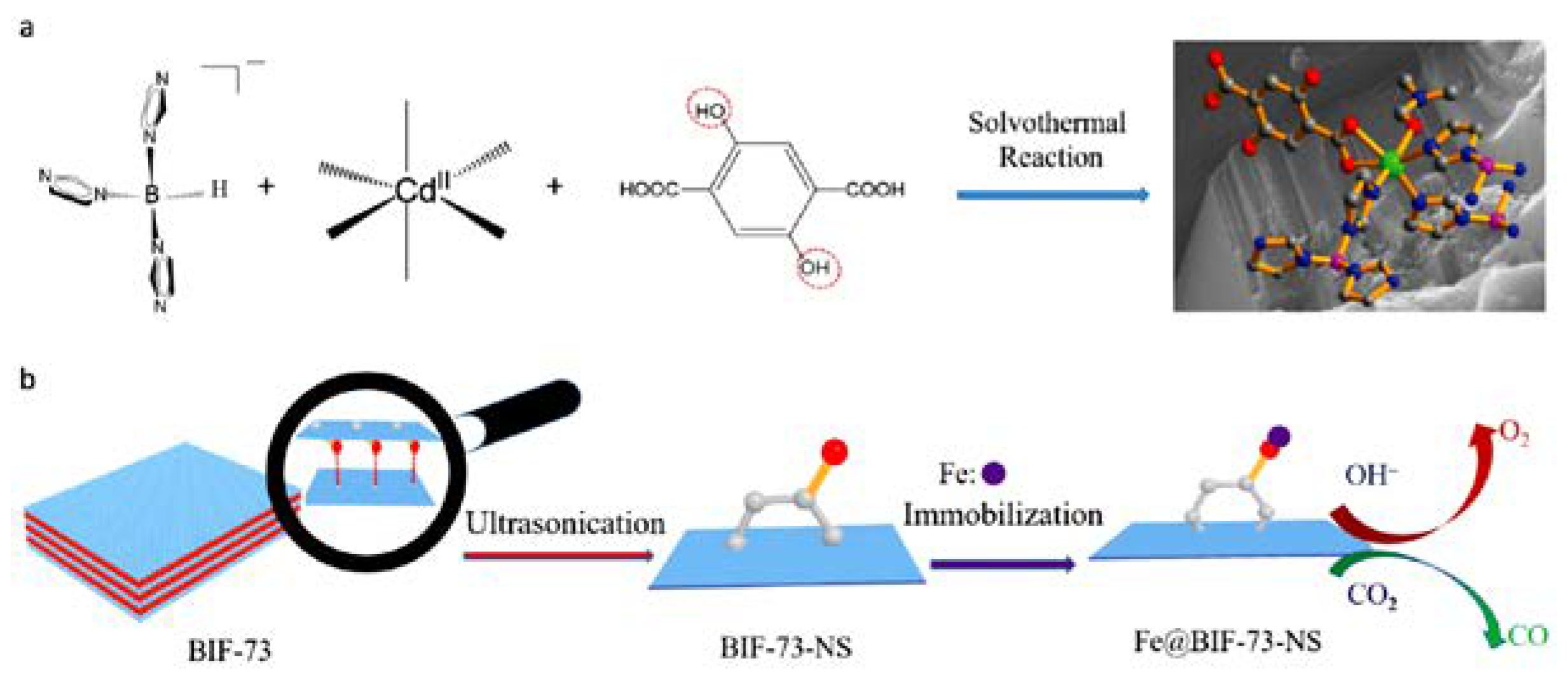
| BIF-73 | CdBH(im)3.(dobdc)0.5. DMF | 2D Stacked layered | 0.334 | Electrocatalysis in the 2D nanosheet form and doped with Fe3+ | [51] |
| BIF-77 | CdB(im)4 0.5(abdc) | tcj | Ag-NP@BIF-77 nanohybrid for photocatalytic degradation of MB | [52] | |
| BIF-80 | CdB(im)4 (dht)0.5 | tcs | [53] | ||
| BIF-81 | Cd3[B(im)4]2(btc)3 | {42.6}{43}{44.62}2{46.66.83}{47.611.810} Topology | Photoluminescence properties with La3+ ions | [53] | |
| BIF-89 | CdB(im)4(btec)0.5·H2O | 3D Structure | Electrocatalysis | [54] | |
| BIF-91 | CoB(im)4(ndc)0.5 | 3D Structure | Electrocatalysis | [40] | |
| BIF-101 | Co1.5(OiPr)0.5(OH)0.5 (H2O)(HB(mim)3) (nbdc) | 3D Pillar-layered structure | Heterogeneous co-catalyst exhibits efficient CO2 photocatalytic reduction | [55] | |
| Zn-BIF | Zn2(BH(2-mim)3)2(obb) | 3D Structure | Large cavities | M-NP@ Zn-BIF Catalytic reduction, antibacterial properties | [56] |
| BIF | Structure | CO2, 1 bar | H2, 1 atm | Hydrocarbon, 1 bar | Reference |
|---|---|---|---|---|---|
| BIF-20 | Tetrahedral framework with debonded α and β cages | 53.9 cm3/g (2.41 mmol/g), 273 K; 34.8 cm3/g (1.55 mmol/g), 298 K | 1.43 wt.%, 77 K | [41] | |
| BIF-20/@g−C3N4 | Composite 2D nanosheet | 28.2 cm3/g, 296.15 K | [81] | ||
| BIF-24 (s) | Tetrahedral Zn nodes, trianglar B nodes | 104.3 cm3 g−1 (4.64 mmol g−1), 273 K 66.4 cm3 g−1 (2.96 mmolg−1), 298 K | 1.88 (211.6 cm3 g−1), 77 K; 1.30 wt.% (145.3 cm3 g−1), 87 K | C3H8 (88.5 cm3 g−1, 174.2 mg g−-1), C2H6 (84.9 cm3 g−1, 113.9 mg g−1), C2H4 (75.1 cm3 g−1, 94.0 mg g−1, 273 K; C3H8 (80.7 cm3 g−1, 158.8 mg g−1), C2H6 (70.8 cm3 g−1, 95.09 mg g−1), C2H4 (61.9 cm3 g−1, 77.4 mg g−1), CH4 (14.6 cm3 g−1, 10.4 mg g−1), 298 K | [35] |
| BIF-41 | pore space partition via carboxylate ligands | 77.0 cm3 g−1 (3.44 mmol g-1), 273 K; 63.5 cm3 g-1 (2.83 mmol g−1), 294 K | 106.4 cm3 g−1, 77K; 83.4 cm3 g−1, 87 K | [42] | |
| BIF-101 | crosslinking 2D layers | 41.9 cm3 g−1, 273 K; 25.8 cm3 g−1, 298 K | [55] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Isaeva, V.I.; Papathanasiou, K.E.; Kustov, L.M. Zeolite-Like Boron Imidazolate Frameworks (BIFs): Synthesis and Application. Crystals 2020, 10, 617. https://doi.org/10.3390/cryst10070617
Isaeva VI, Papathanasiou KE, Kustov LM. Zeolite-Like Boron Imidazolate Frameworks (BIFs): Synthesis and Application. Crystals. 2020; 10(7):617. https://doi.org/10.3390/cryst10070617
Chicago/Turabian StyleIsaeva, Vera I., Kostantinos E. Papathanasiou, and Leonid M. Kustov. 2020. "Zeolite-Like Boron Imidazolate Frameworks (BIFs): Synthesis and Application" Crystals 10, no. 7: 617. https://doi.org/10.3390/cryst10070617
APA StyleIsaeva, V. I., Papathanasiou, K. E., & Kustov, L. M. (2020). Zeolite-Like Boron Imidazolate Frameworks (BIFs): Synthesis and Application. Crystals, 10(7), 617. https://doi.org/10.3390/cryst10070617






