Water Based Synthesis of ZIF-8 Assisted by Hydrogen Bond Acceptors and Enhancement of CO2 Uptake by Solvent Assisted Ligand Exchange
Abstract
1. Introduction
2. Materials and Methods
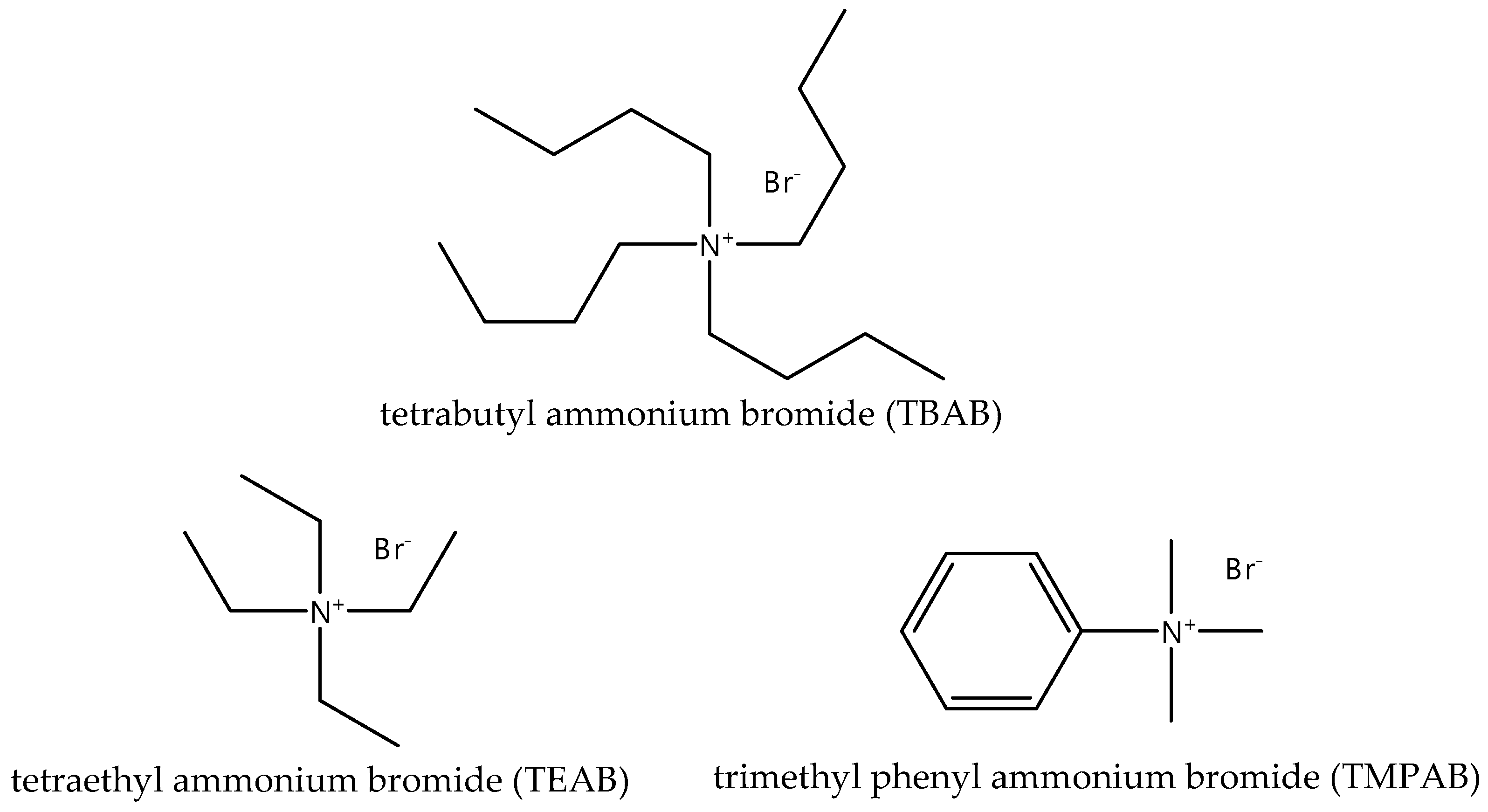
2.1. Materials
2.2. ZIF-8 Synthesis by Water Based Synthesis Assisted by Hydrogen Bond Acceptors
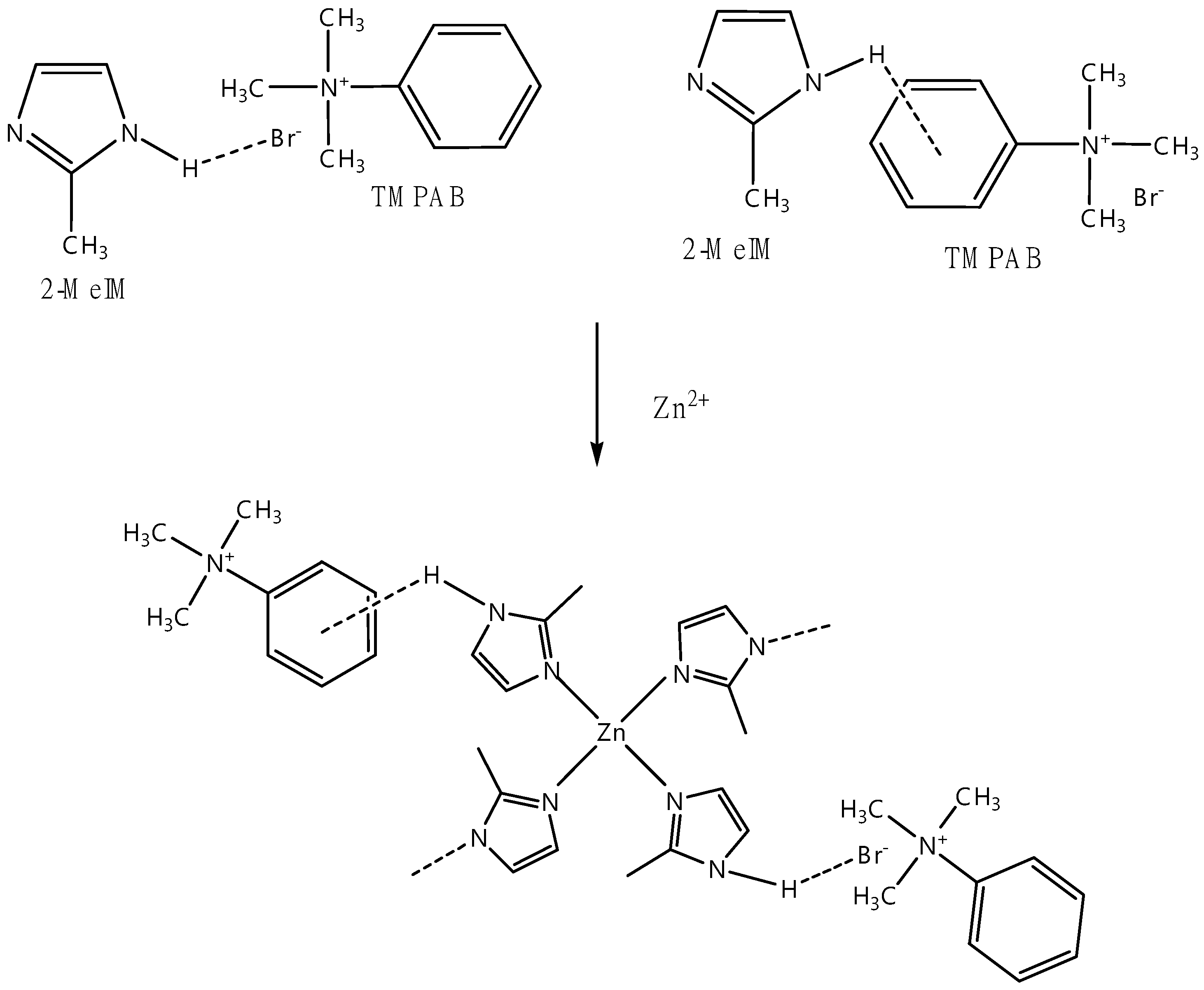
2.3. Modification of Synthesized ZIF-8 by Solvent-assisted Ligand Exchange (SALE) Method
2.4. Characterization
2.5. CO2 Adsorption Study
3. Results and Discussion
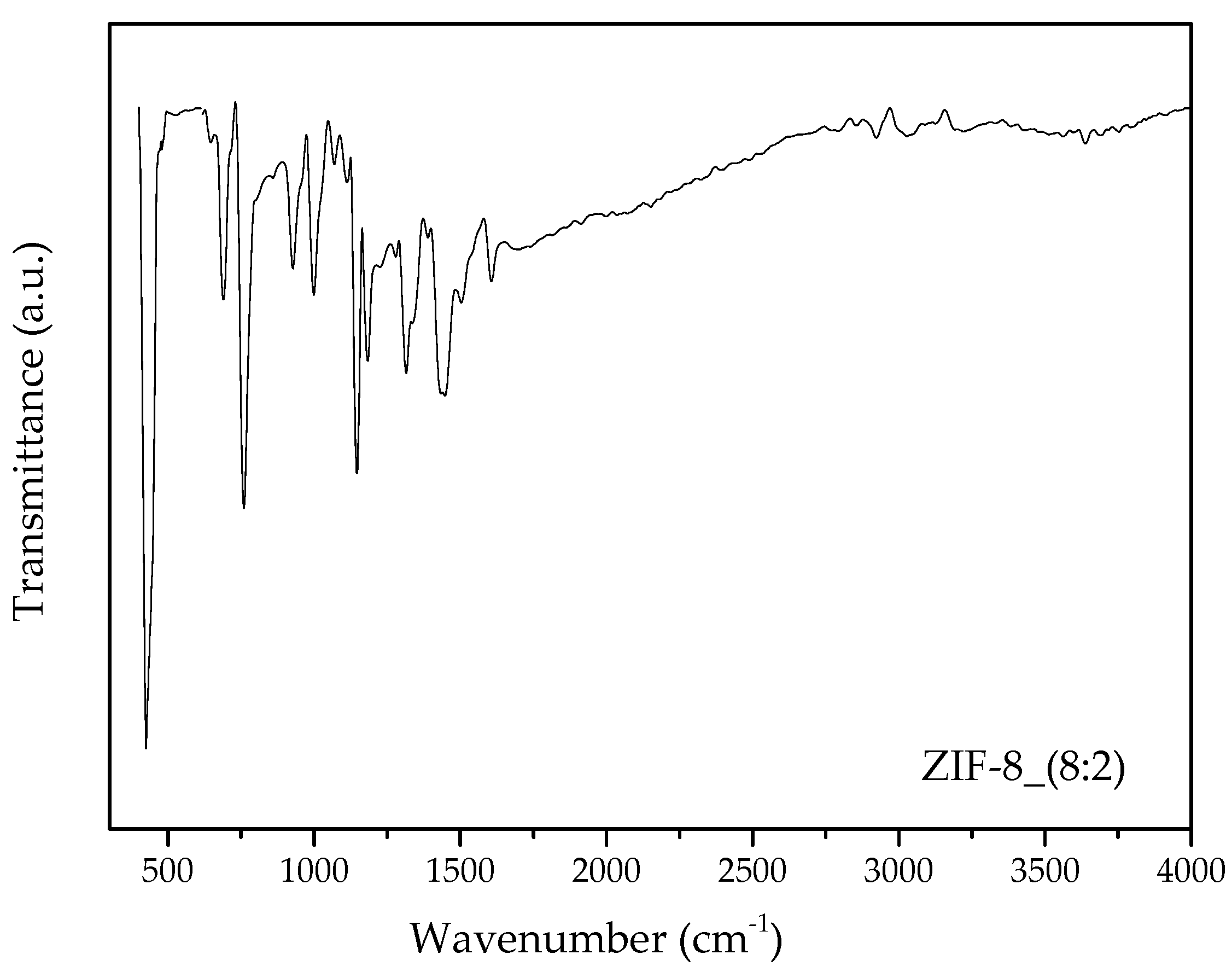
3.1. ZIF-8 Synthesis by Water Based Synthesis Assisted by Hydrogen Bond Acceptors
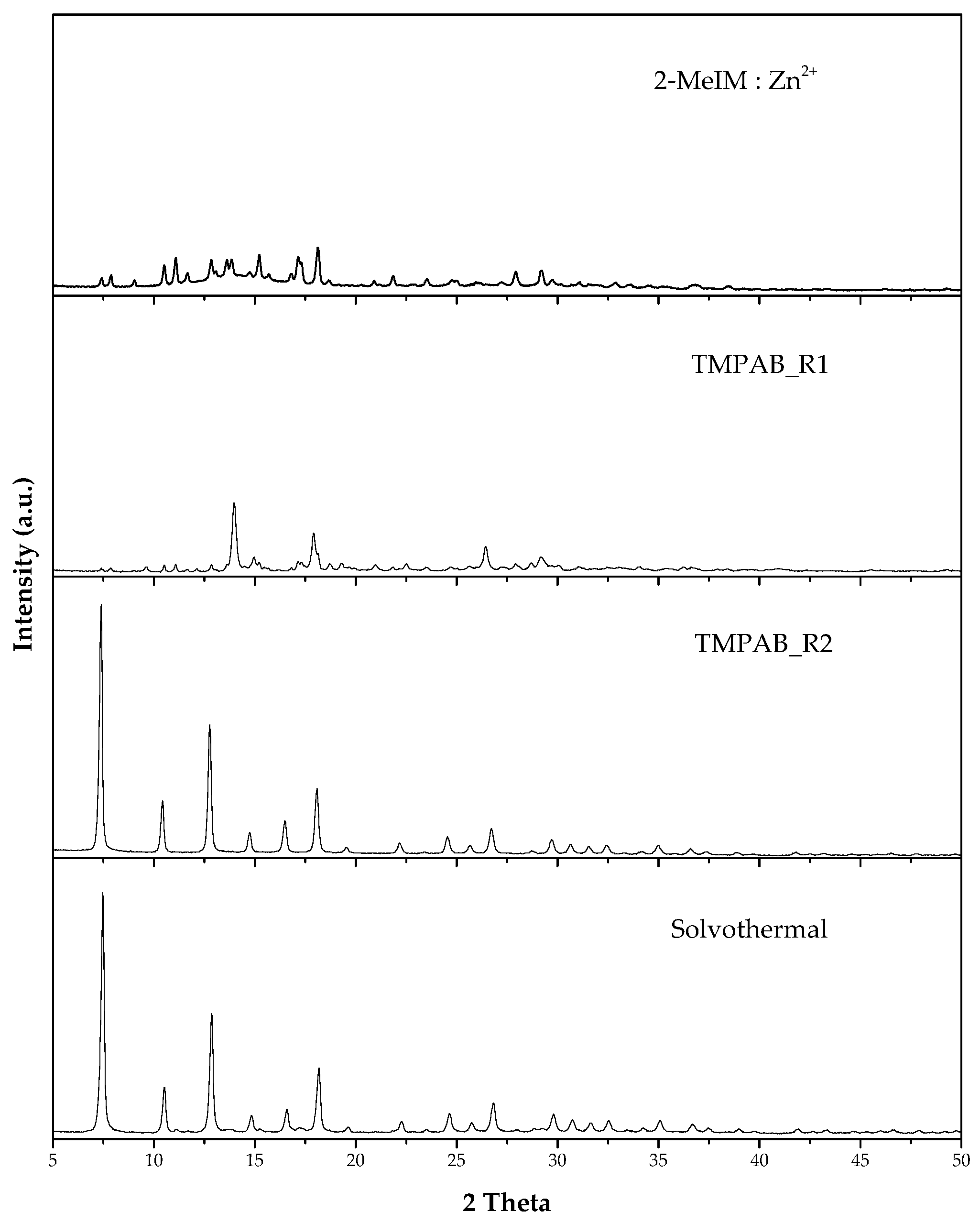
3.1.1. Effect of Mixed Ordering Step on ZIF-8 Properties
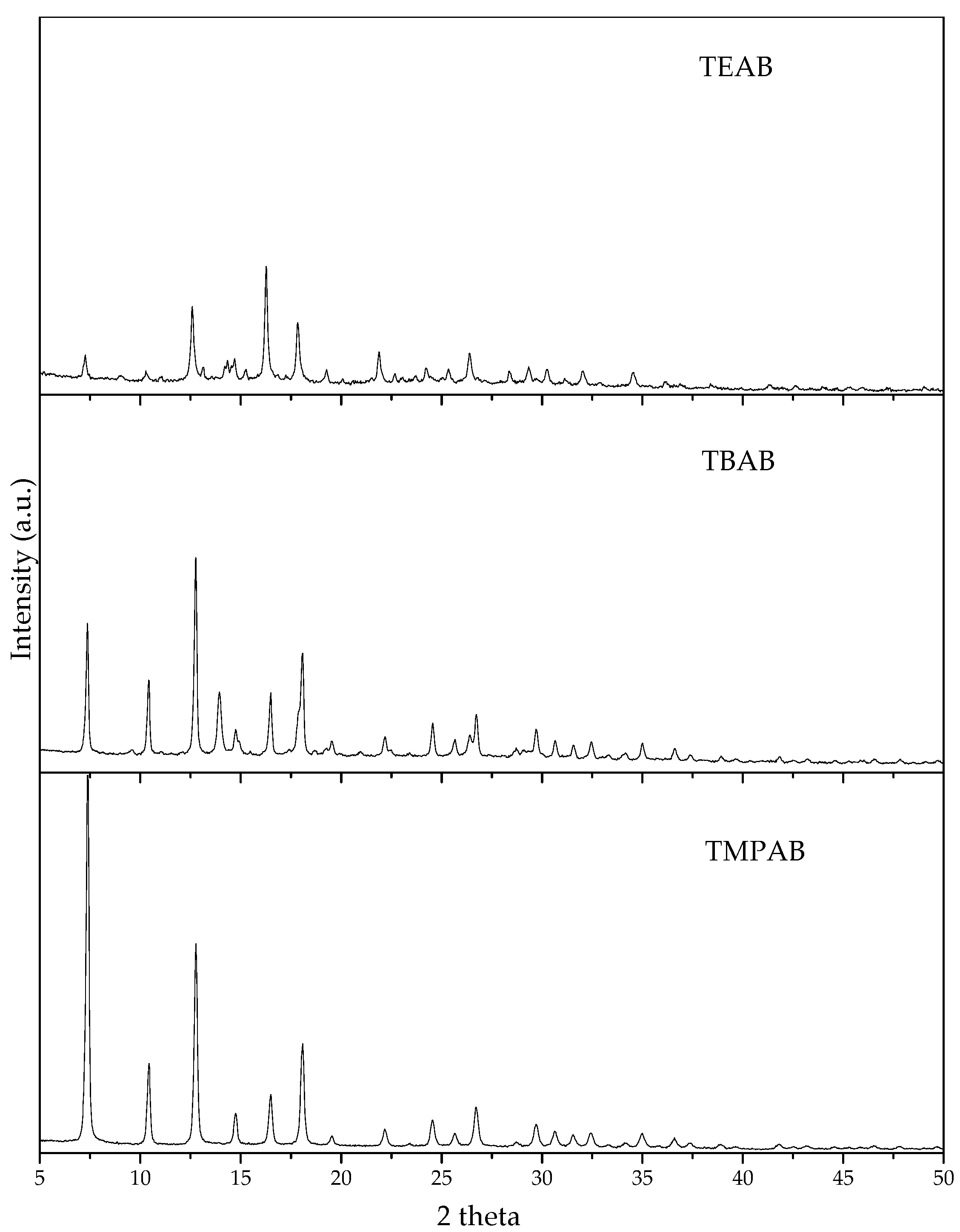
3.1.2. Effect of Different Quaternary Ammonium Salts
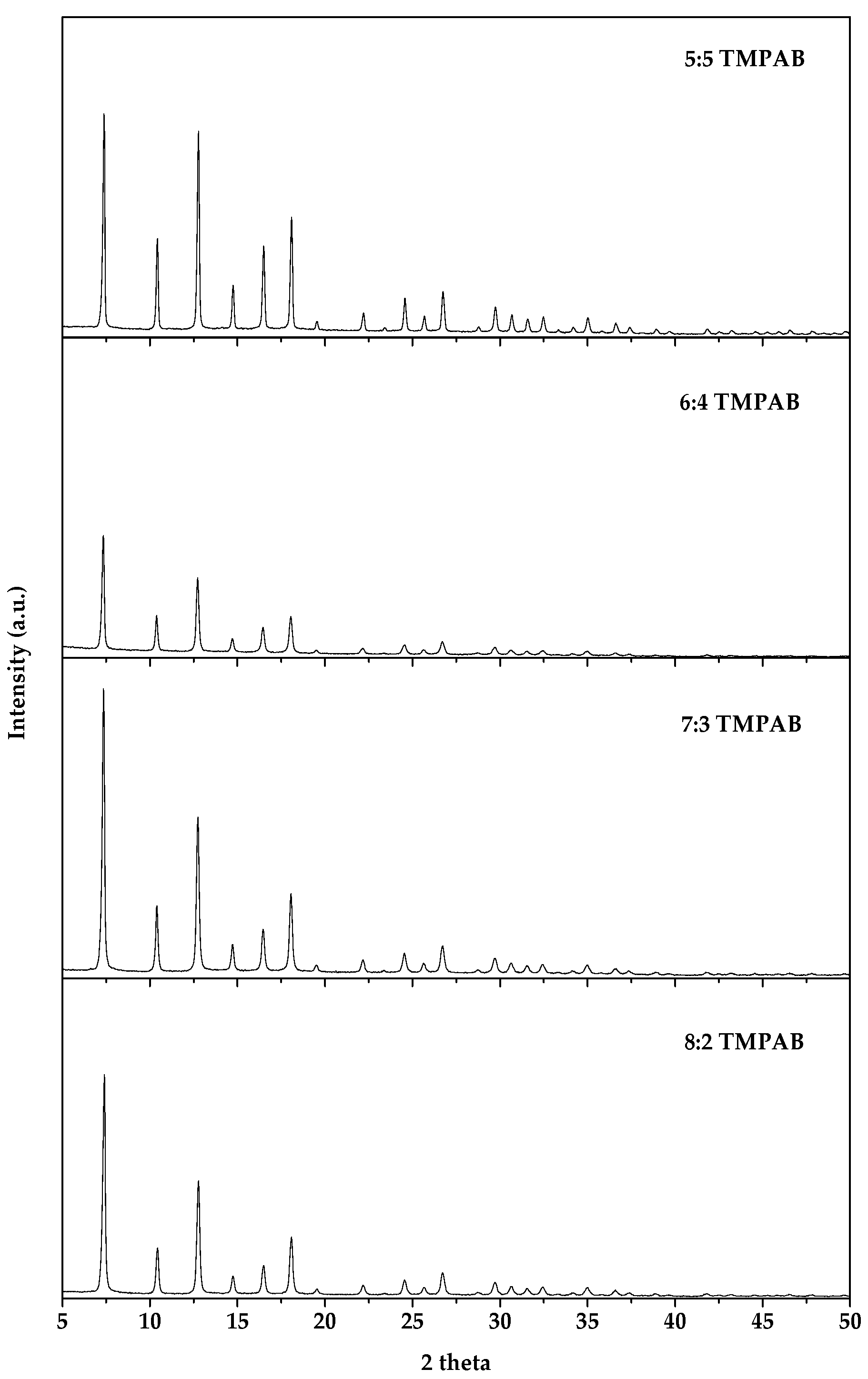
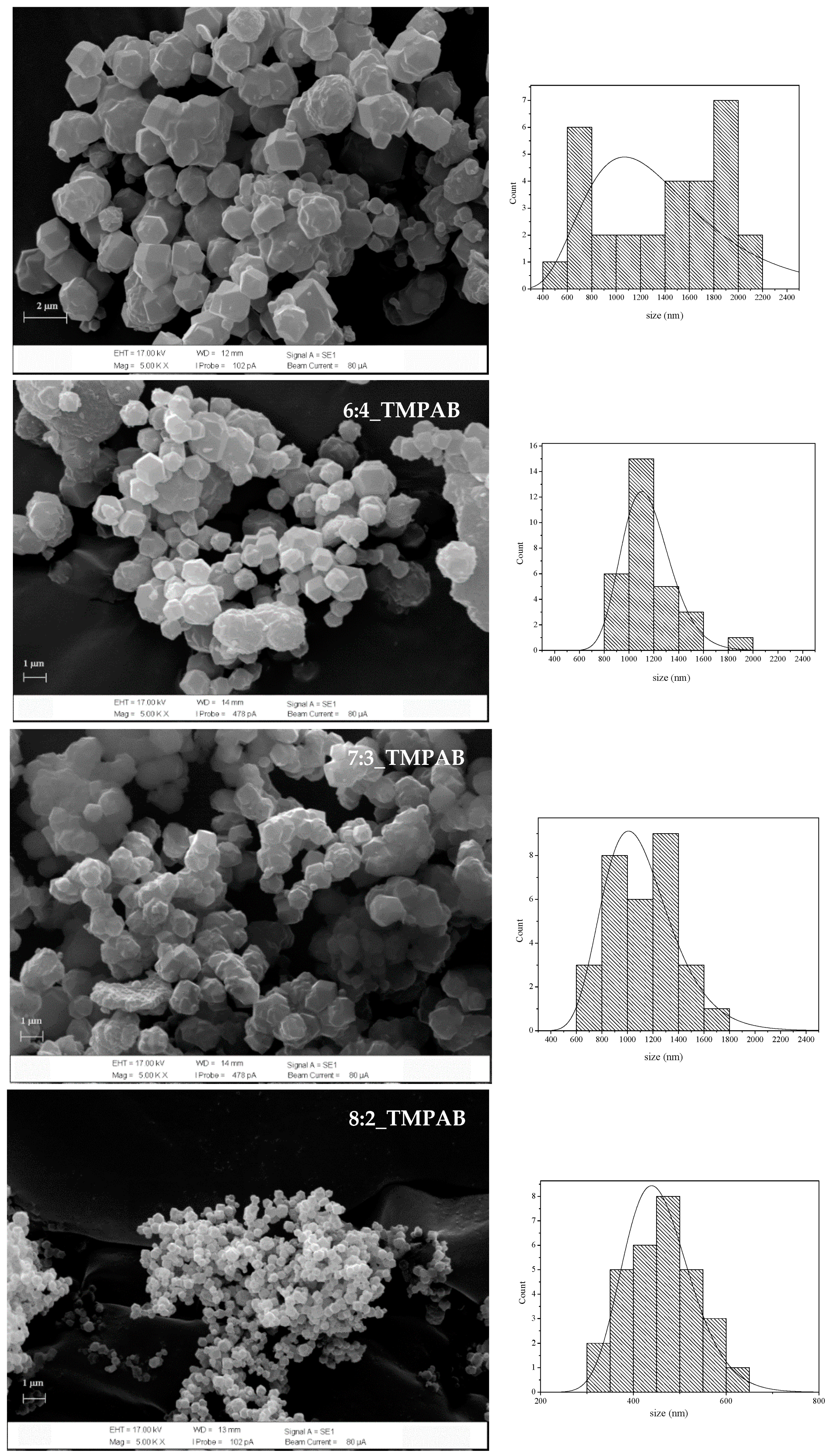
3.1.3. Effect of Ratio between 2-MeIM and QAS
3.2. Modification of Synthesized ZIF-8 by Solvent-assisted Ligand Exchange
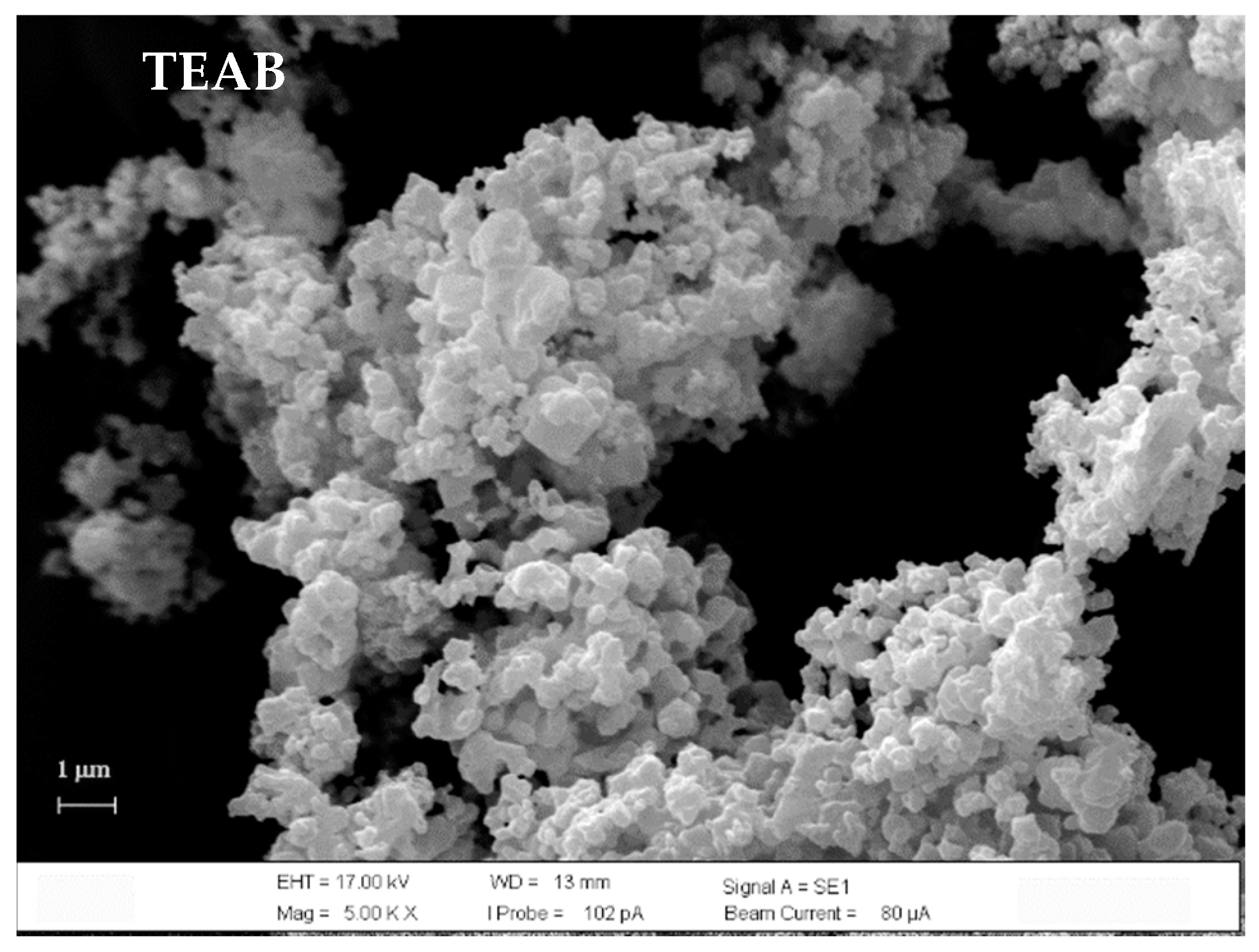
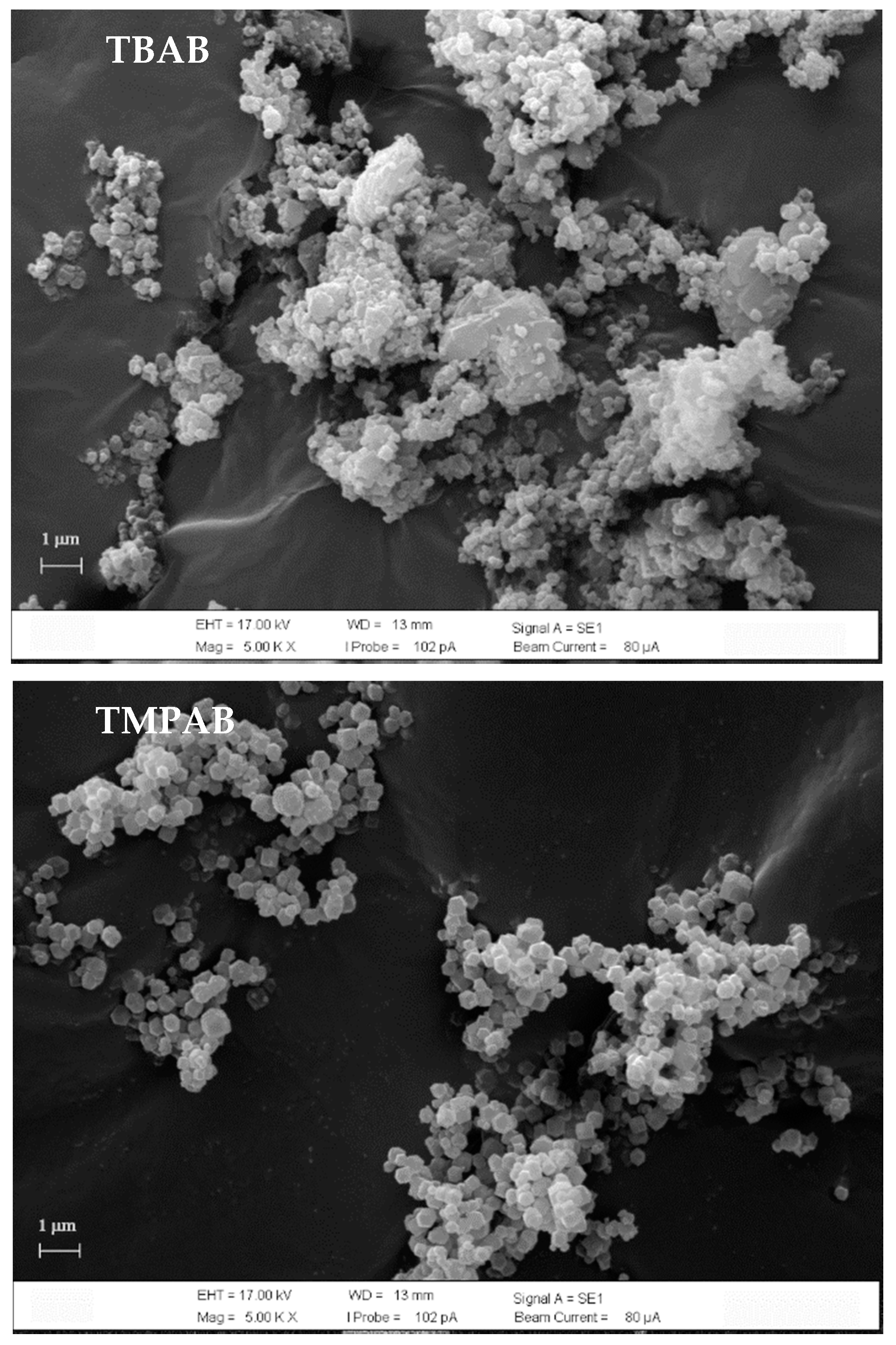
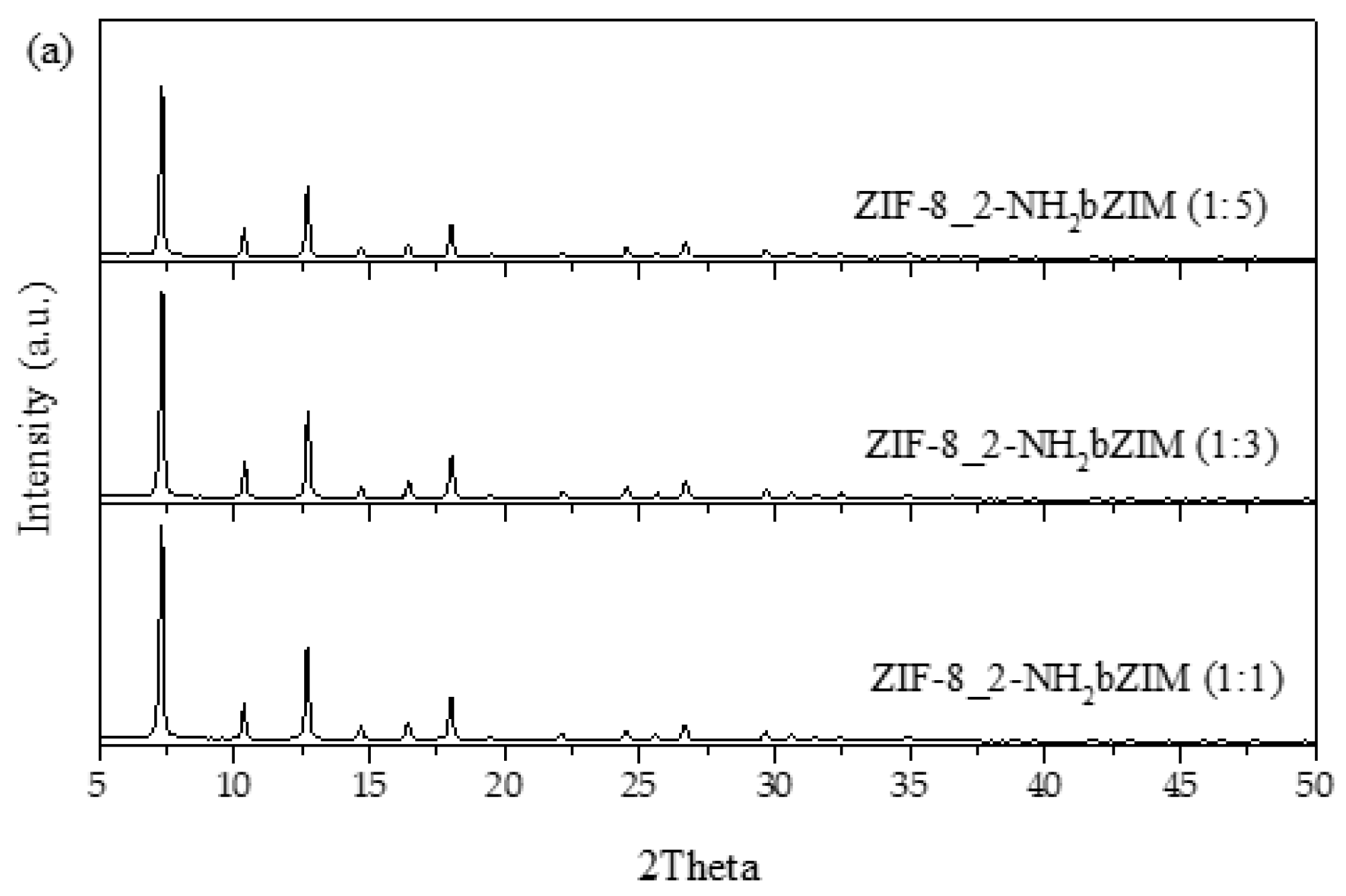
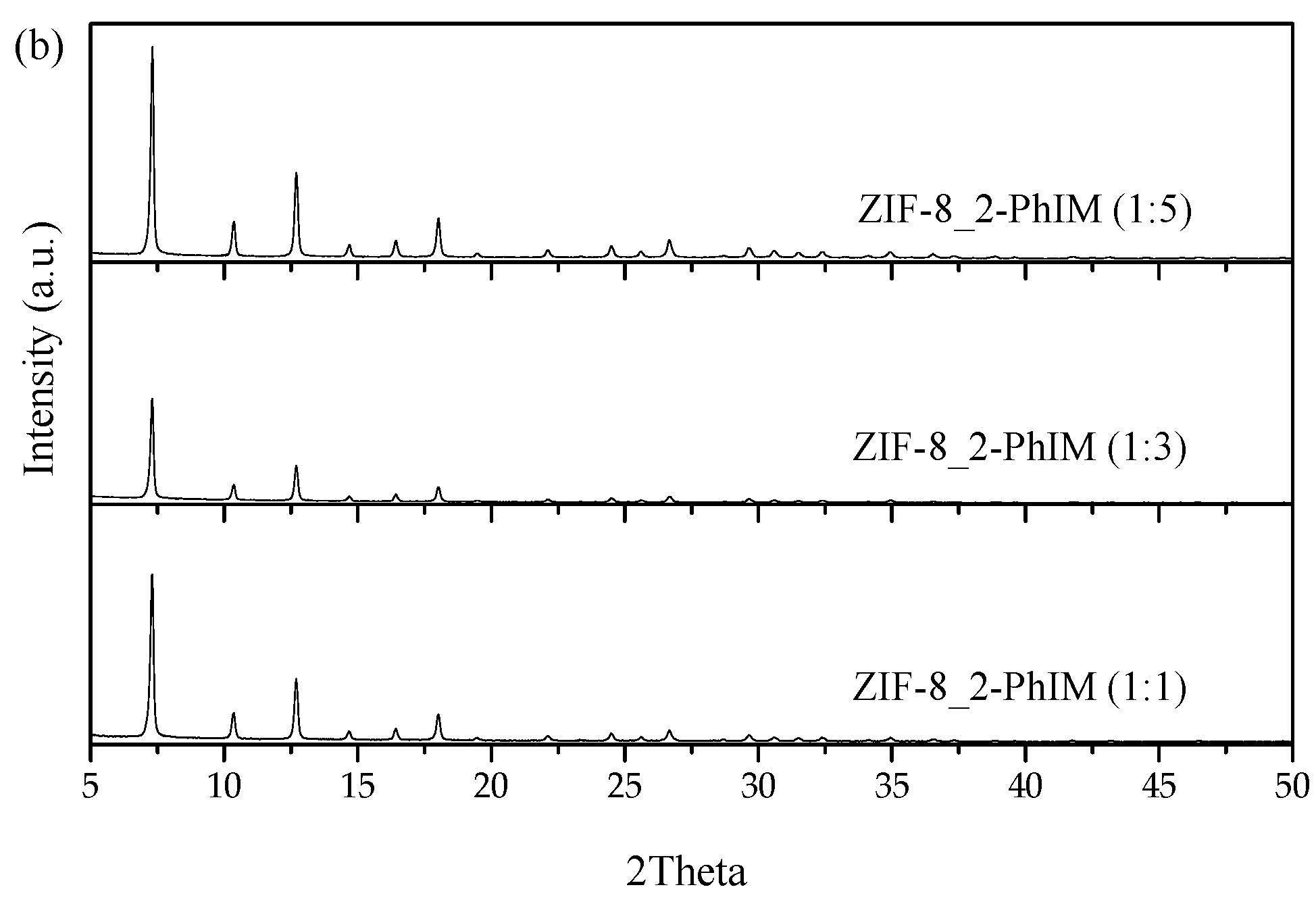
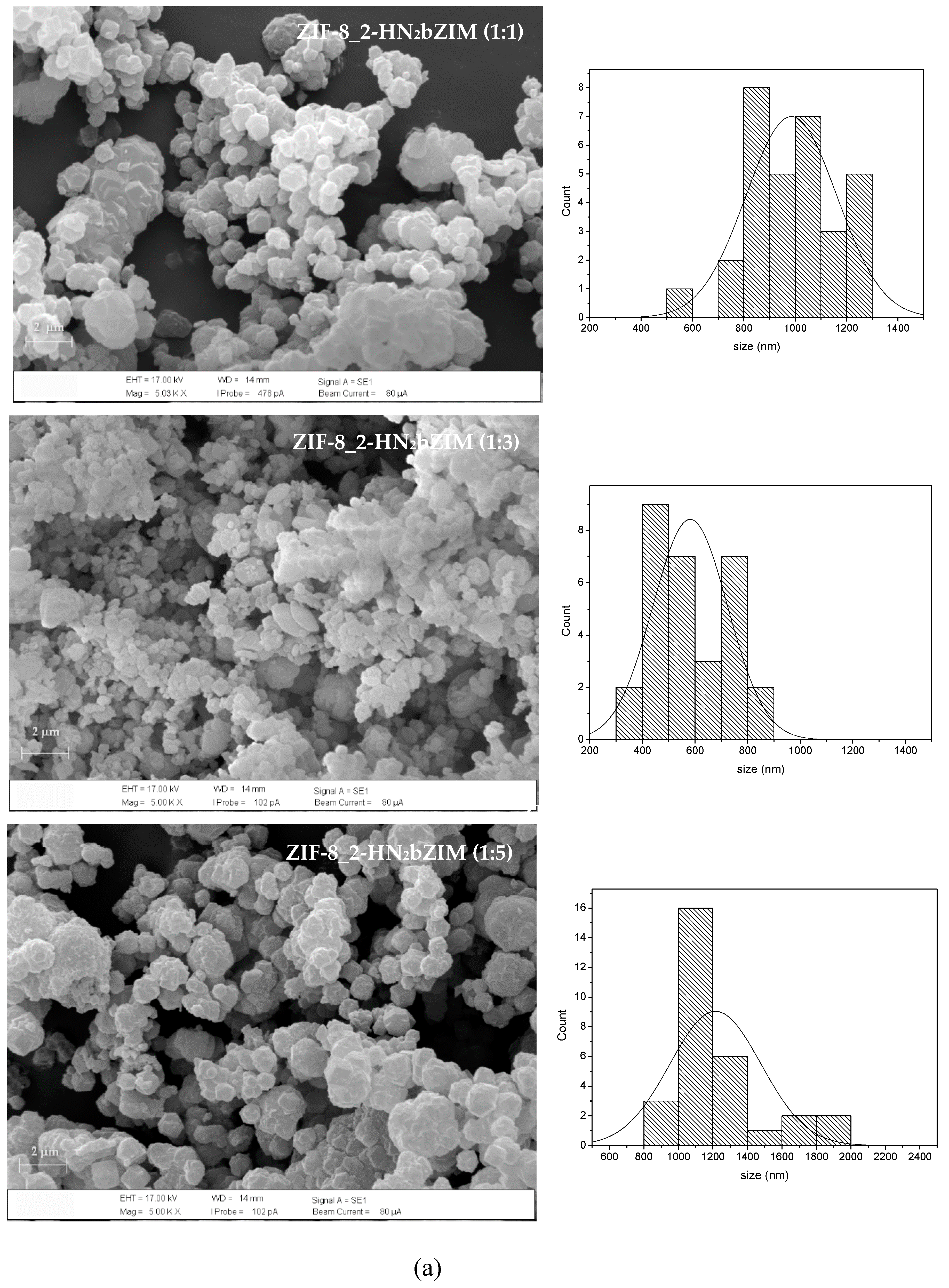
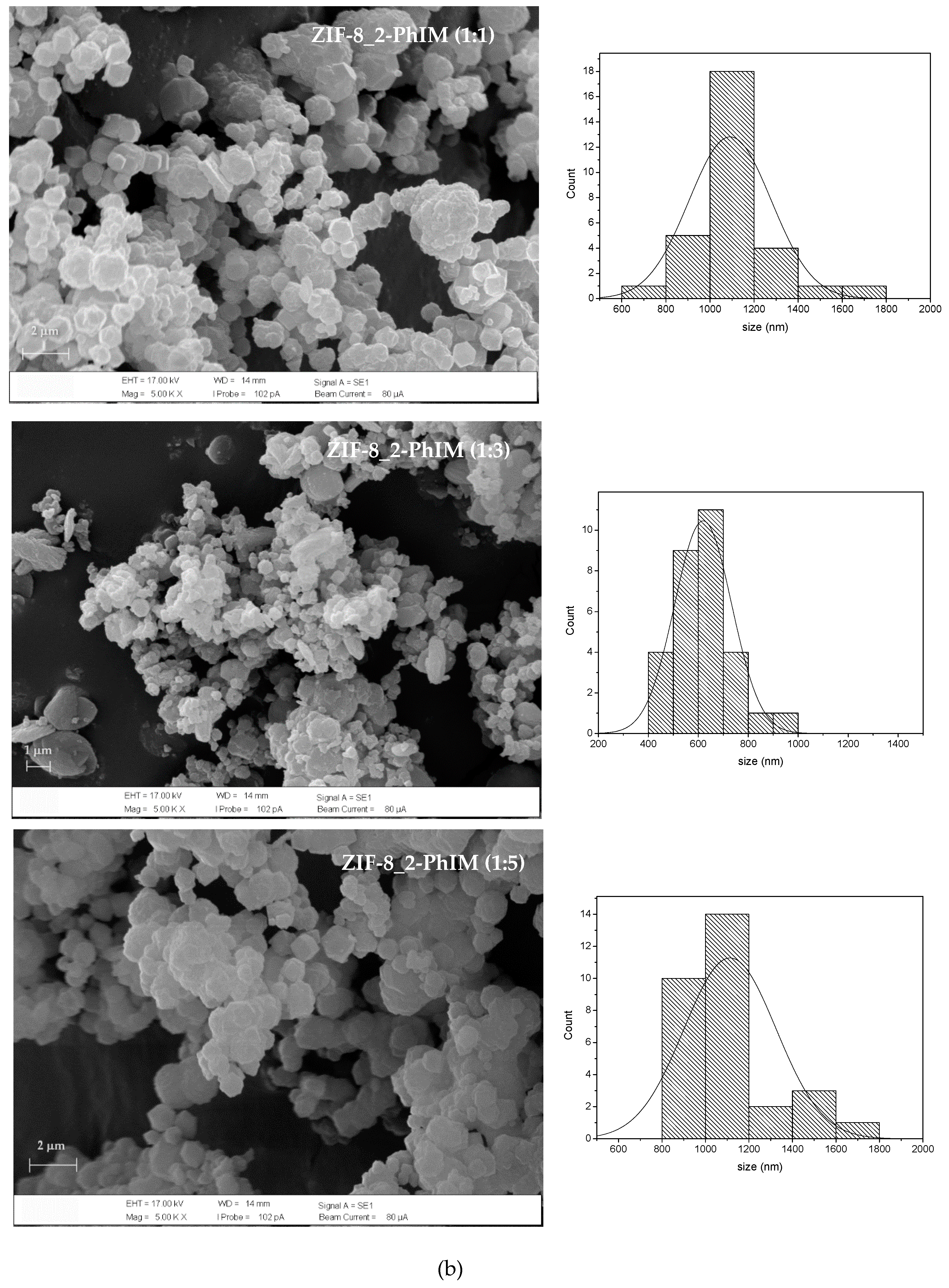
3.2.1. XRD and SEM
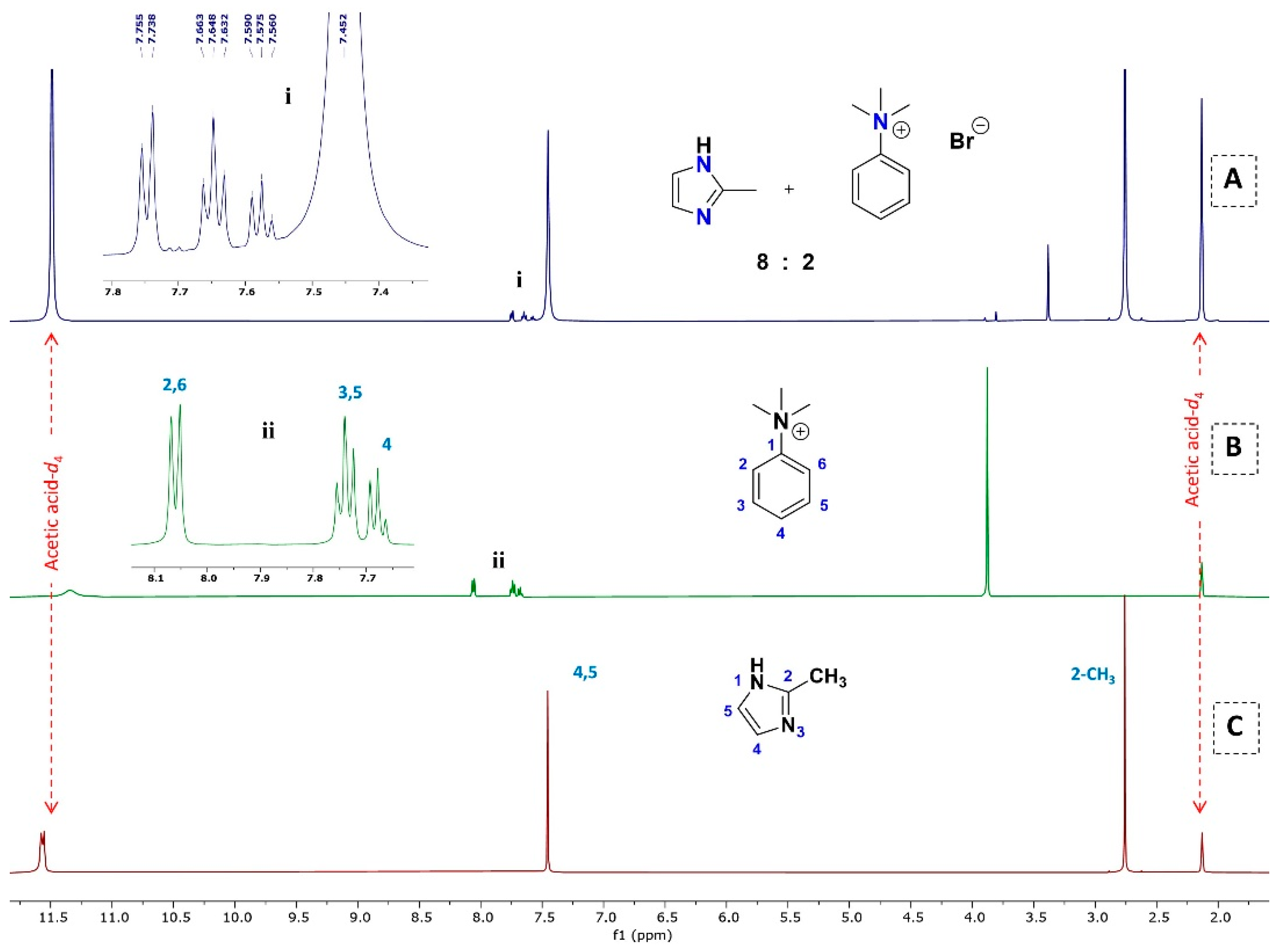
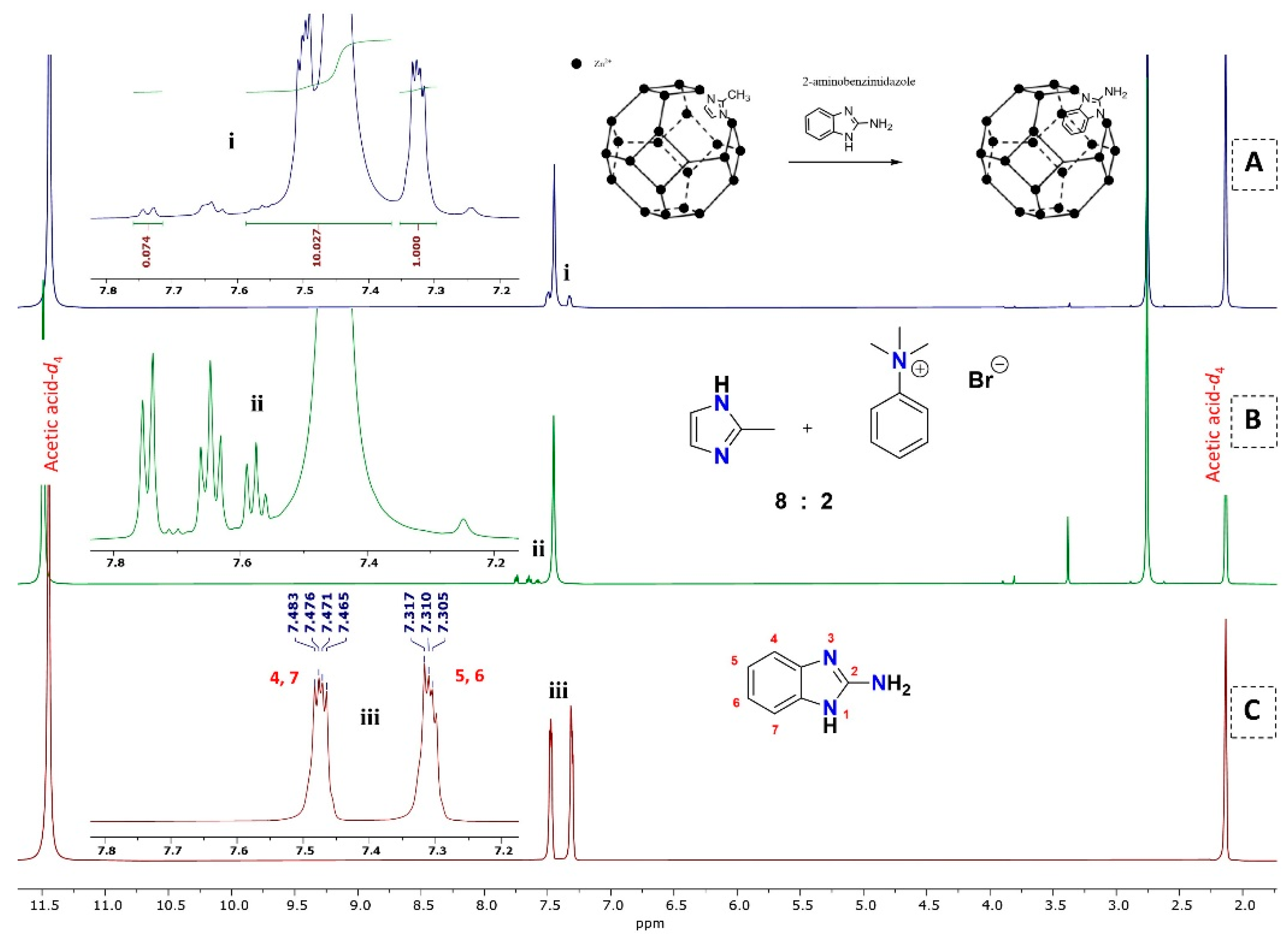
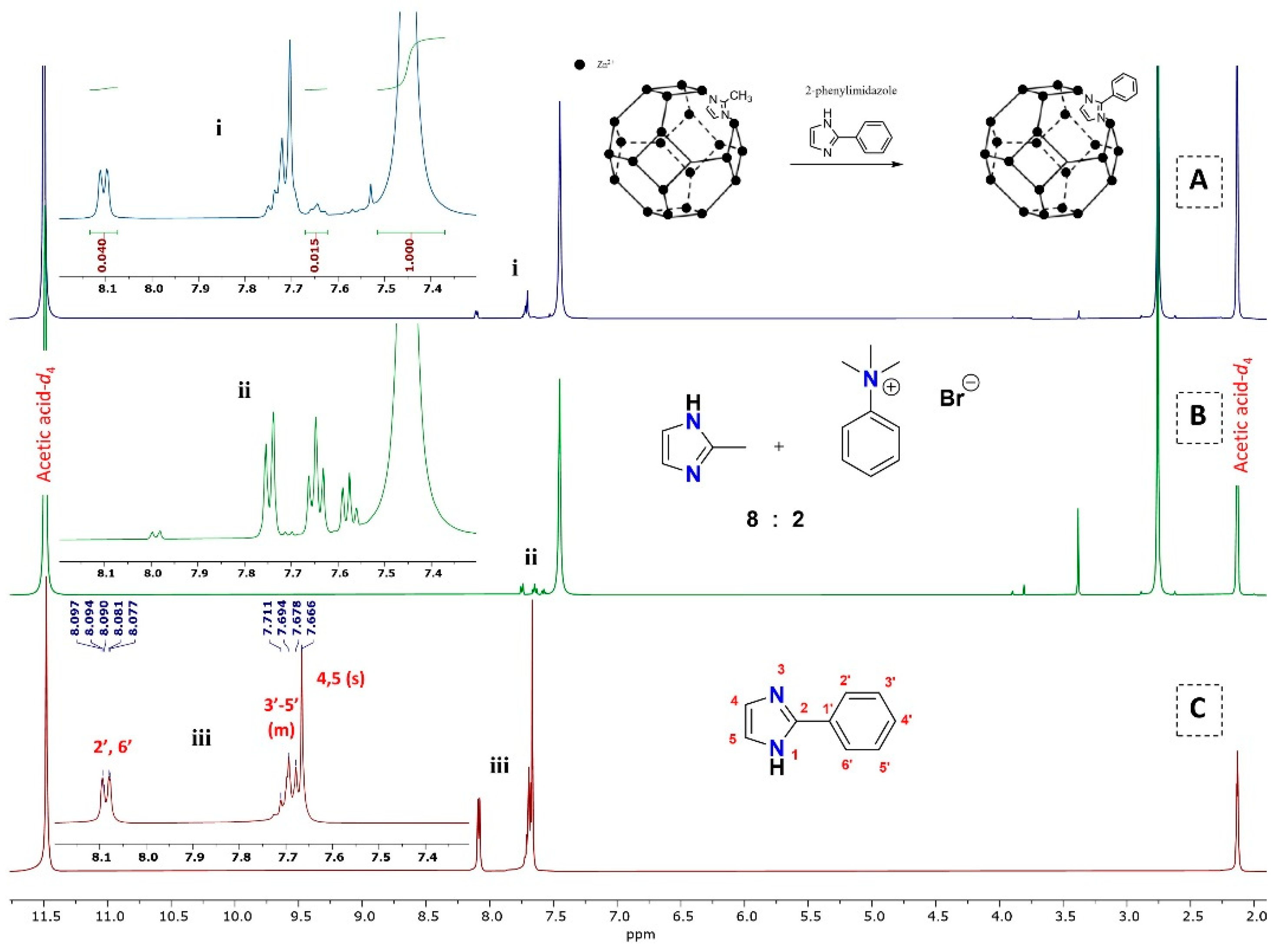
3.2.2. 1H NMR Analysis for the Replacement of Organic Linkers
3.3. CO2 Uptake
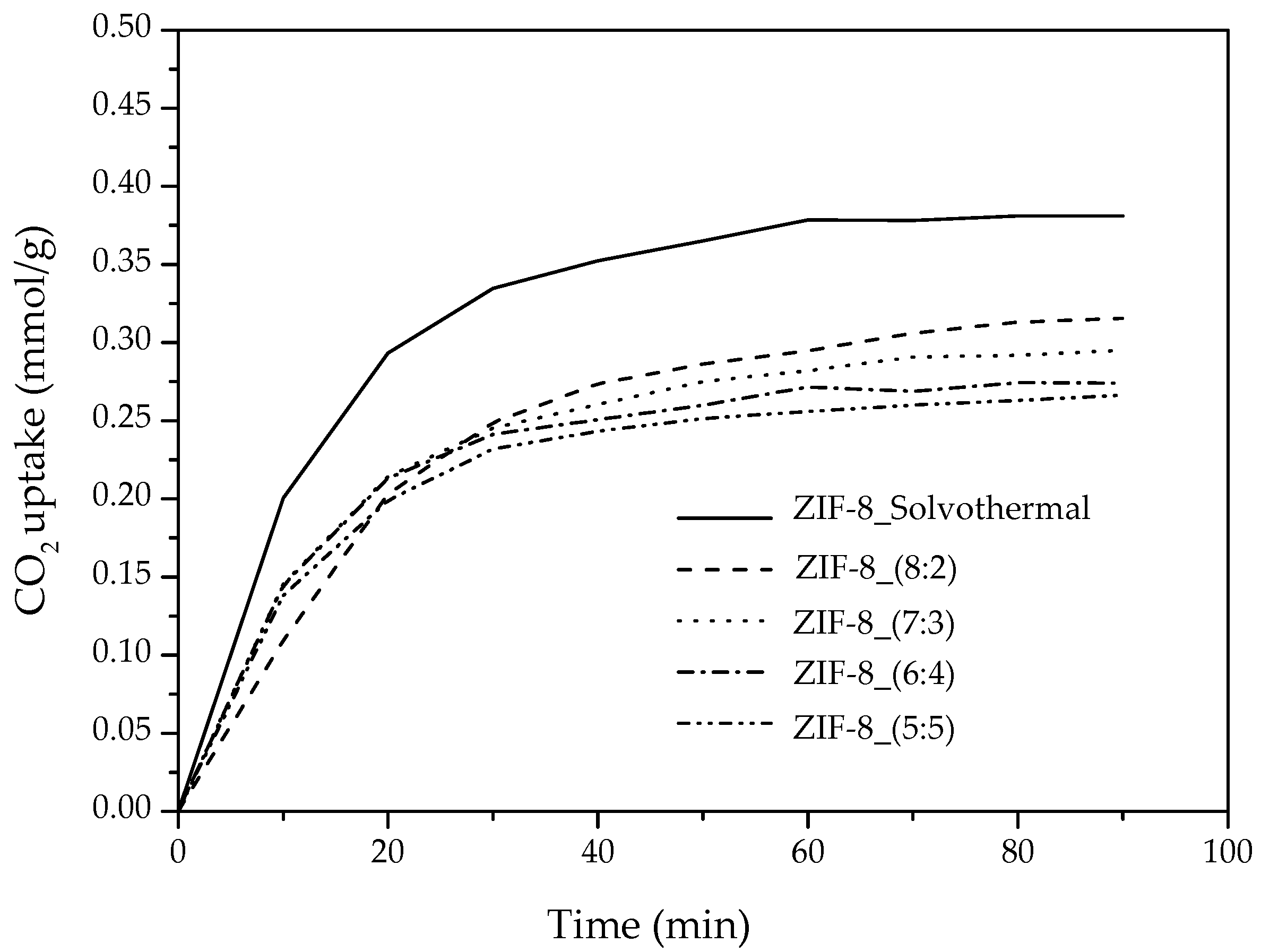
3.3.1. CO2 Uptake of ZIF-8 Synthesized from Water based Synthesis Assisted by Hydrogen Bond Acceptors
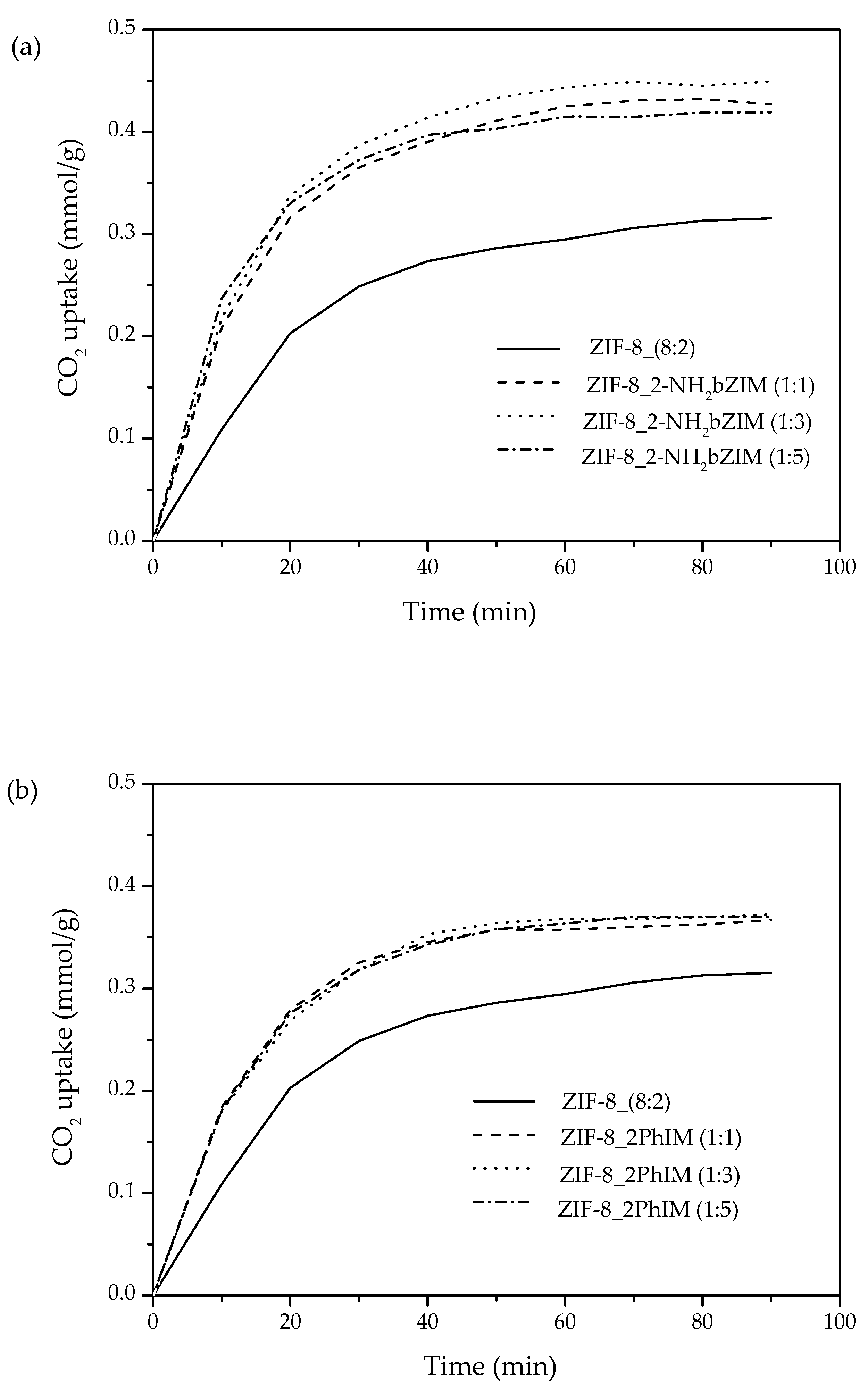
3.3.2. CO2 Uptake of Exchanged ZIF-8 by Imidazole Derivatives
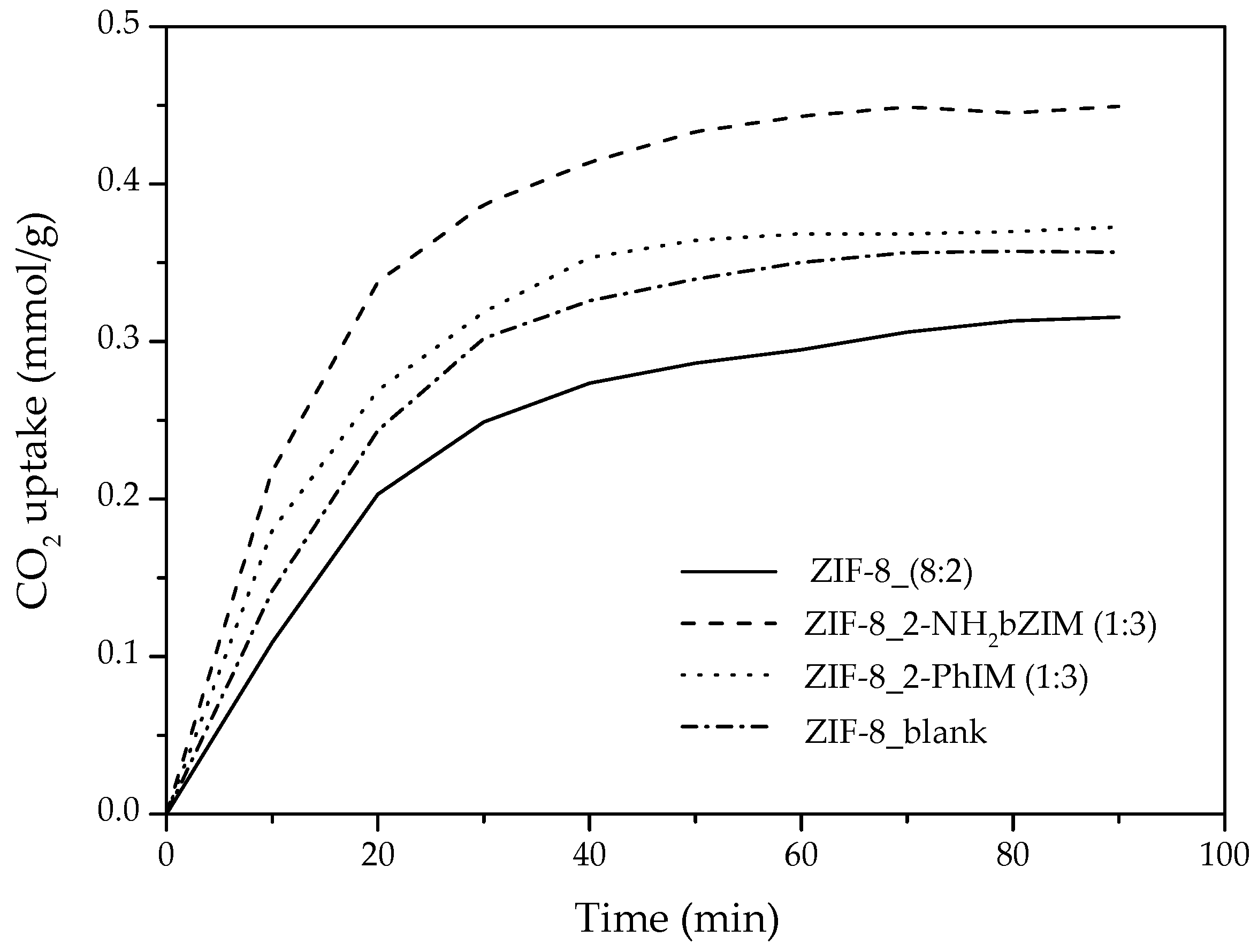
3.3.3. CO2 Adsorption Mechanism
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Xu, G.; Li, L.; Yang, Y.; Tian, L.; Liu, T.; Zhang, K. A novel CO2 cryogenic liquefaction and separation system. Energy 2012, 42, 522–529. [Google Scholar] [CrossRef]
- Knapik, E.; Kosowski, P.; Stopa, J. Cryogenic liquefaction and separation of CO2 using nitrogen removal unit cold energy. Chem. Eng. Res. Des. 2018, 131, 66–79. [Google Scholar] [CrossRef]
- Maqsood, K.; Mullick, A.; Ali, A.; Kargupta, K.; Ganguly, S. Cryogenic carbon dioxide separation from natural gas: A review based on conventional and novel emerging technologies. Rev. Chem. Eng. 2014, 30, 453–477. [Google Scholar] [CrossRef]
- Brunetti, A.; Scura, F.; Barbieri, G.; Drioli, E. Membrane technologies for CO2 separation. J. Membr. Sci. 2010, 359, 115–125. [Google Scholar] [CrossRef]
- Rezakazemi, M.; Amooghin, A.E.; MehdiMontazer-Rahmati, M.; FauziIsmail, A.; Matsuura, T. State-of-the-art membrane based CO2 separation using mixed matrix membranes (MMMs): An overview on current status and future directions. Prog. Polym. Sci. 2014, 39, 817–861. [Google Scholar] [CrossRef]
- Xu, J.; Wu, H.; Wang, Z.; Qiao, Z.; Zhao, S.; Wang, J. Recent advances on the membrane processes for CO2 separation. Chin. J. Chem. Eng. 2018, 26, 2280–2291. [Google Scholar] [CrossRef]
- Rochelle, G.T. Amine scrubbing for CO2 capture. Science 2009, 325, 1652–1654. [Google Scholar] [CrossRef]
- Chen, P.C.; Lin, S.-Z. Optimization in the Absorption and Desorption of CO2 Using Sodium Glycinate Solution. Appl. Sci. 2018, 8, 2041. [Google Scholar] [CrossRef]
- Haider, M.B.; Hussain, Z.; Kumar, R. CO2 absorption and kinetic study in ionic liquid amine blends. J. Mol. Liq. 2016, 224, 1025–1031. [Google Scholar] [CrossRef]
- Mello, M.R.; Phanon, D.; Silveira, G.Q.; Llewellyn, P.L.; Ronconi, C.M. Amine-modified MCM-41 mesoporous silica for carbon dioxide capture. Microporous Mesoporous Mater. 2011, 143, 174–179. [Google Scholar] [CrossRef]
- Younas, M.; Sohail, M.; Leong, L.K.; Bashir, M.J.K.; Sumathi, S. Feasibility of CO2 adsorption by solid adsorbents: A review on low-temperature systems. Int. J. Environ. Sci. Technol. 2016, 13, 1839–1860. [Google Scholar] [CrossRef]
- Wang, J.; Huang, L.; Yang, R.; Zhang, Z.; Wu, J.; Gao, Y.; Wang, Q.; O’Hare, D.; Zhong, Z. Recent advances in solid sorbents for CO2 capture and new development trends. Energy Environ. Sci. 2014, 7, 3478–3518. [Google Scholar] [CrossRef]
- Chen, C.; Ahn, W.S. CO2 adsorption on LTA zeolites: Effect of mesoporosity. Appl. Surf. Sci. 2014, 311, 107–109. [Google Scholar] [CrossRef]
- Gunawan, T.; Wijiyanti, R.; Widiastuti, N. Adsorption–desorption of CO2 on zeolite-Y-templated carbon at various temperatures. RSC Adv. 2018, 8, 41594–41602. [Google Scholar] [CrossRef]
- Megías-Sayago, C.; Bingre, R.; Huang, L.; Lutzweiler, G.; Wang, Q.; Louis, B. CO2 Adsorption Capacities in Zeolites and Layered Double Hydroxide Materials. Front. Chem. 2019, 7, 551. [Google Scholar] [CrossRef]
- Hu, J.; Liu, Y.; Liu, J.; Gu, C.; Wu, D. High CO2 adsorption capacities in UiO type MOFs comprising heterocyclic ligand. Microporous Mesoporous Mater. 2018, 256, 25–31. [Google Scholar] [CrossRef]
- Kukulka, W.; Cendrowski, K.; Michalkiewicz, B.; Mijowska, E. MOF-5 derived carbon as material for CO2 absorption. RSC Adv. 2019, 9, 18527–18537. [Google Scholar] [CrossRef]
- Kochetygov, I.; Bulut, S.; Asgari, M.; Queen, W.L. Selective CO2 adsorption by a new metal–organic framework: Synergy between open metal sites and a charged imidazolinium backbone. Dalton Trans. 2018, 47, 10527–10535. [Google Scholar] [CrossRef]
- Khan, I.U.; Othman, M.H.D.; Jilani, A.; Ismail, A.F.; Hashim, H.; Jaafar, J.; Rehman, G.U. Economical, environmental friendly synthesis, characterization for the production of zeolitic imidazolate framework-8 (ZIF-8) nanoparticles with enhanced CO2 adsorption. Arab. J. Chem. 2018, 11, 1072–1083. [Google Scholar] [CrossRef]
- Song, F.; Cao, Y.; Zhao, Y.; Jiang, R.; Xu, Q.; Yan, J.; Zhong, Q. Ion-Exchanged ZIF-67 Synthesized by One-Step Method for Enhancement of CO2 Adsorption. J. Nanomate. 2020, 1508574. [Google Scholar] [CrossRef]
- Arami-Niya, A.; Birkett, G.; Zhu, Z.; Rufford, T.E. Gate opening effect of zeolitic imidazolate framework ZIF-7 for adsorption of CH4 and CO2 from N2. J. Mater. Chem. A 2017, 5, 21389–21399. [Google Scholar] [CrossRef]
- Lee, Y.R.; Jang, M.S.; Cho, H.Y.; Kwon, H.J.; Kim, S.; Ahn, W.S. ZIF-8: A comparison of synthesis methods. Chem. Eng. J. 2015, 271, 276–280. [Google Scholar] [CrossRef]
- Cravillon, J.; Schröder, C.A.; Bux, H.; Rothkirch, A.; Caro, J.; Wiebcke, M. Formate modulated solvothermal synthesis of ZIF-8 investigated using time-resolved in situ X-ray diffraction and scanning electron microscopy. CrystEngComm 2012, 14, 492–498. [Google Scholar] [CrossRef]
- Sze, L.; Yin, L.; Yeong, F.; Keong, K.; Azmi, L.; Shariff, M. Effect of Synthesis Parameters on the Formation of ZIF-8 Under Microwave-assisted Solvothermal. Procedia Eng. 2016, 148, 35–42. [Google Scholar]
- Sun, W.; Zhai, X.; Zhao, L. Synthesis of ZIF-8 and ZIF-67 nanocrystals with well-controllable size distribution through reverse microemulsions. Chem. Eng. J. 2016, 289, 59–64. [Google Scholar] [CrossRef]
- Butova, V.V.; Budny, A.P.; Bulanova, E.A.; Lamberti, C.; Soldatov, A.V. Water based synthesis of high surface area ZIF-8 with minimal use of TEA. Solid State Sci. 2017, 69, 13–21. [Google Scholar] [CrossRef]
- Oozeerally, R.; Ramkhelawan, S.D.K.; Burnett, D.L.; Tempelman, C.H.L.; Degirmenci, V. ZIF-8 Metal Organic Framework for the Conversion of Glucose to Fructose and 5-Hydroxymethyl Furfural. Catalysts 2019, 9, 812. [Google Scholar] [CrossRef]
- Kida, K.; Okita, M.; Fujita, K.; Tanaka, S.; Miyake, Y. Formation of high crystalline ZIF-8 in an aqueous solution. CrystEngComm 2013, 15, 1794–1801. [Google Scholar] [CrossRef]
- Gross, A.F.; Sherman, E.; Vajo, J.J. Aqueous room temperature synthesis of cobalt and zinc sodalite zeolitic imidizolate frameworks. Dalton Trans. 2012, 41, 5458–5460. [Google Scholar] [CrossRef]
- Yao, J.; He, M.; Wang, K.; Chen, R.; Zhong, Z.; Wang, H. High-yield synthesis of zeolitic imidazolate frameworks from stoichiometric metal and ligand precursor aqueous solutions at room temperature. CrystEngComm 2013, 15, 3601–3606. [Google Scholar] [CrossRef]
- Hu, L.; Chen, L.; Fang, Y.; Wang, A.; Chen, C.; Yan, Z. Facile synthesis of zeolitic imidazolate framework-8 (ZIF-8) by forming imidazole-based deep eutectic solvent. Microporous Mesoporous Mater. 2018, 268, 207–215. [Google Scholar] [CrossRef]
- Karagiaridi, O.; Lalonde, M.B.; Bury, W.; Sarjeant, A.A.; Farha, O.K.; Hupp, J.T. Opening ZIF-8: A catalytically active zeolitic imidazolate framework of sodalite topology with unsubstituted linkers. J. Am. Chem. Soc. 2012, 134, 18790–18796. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, C.J.; Hupp, J.T.; Farha, O.K. Postassembly Transformation of a Catalytically Active Composite Material, Pt@ ZIF-8, via Solvent-Assisted Linker Exchange. Inorg. Chem. 2016, 55, 1361–1363. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Dawson, K.W.; Gelfand, B.S.; Taylor, J.M.; Shimizu, G.K. Enhancing proton conduction in a metal–organic framework by isomorphous ligand replacement. J. Am. Chem. Soc. 2013, 135, 963–966. [Google Scholar] [CrossRef] [PubMed]
- Pullen, S.; Fei, H.; Orthaber, A.; Cohen, S.M.; Ott, S. Enhanced photochemical hydrogen production by a molecular diiron catalyst incorporated into a metal–organic framework. J. Am. Chem. Soc. 2013, 135, 16997–17003. [Google Scholar] [CrossRef]
- Bury, W.; Fairen-Jimenez, D.; Lalonde, M.B.; Snurr, R.Q.; Farha, O.K.; Hupp, J.T. Control over catenation in pillared paddlewheel metal–organic framework materials via solvent-assisted linker exchange. Chem. Mater. 2013, 25, 739–744. [Google Scholar] [CrossRef]
- Tsai, C.W.; Niemantsverdriet, J.W.; Langner, E.H. Enhanced CO2 adsorption in nano-ZIF-8 modified by solvent assisted ligand exchange. Microporous Mesoporous Mater. 2018, 262, 98–105. [Google Scholar] [CrossRef]
- Laurence, C.; Berthelot, M. Observations on the strength of hydrogen bonding. Perspect. Drug Discov. Des. 2000, 18, 39–60. [Google Scholar] [CrossRef]
- Levitt, M.; Perutz, M.F. Aromatic rings act as hydrogen bond acceptors. J. Mol. Biol. 1988, 201, 751–754. [Google Scholar] [CrossRef]
- Zhang, Y.; Jia, Y.; Hou, L. Synthesis of zeolitic imidazolate framework-8 on polyester fiber for PM2.5 removal. RSC Adv. 2018, 8, 31471–31477. [Google Scholar] [CrossRef]
- Tanaka, S.; Tanaka, Y. Simple Step toward Enhancing Hydrothermal Stability of ZIF-8. ACS Omega 2019, 4, 19905–19912. [Google Scholar] [CrossRef] [PubMed]
- Xiang, L.; Sheng, L.; Wang, C.; Zhang, L.; Pan, Y.; Li, Y. Amino-Functionalized ZIF-7 Nanocrystals: Improved Intrinsic Separation Ability and Interfacial Compatibility in Mixed-Matrix Membranes for CO2/CH4 Separation. Adv. Mater. 2017, 29, 1606999. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Wu, Y.; Xia, Q.; Li, Z.; Xi, H. Experimental and molecular simulation studies of CO2 adsorption on zeolitic imidazolate frameworks: ZIF-8 and amine-modified ZIF-8. Adsorption 2013, 19, 25–37. [Google Scholar] [CrossRef]
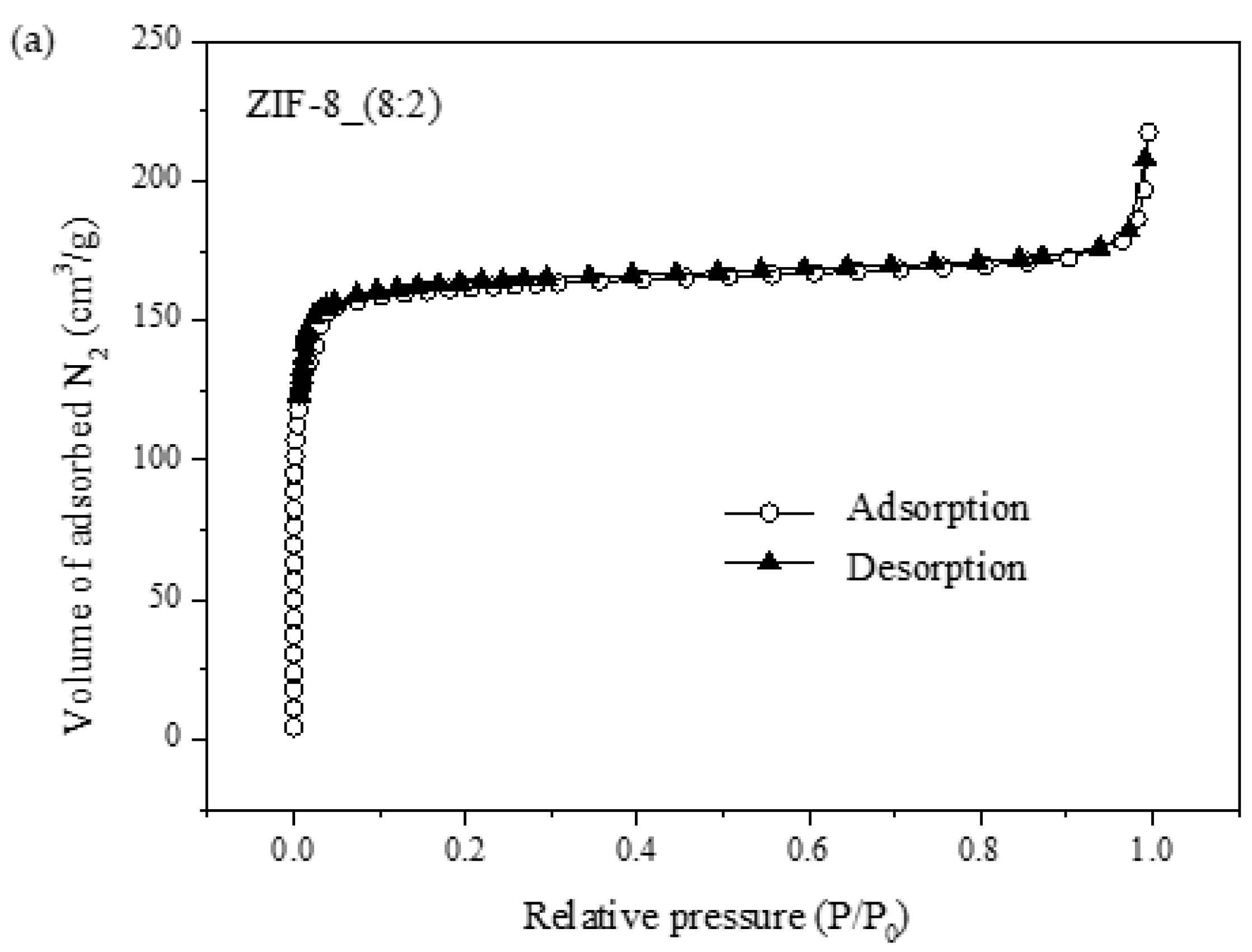

| Sample | Surface Area (m2/g) | Average Pore Diameter (nm) | Pore Volume (cm2/g) |
|---|---|---|---|
| ZIF-8_Solvothermal | 1539 | 2.280 | 0.6469 |
| ZIF-8_(8:2) | 634.0 | 1.920 | 0.3043 |
| ZIF-8_2-NH2bZIM(1:3) | 745.2 | 1.985 | 0.3698 |
| ZIF-8_2-PhIM (1:3) | 775.8 | 1.928 | 0.3739 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kenyotha, K.; Chanapattharapol, K.C.; McCloskey, S.; Jantaharn, P. Water Based Synthesis of ZIF-8 Assisted by Hydrogen Bond Acceptors and Enhancement of CO2 Uptake by Solvent Assisted Ligand Exchange. Crystals 2020, 10, 599. https://doi.org/10.3390/cryst10070599
Kenyotha K, Chanapattharapol KC, McCloskey S, Jantaharn P. Water Based Synthesis of ZIF-8 Assisted by Hydrogen Bond Acceptors and Enhancement of CO2 Uptake by Solvent Assisted Ligand Exchange. Crystals. 2020; 10(7):599. https://doi.org/10.3390/cryst10070599
Chicago/Turabian StyleKenyotha, Kasama, Kingkaew Chayakul Chanapattharapol, Sirirath McCloskey, and Phongphan Jantaharn. 2020. "Water Based Synthesis of ZIF-8 Assisted by Hydrogen Bond Acceptors and Enhancement of CO2 Uptake by Solvent Assisted Ligand Exchange" Crystals 10, no. 7: 599. https://doi.org/10.3390/cryst10070599
APA StyleKenyotha, K., Chanapattharapol, K. C., McCloskey, S., & Jantaharn, P. (2020). Water Based Synthesis of ZIF-8 Assisted by Hydrogen Bond Acceptors and Enhancement of CO2 Uptake by Solvent Assisted Ligand Exchange. Crystals, 10(7), 599. https://doi.org/10.3390/cryst10070599





