Effect of Silver Nanoparticles on the Melting Behavior, Isothermal Crystallization Kinetics and Morphology of Polyoxymethylene
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Synthesis of Ag Nanoparticles
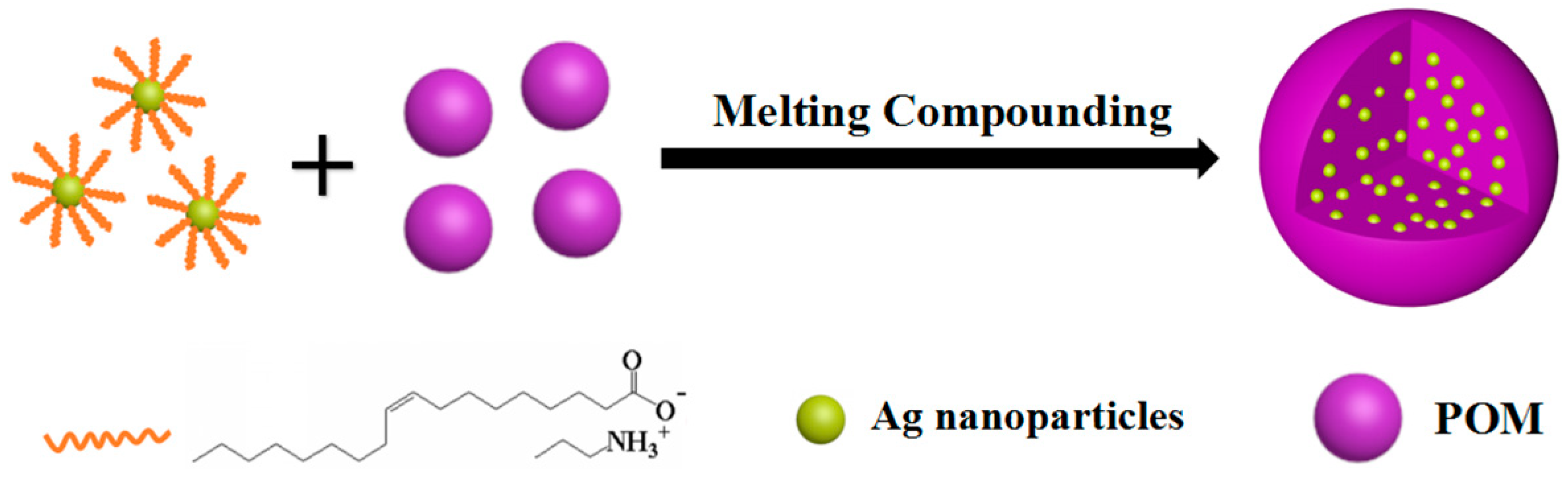
2.3. Preparation of POM/Ag Nanocomposites
2.4. Transmission Electron Microscopy (TEM)
2.5. Differential Scanning Calorimetry (DSC)
2.6. Polarized Light Microscopy (PLM)
3. Results and Discussion
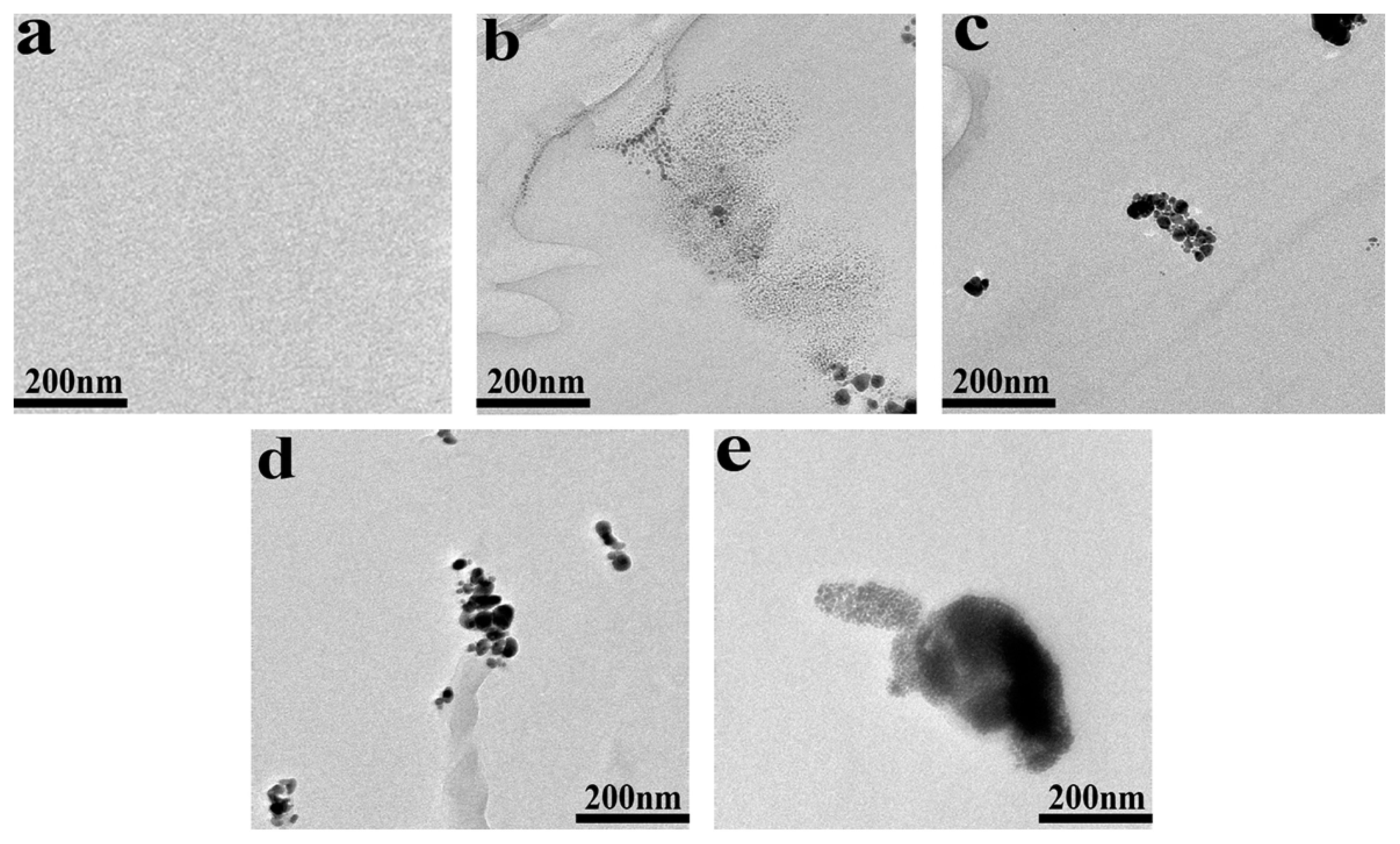
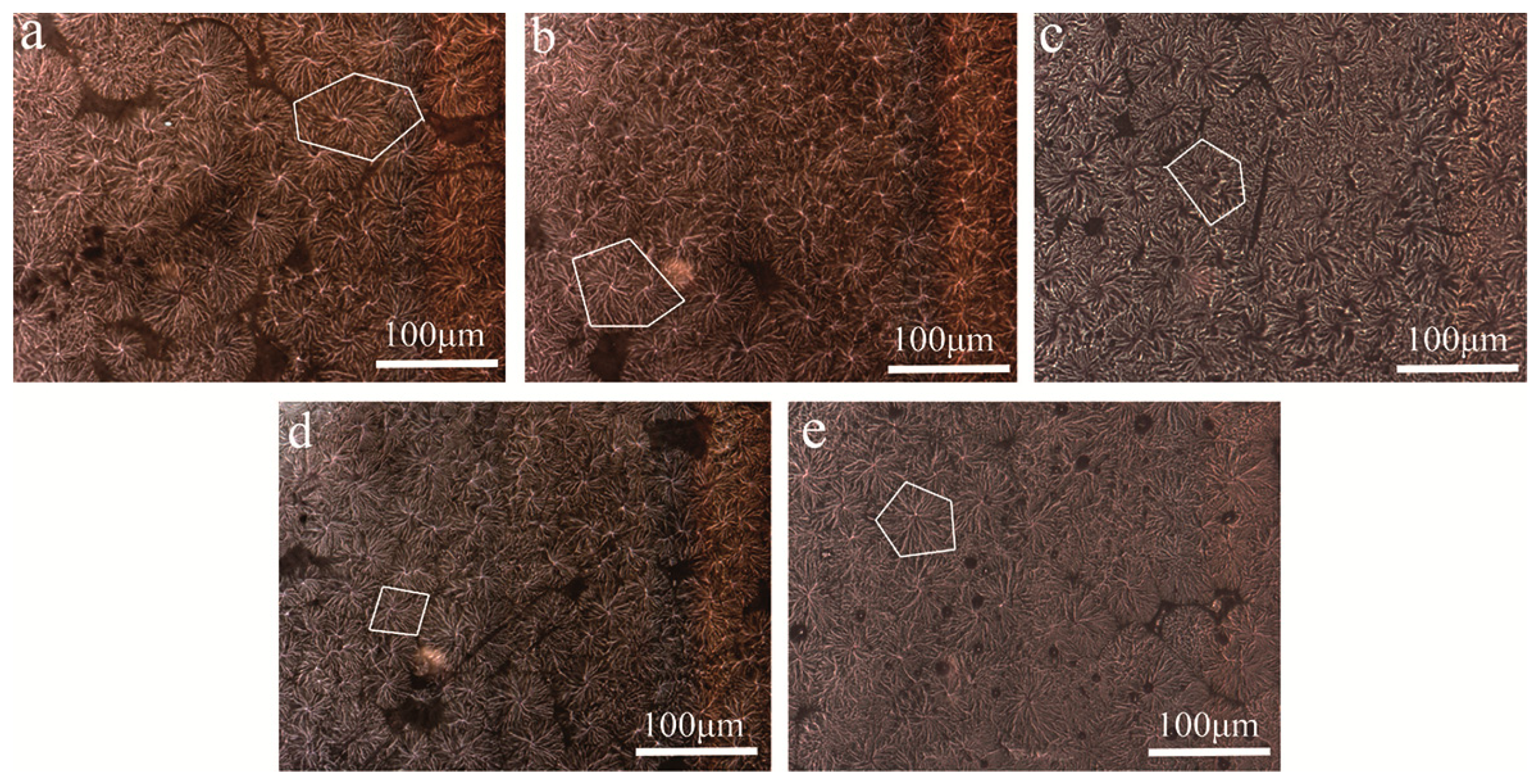
3.1. Morphology of POM/Ag Nanocomposites
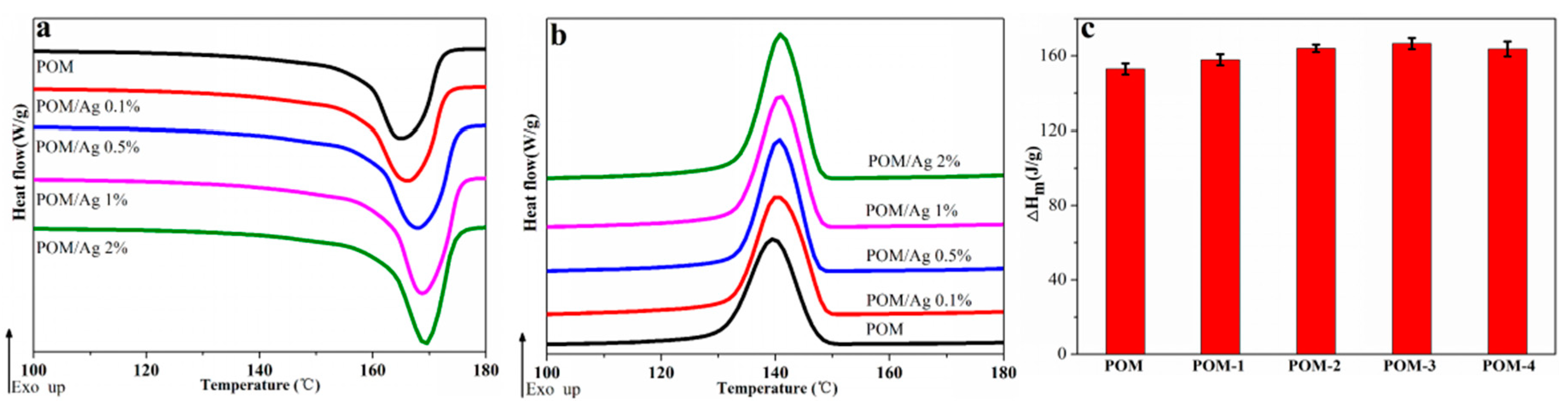
3.2. Melting and Crystallization Behavior of POM/Ag Nanocomposites
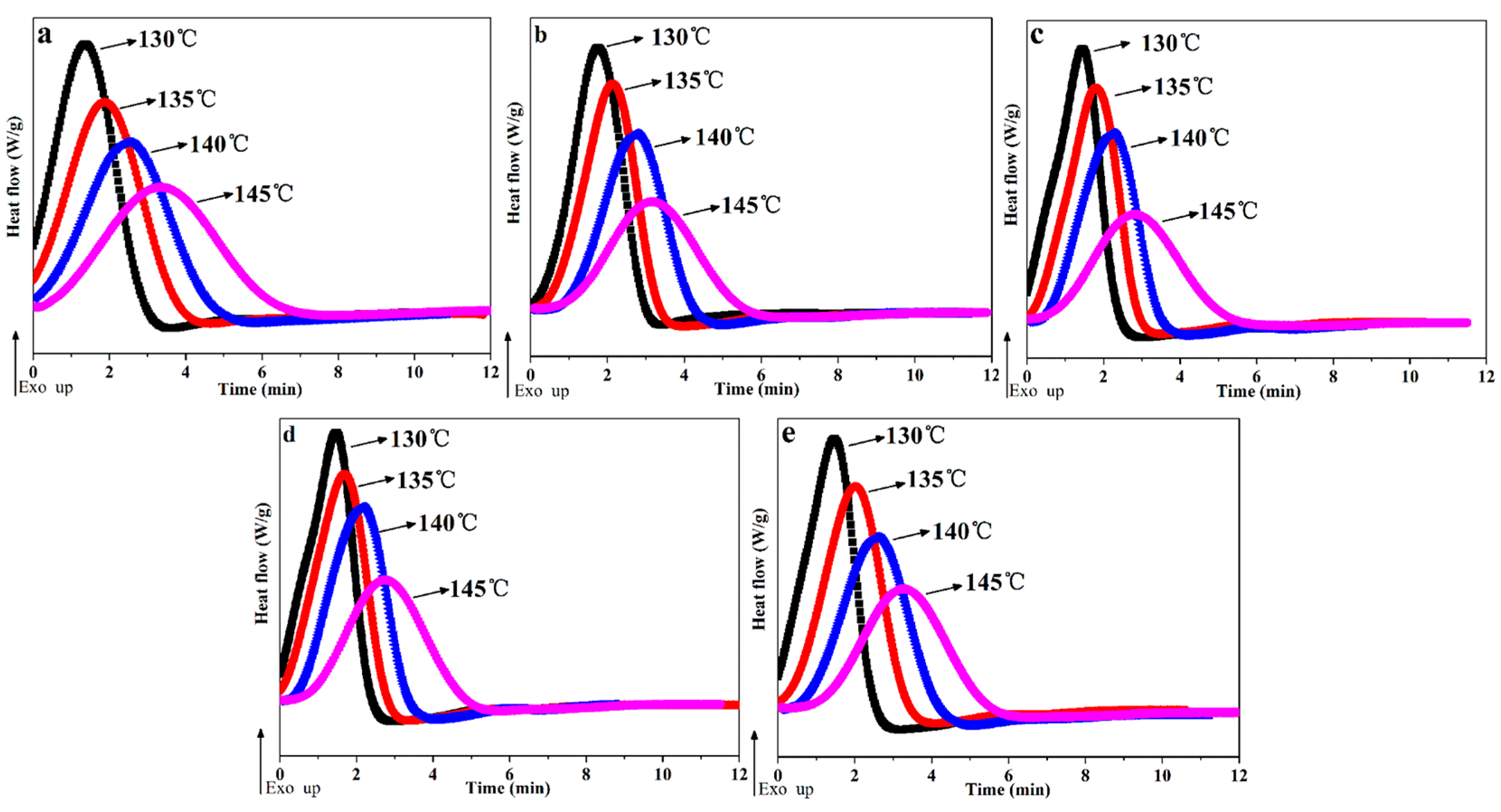
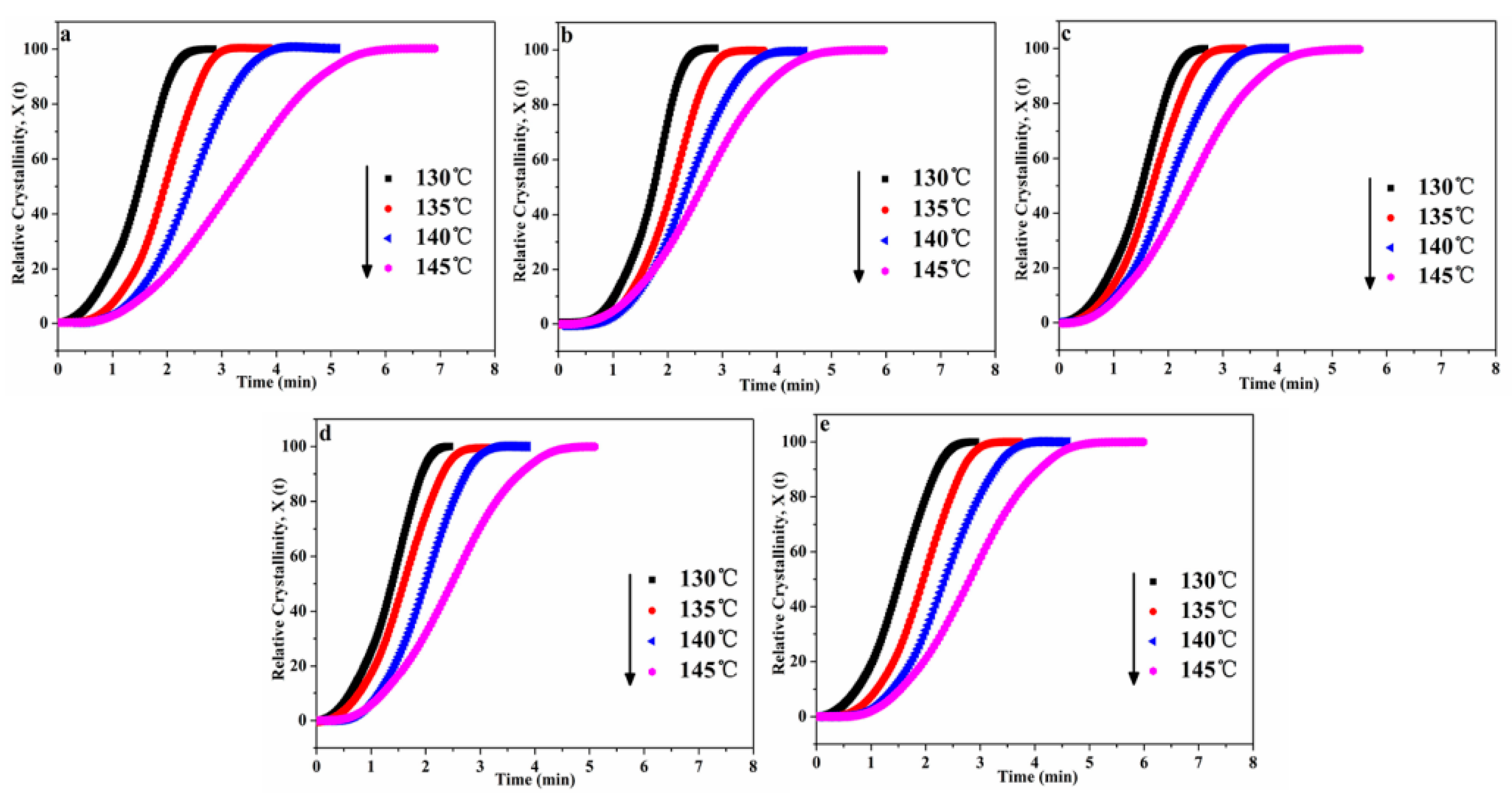
3.3. Isothermal Crystallization Kinetics of POM/Ag Nanocomposites
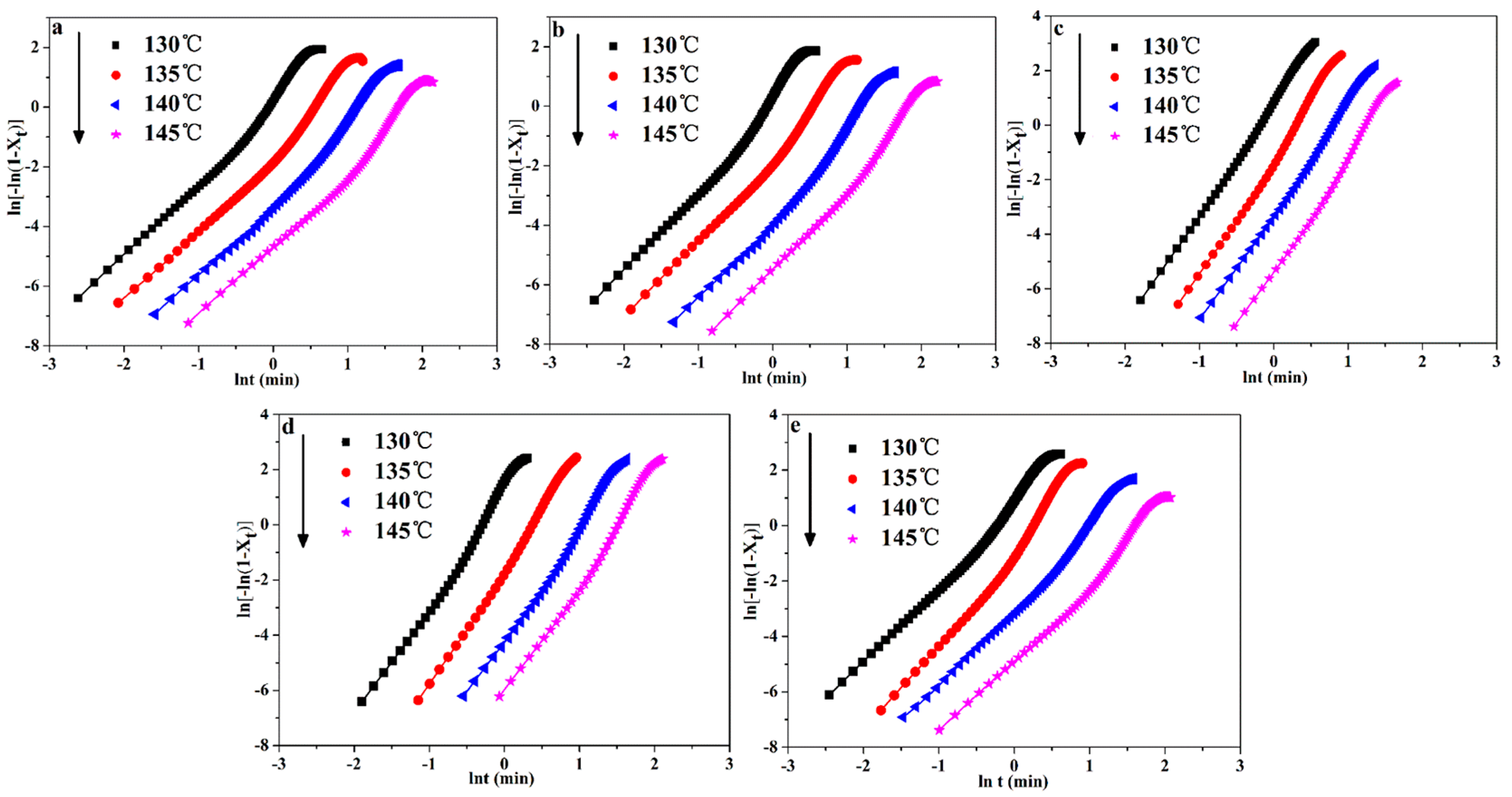
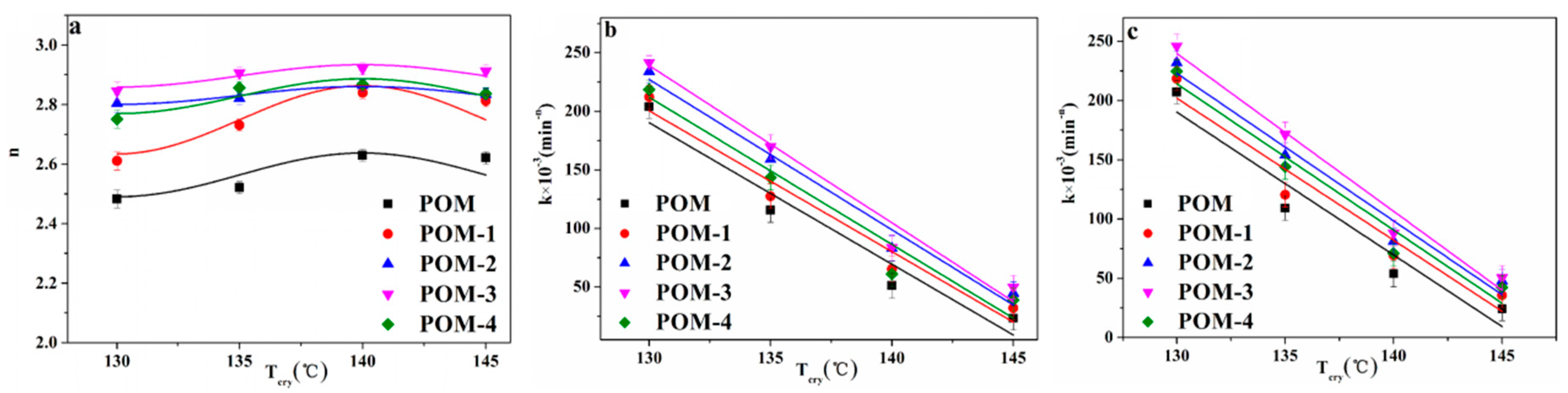
3.3.1. Avrami Model Analysis of POM/Ag Nanocomposites
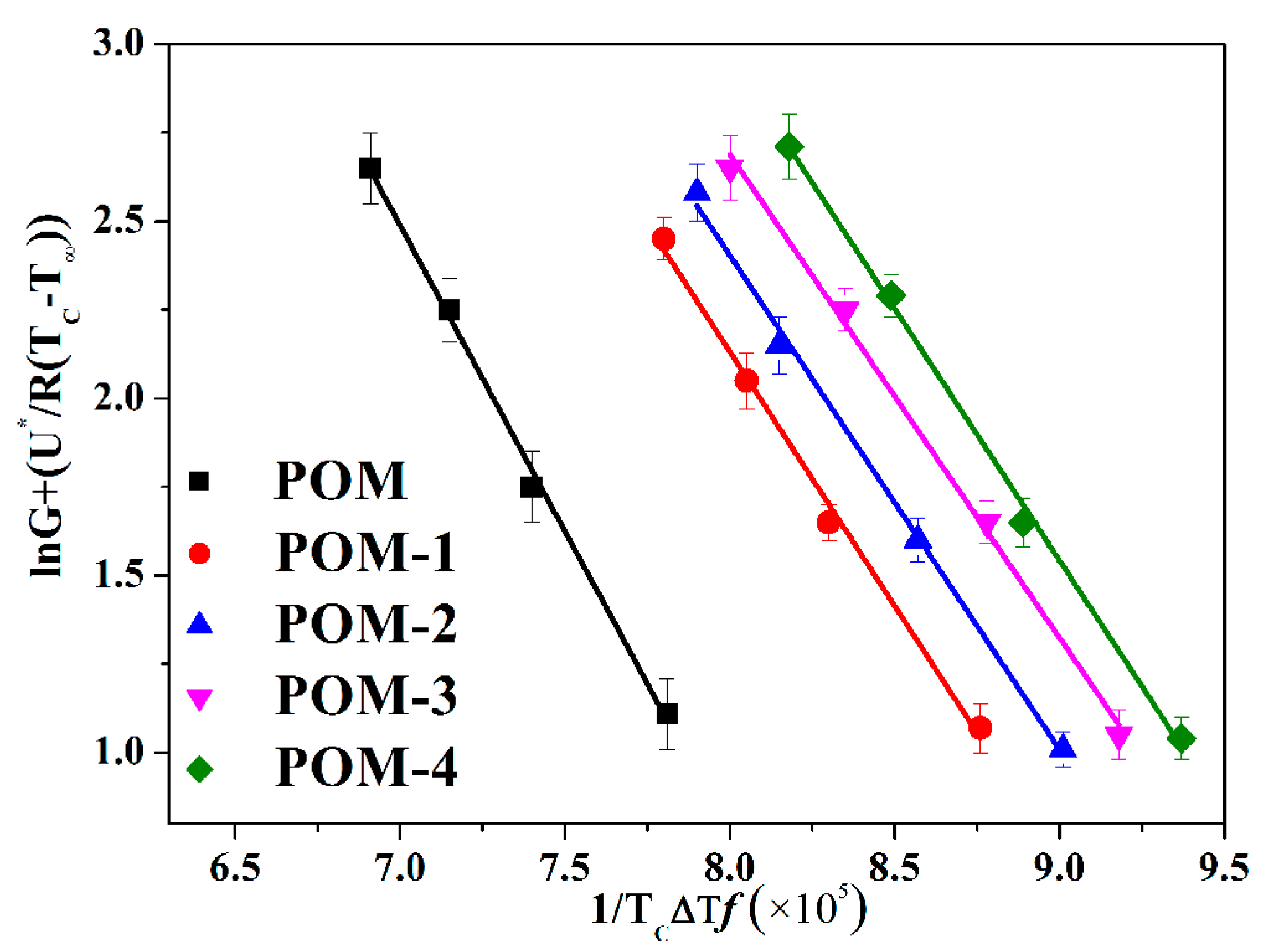
3.3.2. Lauritzen-Hoffman Model Analysis of POM/Ag Nanocomposites
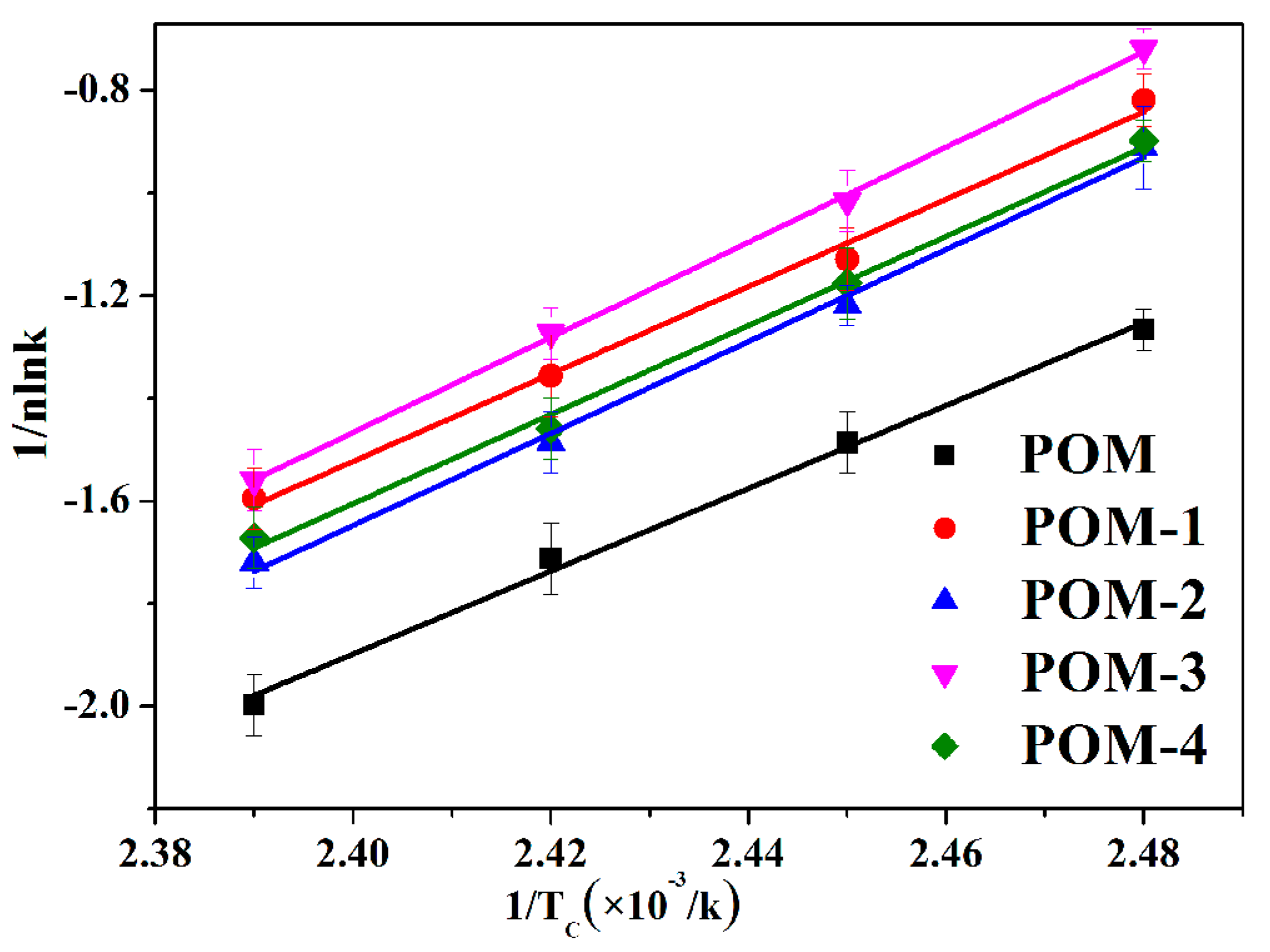
3.3.3. Isothermal Crystallization Activation Energy Analysis of POM/Ag Nanocomposites
3.4. Morphology of POM/Ag Nanocomposites
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Andrzejewski, J.; Skórczewska, K.; Kloziński, A. Improving the toughness and thermal resistance of polyoxymethylene/poly(lactic acid) blends: Evaluation of structure-properties correlation for reactive processing. Polymers 2020, 12, 307. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.C.; Tran, T.M.; Trinh, A.T.; Nguyen, A.H.; Dam, X.T.; Vu, Q.T.; Tran, D.L.; Nguyen, D.T.; Le, T.G.; Thai, H. Polyoxymethylene/silica/polylactic acid-grafted polyethylene glycol nanocomposites: Structure, morphology, and mechanical properties and ozone and UV durability. RSC Adv. 2020, 10, 2691–2702. [Google Scholar] [CrossRef]
- Fang, X.; Wyatt, T.; Shi, J.; Yao, D. Fabrication of high-strength polyoxymethylene fibers by gel spinning. J. Mater. Sci. 2018, 53, 11901–11916. [Google Scholar] [CrossRef]
- Kuciel, S.; Bazan, P.; Liber-Kneć, A.; Gądek-Moszczak, A. Physico-mechanical properties of the poly(oxymethylene) composites reinforced with glass fibers under dynamical loading. Polymers 2019, 11, 2064. [Google Scholar] [CrossRef]
- Yang, J.; Yang, W.; Wang, X.; Dong, M.; Liu, H.; Wujcik, E.K.; Shao, Q.; Wu, S.; Ding, T.; Guo, Z. Synergistically toughening polyoxymethylene by methyl methacrylate-butadiene-styrene copolymer and thermoplastic polyurethane. Macromol. Chem. Phys. 2019, 220, 1800567. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, X.; Wang, H.; Wei, F.; Zhang, Y. Experimental and numerical analysis of interfacial bonding strength of polyoxymethylene reinforced cement composites. Constr. Build. Mater. 2019, 207, 1–9. [Google Scholar] [CrossRef]
- Bazan, P.; Kuciel, S.; Nykiel, M. Characterization of composites based on polyoxymethylene and effect of silicone addition on mechanical and tribological behavior. Polym. Eng. Sci. 2019, 59, 935–940. [Google Scholar] [CrossRef]
- Li, J.H.; Wang, Y.T.; Wang, X.D.; Wu, D.Z. Development of Polyoxymethylene/polylactide blends for a potentially biodegradable material: Crystallization kinetics, lifespan prediction, and enzymatic degradation behavior. Polymers 2019, 11, 1516. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, J.; Huang, J. Poly(lactic acid)/polyoxymethylene blends: Morphology, crystallization, rheology, and thermal mechanical properties. Polymer 2015, 69, 103–109. [Google Scholar] [CrossRef]
- Lguchi, M. Memoir: A polymer whisker-the needle-shaped single crystal of polyoxymethylene. Polymer 2019, 168, 255–268. [Google Scholar] [CrossRef]
- Jasiurkowska-Delaporte, M.; Rozwadowski, T.; Juszyńska-Gałązka, E. Kinetics of non-isothermal and isothermal crystallization in a liquid crystal with highly ordered smectic phase as reflected by differential scanning calorimetry, polarized optical microscopy and broadband dielectric spectroscopy. Crystals 2019, 9, 205. [Google Scholar] [CrossRef]
- Abushammala, H.; Mao, J. Impact of the Surface Properties of cellulose nanocrystals on the crystallization kinetics of poly(butylene succinate). Crystals 2020, 10, 196. [Google Scholar] [CrossRef]
- Tomara, G.N.; Karahaliou, P.K.; Psarras, G.C.; Georga, S.N.; Krontiras, C.A.; Siengchin, S.; Karger-Kocsis, J. Dielectric relaxation mechanisms in polyoxymethylene/polyurethane/layered silicates hybrid nanocomposites. Eur. Polym. J. 2017, 95, 304–313. [Google Scholar] [CrossRef][Green Version]
- Kunnan Singh, J.S.; Ching, Y.C.; Abdullah, L.C.; Ching, K.Y.; Razali, S.; Gan, S.N. Optimization of mechanical properties for polyoxymethylene/glass fiber/polytetrafluoroethylene composites using response surface methodology. Polymers 2018, 10, 338. [Google Scholar] [CrossRef]
- Yu, C.; Xie, Q.; Bao, Y.; Shan, G.; Pan, P. Crystalline and spherulitic morphology of polymers crystallized in confined systems. Crystals 2017, 7, 147. [Google Scholar] [CrossRef]
- Hu, R.R.; Gao, E.L.; Xu, Z.P.; Liu, L.Q.; Wang, G.R.; Zhu, H.W.; Zhang, Z. Hierarchical-structure-dependent high ductility of electrospun polyoxymethylene nanofibers. J. Appl. Polym. Sci. 2018, 136, 47086. [Google Scholar] [CrossRef]
- Durmus, A.; Kasgoz, A.; Ercan, N.; Akın, D.; Sanlı, S. Effect of polyhedral oligomeric silsesquioxane (POSS) reinforced polypropylene (PP) nanocomposite on the microstructure and isothermal crystallization kinetics of polyoxymethylene (POM). Polymer 2012, 53, 5347–5357. [Google Scholar] [CrossRef]
- Tan, C.B.; Bai, S.B.; Wang, Q. The crystallization morphology evolution of polyoxymethylene/poly(ethylene oxide) blend micropart prepared under microinjection molding conditions. J. Appl. Polym. Sci. 2014, 131, 40538. [Google Scholar] [CrossRef]
- Czarnecka-Komorowska, D.; Sterzynski, T. Effect of polyhedral oligomeric silsesquioxane on the melting, structure, and mechanical behavior of polyoxymethylene. Polymers 2018, 10, 203. [Google Scholar] [CrossRef]
- Jiao, Q.; Chen, Q.; Wang, L.; Chen, H.; Li, Y. Investigation on the crystallization behaviors of polyoxymethylene with a small amount of ionic liquid. Nanomaterials 2019, 9, 206. [Google Scholar] [CrossRef]
- Liang, Z.; Tian, Q.; Zhang, H.; Hu, J.; He, P.; Li, H.; Bao, S.; Wang, P.; Huang, H.; Song, F. Exploring the adsorption mechanism of tetracene on Ag(110) by STM and dispersion-corrected DFT. Crystals 2020, 10, 13. [Google Scholar] [CrossRef]
- Cao, L.; Liao, B.; Wu, X.; Li, C.; Huang, G.; Cheng, N. Hot Deformation behavior and microstructure characterization of an Al-Cu-Li-Mg-Ag alloy. Crystals 2020, 10, 416. [Google Scholar] [CrossRef]
- Li, Z.Q.; Wang, X.L.; Yin, Z.K.; Zhao, J.L.; Song, M.; Wu, Z.G.; Li, H.Y.; Wang, X.X. Ag nanoparticles decorated N/S dual-doped graphene nanohybrids for high-performance asymmetric supercapacitors. Carbon 2020, 161, 726–735. [Google Scholar] [CrossRef]
- Abdalla, J.T.; Wang, J.; Wang, D. Effect of Ag/rGO on the optical properties of plasmon-modified SnO2 composite and its application in self-powered UV photodetector. Crystals 2019, 9, 648. [Google Scholar] [CrossRef]
- Qi, L.B.; Zhang, K.M.; Qin, W.; Hu, Y.X. Highly efficient flow-through catalytic reduction of methylene blue using silver nanoparticles functionalized cotton. Chem. Eng. J. 2020, 388, 124252. [Google Scholar] [CrossRef]
- Silvestri, D.; Waclawek, S.; Venkateshaiah, A.; Krawczyk, K.; Sobel, B.; Padil, V.V.T.; Cernik, M.; Varma, R.S. Synthesis of Ag nanoparticles by a chitosan-poly(3-hydroxybutyrate) polymer conjugate and their superb catalytic activity. Carbohyd. Polym. 2020, 232, 115806. [Google Scholar] [CrossRef] [PubMed]
- Martínez Espinosa, J.C.; Carrera Cerritos, R.; Ramírez Morales, M.A.; Sánchez Guerrero, K.P.; Silva Contreras, R.A.; Macías, J.H. Characterization of silver nanoparticles obtained by a green route and their evaluation in the bacterium of pseudomonas aeruginosa. Crystals 2020, 10, 395. [Google Scholar] [CrossRef]
- Zare, E.N.; Makvandi, P.; Borzacchiello, A.; Tay, F.R.; Ashtari, B.; Padil, V.V.T. Antimicrobial gum bio-based nanocomposites and their industrial and biomedical applications. Chem. Commun. 2019, 55, 14871–14885. [Google Scholar] [CrossRef]
- Liao, G.F.; Fang, J.S.; Li, Q.; Li, S.H.; Xu, Z.S.; Fang, B.Z. Ag-Based nanocomposites: Synthesis and applications in catalysis. Nanoscale 2019, 11, 7062–7096. [Google Scholar] [CrossRef]
- Guan, X.; Cao, L.; Huang, Q.; Kong, D.; Zhang, P.; Lin, H.; Li, W.; Lin, Z.; Yuan, H. Direct writing supercapacitors using a carbon nanotube/Ag nanoparticle-based ink on cellulose acetate membrane paper. Polymers 2019, 11, 973. [Google Scholar] [CrossRef]
- Dhiman, M. Plasmonic nanocatalysis for solar energy harvesting and sustainable chemistry. J. Mater. Chem. A 2020, 8, 10074–10095. [Google Scholar] [CrossRef]
- Mei, S.X.; Pan, M.W.; Gao, S.S.; Song, S.F.; Wang, J.; Liu, G. Organic–inorganic bimetallic hybrid particles with controllable morphology for the catalytic degradation of organic dyes. New J. Chem. 2020, 44, 8366–8378. [Google Scholar] [CrossRef]
- Makvandi, P.; Ali, G.W.; Sala, F.D.; Abdel-Fattah, W.I.; Borzacchiello, A. Biosynthesis and characterization of antibacterial thermosensitive hydrogels based on corn silk extract, hyaluronic acid and nanosilver for potential wound healing. Carbohyd. Polym. 2019, 223, 115023. [Google Scholar] [CrossRef] [PubMed]
- Rangraz, Y.; Nemati, F.; Elhampour, A. Selenium-doped graphitic carbon nitride decorated with Ag NPs as a practical and recyclable nanocatalyst for the hydrogenation of nitro compounds in aqueous media. Appl. Surf. Sci. 2020, 507, 145164. [Google Scholar] [CrossRef]
- Shi, Y.; Sun, B.; Zhou, Z.; Wangatia, L.M.; Chen, L.; Zhu, M. Polypropylene nanocomposites based on synthetic organic-soluble Ag nanocrystals with prominent β-nucleating effect: Quiescent crystallization and melting behavior. J. Macromol. Sci. Phys. 2012, 51, 2505–2518. [Google Scholar] [CrossRef]
- Makvandi, P.; Ali, G.W.; Sala, F.D.; Abdel-Fattah, W.I.; Borzacchiello, A. Hyaluronic acid/corn silk extract based injectable nanocomposite: A biomimetic antibacterial scaffold for bone tissue regeneration. Mater. Sci. Eng. C 2020, 107, 110195. [Google Scholar] [CrossRef]
- Zeng, Y.C.; Liu, Y.; Wang, L.M.; Huang, H.L.; Zhang, X.; Liu, Y.L.; Min, M.H.; Li, Y. Effect of silver nanoparticles on the microstructure, non-isothermal crystallization behavior and antibacterial activity of polyoxymethylene. Polymers 2020, 12, 424. [Google Scholar] [CrossRef]
- Kumar, G.; Neelakantan, N.R.; Subramanian, N. Polyacetal and thermoplastic polyurethane elastomer toughened polyacetal: Crystallinity and fracture mechanics. J. Mater. Sci. 1995, 30, 1480–1486. [Google Scholar] [CrossRef]
- Ouladsmane, M.; Saeed, W.S.; Al-Odayni, A.-B.; Ahmed, A.Y.B.H.; Alghamdi, A.A.; Al-Kahtani, A.; Aouak, T. Non isothermal crystallization kinetics and isothermal decomposition of poly(ethylene-co-vinylalcohol/poly(D,L-lactic-co-glycolic acid) blend. Crystals 2020, 10, 425. [Google Scholar] [CrossRef]
- Avrami, M.J. Kinetics of phase change. I general theory. J. Chem Phys. 1939, 7, 1103–1112. [Google Scholar] [CrossRef]
- Chen, Y.H.; Ranganathan, P.; Chen, C.W.; Lee, Y.H.; Rwei, S.P. Effect of bis (2-Aminoethyl) adipamide/adipic acid segment on polyamide 6: Crystallization kinetics study. Polymers 2020, 12, 1067. [Google Scholar] [CrossRef] [PubMed]
- Lauritzen, J.L.; Hoffman, J.D. Extension of theory of growth of chain-folded polymer crystals to large undercoolings. J. Appl. Phys. 1973, 44, 4340–4352. [Google Scholar] [CrossRef]
- Hoffman, J.D. Regime III crystallization in melt-crystallized polymers: The variable cluster model of chain folding. Polymer 1983, 24, 3–26. [Google Scholar] [CrossRef]
- Grigoras, V.C. Aliphatic copolyamide nanocomposites: Isothermal crystallization and crystalline morphology induced by clays. Thermochim. Acta. 2019, 675, 77–83. [Google Scholar] [CrossRef]
- Deng, Y.; He, M.Y.; Li, J.H. Non-isothermal crystallization behavior of polyethylene glycol/expanded vermiculite form-stable composite phase change material. Mater. Lett. 2019, 234, 17–20. [Google Scholar] [CrossRef]
- Wang, Z.Q.; Hu, G.S.; Zhang, J.T.; Xu, J.S.; Shi, W.B. Non-isothermal crystallization kinetics of nylon 10T and nylon 10T/1010 copolymers: Effect of sebacic acid as a third comonomer. Chin. J. Chem. Eng. 2017, 25, 963–970. [Google Scholar] [CrossRef]
- Zhu, B.; Li, W.; Song, J.; Wang, J. Structure and properties of polypropylene/polyolefin elastomer/organic montmorillonite nanocomposites. J. Macromol. Sci. B 2019, 58, 73–87. [Google Scholar] [CrossRef]

| Samples | POM (wt%) | Ag Nanoparticles (wt%) |
|---|---|---|
| POM | 100 | 0 |
| POM-1 | 99.9 | 0.1 |
| POM-2 | 99.5 | 0.5 |
| POM-3 | 99.0 | 1.0 |
| POM-4 | 98.0 | 2.0 |
| Samples | Tm (°C) | TC (°C) | ∆Hm (J/g) | XC (%) |
|---|---|---|---|---|
| POM | 166.24 ± 0.24 | 139.64 ± 0.14 | 152.94 ± 3.2 | 46.91 ± 0.9 |
| POM-1 | 166.83 ± 0.31 | 141.83 ± 0.12 | 157.89 ± 1.9 | 48.43 ± 0.5 |
| POM-2 | 167.91 ± 0.37 | 142.81 ± 0.21 | 164.08 ± 2.4 | 50.33 ± 0.7 |
| POM-3 | 169.23 ± 0.35 | 142.83 ± 0.32 | 166.64 ± 2.8 | 51.12 ± 0.8 |
| POM-4 | 169.81 ± 0.11 | 141.71 ± 0.29 | 163.71 ± 2.1 | 50.21 ± 0.6 |
| Samples | tTcry =130 °C (min) | tTcry =135 °C (min) | tTcry =140 °C (min) | tTcry =145 °C (min) |
|---|---|---|---|---|
| POM | 3.49 ± 0.2 | 4.45 ± 0.2 | 5.45 ± 0.1 | 7.74 ± 0.2 |
| POM-1 | 3.28 ± 0.1 | 3.76 ± 0.1 | 4.81 ± 0.1 | 6.41 ± 0.1 |
| POM-2 | 2.74 ± 0.2 | 3.37 ± 0.1 | 4.23 ± 0.2 | 5.96 ± 0.1 |
| POM-3 | 2.53 ± 0.1 | 3.17 ± 0.1 | 3.86 ± 0.1 | 5.49 ± 0.2 |
| POM-4 | 2.78 ± 0.2 | 3.78 ± 0.2 | 4.89 ± 0.2 | 6.21 ± 0.2 |
| Samples | Tcry (°C) | t0.5 (min) | n | τ0.5 (min−1) | k × 10−3 (min−n) a | k × 10−3 (min−n) b |
|---|---|---|---|---|---|---|
| POM | 130 | 1.614 ± 0.02 | 2.483 ± 0.03 | 0.619 ± 0.01 | 201.93 ± 3.2 | 207.15 ± 2.2 |
| 135 | 1.971 ± 0.01 | 2.491 ± 0.02 | 0.507 ± 0.02 | 109.76 ± 4.1 | 109.29 ± 3.1 | |
| 140 | 2.453 ± 0.01 | 2.630 ± 0.01 | 0.408 ± 0.03 | 51.31 ± 3.4 | 53.83 ± 2.5 | |
| 145 | 3.426 ± 0.03 | 2.621 ± 0.02 | 0.291 ± 0.01 | 23.36 ± 3.8 | 24.30 ± 2.8 | |
| POM-1 | 130 | 1.611 ± 0.02 | 2.611 ± 0.03 | 0.621 ± 0.02 | 212.34 ± 2.9 | 216.58 ± 3.2 |
| 135 | 1.965 ± 0.02 | 2.731 ± 0.02 | 0.508 ± 0.02 | 127.58 ± 2.1 | 119.58 ± 3.3 | |
| 140 | 2.388 ± 0.01 | 2.842 ± 0.02 | 0.418 ± 0.01 | 65.41 ± 2.5 | 69.45 ± 3.4 | |
| 145 | 2.766 ± 0.01 | 2.813 ± 0.02 | 0.362 ± 0.02 | 32.08 ± 1.9 | 35.67 ± 3.5 | |
| POM-2 | 130 | 1.514 ± 0.01 | 2.804 ± 0.02 | 0.661 ± 0.03 | 234.12 ± 2.5 | 231.96 ± 3.4 |
| 135 | 1.722 ± 0.01 | 2.821 ± 0.03 | 0.581 ± 0.02 | 159.05 ± 3.1 | 154.03 ± 2.8 | |
| 140 | 2.079 ± 0.01 | 2.864 ± 0.01 | 0.481 ± 0.02 | 82.89 ± 3.2 | 81.13 ± 2.8 | |
| 145 | 2.513 ± 0.02 | 2.834 ± 0.02 | 0.397 ± 0.01 | 44.71 ± 2.8 | 47.77 ± 2.6 | |
| POM-3 | 130 | 1.384 ± 0.01 | 2.846 ± 0.02 | 0.723 ± 0.02 | 241.71 ± 2.9 | 245.94 ± 3.1 |
| 135 | 1.591 ± 0.02 | 2.906 ± 0.01 | 0.628 ± 0.02 | 169.74 ± 3.6 | 171.48 ± 3.0 | |
| 140 | 1.988 ± 0.02 | 2.921 ± 0.03 | 0.503 ± 0.03 | 83.76 ± 3.1 | 85.14 ± 2.9 | |
| 145 | 2.464 ± 0.01 | 2.913 ± 0.01 | 0.406 ± 0.01 | 49.89 ± 2.8 | 50.61 ± 3.5 | |
| POM-4 | 130 | 1.438 ± 0.01 | 2.751 ± 0.03 | 0.695 ± 0.02 | 218.71 ± 2.8 | 224.71 ± 2.2 |
| 135 | 1.812 ± 0.01 | 2.857 ± 0.02 | 0.552 ± 0.02 | 143.63 ± 2.6 | 146.09 ± 3.1 | |
| 140 | 2.274 ± 0.02 | 2.868 ± 0.02 | 0.439 ± 0.02 | 68.21 ± 2.9 | 71.11 ± 3.3 | |
| 145 | 2.574 ± 0.01 | 2.837 ± 0.01 | 0.388 ± 0.01 | 38.98 ± 3.0 | 41.33 ± 3.4 |
| Samples | POM | POM-1 | POM-2 | POM-3 | POM-4 |
|---|---|---|---|---|---|
| Kg (×105 K2) | 2.48 ± 0.02 | 2.23 ± 0.03 | 2.15 ± 0.05 | 2.11 ± 0.06 | 2.27 ± 0.04 |
| δe (J/m2) | 1.02 ± 0.03 | 0.92 ± 0.03 | 0.88 ± 0.01 | 0.87 ± 0.02 | 0.93 ± 0.01 |
| N (/m3) | (5.63 ± 0.2) × 1010 | (1.29 ± 0.3) × 1011 | (5.71 ± 0.1) × 1011 | (2.16 ± 0.3) × 1012 | (9.15 ± 0.2) × 1011 |
| Samples | POM | POM-1 | POM-2 | POM-3 | POM-4 |
|---|---|---|---|---|---|
| Activation energy ∆E (kJ/mol) | −65.14 ± 0.12 | −70.81 ± 0.20 | −74.14 ± 0.19 | −74.21 ± 0.17 | −74.39 ± 0.14 |
| r | 0.997 | 0.996 | 0.998 | 0.998 | 0.997 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, Y.; Liu, Y.; Zhang, X.; Wang, L.; Huang, H.; Liu, Y.; Qi, G.; Min, M.; Li, Y. Effect of Silver Nanoparticles on the Melting Behavior, Isothermal Crystallization Kinetics and Morphology of Polyoxymethylene. Crystals 2020, 10, 594. https://doi.org/10.3390/cryst10070594
Zeng Y, Liu Y, Zhang X, Wang L, Huang H, Liu Y, Qi G, Min M, Li Y. Effect of Silver Nanoparticles on the Melting Behavior, Isothermal Crystallization Kinetics and Morphology of Polyoxymethylene. Crystals. 2020; 10(7):594. https://doi.org/10.3390/cryst10070594
Chicago/Turabian StyleZeng, Yicheng, Yang Liu, Xun Zhang, Lumin Wang, Hongliang Huang, Yongli Liu, Guangrui Qi, Minghua Min, and Ying Li. 2020. "Effect of Silver Nanoparticles on the Melting Behavior, Isothermal Crystallization Kinetics and Morphology of Polyoxymethylene" Crystals 10, no. 7: 594. https://doi.org/10.3390/cryst10070594
APA StyleZeng, Y., Liu, Y., Zhang, X., Wang, L., Huang, H., Liu, Y., Qi, G., Min, M., & Li, Y. (2020). Effect of Silver Nanoparticles on the Melting Behavior, Isothermal Crystallization Kinetics and Morphology of Polyoxymethylene. Crystals, 10(7), 594. https://doi.org/10.3390/cryst10070594






