Chemical Vapor Deposition of IrTe2 Thin Films
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. CVD Growth of IrTe2 Films
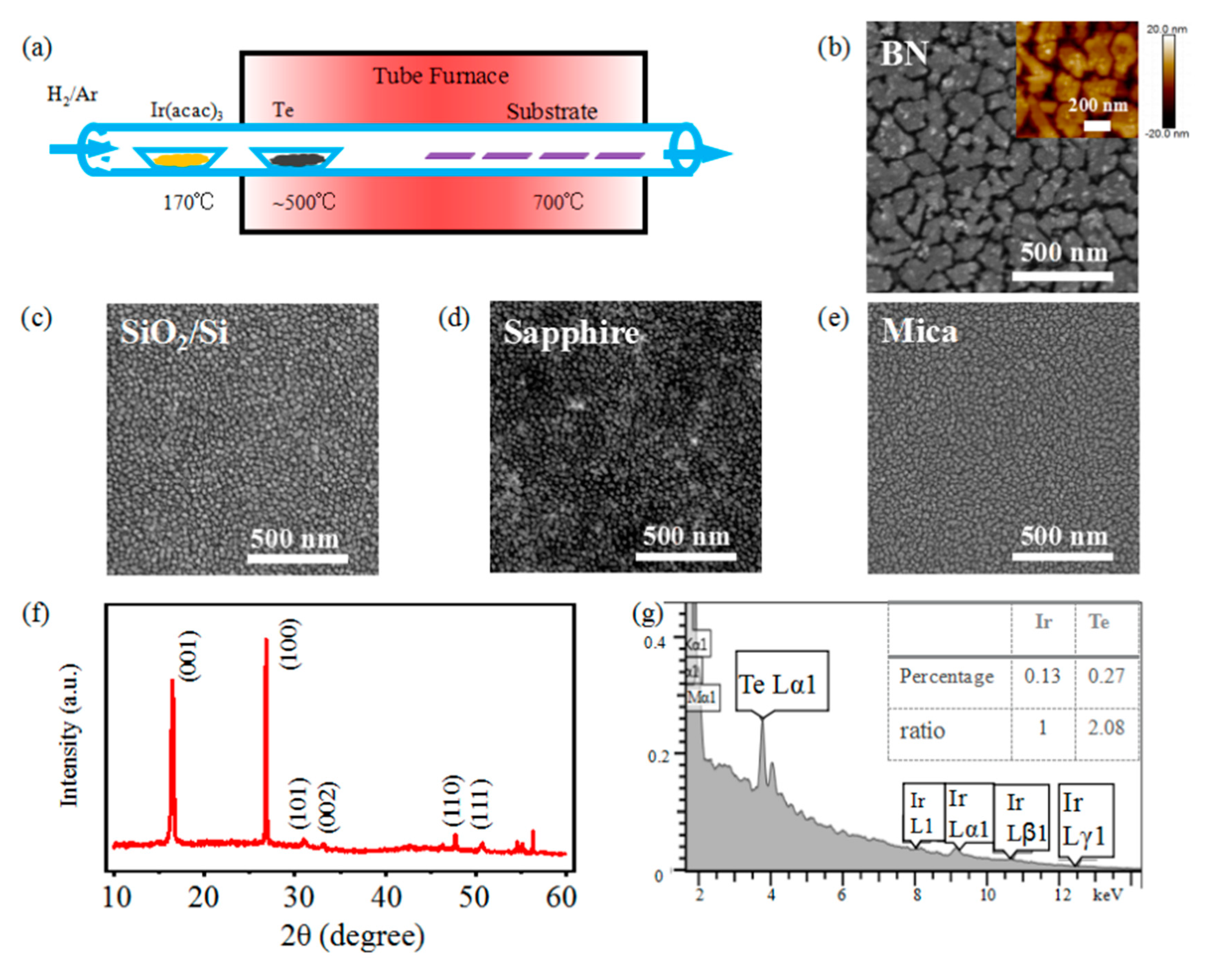
2.2.1. IrTe2 Growth Using Ir(acac)3 Precursor
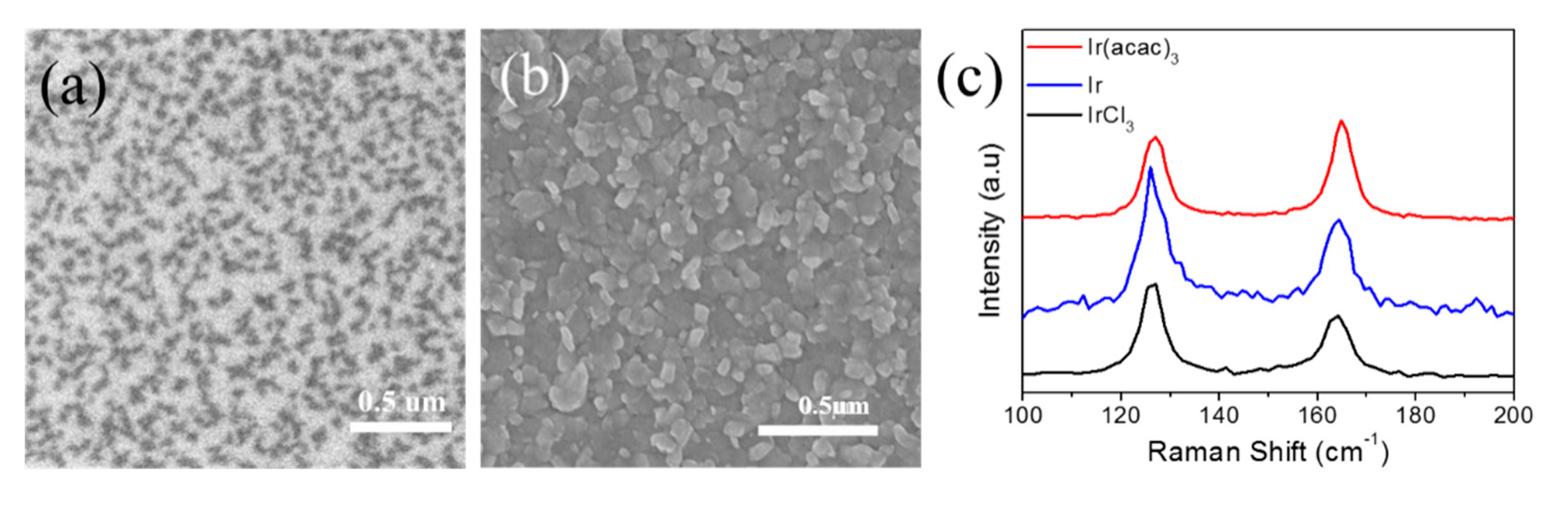
2.2.2. IrTe2 Growth Using IrCl3 Precursor
2.2.3. IrTe2 Growth Using Element Ir as the Precursor
2.3. Characterization
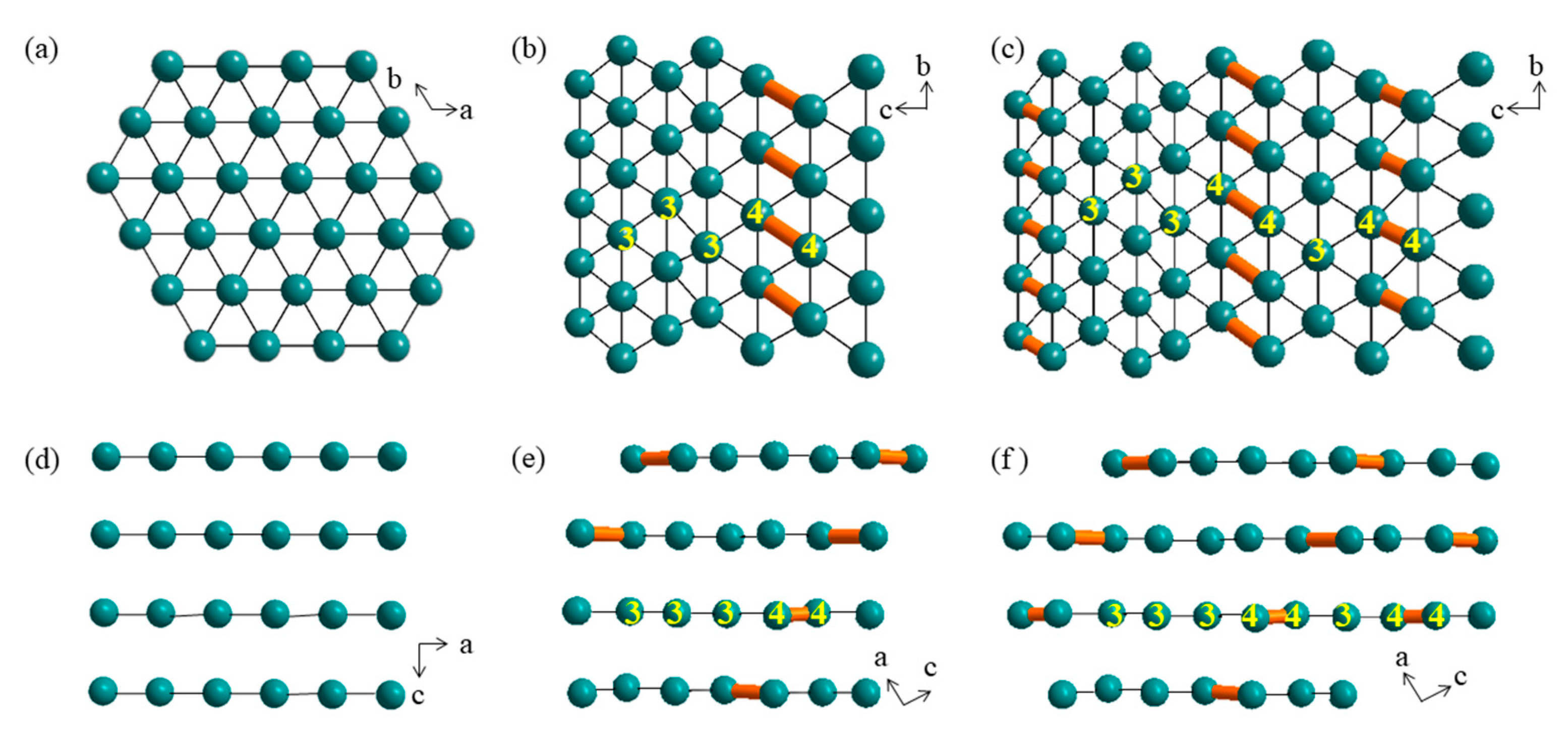
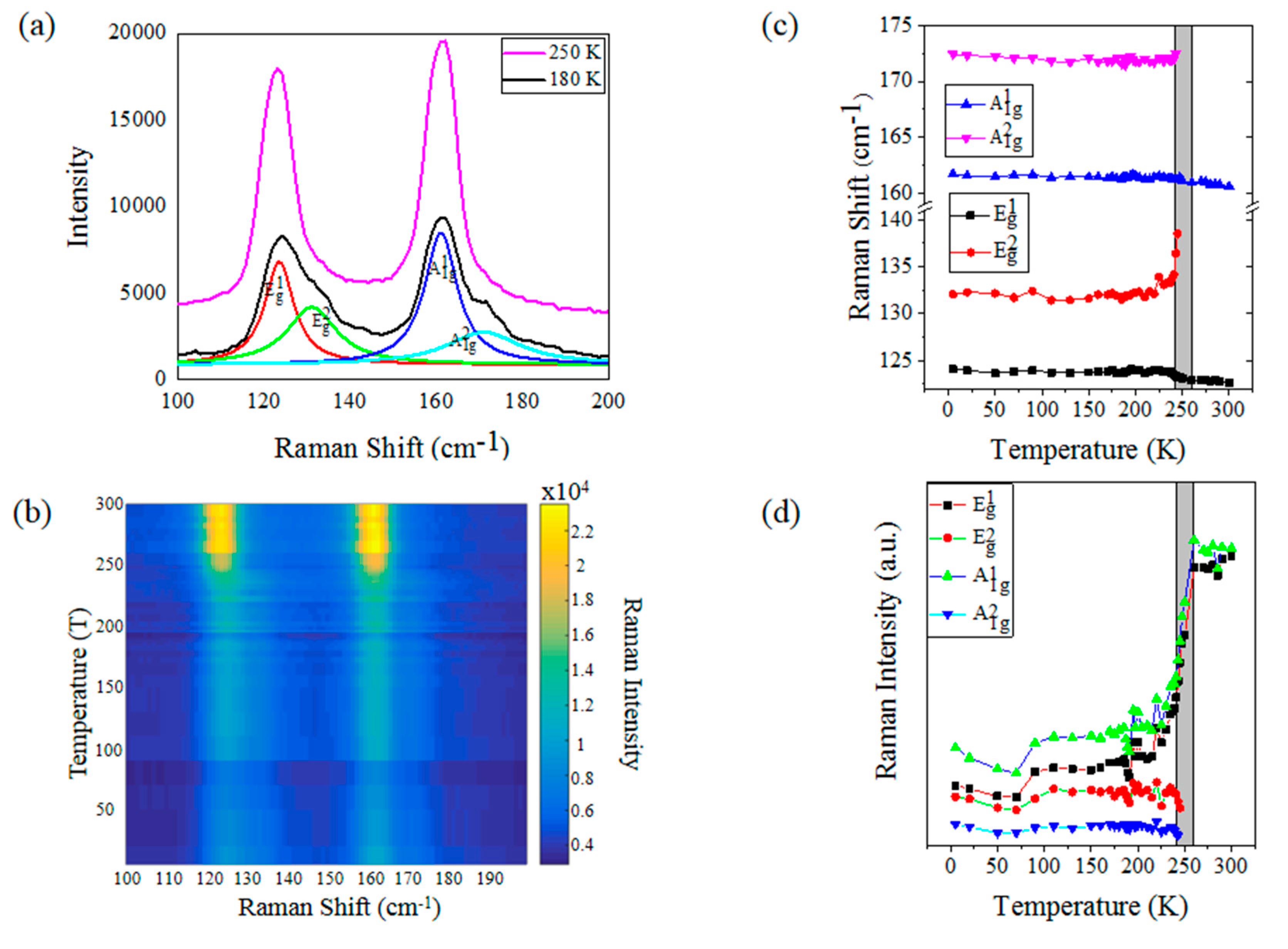
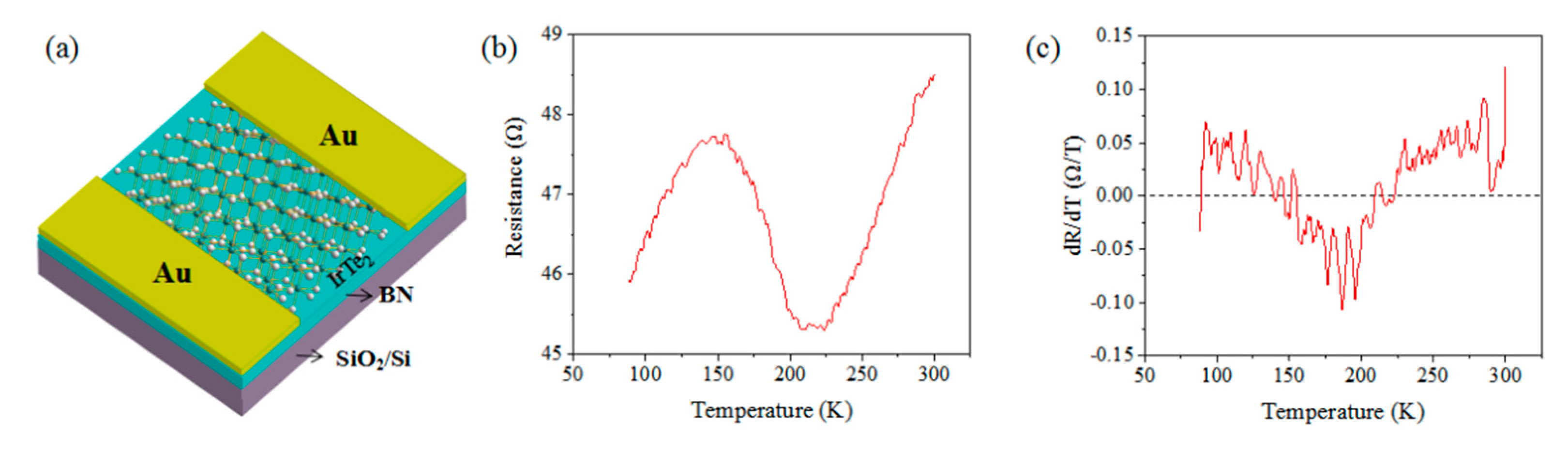
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Pyon, S.; Kudo, K.; Nohara, M. Superconductivity Induced by Bond Breaking in the Triangular Lattice of IrTe2. J. Phys. Soc. Jpn. 2012, 81, 053701. [Google Scholar] [CrossRef]
- Kiswandhi, A.; Brooks, J.S.; Cao, H.B.; Yan, J.Q.; Mandrus, D.; Jiang, Z.; Zhou, H.D. Competition between the structural phase transition and superconductivity in Ir1−xPtxTe2 as revealed by pressure effects. Phys. Rev. B 2013, 87, 121107. [Google Scholar] [CrossRef]
- Oh, Y.S.; Yang, J.J.; Horibe, Y.; Cheong, S.W. Anionic Depolymerization Transition in IrTe2. Phys. Rev. Lett. 2013, 110, 127209. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Kim, S.; Min, B.I. Optical and transport properties and the structural identification of IrTe2. Phys. Rev. B 2014, 90, 195136. [Google Scholar] [CrossRef]
- Wen, W.; Dang, C.H.; Xie, L.M. Photoinduced phase transitions in two-dimensional charge-density-wave 1T-TaS2. Chin. Phys. B 2019, 28, 058504. [Google Scholar] [CrossRef]
- Ootsuki, D.; Wakisaka, Y.; Pyon, S.; Kudo, K.; Nohara, M.; Arita, M.; Anzai, H.; Namatame, H.; Taniguchi, M.; Saini, N.L.; et al. Orbital degeneracy and Peierls instability in the triangular-lattice superconductor Ir1−xPtxTe2. Phys. Rev. B 2012, 86, 014519. [Google Scholar] [CrossRef]
- Ma, L.; Ye, C.; Yu, Y.; Lu, X.F.; Niu, X.; Kim, S.; Feng, D.; Tomanek, D.; Son, Y.-W.; Chen, X.H.; et al. A metallic mosaic phase and the origin of Mott-insulating state in 1T-TaS2. Nat. Commun. 2016, 7, 10956. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.X.; Debnath, B.; Pope, T.R.; Salguero, T.T.; Lake, R.K.; Balandin, A.A. A charge-density-wave oscillator based on an integrated tantalum disulfide-boron nitride-graphene device operating at room temperature. Nat. Nanotech. 2016, 11, 844–850. [Google Scholar] [CrossRef]
- Hor, Y.S.; Williams, A.J.; Checkelsky, J.G.; Roushan, P.; Seo, J.; Xu, Q.; Zandbergen, H.W.; Yazdani, A.; Ong, N.P.; Cava, R.J. Superconductivity in CuxBi2Se3 and its Implications for Pairing in the Undoped Topological Insulator. Phys. Rev. Lett. 2010, 104, 057001. [Google Scholar] [CrossRef]
- Ko, K.T.; Lee, H.H.; Kim, D.H.; Yang, J.J.; Cheong, S.W.; Eom, M.J.; Kim, J.S.; Gammag, R.; Kim, K.S.; Kim, H.S.; et al. Charge-ordering cascade with spin-orbit Mott dimer states in metallic iridium ditelluride. Nat. Commun. 2015, 6, 7342. [Google Scholar] [CrossRef]
- Eom, M.J.; Kim, K.; Jo, Y.J.; Yang, J.J.; Choi, E.S.; Min, B.I.; Park, J.H.; Cheong, S.W.; Kim, J.S. Dimerization-Induced Fermi-Surface Reconstruction in IrTe2. Phys. Rev. Lett. 2014, 113, 266406. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, N.; Taniguchi, K.; Endoh, R.; Takano, H.; Nagata, S. Resistance and Susceptibility Anomalies in IrTe2 and CuIr2Te4. J. Low Temp. Phys. 1999, 117, 1129–1133. [Google Scholar] [CrossRef]
- Ivashko, O.; Yang, L.; Destraz, D.; Martino, E.; Chen, Y.; Guo, C.Y.; Yuan, H.Q.; Pisoni, A.; Matus, P.; Pyon, S.; et al. Charge-stripe order and superconductivity in Ir(1−x)PtxTe(2). Sci. Rep. 2017, 7, 17157. [Google Scholar] [CrossRef]
- Yoshida, M.; Kudo, K.; Nohara, M.; Iwasa, Y. Metastable superconductivity in two-dimensional IrTe2 crystals. Nano Lett. 2018, 18, 3113–3117. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.J.; Choi, Y.J.; Oh, Y.S.; Hogan, A.; Horibe, Y.; Kim, K.; Min, B.I.; Cheong, S.W. Charge-Orbital Density Wave and Superconductivity in the Strong Spin-Orbit Coupled IrTe2:Pd. Phys. Rev. Lett. 2012, 108, 116402. [Google Scholar] [CrossRef]
- Fang, A.F.; Xu, G.; Dong, T.; Zheng, P.; Wang, N.L. Structural phase transition in IrTe2: A combined study of optical spectroscopy and band structure calculations. Sci. Rep. 2013, 3, 1153. [Google Scholar] [CrossRef]
- Liu, Y.; Lei, H.; Wang, K.; Abeykoon, M.; Warren, J.B.; Bozin, E.; Petrovic, C. Thermoelectric studies of Ir1-xRhxTe2 (0 ≤ x ≤ 0.3). Phys. Rev. B 2018, 98, 094519. [Google Scholar] [CrossRef]
- Li, X.; Yan, J.Q.; Singh, D.J.; Goodenough, J.B.; Zhou, J.S. Synthesis of monoclinic IrTe2 under high pressure and its physical properties. Phys. Rev. B 2015, 92, 155118. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, Z.; Dong, J.; Yi, D.; Niu, J.; Wu, M.; Lin, L.; Yin, R.; Li, M.; Zhou, J.; et al. Ultrafast epitaxial growth of metre-sized single-crystal graphene on industrial Cu foil. Sci. Bull. 2017, 62, 1074–1080. [Google Scholar] [CrossRef]
- Van Luan, N.; Shin, B.G.; Dinh Loc, D.; Kim, S.T.; Perello, D.; Lim, Y.J.; Yuan, Q.H.; Ding, F.; Jeong, H.Y.; Shin, H.S.; et al. Seamless Stitching of Graphene Domains on Polished Copper (111) Foil. Adv. Mater. 2015, 27, 1376–1382. [Google Scholar] [CrossRef]
- Zhang, M.; Zhu, Y.; Wang, X.; Feng, Q.; Qiao, S.; Wen, W.; Chen, Y.; Cui, M.; Zhang, J.; Cai, C.; et al. Controlled Synthesis of ZrS2 Mono layer and Few Layers on Hexagonal Boron Nitride. J. Am. Chem. Soc. 2015, 137, 7051–7054. [Google Scholar] [CrossRef] [PubMed]
- Qian, Z.Y.; Jiao, L.Y.; Xie, L.M. Phase Engineering of Two-Dimensional Transition Metal Dichalcogenides. Chin. J. Chem. 2020, 38, 753–760. [Google Scholar] [CrossRef]
- Najmaei, S.; Liu, Z.; Zhou, W.; Zou, X.; Shi, G.; Lei, S.; Yakobson, B.I.; Idrobo, J.-C.; Ajayan, P.M.; Lou, J. Vapour phase growth and grain boundary structure of molybdenum disulphide atomic layers. Nat. Mater. 2013, 12, 754–759. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wang, L.; Qi, J.; Liu, K. Designed Growth of Large-Size 2D Single Crystals. Adv. Mater. 2020, 32, 2000046. [Google Scholar] [CrossRef] [PubMed]
- Yan, A.; Velasco, J.; Kahn, S.; Watanabe, K.; Taniguchi, T.; Wang, F.; Crommie, M.F.; Zettl, A. Direct growth of single- and few-layer MoS2 on h-BN with preferred relative rotation angles. Nano Lett. 2015, 15, 6324–6331. [Google Scholar] [CrossRef] [PubMed]
- Okada, M.; Sawazaki, T.; Watanabe, K.; Taniguch, T.; Hibino, H.; Shinohara, H.; Kitaura, R. Direct Chemical Vapor Deposition Growth of WS2 Atomic Layers on Hexagonal Boron Nitride. ACS Nano 2014, 8, 8273–8277. [Google Scholar] [CrossRef]
- Zhang, L.; Zhu, X.; Ling, L.; Zhang, C.; Pi, L.; Zhang, Y. Lattice dynamics study of the structural transition in IrTe2. Philos. Mag. 2014, 94, 439–446. [Google Scholar] [CrossRef]
- Glamazda, A.; Choi, K.Y.; Lemmens, P.; Yang, J.J.; Cheong, S.W. Proximity to a commensurate charge modulation in IrTe2−xSex (x = 0 and 0.45) revealed by Raman spectroscopy. New J. Phys. 2014, 16, 093061. [Google Scholar] [CrossRef]
- Lazarevic, N.; Bozin, E.S.; Scepanovic, M.; Opacic, M.; Lei, H.C.; Petrovic, C.; Popovic, Z.V. Probing IrTe2 crystal symmetry by polarized Raman scattering. Phys. Rev. B 2014, 89, 224301. [Google Scholar] [CrossRef]
- Pascut, G.L.; Haule, K.; Gutmann, M.J.; Barnett, S.A.; Kiryukhin, V. Dimerization-Induced Cross-Layer Quasi-Two-Dimensionality in Metallic IrTe2. Phys. Rev. Lett. 2014, 112, 086402. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, R.; Zhao, Z.; Wu, J.; Xie, L. Chemical Vapor Deposition of IrTe2 Thin Films. Crystals 2020, 10, 575. https://doi.org/10.3390/cryst10070575
Zhou R, Zhao Z, Wu J, Xie L. Chemical Vapor Deposition of IrTe2 Thin Films. Crystals. 2020; 10(7):575. https://doi.org/10.3390/cryst10070575
Chicago/Turabian StyleZhou, Rui, Zhaoyang Zhao, Juanxia Wu, and Liming Xie. 2020. "Chemical Vapor Deposition of IrTe2 Thin Films" Crystals 10, no. 7: 575. https://doi.org/10.3390/cryst10070575
APA StyleZhou, R., Zhao, Z., Wu, J., & Xie, L. (2020). Chemical Vapor Deposition of IrTe2 Thin Films. Crystals, 10(7), 575. https://doi.org/10.3390/cryst10070575




