The Dynamic State of a Pseudo-Crystalline Structure of B42 Molecules
Abstract
1. Introduction
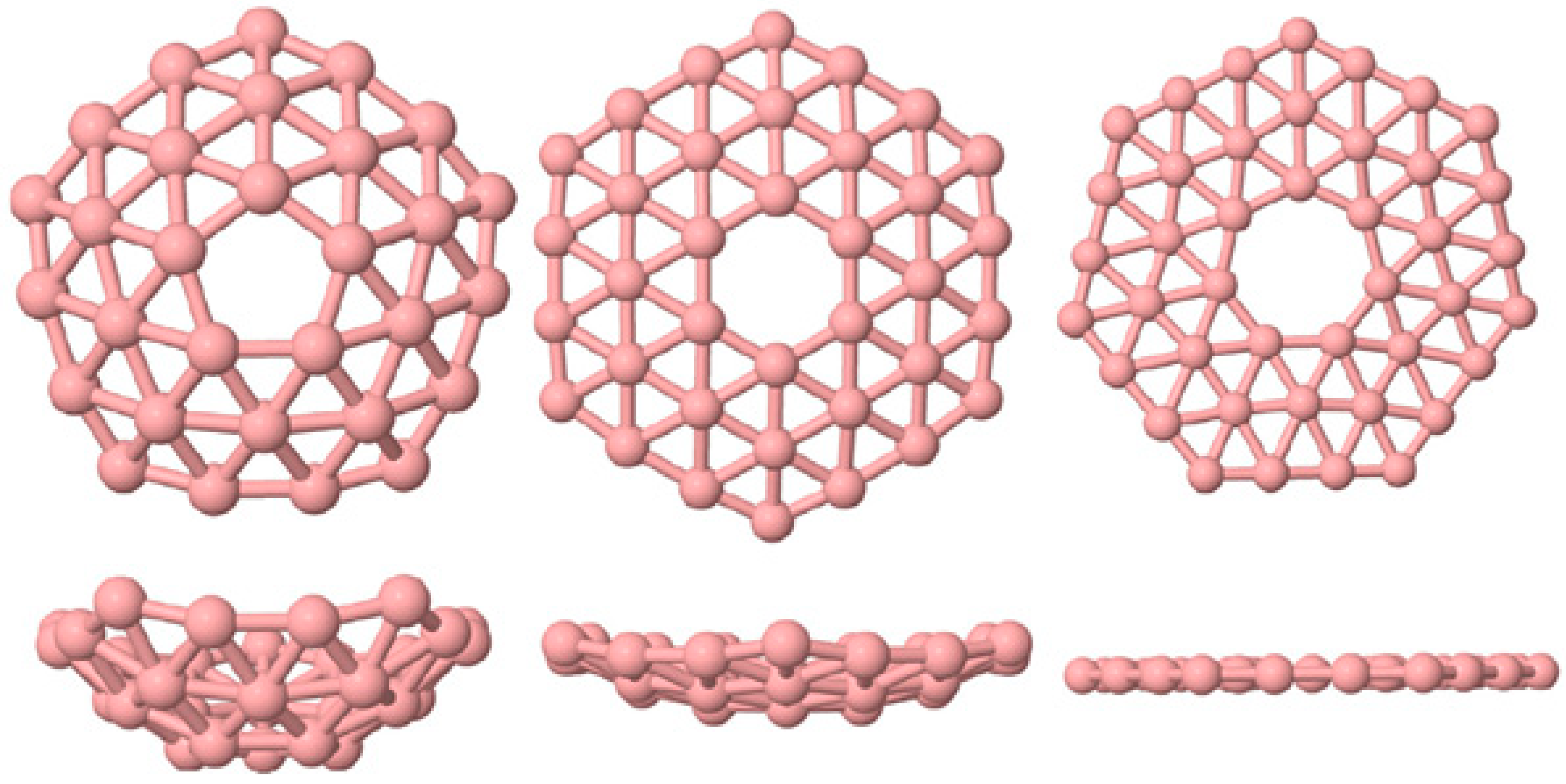
2. Materials and Methods
Algorithm Testing
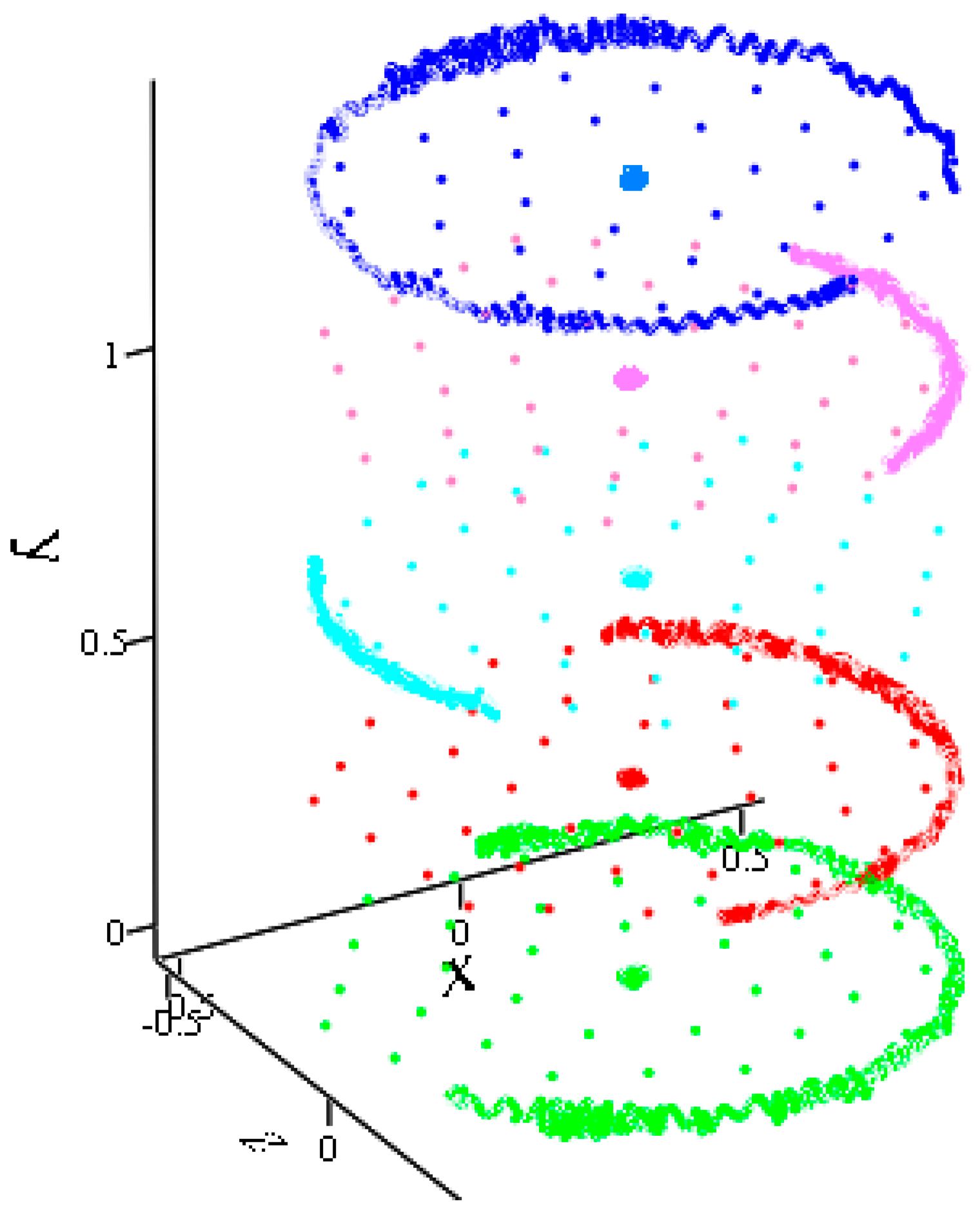
- Inertia rotation of the free disk B42 in three different planes of the coordinate space. If there are no torques, then the initial speed remains constant throughout the movement.
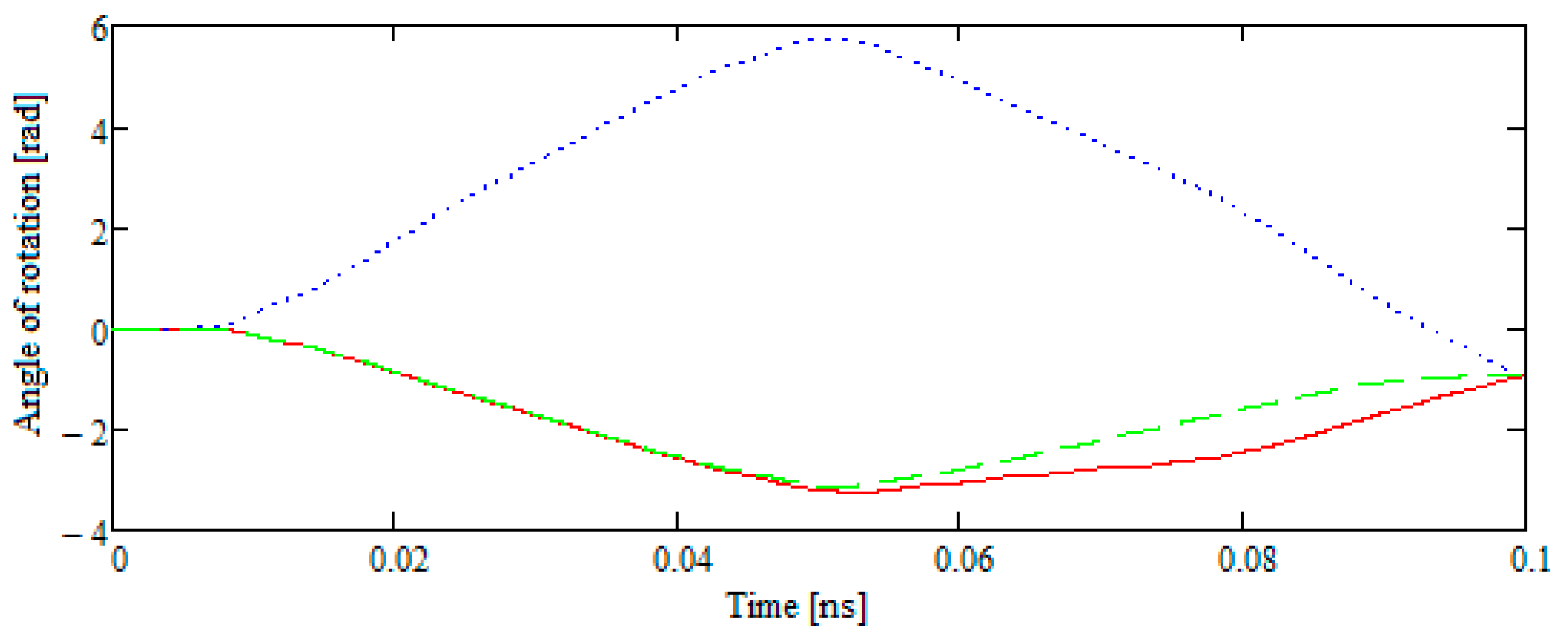
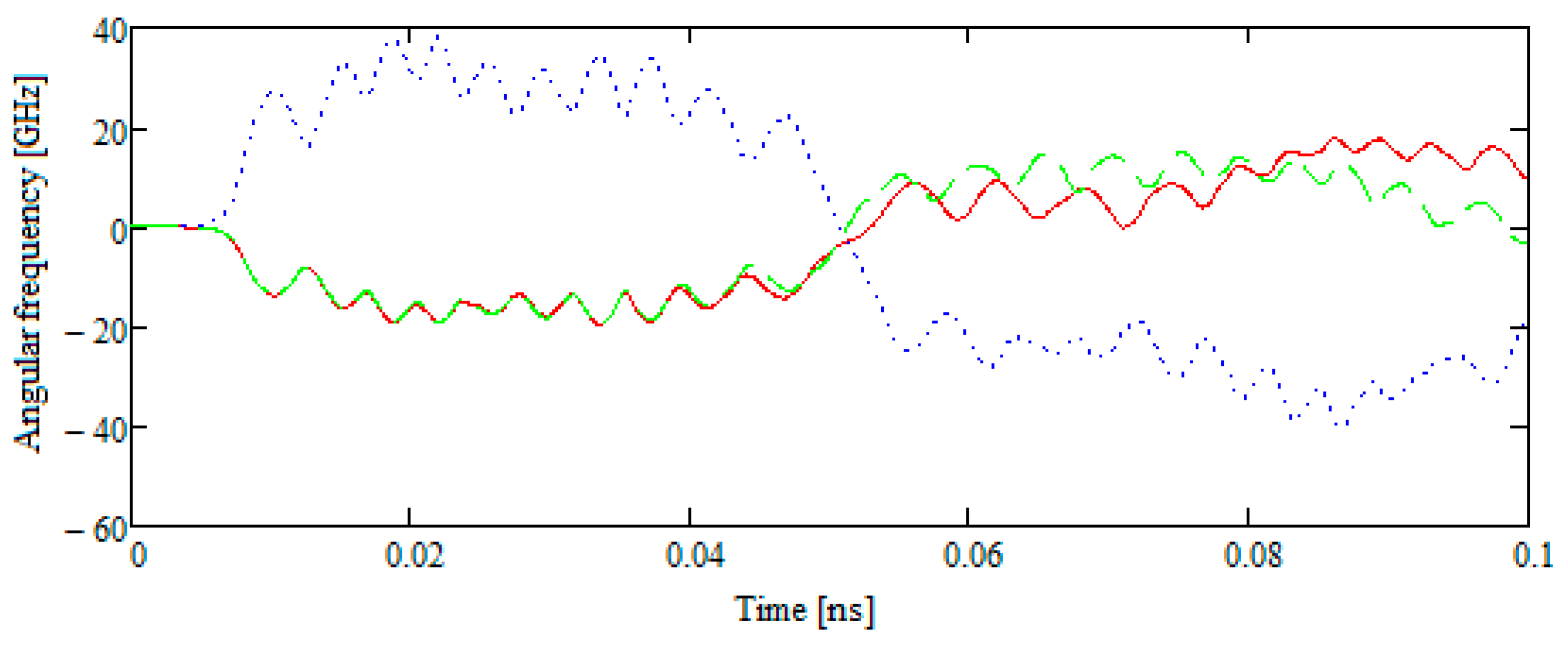
- Let the velocity vector of the test molecule representing the external action be in the plane of the disk B42 and not directed to its center of mass, which has a fixed position. Then, at the particle approaching site, the disk rotates at a certain angle, and, approaching according to the defined distance, the particle returns to its original position.
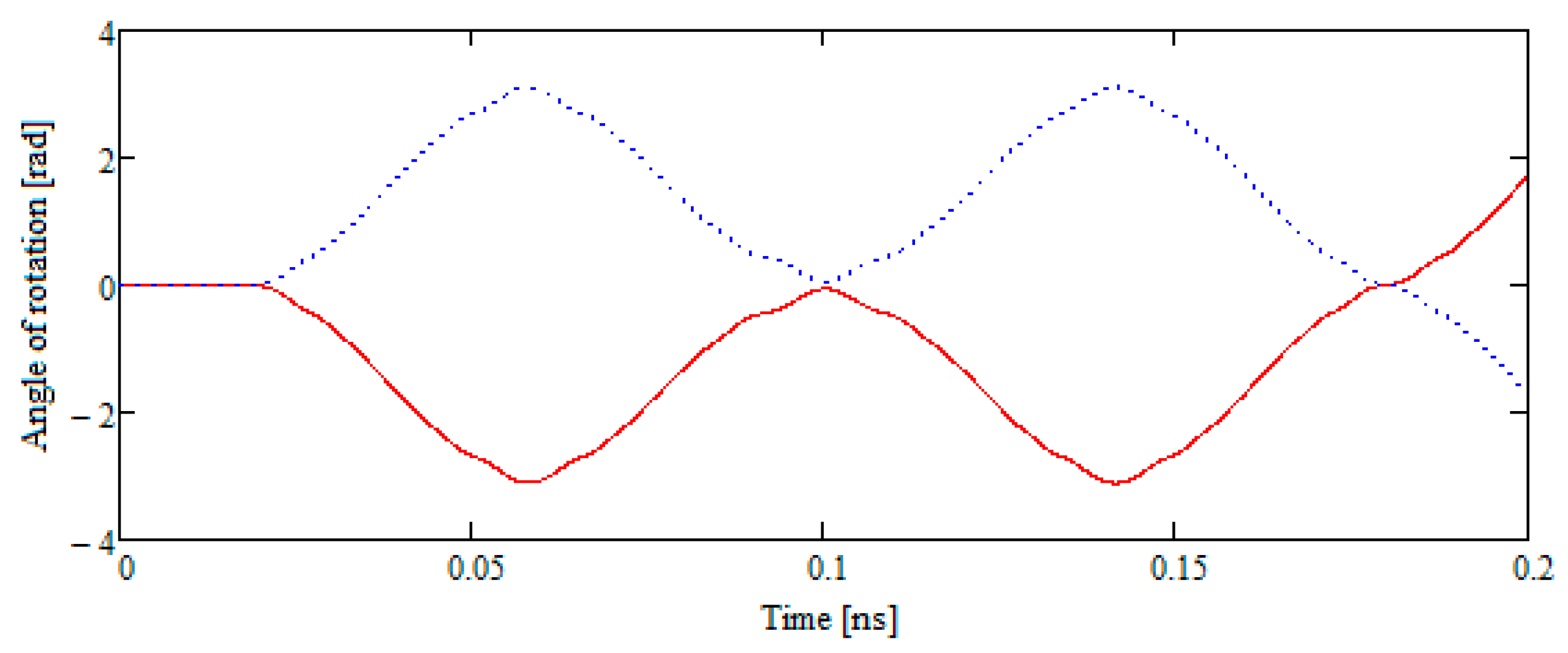
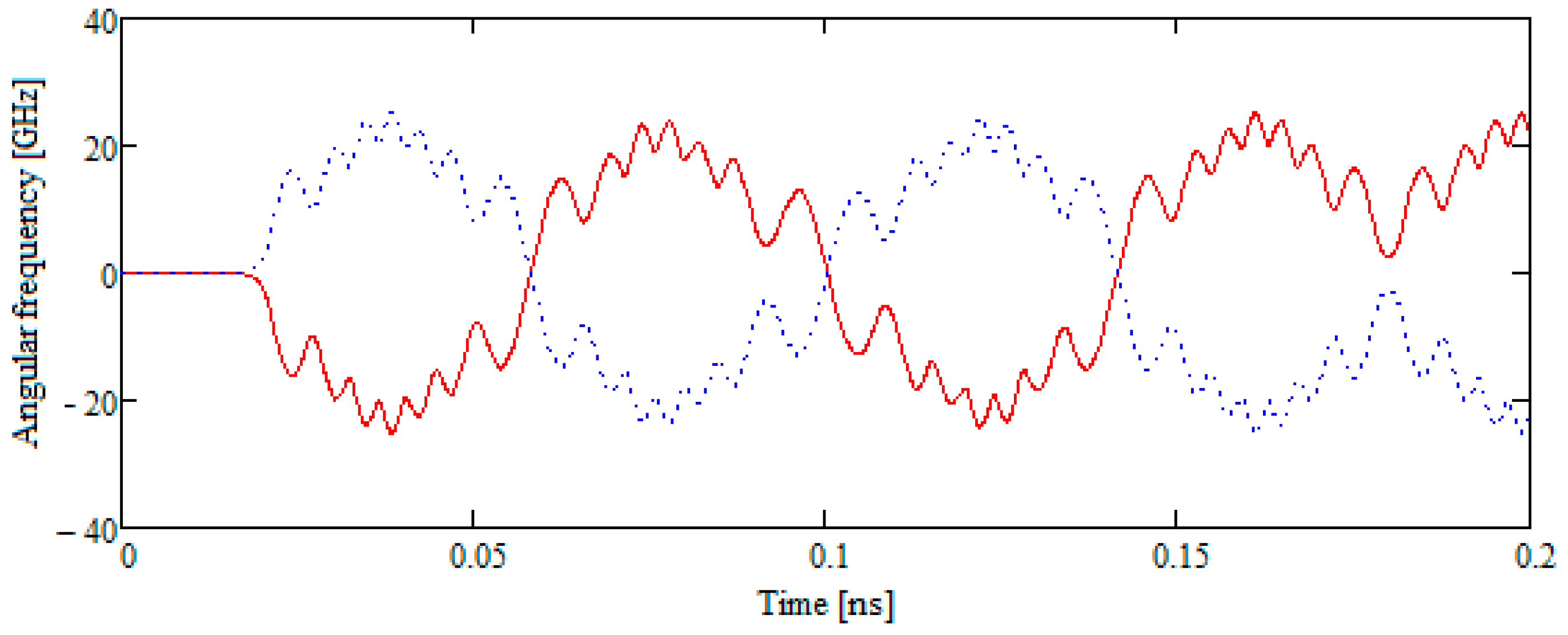
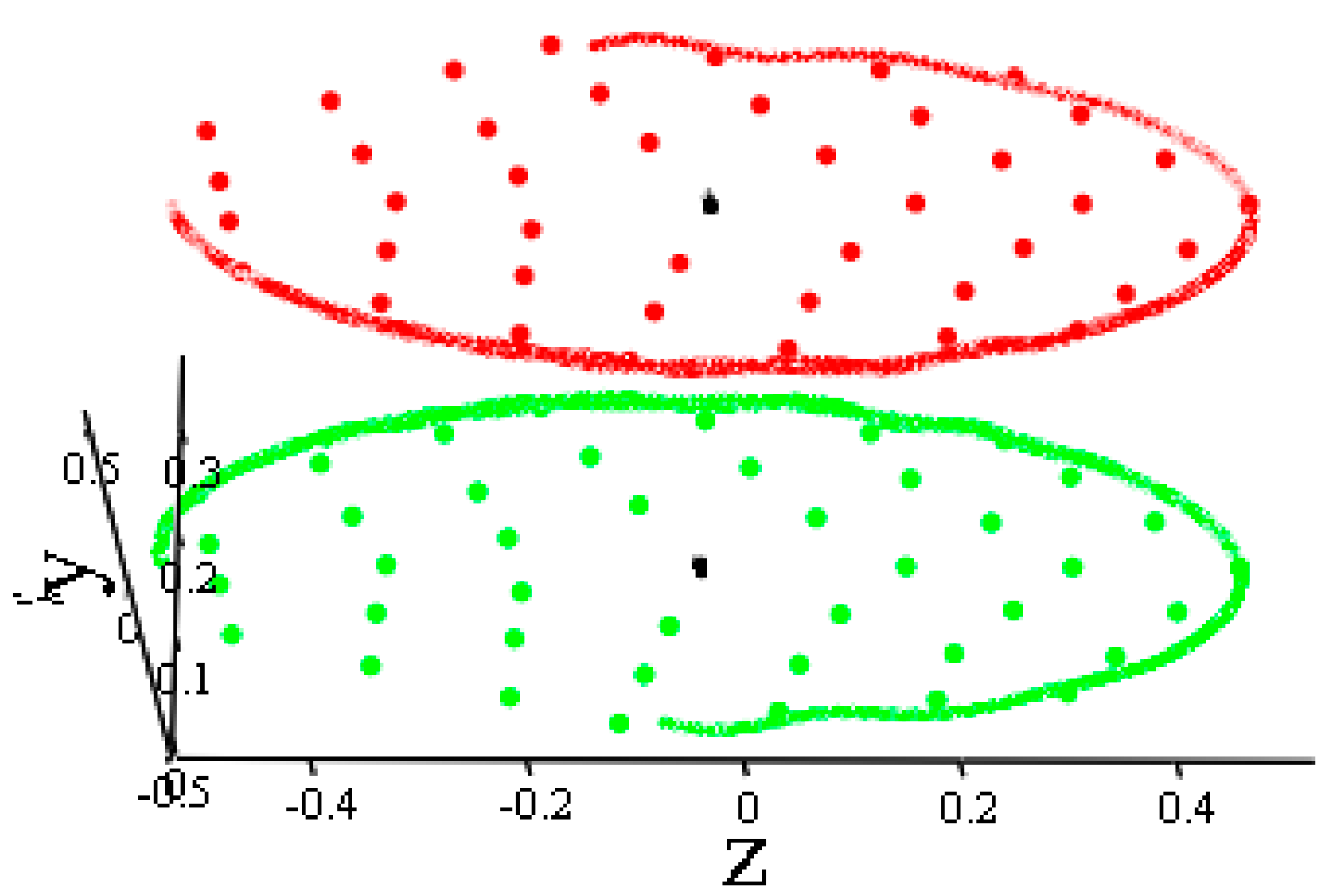
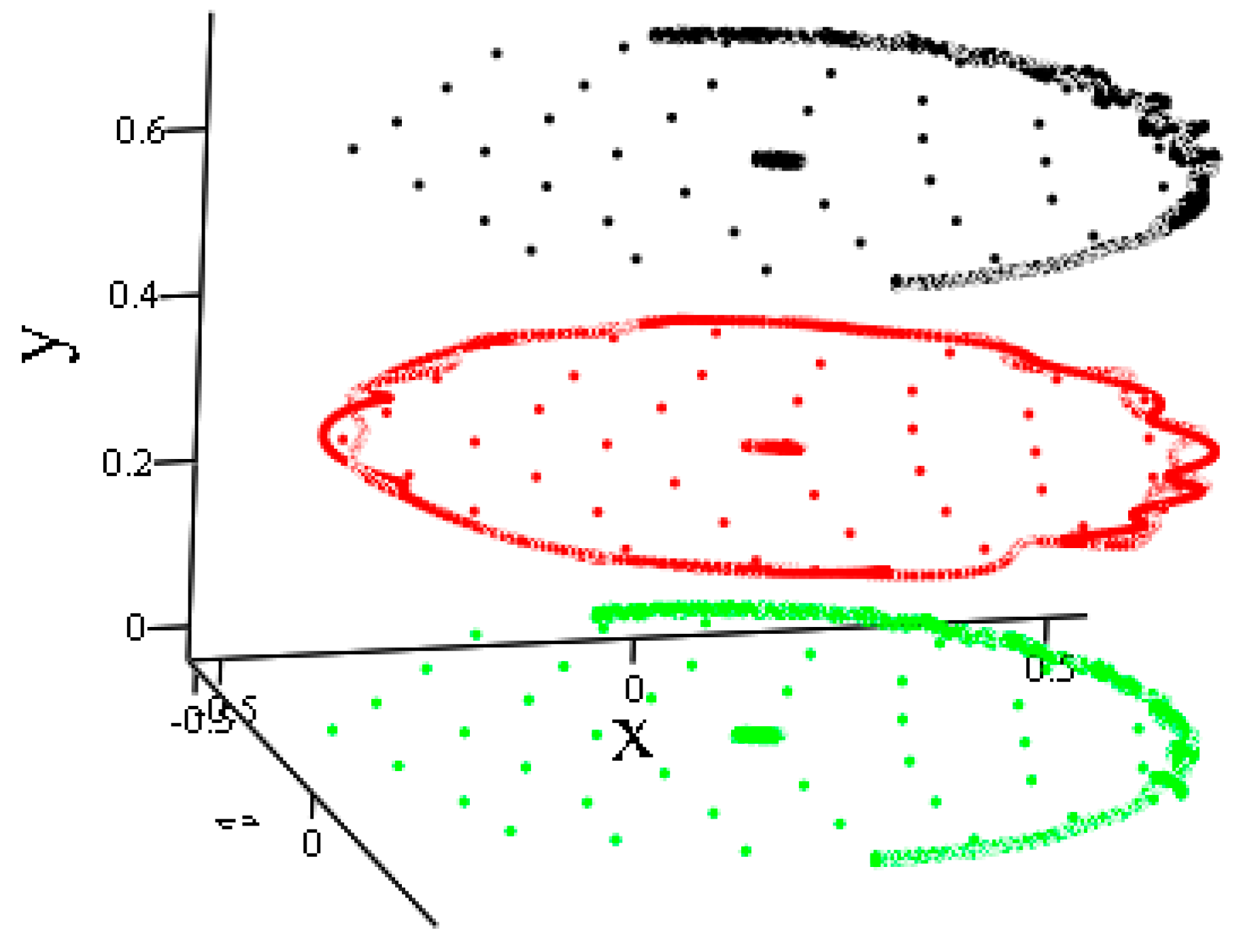
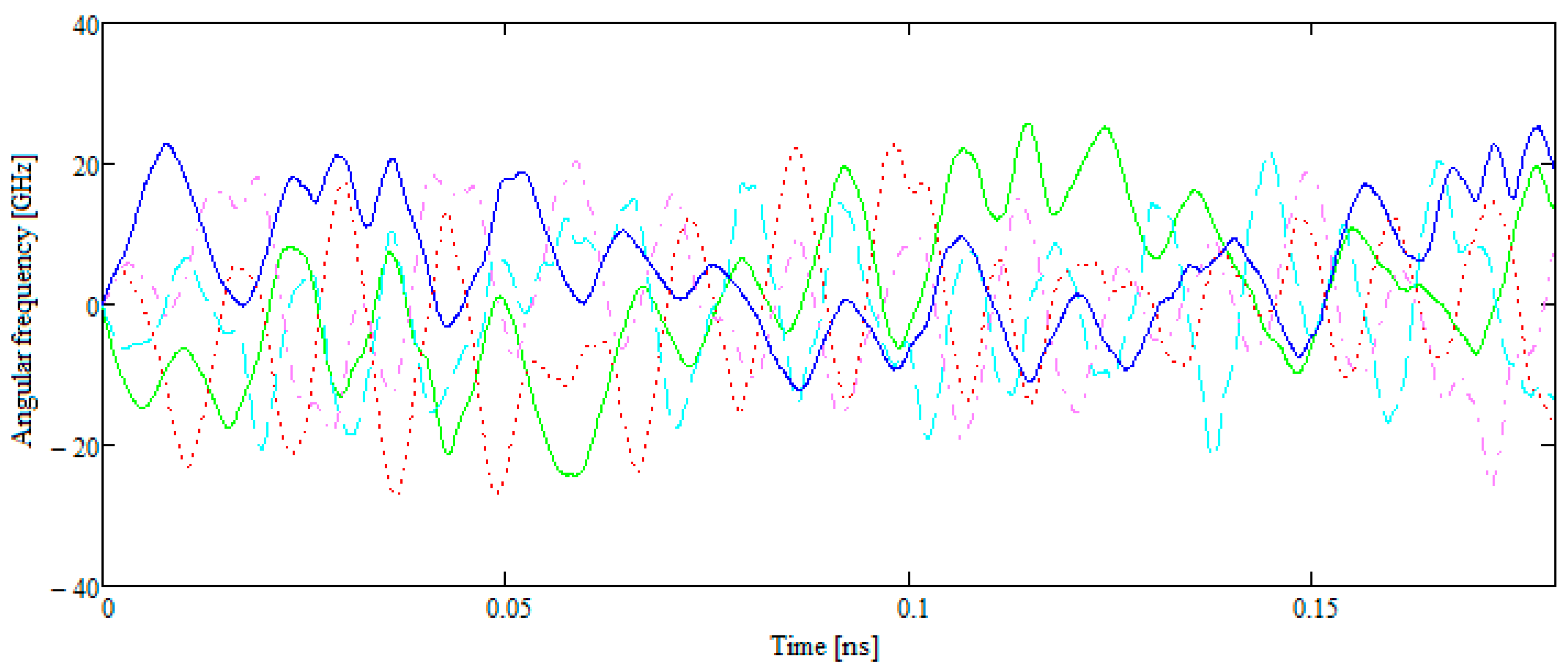
- Two B42 plates located in the same plane but separated by a sufficient distance begin to move towards each other. Subsequently, the movement remains flat. The total energy of the system remains constant throughout the movement. The kinetic energy at the beginning of the test is equal to the kinetic energy of two molecules when they are removed from each other.
3. Results
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hosmane, N.S. Boron Science, 1st ed.; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Hosmane, N.S.; Eagling, R. Handbook of Boron Science, 1st ed.; World Scientific Publishing Europe Ltd.: London, UK, 2018. [Google Scholar]
- Pham, H.T.; Duong, L.; Tam, N.M.; Pham-Ho, M.; Nguyen, M.T. The boron conundrum: Bonding in the bowl B30 and B36, fullerene B40 and triple ring B42 clusters. Chem. Phys. Lett. 2014, 608, 295–302. [Google Scholar] [CrossRef]
- Hu, T.; Mei, X.; Wang, Y.; Weng, X.; Liang, R.; Wei, M. Two-dimensional nanomaterials: Fascinating materials in biomedical field. Sci. Bull. 2019, 64, 1707–1727. [Google Scholar] [CrossRef]
- Wang, Y.; Qiu, M.; Won, M.; Jung, E.; Fan, T.; Xie, N.; Chi, S.-G.; Zhang, H.; Kim, J.S. Emerging 2D material-based nanocarrier for cancer therapy beyond graphene. Coord. Chem. Rev. 2019, 400, 213041. [Google Scholar] [CrossRef]
- Li, S.; Ma, L.; Zhou, M.; Li, Y.; Xia, Y.; Fan, X.; Cheng, C.; Luo, H. New opportunities for emerging 2D materials in bioelectronics and biosensors. Curr. Opin. Biomed. Eng. 2020, 13, 32–41. [Google Scholar] [CrossRef]
- Vatanparast, M.; Shariatinia, Z. Hexagonal boron nitride nanosheet as novel drug delivery system for anticancer drugs: Insights from DFT calculations and molecular dynamics simulations. J. Mol. Graph. Model. 2019, 89, 50–59. [Google Scholar] [CrossRef]
- Sun, T.; Zhang, G.; Xu, N.; Lian, X.; Li, H.; Chen, W.; Su, C. Defect chemistry in 2D materials for electrocatalysis. Mater. Today Energy 2019, 12, 215–238. [Google Scholar] [CrossRef]
- Muratore, C.; Voevodin, A.A.; Glavin, N.R. Physical vapor deposition of 2D Van der Waals materials: A review. Thin Solid Films 2019, 688, 137500. [Google Scholar] [CrossRef]
- Sharma, V.; Kagdada, H.L.; Jha, P.K.; Śpiewak, P.; Kurzydłowski, K.J. Thermal transport properties of boron nitride based materials: A review. Renew. Sustain. Energy Rev. 2020, 120, 109622. [Google Scholar] [CrossRef]
- Si, N.; Niu, T. Epitaxial growth of elemental 2D materials: What can we learn from the periodic table? Nano Today 2020, 30, 100805. [Google Scholar] [CrossRef]
- Yao, J.; Zheng, Z.; Yang, G. Production of large-area 2D materials for high-performance photodetectors by pulsed-laser deposition. Prog. Mater. Sci. 2019, 106, 100573. [Google Scholar] [CrossRef]
- Wu, L.; Gao, J.; Lu, X.; Huang, C.; Dhanjai; Chen, J. Graphdiyne: A new promising member of 2D all-carbon nanomaterial as robust electrochemical enzyme biosensor platform. Carbon 2020, 156, 568–575. [Google Scholar] [CrossRef]
- Guerra, T.; Leite, L.; Azevedo, S. Graphene monolayers and nanoribbons with controlled domain sizes of hexagonal boron nitride: An ab initio calculations. Solid State Commun. 2019, 289, 5–11. [Google Scholar] [CrossRef]
- Yin, J.; Yang, Z.; Bi, L.; Ren, S.; Yan, G.; Wang, Y.; Huang, X. Electronic and magnetic properties of Fe-doped narrow zigzag boron nitride nanoribbons. Mater. Today Commun. 2020, 22, 100753. [Google Scholar] [CrossRef]
- Eshkalak, K.E.; Sadeghzadeh, S.; Jalaly, M. Thermal resistance analysis of hybrid graphene-boron nitride nanosheets: The effect of geometry, temperature, size, strain and structural defects. Comput. Mater. Sci. 2020, 174, 109484. [Google Scholar] [CrossRef]
- Xiao, G.; Di, J.; Li, H.; Wang, J. Highly thermally conductive, ductile biomimetic boron nitride/aramid nanofiber composite film. Compos. Sci. Technol. 2020, 189, 108021. [Google Scholar] [CrossRef]
- Yin, X.; Kou, Z.; Wang, Z.; Liu, T.; Liang, A.; Yang, M.; Guan, S.; Zhang, Y.; Chen, S.; Jiang, M.; et al. Micro-sized polycrystalline cubic boron nitride with properties comparable to nanocrystalline counterparts. Ceram. Int. 2020, 46, 8806–8810. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, W.; Zhao, Z. Recent progress and remaining challenges of 2D material-based terahertz detectors. Infrared Phys. Technol. 2019, 102, 103024. [Google Scholar] [CrossRef]
- Zhang, S.; Legut, D.; Fu, Z.; Germann, T.C.; Zhang, R. High-throughput screening for superhard carbon and boron nitride allotropes with superior stiffness and strength. Carbon 2018, 137, 156–164. [Google Scholar] [CrossRef]
- Chauhan, N.P.S.; Hosmane, N.; Mozafari, M. Boron-based polymers: Opportunities and challenges. Mater. Today Chem. 2019, 14, 100184. [Google Scholar] [CrossRef]
- Korkmaz, M. Boron: Environmental Exposure and Human Health. In Encyclopedia of Environmental Health, 2nd ed.; Nriagu, J., Ed.; Elsevier: Amsterdam, The Netherlands, 2011; pp. 456–459. [Google Scholar]
- Yuan, W.; Wu, Y.; Wu, C.; Zhao, S.; Liu, X. Evolution of amorphous boron transformed into crystal nanospheres under electron beam irradiation. Results Phys. 2020, 16, 102841. [Google Scholar] [CrossRef]
- Rozas, S.; Alcalde, R.; Atilhan, M.; Aparicio, S. A theoretical study on the adsorption of acid gases by boron nitride-based nanomaterials. Appl. Surf. Sci. 2019, 480, 83–95. [Google Scholar] [CrossRef]
- Abbas, S.; Abbas, A.; Liu, Z.; Tang, C. The two-dimensional boron nitride hierarchical nanostructures: Controllable synthesis and superhydrophobicity. Mater. Chem. Phys. 2020, 240, 122145. [Google Scholar] [CrossRef]
- Muya, J.T.; Ramanantoanina, H.; Daul, C.; Nguyen, M.T.; Gopakumar, G.; Ceulemans, A. Jahn–Teller instability in cationic boron and carbon buckyballs B80+ and C60+: A comparative study. Phys. Chem. Chem. Phys. 2013, 15, 2829. [Google Scholar] [CrossRef] [PubMed]
- Gamage, G.A.; Chen, K.; Chen, G.; Tian, F.; Ren, Z. Effect of nucleation sites on the growth and quality of single-crystal boron arsenide. Mater. Today Phys. 2019, 11, 100160. [Google Scholar] [CrossRef]
- Chen, S.; Feng, F.; Yin, Y.; Lizo, X.; Ma, Z.-F. Plastic crystal polymer electrolytes containing boron based anion acceptors for room temperature all-solid-state sodium-ion batteries. Energy Storage Mater. 2019, 22, 57–65. [Google Scholar] [CrossRef]
- Gurubasavaraj, P.M.; Gao, S.; Hosmane, N. Boron. In Comprehensive Energy Systems, 1st ed.; Dincer, I., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; Volume 2, pp. 72–87. [Google Scholar]
- Johnson, R.D.; Yannoni, C.S.; Dorn, H.C.; Salem, J.R.; Bethune, D.S. C60 Rotation in the Solid State: Dynamics of a Faceted Spherical Top. Science 1992, 255, 1235–1238. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bubenchikov, A.; Bubenchikov, M.; Mamontov, D. The Dynamic State of a Pseudo-Crystalline Structure of B42 Molecules. Crystals 2020, 10, 510. https://doi.org/10.3390/cryst10060510
Bubenchikov A, Bubenchikov M, Mamontov D. The Dynamic State of a Pseudo-Crystalline Structure of B42 Molecules. Crystals. 2020; 10(6):510. https://doi.org/10.3390/cryst10060510
Chicago/Turabian StyleBubenchikov, Alexey, Mikhail Bubenchikov, and Dmitriy Mamontov. 2020. "The Dynamic State of a Pseudo-Crystalline Structure of B42 Molecules" Crystals 10, no. 6: 510. https://doi.org/10.3390/cryst10060510
APA StyleBubenchikov, A., Bubenchikov, M., & Mamontov, D. (2020). The Dynamic State of a Pseudo-Crystalline Structure of B42 Molecules. Crystals, 10(6), 510. https://doi.org/10.3390/cryst10060510




