Crystal Structure and Hirshfeld Surface Analysis of Bis(Triethanolamine)Nickel(II) Dinitrate Complex and a Revelation of Its Characteristics via Spectroscopic, Electrochemical and DFT Studies Towards a Promising Precursor for Metal Oxides Synthesis
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis and Crystallization of the Ni-TEA Complex
2.2. Single-Crystal X-Ray Diffraction
2.3. Characterization of the Ni-TEA Complex
2.4. Computational Calculation
2.5. Electrochemical Study of the Ni-TEA Complex
3. Results and Discussion
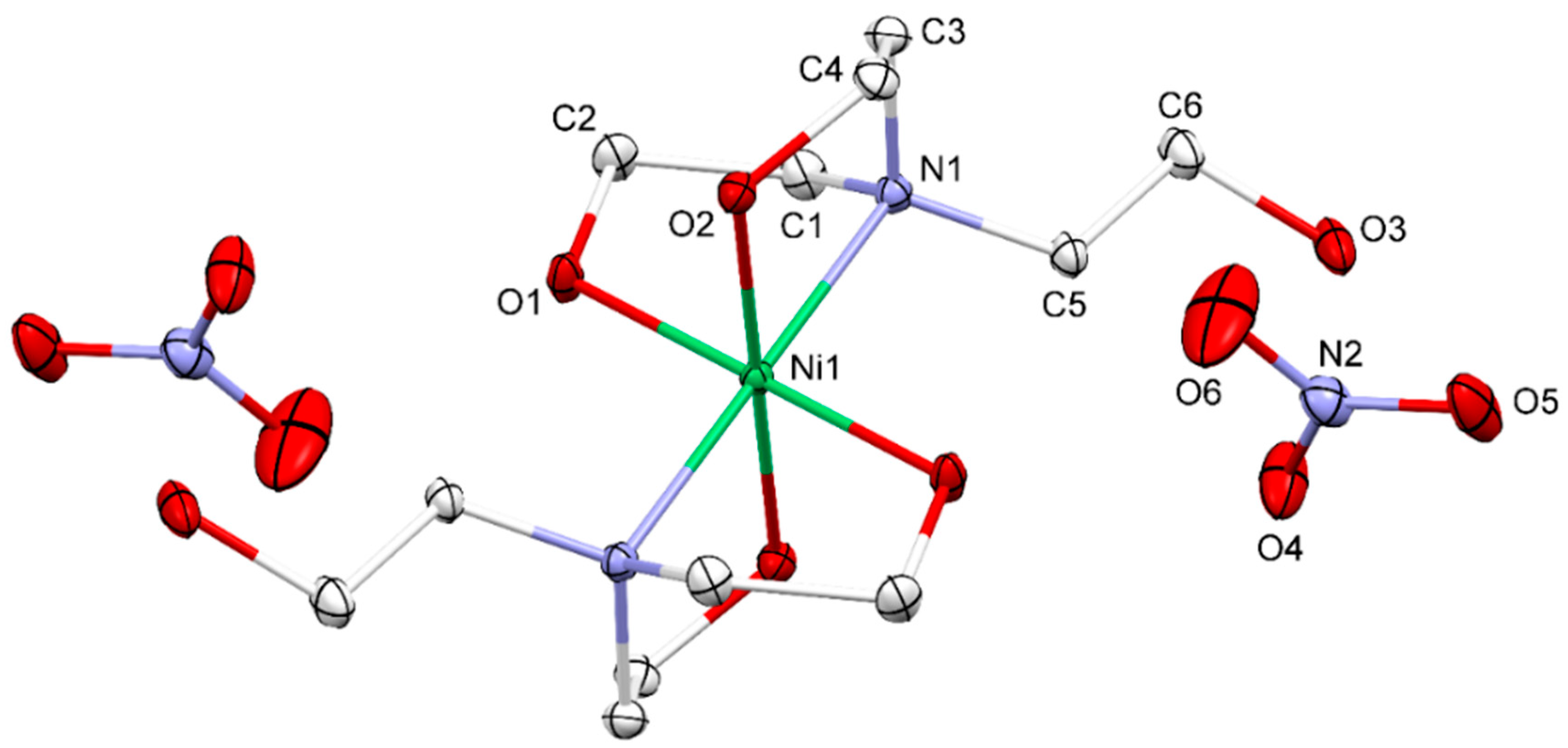
3.1. Crystal Structure
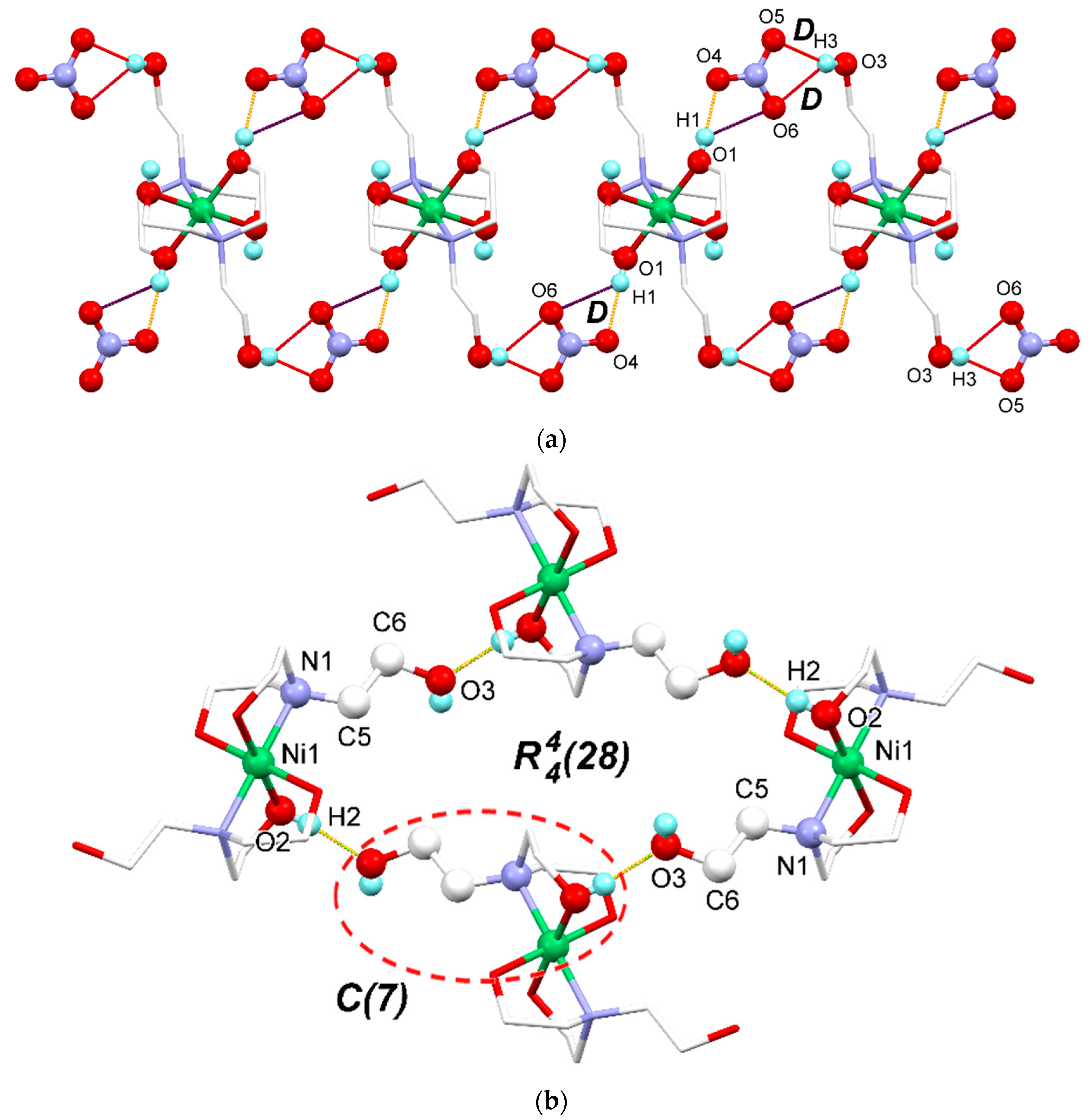
3.2. Supramolecular Features
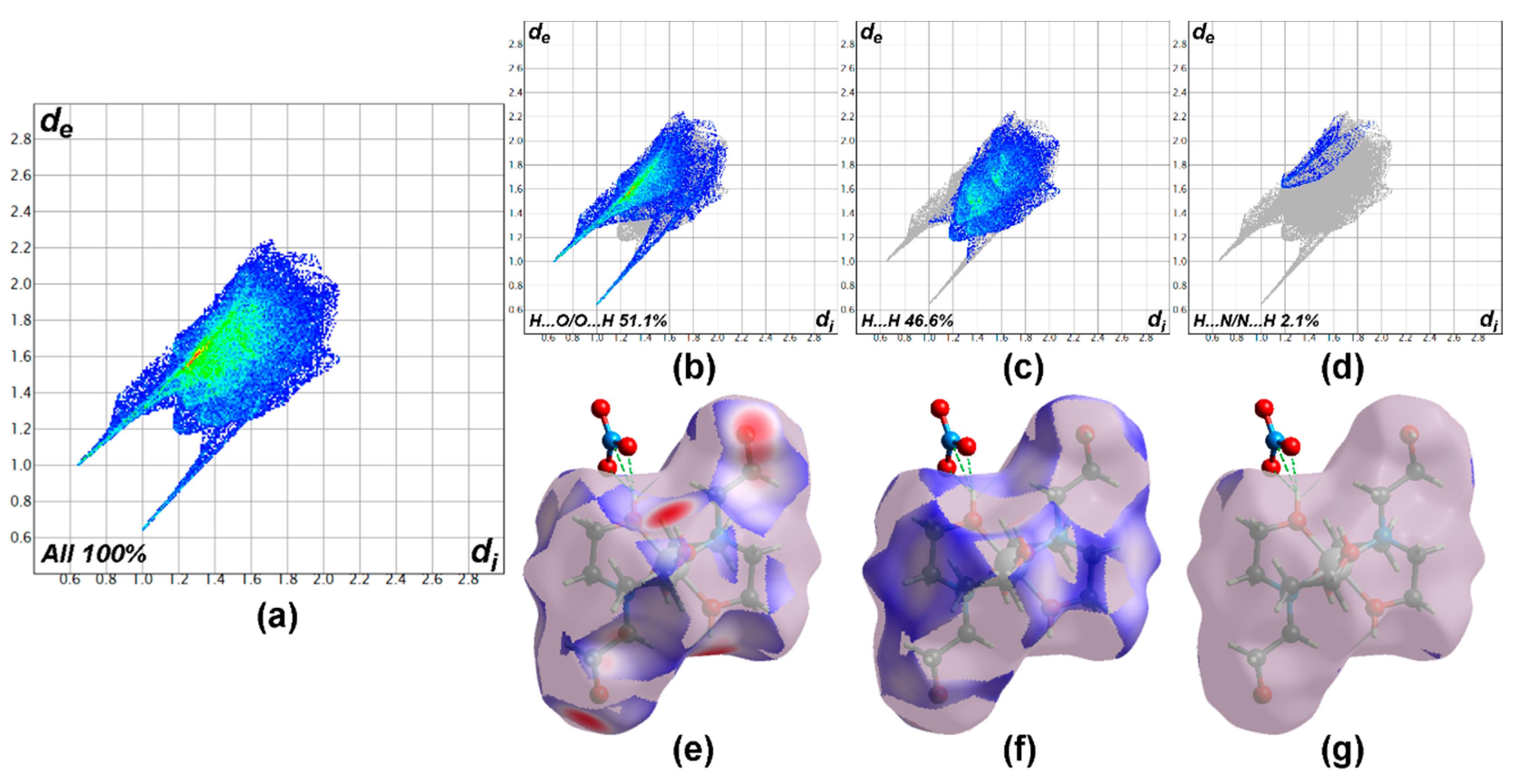
3.3. Hirshfeld Surface Analysis
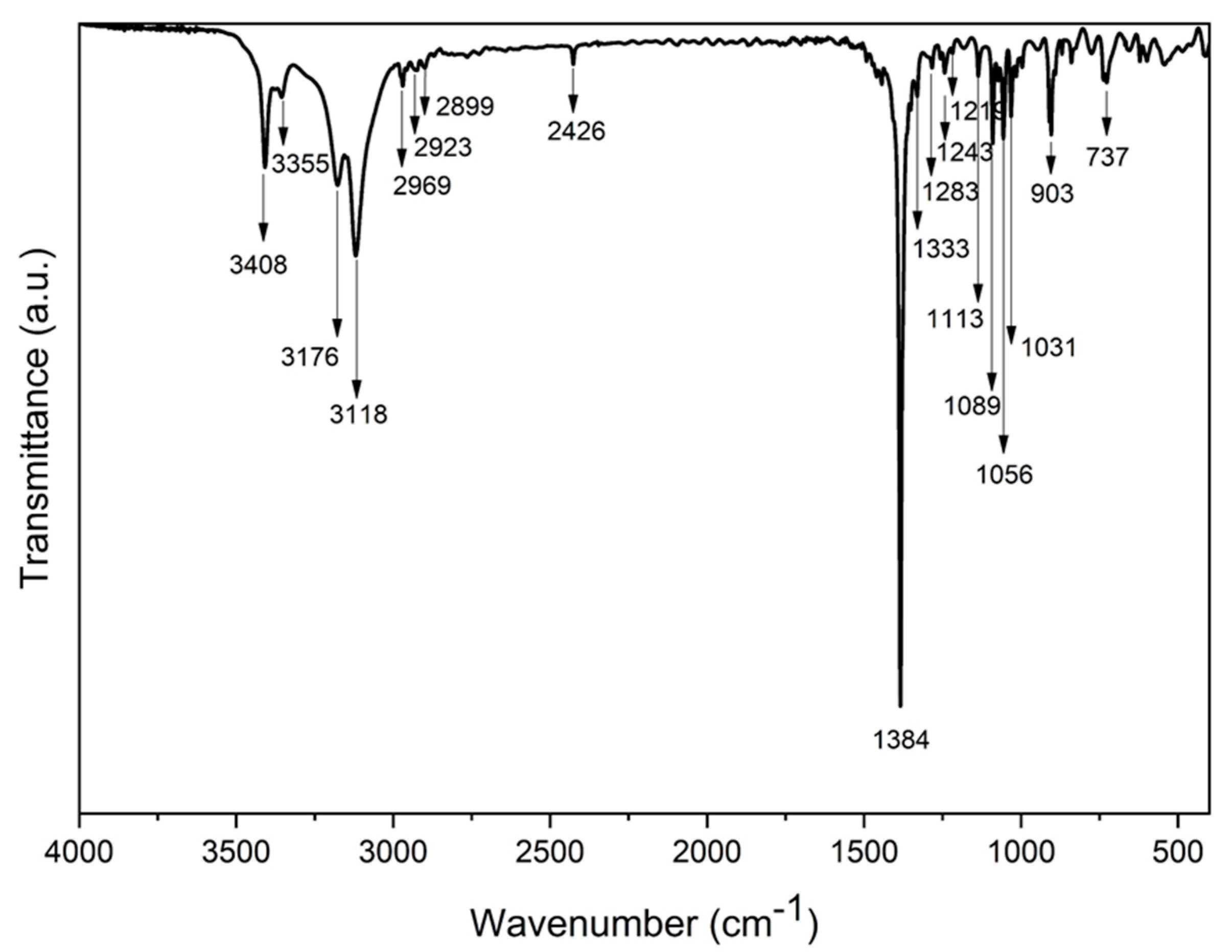
3.4. Characteristic Features of the Ni-TEA Complex
3.5. Quantum Chemical Calculations
3.6. Thermal Analysis
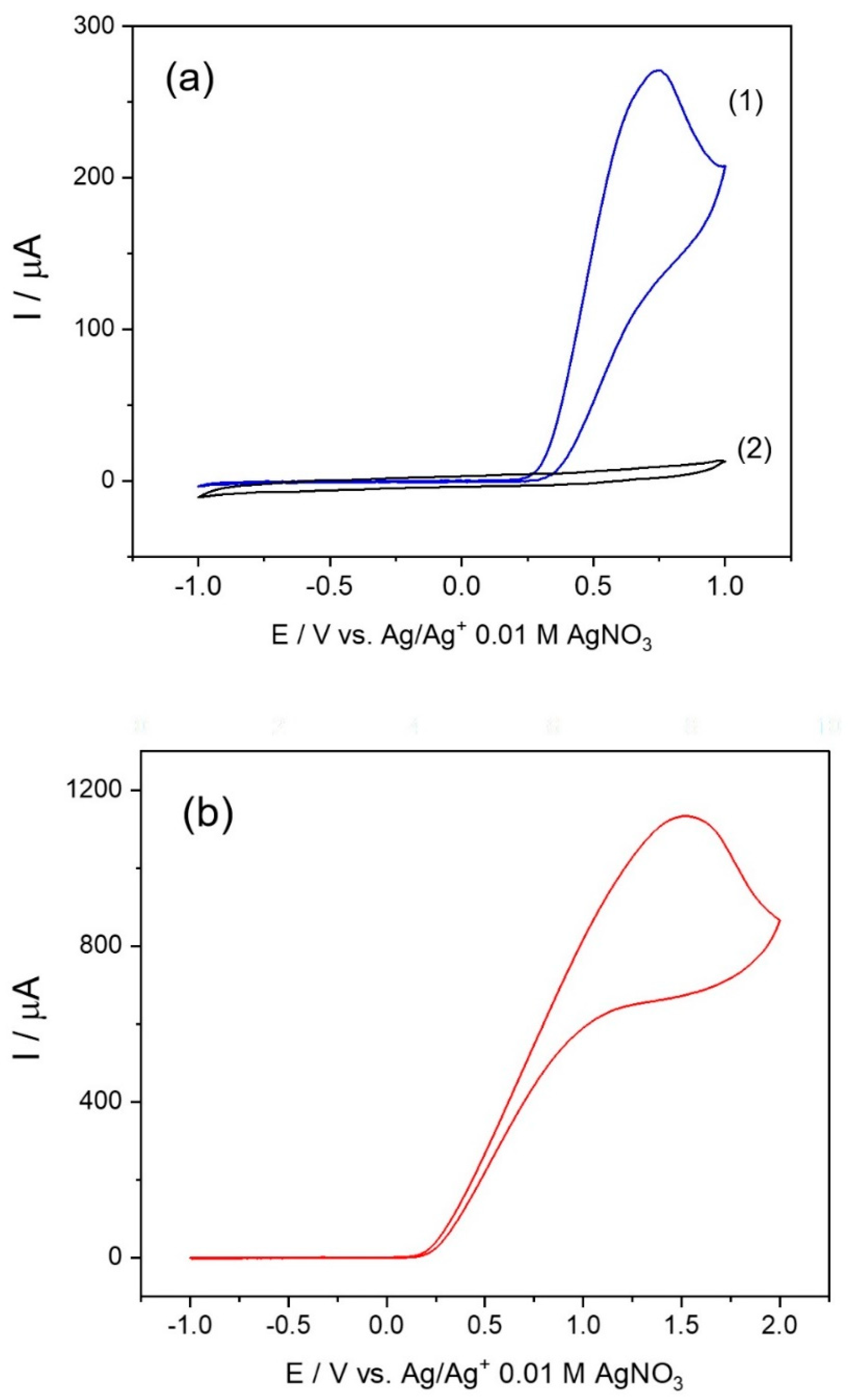
3.7. Electrochemical Study
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Seisenbaeva, G.A.; Kessler, V.G. Precursor directed synthesis—“molecular” mechanisms in the Soft Chemistry approaches and their use for template-free synthesis of metal, metal oxide and metal chalcogenide nanoparticles and nanostructures. Nanoscale 2014, 6, 6229–6244. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Fatah, S.M.; Sánchez, M.D.; Díaz-García, D.; Prashar, S.; Abdel-Rahman, L.H.; Gómez-Ruiz, S. Nanostructured metal oxides prepared from schiff base metal complexes: Study of the catalytic activity in selective oxidation and C–C coupling reactions. J. Inorg. Organomet. Polym. Mater. 2019, 30, 1293–1305. [Google Scholar] [CrossRef]
- Lu, H.; Wright, D.S.; Pike, S.D. The use of mixed-metal single source precursors for the synthesis of complex metal oxides. Chem. Commun. 2020, 56, 854–871. [Google Scholar] [CrossRef]
- Polarz, S.; Orlov, A.V.; Berg, M.W.E.V.D.; Driess, M. Molecular encoding at the nanoscale: From complex cubes to bimetallic oxides. Angew. Chem. Int. Ed. 2005, 44, 7892–7896. [Google Scholar] [CrossRef] [PubMed]
- Pfrommer, J.; Lublow, M.; Azarpira, A.; Göbel, C.; Lücke, M.; Steigert, A.; Pogrzeba, M.; Menezes, P.W.; Fischer, A.; Schedel-Niedrig, T.; et al. A molecular approach to self-supported cobalt-substituted ZnO materials as remarkably stable electrocatalysts for water oxidation. Angew. Chem. Int. Ed. 2014, 53, 5183–5187. [Google Scholar] [CrossRef]
- Pfrommer, J.; Azarpira, A.; Steigert, A.; Olech, K.; Menezes, P.W.; Duarte, R.F.; Liao, X.; Wilks, R.G.; Bär, M.; Schedel-Niedrig, T.; et al. Active and stable nickel-based electrocatalysts based on the ZnO:Ni system for water oxidation in alkaline media. ChemCatChem 2016, 9, 672–676. [Google Scholar] [CrossRef]
- Rao, C.N.R. Chemical approaches to the design of oxide materials. Pure Appl. Chem. 1994, 66, 1765–1772. [Google Scholar] [CrossRef]
- Huang, X.; Zhao, G.; Wang, G.; Irvine, J.T.S. Synthesis and applications of nanoporous perovskite metal oxides. Chem. Sci. 2018, 9, 3623–3637. [Google Scholar] [CrossRef]
- Naiini, A.A.; Pinkas, J.; Plass, W.; Young, V.G.; Verkade, J.G. Triethanolamine Complexes of H+, Li+, Na+, Sr2+, and Ba2+ Perchlorates. Inorg. Chem. 1994, 33, 2137–2141. [Google Scholar] [CrossRef]
- Naiini, A.A.; Young, V.G.; Verkade, J.G. Alkali and alkaline earth metal chloride complexes of triethanolamine: The structure of [Sr(TEA)2]Cl2. Polyhedron 1997, 16, 2087–2092. [Google Scholar] [CrossRef]
- Yilmaz, V.T.; Senel, E.; Thöne, C. Synthesis, spectral, thermal and structural characterization of {[Cu(H2O)3][Cu(MAL)2]·2H2O}∞ and [Cu(MAL)(TEA)]·H2O (MAL = malonate and TEA = triethanolamine). Transit. Met. Chem. 2004, 29, 336–342. [Google Scholar] [CrossRef]
- Ashurov, J.; Ibragimov, A.; Ibragimov, B. Mixed-ligand complexes of Zn(II), Cd(II) and Cu(II) with triethanolamine and p-nitrobenzoic acid: Syntheses and crystal structures. Polyhedron 2015, 102, 441–446. [Google Scholar] [CrossRef]
- Wattanathana, W.; Nootsuwan, N.; Veranitisagul, C.; Koonsaeng, N.; Laosiripojana, N.; Laobuthee, A. Simple cerium-triethanolamine complex: Synthesis, characterization, thermal decomposition and its application to prepare ceria support for platinum catalysts used in methane steam reforming. J. Mol. Struct. 2015, 1089, 9–15. [Google Scholar] [CrossRef]
- Naiini, A.A.; Young, V.; Verkade, J.G. New complexes of thriethanolamine (Tea): Novel structural features of [Y(TEA)2](ClO4)3·3C5H5N and [Cd(TEA)2](NO3)2. Polyhedron 1995, 14, 393–400. [Google Scholar] [CrossRef]
- Ashurov, J.M. Crystal structure of the salt bis-(tri-ethano-lamine-κ(4) N,O,O′,O″)cadmium bis[2-(2-oxo-2,3-di-hydro-1,3-benzo-thia-zol-3-yl)acetate]. Acta Crystallogr. Sect. E Crystallogr. Commun. 2016, 72, 526–529. [Google Scholar] [CrossRef] [PubMed]
- Krabbes, I.; Seichter, W.; Gloe, K. Bis(triethanolamine-O,O′)nickel(II) diacetate. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 2000, 56. [Google Scholar] [CrossRef]
- Topcu, Y.; Andaç, Ö.; Yilmaz, V.T.; Harrison, W.T.A. Bis(triethanolamine-N,O,O′)nickel(II) bis(saccharinate). Acta Crystallogr. Sect. E Struct. Rep. Online 2001, 57, m82–m84. [Google Scholar] [CrossRef]
- Topcu, Y.; Andaç, Ö.; Yilmaz, V.T.; Harrison, W.; Yılmaz, V. Synthesis, characterization and spectral studies of triethanolamine complexes of metal saccharinates. Crystal structures of [Co(TEA)2](SAC)2 AND [Cu2(μ-TEA)2(SAC)2]·2(CH3OH). J. Coord. Chem. 2002, 55, 805–815. [Google Scholar] [CrossRef]
- Uçar, I.; Yeşilel, O.Z.; Bulut, A.; Icbudak, H.; Ölmez, H.; Kazak, C. Bis(triethanolamine-κ 3N,O,O′)copper(II) squarate. Acta Crystallogr. Sect. E Struct. Rep. Online 2004, 60. [Google Scholar] [CrossRef]
- Haukka, M.; Kirillov, A.M.; Kopylovich, M.N.; Pombeiro, A.J.L. Bis(triethanolamine-κ 3N, O, O′)nickel(II) benzene-1,4-dicarboxylate. Acta Crystallogr. Sect. E Struct. Rep. Online 2005, 61. [Google Scholar] [CrossRef]
- Yeşilela, O.Z.; Ölmez, H.; Uçar, I.; Bulut, A.; Kazak, C. Synthesis, spectrothermal behaviour and molecular structure of Aquaorotatotriethanolaminenickel(II) monohydrate. Zeitschrift für anorganische und allgemeine Chemie 2005, 631, 3100–3103. [Google Scholar] [CrossRef]
- Ashurov, J.M.; Obidova, N.J.; Abdireymov, H.B.; Ibragimov, B. Crystal structure of the salt bis-(tri-ethano-lamine-κ(3) N,O,O′)cobalt(II) bis-[2-(2-oxo-2,3-di-hydro-1,3-benzo-thia-zol-3-yl)acetate]. Acta Crystallogr. Sect. E Crystallogr. Commun. 2016, 72, 420–423. [Google Scholar] [CrossRef]
- Andaç, Ö.; Topcu, Y.; Yilmaz, V.T.; Guven, K. Bis(triethanolamine)cadmium(II) and -mercury(II) saccharinates: Seven-coordinate complexes containing both tri- and tetradentate triethanolamine ligands. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 2001, 57, 1381–1384. [Google Scholar] [CrossRef] [PubMed]
- Atria, A.M.; Parada, J.; Garland, M.T.; Baggio, R. A Cu(II) polymeric complex surveying triethanolamine and 1,2-di(4-pyridyl)ethylene as bridging ligands. J. Chil. Chem. Soc. 2015, 60, 3059–3062. [Google Scholar] [CrossRef]
- Kirillov, A.M.; Kopylovich, M.N.; Kirillova, M.V.; Haukka, M.; Da Silva, M.F.C.G.; Pombeiro, A.J.L. Multinuclear copper triethanolamine complexes as selective catalysts for the peroxidative oxidation of alkanes under mild conditions. Angew. Chem. Int. Ed. 2005, 44, 4345–4349. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Wei, X.; Li, B.; Yuan, Y. Copper-triethanolamine complex as efficient and active catalyst for selective oxidation of alkylarenes to phenyl ketones by tert-butylhydroperoxide. Tetrahedron Lett. 2007, 48, 9108–9111. [Google Scholar] [CrossRef]
- Sampaio, R.N.; Grills, D.C.; Polyansky, D.E.; Szalda, D.J.; Fujita, E. Unexpected roles of triethanolamine in the photochemical reduction of CO2 to formate by ruthenium complexes. J. Am. Chem. Soc. 2019, 142, 2413–2428. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, T.; Nakajima, T.; Sawa, S.; Nakanishi, R.; Imori, D.; Ishitani, O. CO2 capture by a Rhenium(I) complex with the aid of triethanolamine. J. Am. Chem. Soc. 2013, 135, 16825–16828. [Google Scholar] [CrossRef]
- Koizumi, H.; Chiba, H.; Sugihara, A.; Iwamura, M.; Nozaki, K.; Ishitani, O. CO2 capture by Mn(i) and Re(i) complexes with a deprotonated triethanolamine ligand. Chem. Sci. 2019, 10, 3080–3088. [Google Scholar] [CrossRef]
- Boulsourani, Z.; Geromichalos, G.; Repana, K.; Yiannaki, E.; Psycharis, V.; Raptopoulou, C.P.; Hadjipavlou-Litina, D.; Pontiki, E.; Dendrinou-Samara, C. Preparation and pharmacochemical evaluation of mixed ligand copper(II) complexes with triethanolamine and thiophenyl-2 saturated carboxylic acids. J. Inorg. Biochem. 2011, 105, 839–849. [Google Scholar] [CrossRef]
- Park, Y.; Kim, C.; Lee, H. Effects of catalyst and solvent on PbTiO3 fibers prepared from triethanolamine complexed titanium isopropoxide. J. Sol-Gel Sci. Technol. 1999, 14, 149–162. [Google Scholar] [CrossRef]
- Laobuthee, A.; Wongkasemjit, S.; Traversa, E.; Laine, R.M. MgAl2O4 spinel powders from oxide one pot synthesis (OOPS) process for ceramic humidity sensors. J. Eur. Ceram. Soc. 2000, 20, 91–97. [Google Scholar] [CrossRef]
- Wattanathana, W.; Lakkham, A.; Kaewvilai, A.; Koonsaeng, N.; Laobuthee, A.; Veranitisagul, C. Preliminary study of Pd/CeO2 derived from cerium complexes as solid support catalysts for hydrogenation reaction in a micro-reactor. Energy Procedia 2011, 9, 568–574. [Google Scholar] [CrossRef]
- Wattanathana, W.; Wannapaiboon, S.; Veranitisagul, C.; Laosiripojana, N.; Koonsaeng, N.; Laobuthee, A. Preparation of palladium-impregnated ceria by metal complex decomposition for methane steam reforming catalysis. Adv. Mater. Sci. Eng. 2017, 2017, 1–10. [Google Scholar] [CrossRef]
- Wattanathana, W.; Veranitisagul, C.; Wannapaiboon, S.; Klysubun, W.; Koonsaeng, N.; Laobuthee, A. Samarium doped ceria (SDC) synthesized by a metal triethanolamine complex decomposition method: Characterization and an ionic conductivity study. Ceram. Int. 2017, 43, 9823–9830. [Google Scholar] [CrossRef]
- Nantharak, W.; Wattanathana, W.; Klysubun, W.; Rimpongpisarn, T.; Veranitisagul, C.; Koonsaeng, N.; Laobuthee, A. Effect of local structure of Sm3+ in MgAl2O4:Sm3+ phosphors prepared by thermal decomposition of triethanolamine complexes on their luminescence property. J. Alloy. Compd. 2017, 701, 1019–1026. [Google Scholar] [CrossRef]
- Haron, W.; Thaweechai, T.; Wattanathana, W.; Laobuthee, A.; Manaspiya, H.; Veranitisagul, C.; Koonsaeng, N. Structural characteristics and dielectric properties of La1-xCoxFeO3 and LaFe1-xCoxO3 synthesized via metal organic complexes. Energy Procedia 2013, 34, 791–800. [Google Scholar] [CrossRef]
- Rimpongpisarn, T.; Wattanathana, W.; Sukthavorn, K.; Nootsuwan, N.; Hanlumyuang, Y.; Veranitisagul, C.; Laobuthee, A. Novel luminescent PLA/MgAl2O4:Sm3+ composite filaments for 3D printing application. Mater. Lett. 2019, 237, 270–273. [Google Scholar] [CrossRef]
- Laobuthee, A.; Veranitisagul, C.; Wattanathana, W.; Koonsaeng, N.; Laosiripojana, N. Activity of Fe supported by Ce 1−x Sm x O 2−δ derived from metal complex decomposition toward the steam reforming of toluene as biomass tar model compound. Renew. Energy 2015, 74, 133–138. [Google Scholar] [CrossRef]
- Veranitisagul, C.; Wattanathana, W.; Nantharak, W.; Jantaratana, P.; Laobuthee, A.; Koonsaeng, N. BaFe 12 O 19 from thermal decomposition of bimetallic triethanolamine complex as magnetic filler for bioplastics. Mater. Chem. Phys. 2016, 177, 48–55. [Google Scholar] [CrossRef]
- Hieber, W.; Levy, E. Das komplexchemische Verhalten der Äthylolamine. II. Zeitschrift für anorganische und allgemeine Chemie 1934, 219, 225–237. [Google Scholar] [CrossRef]
- Nielsen, K.; Hazell, R.G.; Rasmussen, S.E.; Larsson, R.; Norden, B.; Sundbom, M. The crystal structure of Di-triethanolamine-Ni(II)-dinitrate. Acta Chem. Scand. 1972, 26, 889–896. [Google Scholar] [CrossRef]
- SAINT. Version 8.34A 2013; Bruker AXS: Madison, WI, USA, 2013. [Google Scholar]
- Sheldrick, G.M. SADABS.; University of Göttingen: Göttingen, Germany, 1996. [Google Scholar]
- Dolomanov, O.; Bourhis, L.J.; Gildea, R.; Howard, J.A.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Macrae, C.; Edgington, P.R.; McCabe, P.E.; Pidcock, E.; Shields, G.; Taylor, R.; Towler, M.; Van De Streek, J. Mercury: Visualization and analysis of crystal structures. J. Appl. Crystallogr. 2006, 39, 453–457. [Google Scholar] [CrossRef]
- Barboza, C.A.; Germino, J.C.; Santana, A.M.; Quites, F.J.; Vazquez, P.A.M.; Atvars, T.D.Z. Structural correlations between luminescent properties and excited state internal proton transfer in some Zinc(II)N,N′-Bis(salicylidenes). J. Phys. Chem. C 2015, 119, 6152–6163. [Google Scholar] [CrossRef]
- Vivas, M.; Germino, J.C.; Barboza, C.A.; A Vazquez, P.; De Boni, L.; Atvars, T.D.Z.; Mendonça, C.R. Excited-state and two-photon absorption in salicylidene molecules: The role of Zn(II) planarization. J. Phys. Chem. C 2016, 120, 4032–4039. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Raghavachari, K.; Trucks, G.W. Highly correlated systems. Excitation energies of first row transition metals Sc–Cu. J. Chem. Phys. 1989, 91, 1062–1065. [Google Scholar] [CrossRef]
- Krishnan, R.; Binkley, J.S.; Seeger, R.; Pople, J.A. Self-consistent molecular orbital methods. XX. A basis set for correlated wave functions. J. Chem. Phys. 1980, 72, 650. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision B. 01; Gaussian Inc: Wallingford, CT, USA, 2010. [Google Scholar]
- Scalmani, G.; Frisch, M.J.; Mennucci, B.; Tomasi, J.; Cammi, R.; Barone, V. Geometries and properties of excited states in the gas phase and in solution: Theory and application of a time-dependent density functional theory polarizable continuum model. J. Chem. Phys. 2006, 124, 094107. [Google Scholar] [CrossRef] [PubMed]
- Mennucci, B.; Cappelli, C.; Guido, C.A.; Cammi, R.; Tomasi, J. Structures and properties of electronically excited chromophores in solution from the polarizable continuum model coupled to the time-dependent density functional theory. J. Phys. Chem. A 2009, 113, 3009–3020. [Google Scholar] [CrossRef] [PubMed]
- Içbudak, H.; Yilmaz, V.T.; Howie, R.A.; Andaç, Ö.; Ölmez, H. Bis[tris(2-hydroxyethyl)amine]nickel(II) Chloride. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 1995, 51, 1759–1761. [Google Scholar] [CrossRef]
- Yeşilel, O.Z.; Bulut, A.; Uçar, I.; Icbudak, H.; Ölmez, H.; Büyükgüngör, O. Bis(triethanolamine-κ3N,O,O′) nickel(II) squarate. Acta Crystallogr. Sect. E Struct. Rep. Online 2004, 60, m228–m230. [Google Scholar] [CrossRef]
- Fundamensky, V.; Kochina, T.; Kondratenko, Y.; Zolotarev, A.A.; Vlasov, Y.; Ignatyev, I. Ionic liquids based on triethanolammonium salts of dicarboxylic acids (oxalic, malonic, succinic). Crystal structure and cation-anion interaction. J. Mol. Liq. 2017, 230, 113–120. [Google Scholar] [CrossRef]
- Etter, M.C.; Macdonald, J.C.; Bernstein, J. Graph-set analysis of hydrogen-bond patterns in organic crystals. Acta Crystallogr. Sect. B Struct. Sci. 1990, 46, 256–262. [Google Scholar] [CrossRef]
- Bernstein, J.; Shimoni, L.; Davis, R.E.; Chang, N.-L. Graph set analysis of hydrogen-bond patterns in organic crystals. Recent developments and applications. Acta Crystallogr. Sect. A Found. Crystallogr. 1993, 49, c164. [Google Scholar] [CrossRef]
- Grell, J.; Bernstein, J.; Tinhofer, G. Graph-set analysis of hydrogen-bond patterns: Some mathematical concepts. Acta Crystallogr. Sect. B Struct. Sci. 1999, 55, 1030–1043. [Google Scholar] [CrossRef]
- Hirshfeld, F.L. Bonded-atom fragments for describing molecular charge densities. Theor. Chem. Accounts 1977, 44, 129–138. [Google Scholar] [CrossRef]
- Spackman, M.A.; Jayatilaka, D. Hirshfeld surface analysis. CrystEngComm 2009, 11, 19–32. [Google Scholar] [CrossRef]
- Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Spackman, P.R.; Jayatilaka, D.; Spackman, M.A. Crystal Explorer 17; The University of Western Australia: Perth, Australia, 2017. [Google Scholar]
- McKinnon, J.; Jayatilaka, D.; Spackman, M.A. Towards quantitative analysis of intermolecular interactions with Hirshfeld surfaces. Chem. Commun. 2007, 3814–3816. [Google Scholar] [CrossRef] [PubMed]
- Bellamy, L.; Owen, A. A simple relationship between the infra-red stretching frequencies and the hydrogen bond distances in crystals. Spectrochim. Acta Part A Mol. Spectrosc. 1969, 25, 329–333. [Google Scholar] [CrossRef]
- Ignatyev, I.; Kondratenko, Y.; Fundamensky, V.; Kochina, T. Synthesis and characterization of cobalt(II) complexes with triethanolamine and succinate and/or nitrate anions. Transit. Met. Chem. 2017, 43, 127–136. [Google Scholar] [CrossRef]
- Larabi, L.; Reguig, A.; Mostafa, M.; Harek, Y. Nickel(II) Complexes with Sulphonylhydrazone derivatives: Spectroscopic and electrochemical studies. J. Appl. Sci. 2008, 8, 3191–3198. [Google Scholar] [CrossRef]
- Güveli, Ş.; Koca, A.; Özdemir, N.; Bal-Demirci, T.; Ülküseven, B. Electrochemistry and structural properties of new mixed ligand nickel(ii) complexes based on thiosemicarbazone. New J. Chem. 2014, 38, 5582–5589. [Google Scholar] [CrossRef]
- Hamacher, C.; Hurkes, N.; Kaiser, A.; Klein, A.; Schüren, A. Electrochemistry and spectroscopy of organometallic terpyridine nickel complexes. Inorg. Chem. 2009, 48, 9947–9951. [Google Scholar] [CrossRef]
- Safavi, A.; Fotouhia, L. Electrochemical oxidation of the Ni(II) complex of 2-amino cyclopentene-1-dithiocarboxylate at a Pt electrode. J. Electroanal. Chem. 1997, 434, 93–98. [Google Scholar] [CrossRef]
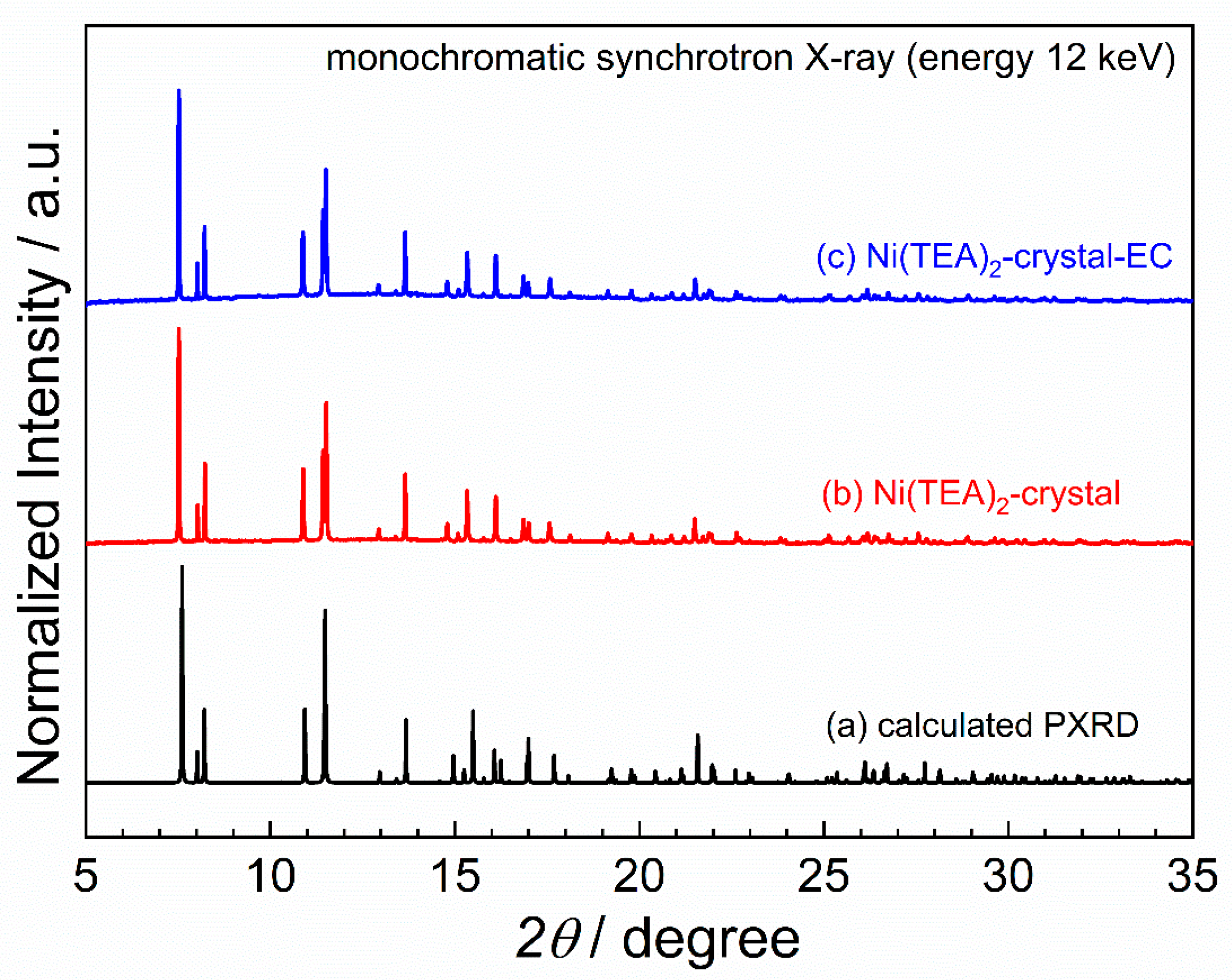
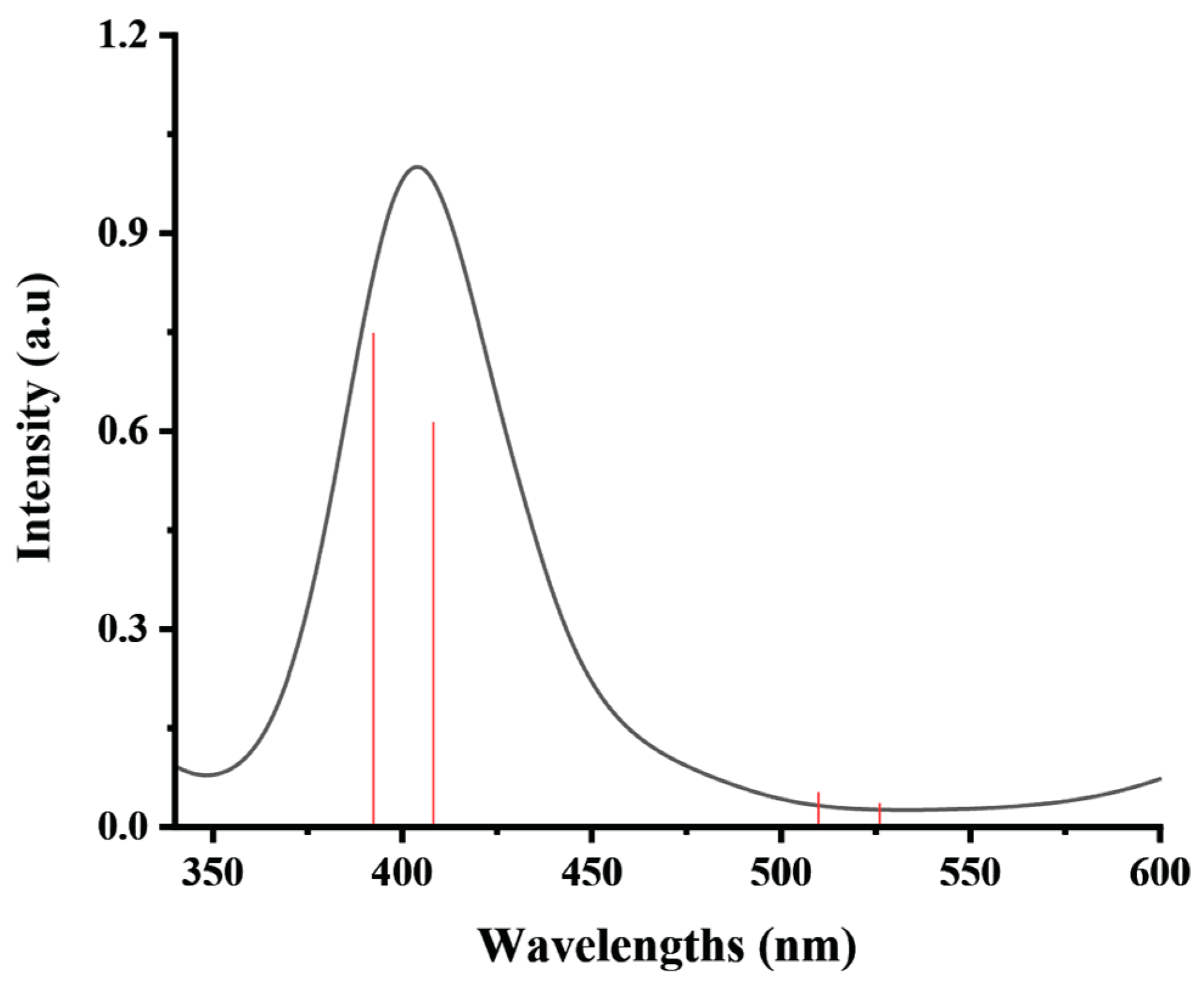
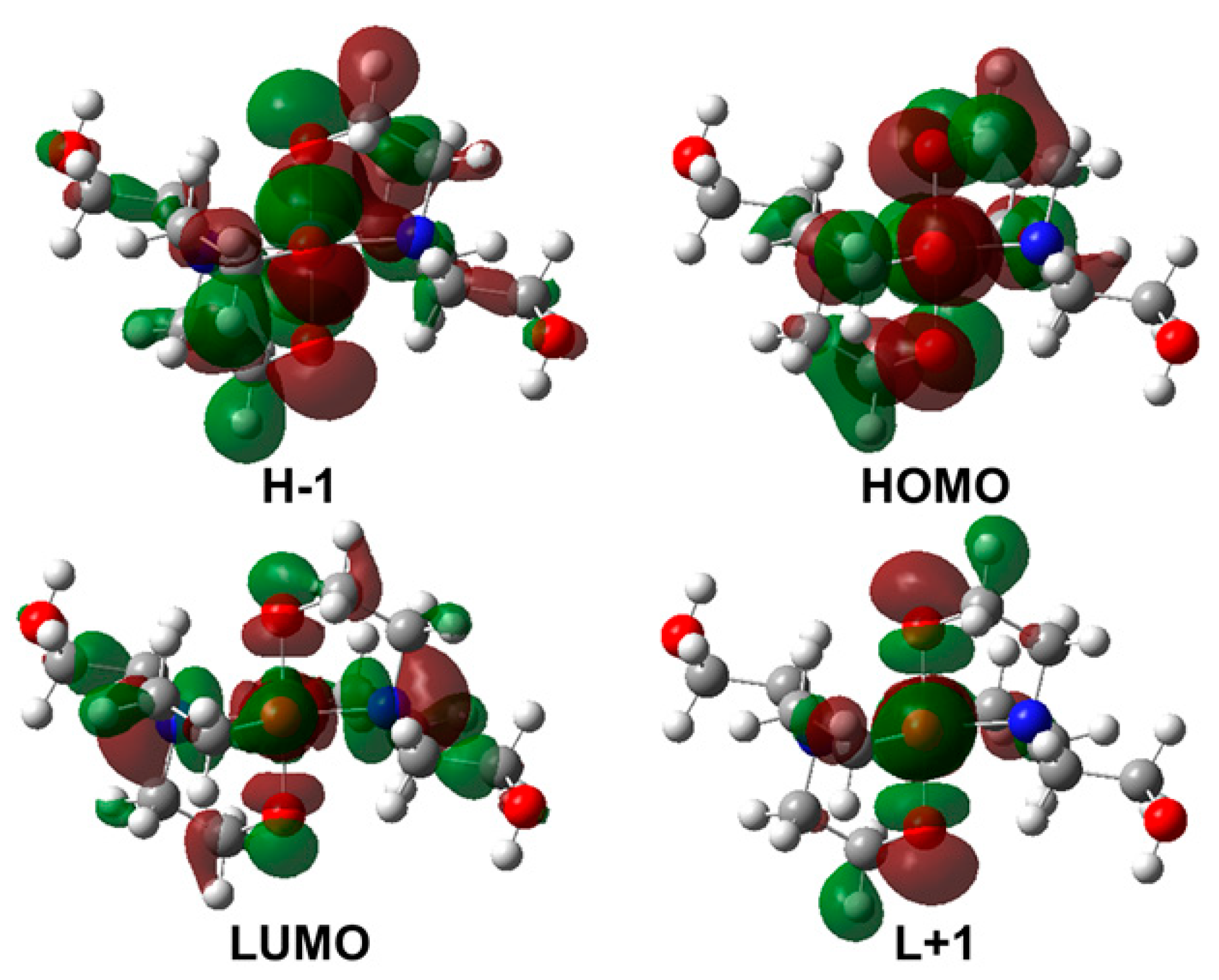
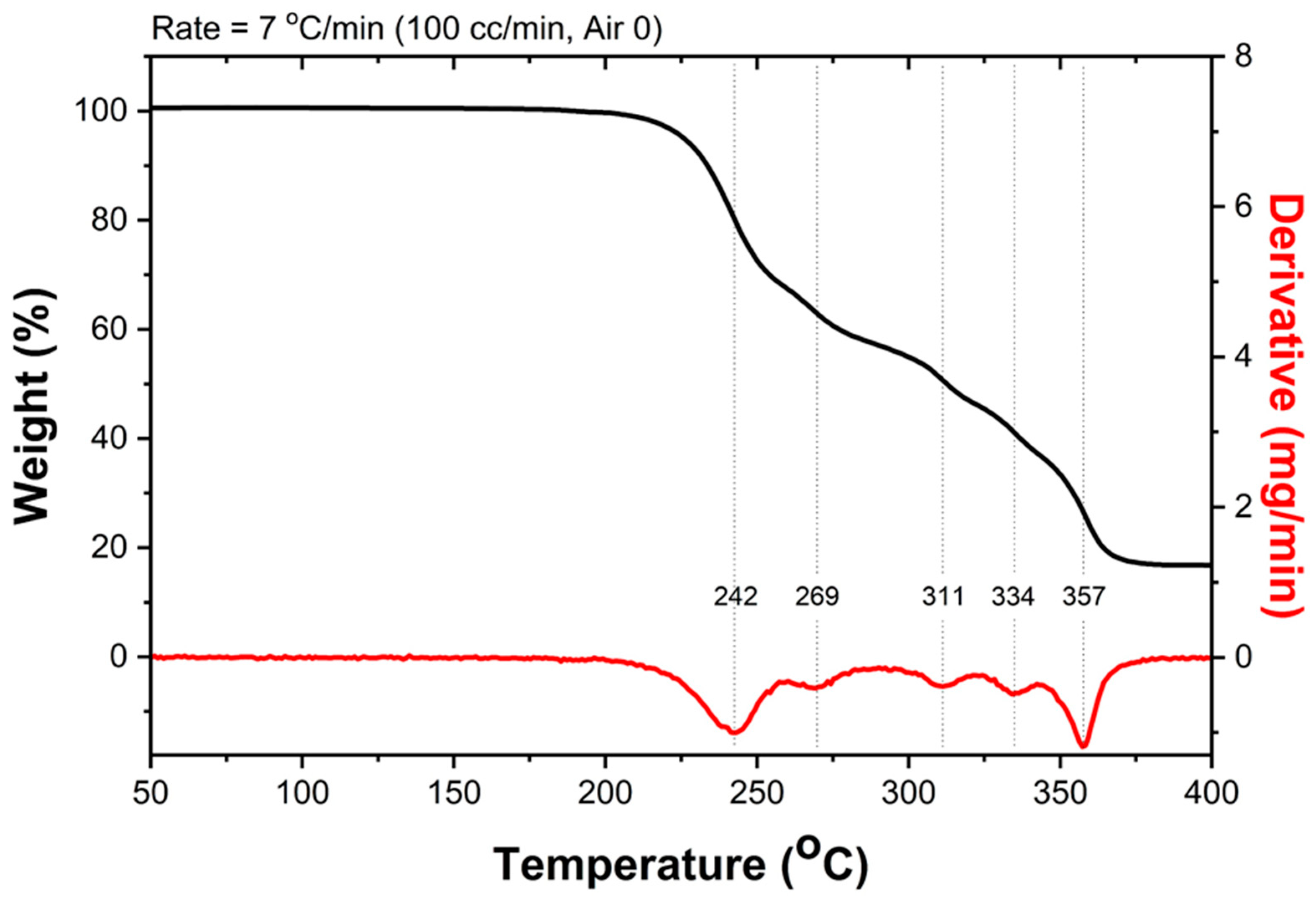
| Crystallographic Data and Structural Refinement Details | Bis(triethanolamine)Nickel(II) Dinitrate |
|---|---|
| Empirical formula | C12H30N4O12Ni |
| Formula weight | 481.11 |
| Temperature/K | 293.15 |
| Crystal system | Monoclinic |
| Space group | P21/c |
| a/Å | 7.224(3) |
| b/Å | 14.777(6) |
| c/Å | 9.173(4) |
| α/° | 90 |
| β/° | 93.198(18) |
| γ/° | 90 |
| Volume/Å3 | 977.7(7) |
| Z | 2 |
| ρcalcg/cm3 | 1.634 |
| μ/mm−1 | 1.063 |
| F(000) | 508.0 |
| Crystal size/mm3 | 0.394 × 0.267 × 0.202 |
| Radiation | Mo Kα (λ = 0.71073) |
| 2θ range for data collection/° | 5.232 to 61.27 |
| Index ranges | –10 ≤ h ≤ 10, –21 ≤ k ≤ 21, –13 ≤ l ≤ 13 |
| Reflections collected | 88,417 |
| Independent reflections | 3016 [Rint = 0.0262, Rsigma = 0.0083] |
| Data/restraints/parameters | 3016/0/143 |
| Goodness of fit on F2 | 1.074 |
| Final R indexes [I ≥ 2σ (I)] | R1 = 0.0221, wR2 = 0.0574 |
| Final R indexes [all data] | R1 = 0.0226, wR2 = 0.0577 |
| Largest difference peak/hole /e Å−3 | 0.51/–0.53 |
| D-H⋅⋅⋅A | d(D-H)/Å | d(H-A)/Å | d(D-A)/Å | D-H-A/° |
|---|---|---|---|---|
| O2-H2⋅⋅⋅O3 a | 0.801(18) | 1.843(18) | 2.6412(13) | 173.5(17) |
| O1-H1⋅⋅⋅O4 | 0.82(2) | 1.86(2) | 2.6689(14) | 168(2) |
| O3-H3⋅⋅⋅O5 b | 0.78(2) | 2.21(2) | 2.9533(15) | 161(2) |
| O3-H3⋅⋅⋅O6 b | 0.78(2) | 2.30(2) | 2.9535(16) | 142.1(19) |
| Elements | Elemental Composition (Excluding H atoms) (% Weight) | ||||
|---|---|---|---|---|---|
| Calculated Data from Single Crystal | XRF Data of the Obtained Ni-TEA Crystals | ||||
| 1st | 2nd | 3rd | Averaged | ||
| C | 31.97 | 29.63 | 31.13 | 31.40 | 30.72 ± 0.78 |
| N | 12.43 | 12.78 | 12.75 | 13.25 | 12.93 ± 0.23 |
| O | 42.58 | 44.34 | 42.73 | 41.26 | 42.77 ± 1.26 |
| Ni | 13.02 | 13.25 | 13.39 | 14.09 | 13.58 ± 0.37 |
| Parameters | X-ray Crystallography | DFT Calculations |
|---|---|---|
| Bond Lengths (Å) | ||
| Ni1-O1 | 2.0519(8) | 1.868 |
| Ni1-O1 1 | 2.0519(9) | 1.864 |
| Ni1-O2 | 2.0751(9) | 1.872 |
| Ni1-O2 1 | 2.0751(9) | 1.867 |
| Ni1-N1 | 2.1021(10) | 1.979 |
| Ni1-N1 1 | 2.1021(10) | 1.971 |
| Bond Angles (°) | ||
| O1-Ni1-O2 | 93.07(4) | 88.12 |
| O1-Ni1-O1 1 | 180.0 | 175.68 |
| O1 1-Ni1-O2 | 86.92(4) | 92.36 |
| C4-O2-Ni1 | 106.51(6) | 110.94 |
| C3-N1-Ni1 | 107.50(6) | 102.38 |
| N1-Ni1-N1 1 | 180.0 | 177.61 |
| States | eV | nm | f | Transition State |
|---|---|---|---|---|
| S0 → S5 | 2.38 | 521 | 0.0015 | HOMO→ LUMO (72%) + HOMO→ L+1 (18%) |
| S0 → S6 | 2.45 | 506 | 0.0028 | H−1→ LUMO (66%) + H−1→ L+1 (23%) |
| S0 → S9 | 3.08 | 402 | 0.0358 | HOMO→ L+1 (33%) + HOMO→ LUMO (22%) |
| S0 → S10 | 3.19 | 388 | 0.0435 | H−1→ LUMO (23%) + H−1→ L+1 (22%) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deeloed, W.; Wannapaiboon, S.; Pansiri, P.; Kumpeerakij, P.; Phomphrai, K.; Laobuthee, A.; Hanlumyuang, Y.; Suramitr, S.; Pinyou, P.; Wattanathana, W. Crystal Structure and Hirshfeld Surface Analysis of Bis(Triethanolamine)Nickel(II) Dinitrate Complex and a Revelation of Its Characteristics via Spectroscopic, Electrochemical and DFT Studies Towards a Promising Precursor for Metal Oxides Synthesis. Crystals 2020, 10, 474. https://doi.org/10.3390/cryst10060474
Deeloed W, Wannapaiboon S, Pansiri P, Kumpeerakij P, Phomphrai K, Laobuthee A, Hanlumyuang Y, Suramitr S, Pinyou P, Wattanathana W. Crystal Structure and Hirshfeld Surface Analysis of Bis(Triethanolamine)Nickel(II) Dinitrate Complex and a Revelation of Its Characteristics via Spectroscopic, Electrochemical and DFT Studies Towards a Promising Precursor for Metal Oxides Synthesis. Crystals. 2020; 10(6):474. https://doi.org/10.3390/cryst10060474
Chicago/Turabian StyleDeeloed, Wanchai, Suttipong Wannapaiboon, Pimporn Pansiri, Pornsawan Kumpeerakij, Khamphee Phomphrai, Apirat Laobuthee, Yuranan Hanlumyuang, Songwut Suramitr, Piyanut Pinyou, and Worawat Wattanathana. 2020. "Crystal Structure and Hirshfeld Surface Analysis of Bis(Triethanolamine)Nickel(II) Dinitrate Complex and a Revelation of Its Characteristics via Spectroscopic, Electrochemical and DFT Studies Towards a Promising Precursor for Metal Oxides Synthesis" Crystals 10, no. 6: 474. https://doi.org/10.3390/cryst10060474
APA StyleDeeloed, W., Wannapaiboon, S., Pansiri, P., Kumpeerakij, P., Phomphrai, K., Laobuthee, A., Hanlumyuang, Y., Suramitr, S., Pinyou, P., & Wattanathana, W. (2020). Crystal Structure and Hirshfeld Surface Analysis of Bis(Triethanolamine)Nickel(II) Dinitrate Complex and a Revelation of Its Characteristics via Spectroscopic, Electrochemical and DFT Studies Towards a Promising Precursor for Metal Oxides Synthesis. Crystals, 10(6), 474. https://doi.org/10.3390/cryst10060474






