Ternary Aluminides of a New Homologous Series—CePt2Al2 and CePt3Al3: Crystal Structures and Thermal Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis
2.2. Energy Dispersive X-Ray Analysis
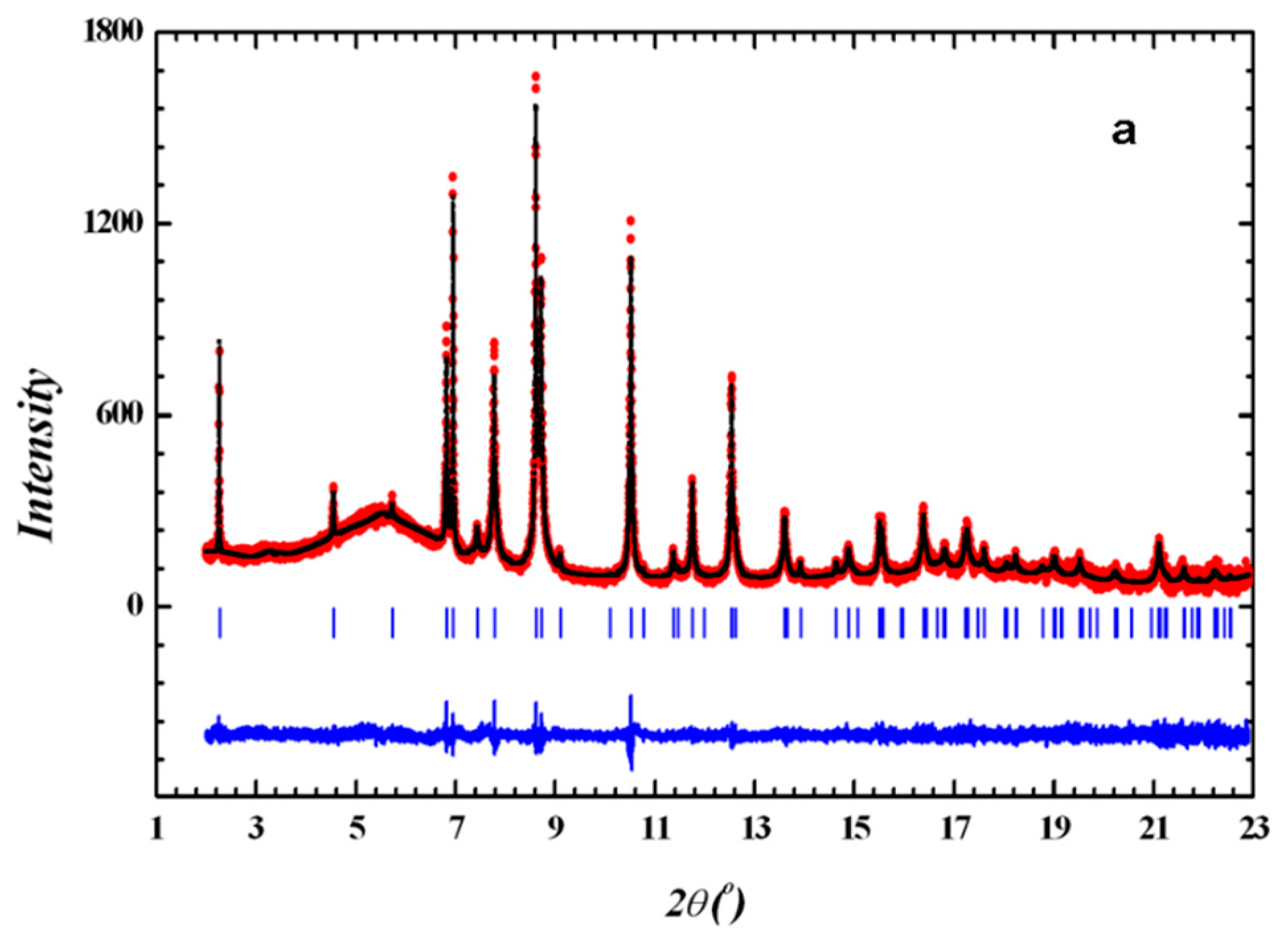
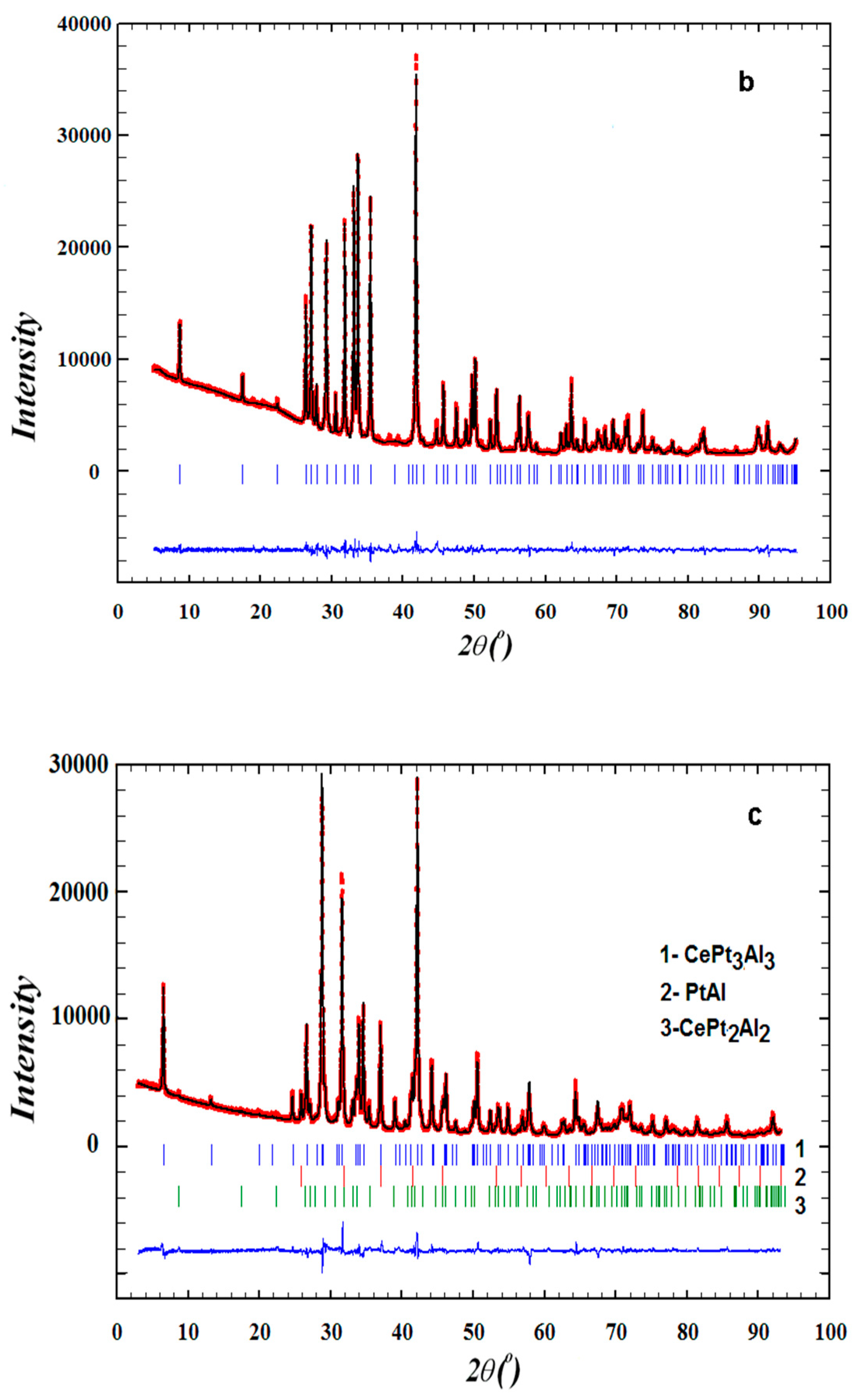
2.3. Powder X-Ray Diffraction
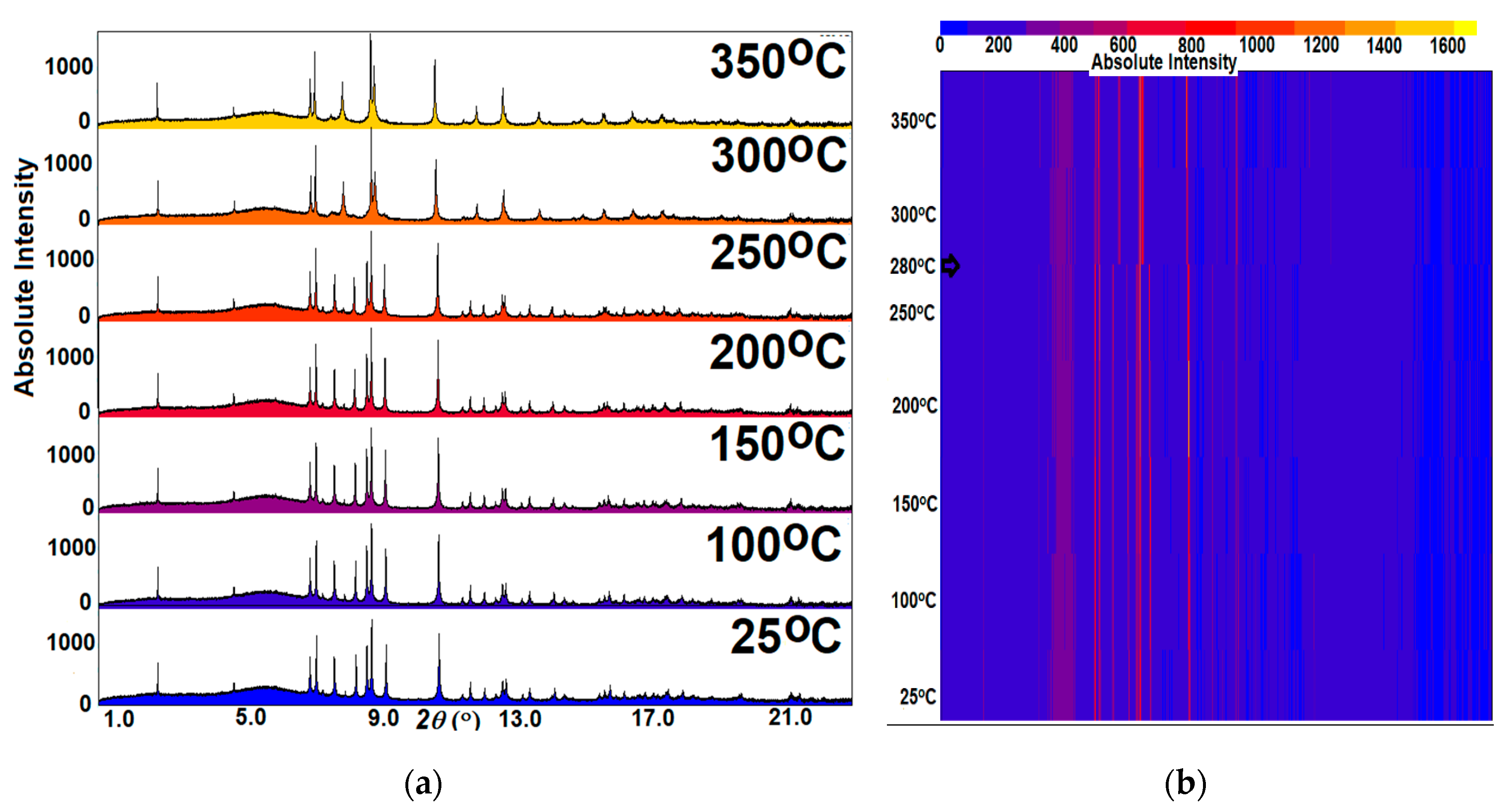
2.4. High Temperature Powder Synchrotron X-Ray Diffraction
2.5. Crystal Structure Determination
2.6. Differential Thermal Analysis
3. Results and Discussion
3.1. Sample Characterization
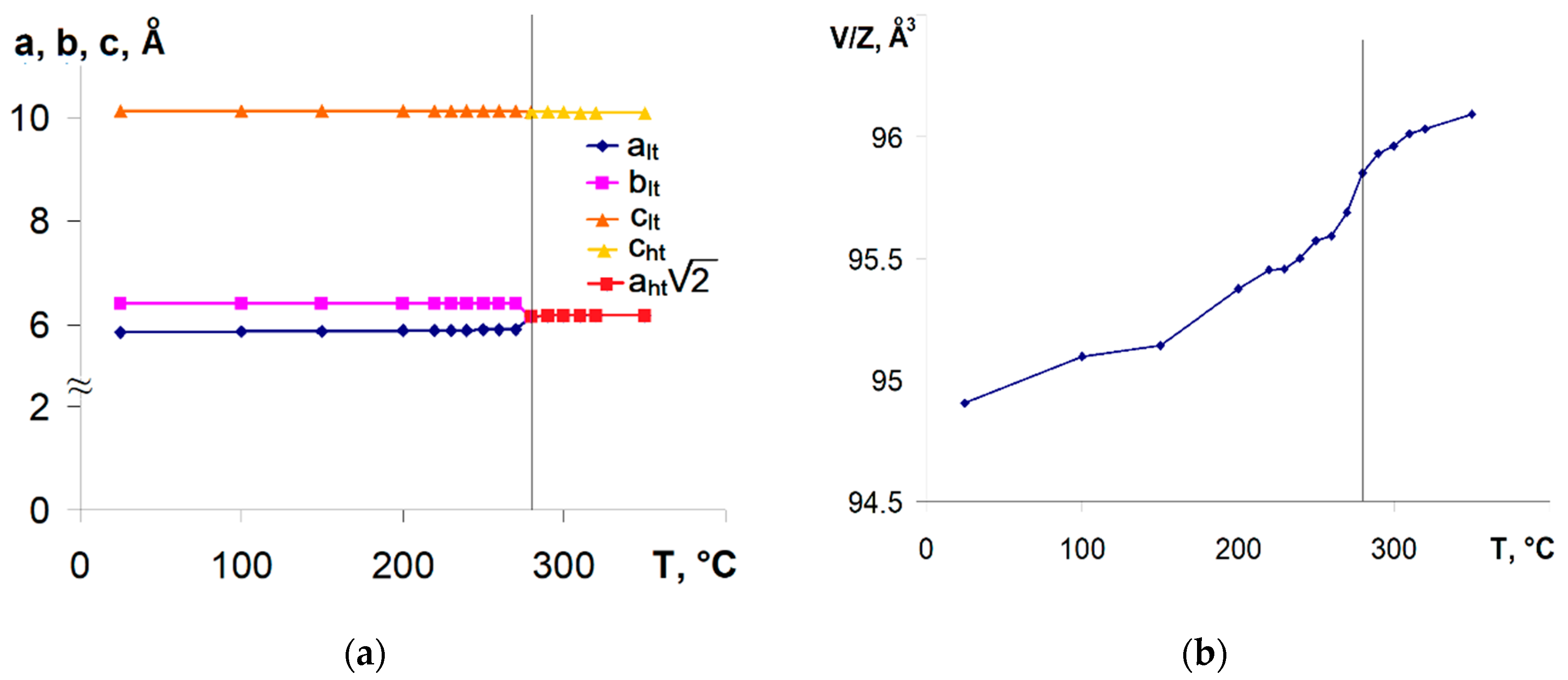
3.2. Thermal Analysis and Temperature-Dependent XRD
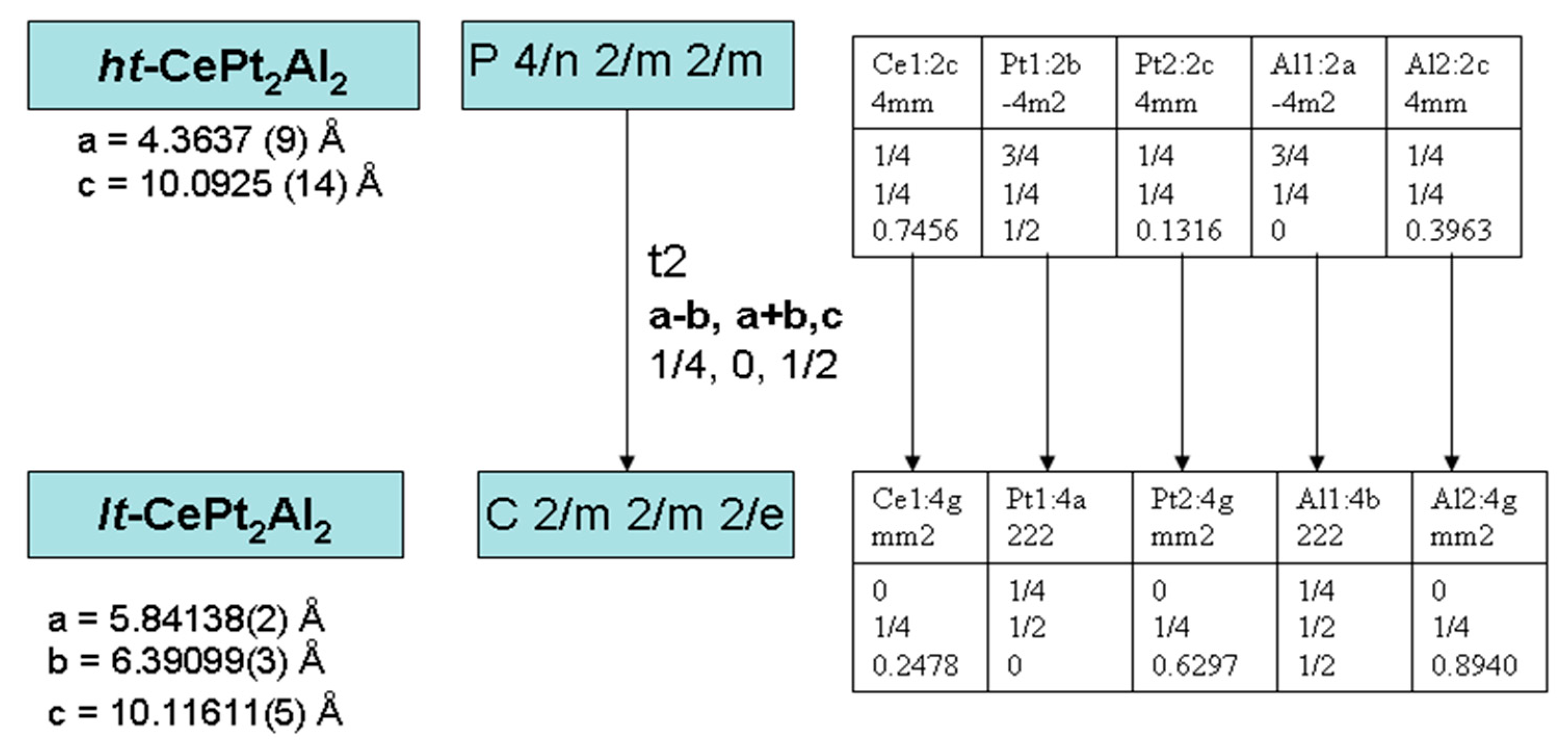
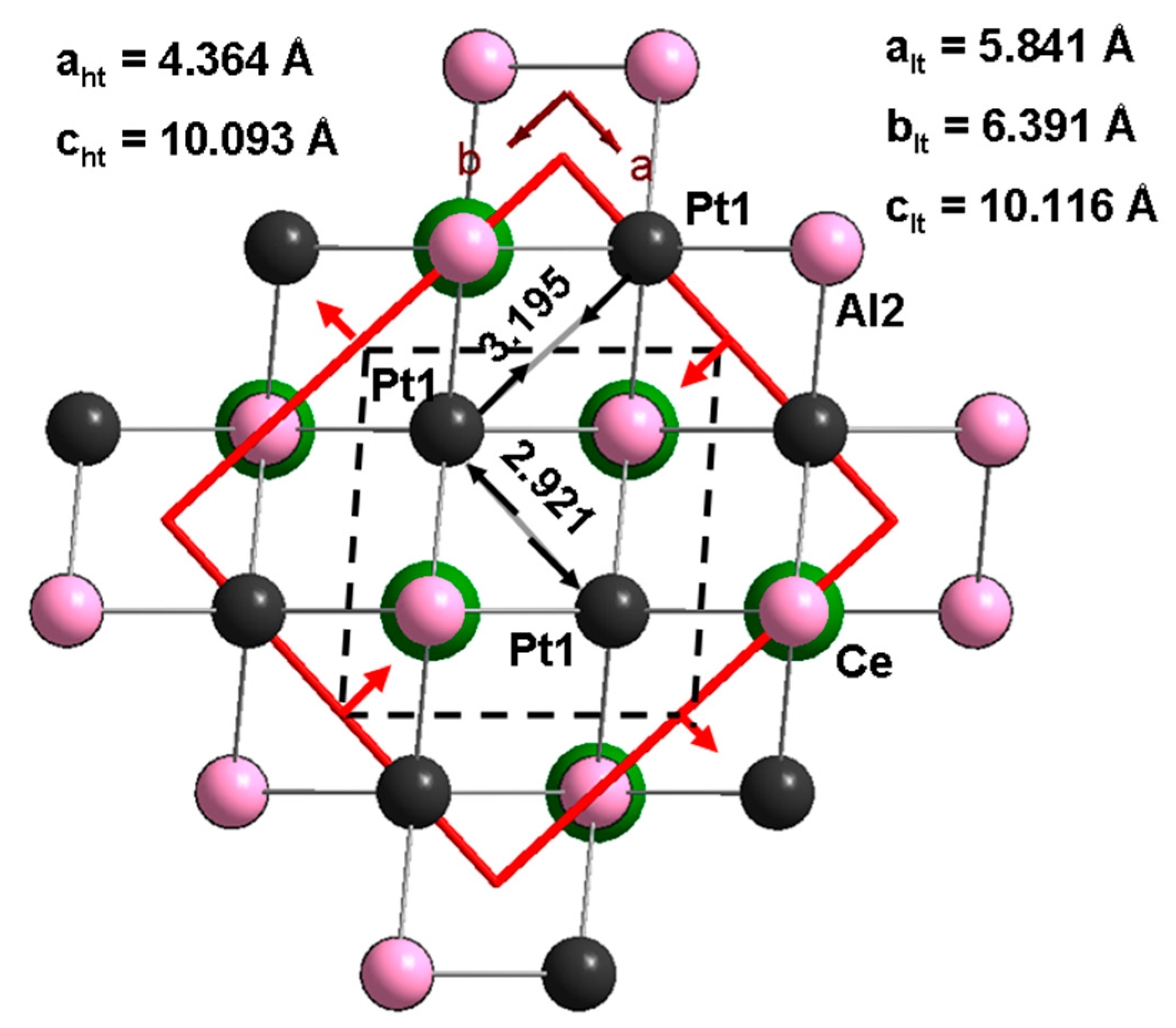
3.3. CePt2Al2 Crystal Structures
3.4. CePt2Al2 Phase Transition
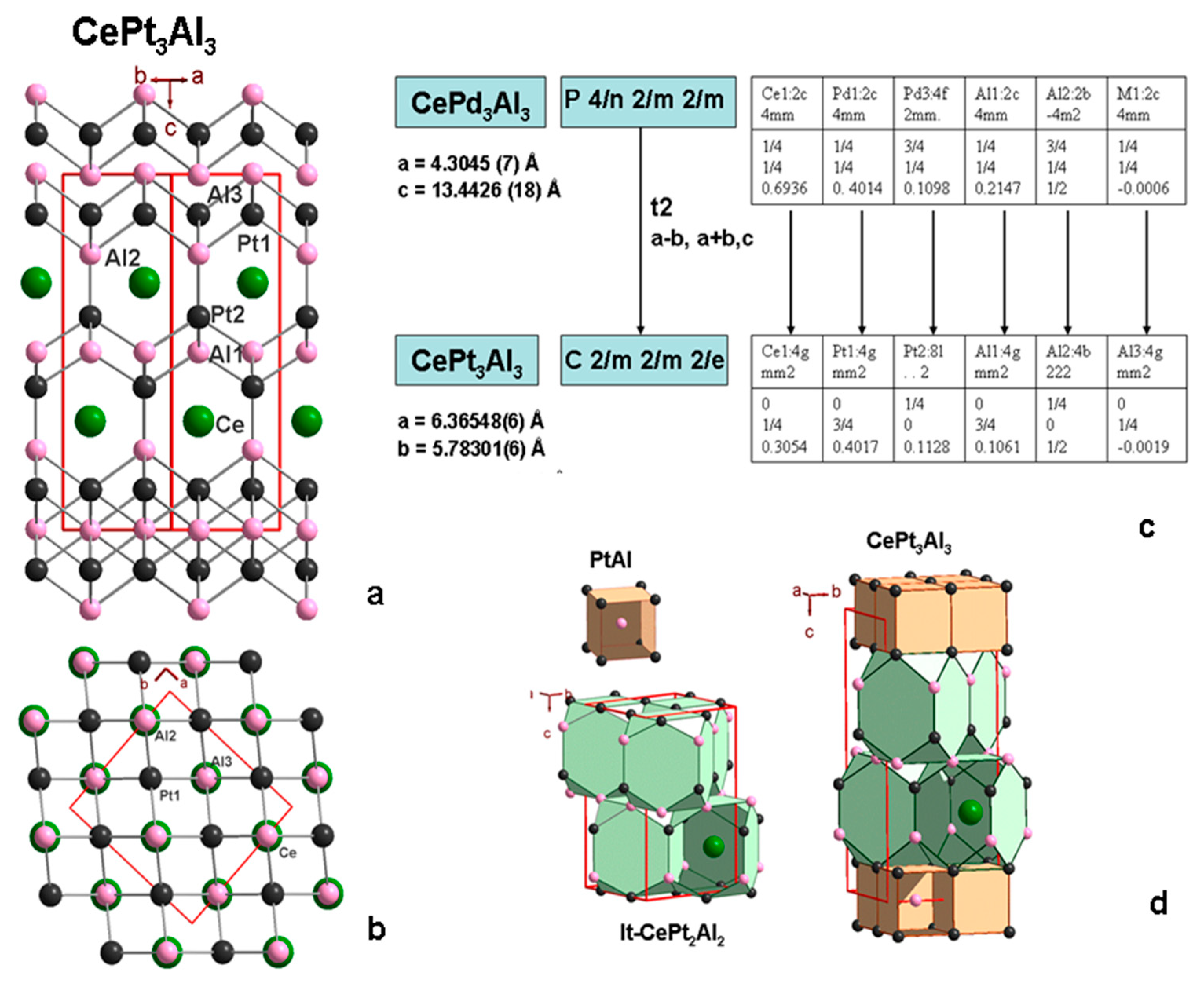
3.5. CePt3Al3 Crystal Structure
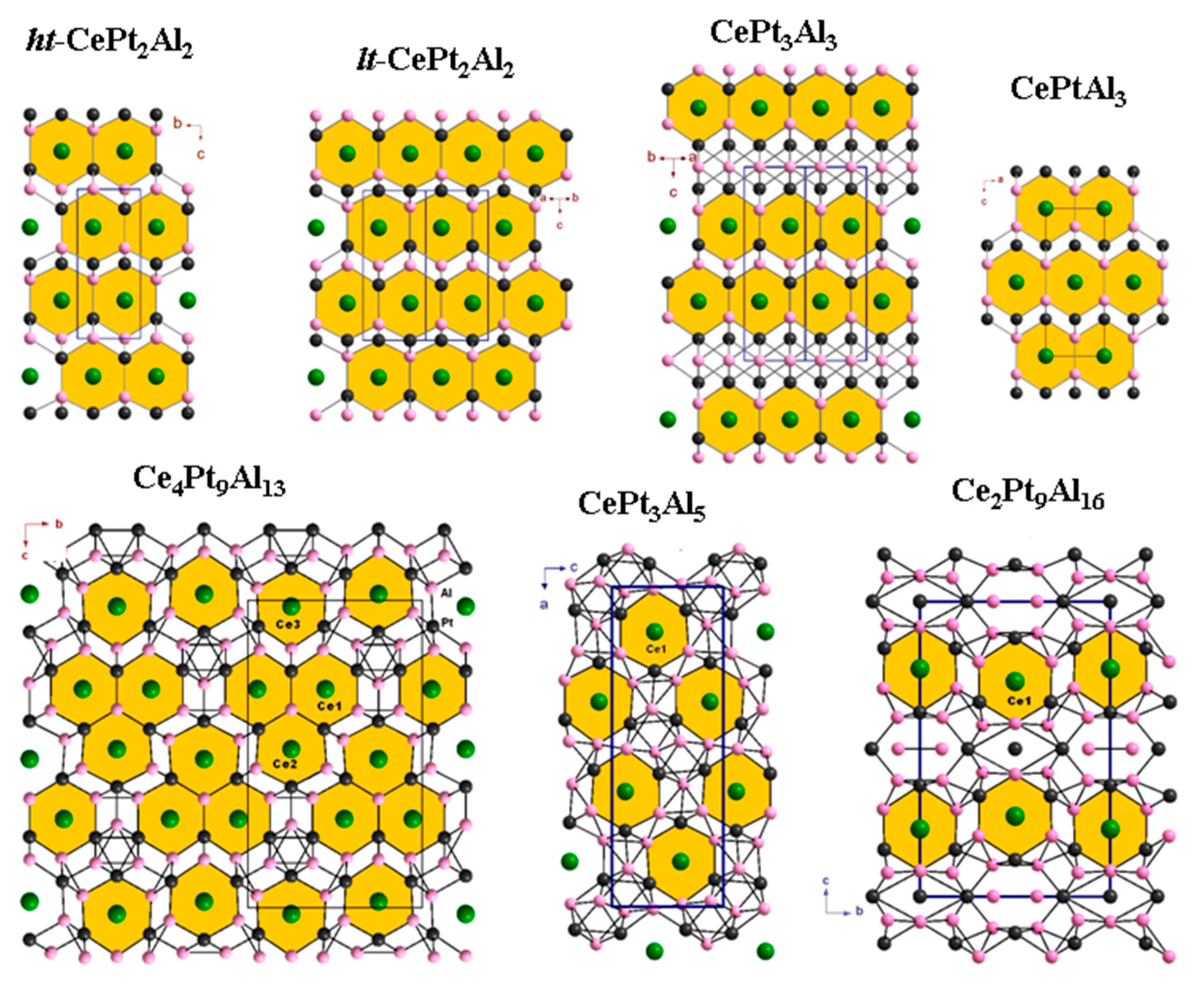
3.6. New Homologous Series
3.7. Crystal Structures of Cerium Platinum Aluminides with High Al Content
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dommann, A.; Hulliger, F.; Ott, H.R.; Gramlich, V. The crystal structure and some properties of CePt2Si2 and CePt2Ge2. J. Less Common Met. 1985, 110, 331–337. [Google Scholar] [CrossRef]
- Beyermann, W.P.; Hundley, M.F.; Canfield, P.C.; Godart, C.; Selsane, M.; Fisk, Z.; Smith, J.L.; Thompson, J.D. Antiferromagnetism and enhanced specific heat in CeM2Sn2 (M = Ni, Ir, Cu, Rh, Pd and Pt). Phys. B Condens. Matter 1991, 171, 373–376. [Google Scholar] [CrossRef]
- Zhang, P.; Zhai, H.F. Superconductivity in 122-type pnictides without iron. Phys. B Condens. Matter 2017, 2, 28. [Google Scholar] [CrossRef]
- Imai, M.; Ibuka, S.; Kikugawa, N.; Terashima, T.; Uji, S.; Yajima, T.; Kageyama, H.; Hase, I. Superconductivity in 122-type antimonide BaPt2Sb2. Phys. Rev. B 2015, 91, 014513. [Google Scholar] [CrossRef]
- Kitagawa, J.; Ishikawa, M. Magnetic properties and structural phase transition of CePd2Ga2 with CaBe2Ge2-type structure. J. Phys. Soc. Jpn. 1999, 68, 2380–2383. [Google Scholar] [CrossRef]
- Takabatake, T.; Tanaka, T.; Bando, Y.; Fudjii, H.; Takeda, N.; Ishikawa, M.; Oguro, I. Magnetic and structural transitions in CeRh2Sb2. Phys. B Condens. Matter 1997, 230–232, 223–225. [Google Scholar] [CrossRef]
- Doležal, P.; Klicpera, M.; Prchal, J.; Yavorský, P. Structural phase transition in CePd2Ga2 under hydrostatic pressure. Acta Phys. Pol. A 2015, 127, 219–221. [Google Scholar] [CrossRef]
- Eisenmann, B.; May, N.; Müller, W.; Schäfer, H. Eine neue strukturelle Variante des BaAl4-Typs: Der CaBe2Ge2-Typ. Z. Nat. B 1972, 27, 1155–1157. [Google Scholar] [CrossRef]
- Ban, Z.; Sikirica, M. The crystal structure of ternary silicides ThM2Si2 (M = Cr, Mn, Fe, Co, Ni, Cu). Acta Crystallogr. 1965, 18, 594–599. [Google Scholar] [CrossRef]
- Bruzzone, G.; Merlo, F. The strontium-aluminium and barium-aluminium systems. J. Less Common Met. 1975, 39, 1–6. [Google Scholar] [CrossRef]
- Shelton, R.N.; Braun, H.F.; Musick, E. Superconductivity and relative phase stability in 1:2:2 ternary transition metal silicides and germanides. Solid State Common 1984, 52, 797–799. [Google Scholar] [CrossRef]
- Hiebl, K.; Rogl, P. Magnetism and structural chemistry of ternary silicides: (RE, Th, U) Pt2Si2 (RE = Rare Earth). J. Magn. Magn. Mater. 1985, 50, 39–48. [Google Scholar] [CrossRef]
- Rossi, D.; Marazza, R.; Ferro, R. Ternary RM2X2 alloys of the rare earths with the precious metals and silicon (or germanium). J. Less Common Met. 1979, 66, 17–25. [Google Scholar] [CrossRef]
- Mayer, I.; Yetor, P.D. MPt2Si2 compounds of the ThCr2Si2 type. J. Less Common Met. 1977, 55, 171–176. [Google Scholar] [CrossRef]
- Venturini, G.; Malaman, B.; Roques, B. A monoclinic variant of the tetragonal CaBe2Ge2-type structure. J. Less Common Met. 1989, 146, 271–278. [Google Scholar] [CrossRef]
- Imre, A.; Hellmann, A.; Mewis, A. LaPt2Ge2 und EuPt2Ge2 – Neubestimmung der Kristallstrukturen. Z. Anorg. Allg.Chem. 2006, 632, 2217–2221. [Google Scholar] [CrossRef]
- Selsane, M.; Lebail, M.; Hamdaoui, N.; Kappler, J.P.; Noel, H.; Achard, J.C.; Godart, C. Structural, magnetic and valence properties of CeM2Sn2 (M = Ni, Cu, Rh, Pd, Ir, Pt). Phys. B Condens. Matter 1990, 163, 213–215. [Google Scholar] [CrossRef]
- Wenski, G.; Mewis, A. BaAl4-Denvative Structures of ARu2X2 (A = Ca, Sr, Ba, Eu; X—P, As) and of APt2P2 (A = Ca, Eu). Z. Nat. B 1986, 41b, 38–43. [Google Scholar] [CrossRef]
- Imre, A.; Hellmann, A.; Wenski, G.; Graf, J.; Johrendt, D.; Mewis, A. Inkommensurabel modulierte Kristallstrukturen und Phasenumwandlungen—Die Verbindungen SrPt2As2 und EuPt2As2. Z. Anorg. Allg. Chem. 2007, 633, 2037–2045. [Google Scholar] [CrossRef]
- Hulliger, F.; Nissen, H.U.; Wessicken, R. On new CaBe2Ge2-type representatives MAu2AI2. J. Alloys Compd. 1994, 206, 263–266. [Google Scholar] [CrossRef]
- Schank, C.; Jfihrling, F.; Luo, L.; Grauel, A.; Wassilew, C.; Borth, R.; Olesch, G.; Bredl, C.D.; Geibel, C.; Steglich, F. 4f-conduction electron hybridization in ternary Ce-TM-A1 compounds. J. Alloys Compd. 1994, 207, 329–332. [Google Scholar] [CrossRef]
- Chapona, L.C.; Goremychkina, E.A.; Osborn, R.; Rainford, B.D.; Short, S. Magnetic and structural instabilities in CePd2Al2 and LaPd2Al2. Phys. B Condens. Matter 2006, 378–380, 819–820. [Google Scholar] [CrossRef]
- Tursina, A.; Khamitcaeva, E.; Gribanov, A.; Gnida, D.; Kaczorowski, D. CePd2Al2, CePd3Al3, and CePd4Al4, A New Homologous Series Built of CaBe2Ge2- and CsCl-type Units. Inorg. Chem. 2015, 54, 3439–3445. [Google Scholar] [CrossRef] [PubMed]
- Bukhanko, N.; Gribanov, A.; Kaldarar, H.; Royanian, E.; Michor, H.; Bauer, E.; Murashova, E.; Seropegin, Y.; Grytsiv, A.; Rogl, P.; et al. Novel CePt2Al2: Crystal structure and physical properties. In Proceedings of the 16th International Conference on Solid Compounds of Transition Elements, Dresden, Germany, 26–31 July 2008; p. 172. [Google Scholar]
- Morozova, Y.S.; Tursina, A.I.; Dunaev, S.F.; Murashova, E.V. Ternary intermetallic compound CePt3Al3. In Proceedings of the VIIIth National Crystal Chemistry Conference, Suzdal, Russia, 30 May–3 June 2016; p. 181. [Google Scholar]
- STOE WinXPOW (Version 2.24); Stoe & Cie GmbH: Darmstadt, Germany, 1999.
- Rodriguez-Carvajal, J. Recent Developments of the Program FULLPROF, in Commission on Powder Diffraction (IUCr). Newsletter 2001, 26, 12–19. [Google Scholar]
- Roisnel, T.; Rodriguez-Carvajal, J. Materials science forum. In Proceedings of the European Powder Diffraction Conference, Warsaw, Poland, 19–22 September 2008; p. 118. [Google Scholar]
- Zlokazov, V.B.; Chernyshev, V.V. MRIA—A program for a full profile analysis of powder multiphase neutron-diffraction time-of-flight (direct and Fourier) spectra. J. Appl. Crystallogr. 1992, 25, 447–451. [Google Scholar] [CrossRef]
- Popa, N.C. The (hkl) dependence of diffraction line broadening caused by strain and size for all Laue groups in Rietveld refinement. J. Appl. Crystallogr. 1998, 31, 176–180. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Bärnighausen, H. Group-subgroup relations between space groups: A useful tool in crystal chemistry. MATCH Commun. Math. Chem. 1980, 9, 139–175. [Google Scholar]
- Müller, U. Kristallographische gruppe-untergruppe-beziehungen und ihre anwendung in der kristallchemie. Z. Anorg. Allg. Chem. 2004, 630, 1519. [Google Scholar] [CrossRef]
- Tursina, A.I.; Gribanov, A.V.; Bukhan’ko, N.G.; Rogl, P.; Seropegin, Y.D. Crystal structure of the novel compound Ce3Pt4Al6. Chem. Met. Alloys 2008, 1, 62–66. [Google Scholar] [CrossRef]
- Mock, S.; Pfleiderer, C.; Lohneysen, H. Low-temperature properties of CeTAl3 (T = Au, Cu, Pt) and CeAuGa3. J. Low Temp. Phys. 1999, 115, 1–14. [Google Scholar] [CrossRef]
- Tursina, A.I.; Bukhan’ko, N.G.; Gribanov, A.V.; Shchelkunov, V.A.; Nelyubina, Y.V. A new ternary aluminide, CePt3Al5. Acta Crystallogr. Sect. E Struct. Rep. Online 2005, 61, i285–i286. [Google Scholar] [CrossRef]
- Morozova, Y.; Gribanov, A.; Murashova, E.; Dunaev, S.; Grytsiv, A.; Rogl, P.; Giester, G.; Kaczorowski, D. Novel ternary compound Ce4Pt9Al13: Crystal structure, physical properties. J. Alloys Compd. 2018, 767, 496–503. [Google Scholar] [CrossRef]
- Tursina, A.; Murashova, E.; Noël, H.; Bukhan’ko, N.; Seropegin, Y. Crystal structure and magnetic properties of the new intermetallic Ce2Pt9Al16. Intermetallics 2009, 17, 780–783. [Google Scholar] [CrossRef]


| Empirical Formula | ht-CePt2Al2 | lt-CePt2Al2 | CePt3Al3 * |
|---|---|---|---|
| Molar mass, g/mol | 584.26 | 584.26 | 806.33 |
| Structure type, Pearson symbol | CaBe2Ge2, tP10 | CePt2Al2, oC20 | CePt3Al3, oC28 |
| Space group, Z | P4/nmm (129), 2 | Cmme (67), 4 | Cmme (67), 4 |
| Unit cell dimensions | |||
| a, Å | 4.3637(9) | 5.84138(2) | 6.36548(6) |
| b, Å | 4.3637(9) | 6.39099 (3), | 5.78301(6) |
| c, Å | 10.0925(14) | 10.11611(5) | 13.36245(19) |
| V, Å3 Calculated density, g/cm3 | 192.18(6) 10.097 | 377.657 (3)10.276 | 491.894(10) 10.888 |
| T, K | 623(1) | 295(1) | 298(2) |
| Radiation, λ, Å | synchrotron, 0.399962(13) | CuKα1, 1.54056 | CuKα1, 1.54056 |
| 2θ range, step° | 2–22.912, 0.002 | 5–95.19, 0.01 | 3–93.09, 0.01 |
| Total no. reflections | 79 | 115 | 162 |
| Refined parameters no. | 35 | 12 | 29 |
| Rietveld reliability factors | |||
| Rp | 0.060 | 0.024 | 0.037 |
| Rwp | 0.069 | 0.035 | 0.050 |
| Rexp | 0.060 | 0.016 | 0.020 |
| χ2 | 1.334 | 5.54 | 5.99 |
| Atom | Multiplicity, Wyckoff Letter, Site Symmetry | x/a | y/b | z/c | Uiso., Å2 |
|---|---|---|---|---|---|
| ht-CePt2Al2 | |||||
| Ce1 | 2c(4mm) | 1/4 | 1/4 | 0.7456(3) | 0.0211(9) |
| Pt1 | 2b(-4m2) | 3/4 | 1/4 | 1/2 | 0.0211(9) |
| Pt2 | 2c(4mm) | 1/4 | 1/4 | 0.1316(3) | 0.0211(9) |
| Al1 | 2a(-4m2) | 3/4 | 1/4 | 0 | 0.0211(9) |
| Al2 | 2c(4mm) | 1/4 | 1/4 | 0.3963(17) | 0.0211(9) |
| lt-CePt2Al2 | |||||
| Ce1 | 4g(mm2) | 0 | 1/4 | 0.24780(15) | 0.0117(4) |
| Pt1 | 4a(222) | 1/4 | 1/2 | 0 | 0.0224(4) |
| Pt2 | 4g(mm2) | 0 | 1/4 | 0.62972(11) | 0.0146(3) |
| Al1 | 4b(222) | 1/4 | 1/2 | 1/2 | 0.019(2) |
| Al2 | 4g(mm2) | 0 | 1/4 | 0.8940(7) | 0.013(2) |
| CePt3Al3 | |||||
| Ce1 | 4g(mm2) | 0 | 1/4 | 0.30545(16) | 0.0097(7) |
| Pt1 | 8l(…2) | 1/4 | 1/2 | 0.11280(8) | 0.0046(4) |
| Pt2 | 4g(mm2) | 0 | 1/4 | 0.59815(12) | 0.0064(5) |
| Al1 | 4b(222) | 1/4 | 1/2 | 1/2 | 0.001(3) |
| Al2 | 4g(mm2) | 0 | 1/4 | 0.7760(8) | 0.039(5) |
| Al3 | 4g(mm2) | 0 | 1/4 | −0.0019(9) | 0.057(5) |
| ht-CePt2Al2 | lt-CePt2Al2 | CePt3Al3 | ||||||
|---|---|---|---|---|---|---|---|---|
| Atom 1 | Atom 2 | d, Å | Atom 1 | Atom 2 | d, Å | Atom 1 | Atom 2 | d, Å |
| Ce1 | 4Pt1 | 3.302(3) | Ce1 | 4Pt1 | 3.3120(11) | Ce1 | 4Pt1 | 3.3540(18) |
| 4Pt2 | 3.3251(18) | 2Pt2 | 3.1727(7) | 2Pt2 | 3.1655(11) | |||
| 4Al1 | 3.369(3) | 2Pt2 | 3.4273(7) | 2Pt2 | 3.4335(10) | |||
| 4Al2 | 3.402(7) | 4Al1 | 3.3458(12) | 4Al1 | 3.3736(16) | |||
| 2Al2 | 3.254(3) | 2Al2 | 3.090(4) | |||||
| 2Al2 | 3.503(3) | 2Al2 | 3.364(4) | |||||
| Pt1 | 4Al2 | 2.420(7) | Pt1 | 4Al2 | 2.416(3) | Pt1 | 2Al3 | 2.611(7) |
| 4Pt1 | 3.0856(6) | 2Pt1 | 2.9207(10) | 2Al2 | 2.614(6) | |||
| 4Ce1 | 3.302(3) | 2Pt1 | 3.1955(15) | 2Al3 | 2.640(7) | |||
| 4Ce | 3.3120(11) | 2Pt1 | 2.8915(13) | |||||
| Pt1 | 3.0146(15) | |||||||
| 2Pt1 | 3.1827(13) | |||||||
| 2Ce1 | 3.3540(18) | |||||||
| Pt2 | 4Al1 | 2.5544(15) | Pt2 | 4Al1 | 2.5313(6) | Pt2 | Al2 | 2.377(11) |
| Al2 | 2.672(17) | Al2 | 2.673(7) | 4Al1 | 2.5185(8) | |||
| 4Ce1 | 3.3251(18) | 2Ce1 | 3.1727(7) | 2Ce1 | 3.1655(11) | |||
| 2Ce1 | 3.4273(7) | 2Ce1 | 3.4335(10) | |||||
| Al1 | 4Pt2 | 2.5544(15) | Al1 | 4Pt2 | 2.5313(6) | Al1 | 4Pt2 | 2.5185(8) |
| 4Al1 | 3.0856(6) | 2Al1 | 2.9207(10) | 2Al1 | 2.8915(13) | |||
| 4Ce1 | 3.369(3) | 2Al1 | 3.1955(15) | 2Al1 | 3.1827(13) | |||
| 4Ce1 | 3.3458(12) | 4Ce | 3.3736(16) | |||||
| Al2 | 4Pt1 | 2.420(7) | Al2 | 4Pt1 | 2.416(3) | Al2 | Pt2 | 2.377(11) |
| Pt2 | 2.672(17) | Pt2 | 2.673(7) | 4Pt1 | 2.614(6) | |||
| 4Ce1 | 3.402(7) | 2Ce1 | 3.254(3) | Al3 | 2.968(16) | |||
| 2Ce1 | 3.503(3) | 2Ce1 | 3.090(4) | |||||
| 2Ce1 | 3.364(4) | |||||||
| Al3 | 4Pt1 | 2.611(7) | ||||||
| 4Pt1 | 2.640(7) | |||||||
| 2Al3 | 2.8920(3) | |||||||
| Al2 | 2.968(16) | |||||||
| 2Al3 | 3.1831(3) | |||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murashova, E.; Morozova, Y.; Dunaev, S.; Kurenbaeva, Z.; Tursina, A. Ternary Aluminides of a New Homologous Series—CePt2Al2 and CePt3Al3: Crystal Structures and Thermal Properties. Crystals 2020, 10, 465. https://doi.org/10.3390/cryst10060465
Murashova E, Morozova Y, Dunaev S, Kurenbaeva Z, Tursina A. Ternary Aluminides of a New Homologous Series—CePt2Al2 and CePt3Al3: Crystal Structures and Thermal Properties. Crystals. 2020; 10(6):465. https://doi.org/10.3390/cryst10060465
Chicago/Turabian StyleMurashova, Elena, Yuliya Morozova, Sergey Dunaev, Zhanafiya Kurenbaeva, and Anna Tursina. 2020. "Ternary Aluminides of a New Homologous Series—CePt2Al2 and CePt3Al3: Crystal Structures and Thermal Properties" Crystals 10, no. 6: 465. https://doi.org/10.3390/cryst10060465
APA StyleMurashova, E., Morozova, Y., Dunaev, S., Kurenbaeva, Z., & Tursina, A. (2020). Ternary Aluminides of a New Homologous Series—CePt2Al2 and CePt3Al3: Crystal Structures and Thermal Properties. Crystals, 10(6), 465. https://doi.org/10.3390/cryst10060465




