Abstract
The BF4− anion is characterised by weak Lewis base properties; it is usually classified as a “non-coordinating anion”. The searches through the Cambridge Structural Database (CSD) were performed and it was found that the BF4− anion often occurs in crystal structures and it is involved in numerous intermolecular interactions; hydrogen bonds are the majority of them. The hydrogen bonds involving the BF4− anion as a proton acceptor are closer to linearity with the increase of the strength of interaction that is in line with the tendency known for other hydrogen bonds. However, even for short contacts between the proton and the Lewis base centre, slight deviations from linearity occur. The MP2/aug-cc-pVTZ calculations on the BF4−…HCN complex and on the BF4−…(HCN)4 cluster were also carried out to characterise corresponding C-H…F hydrogen bonds; such interactions often occur in crystal structures.
1. Introduction
The term ‘non-coordinating anion” was used in earlier [1,2,3,4] and in more recent studies [5] to characterise anions that do not interact with other species. However, it has been described that this term may be misleading since such anions known before as not able to be coordinated reveal properties to interact, at least weakly, with Lewis acid centres [1,4]. It was earlier explained that some ions matching this term were found to be coordinated in the water environment if water is rigorously excluded [6]. The BF4− species was often mentioned among the other so-called “non-coordinating” or “poorly-coordinating”anions [1]. However, it was mentioned that numerous crystal structures show the latter anion as a monodentate or bidentate bridging ligand [1]. It has been recognized early that the BF4− ion is not a completely non-nucleophilic species [7]; its reactivity was analysed recently [8], especially the reactions where the BF4− ion acts as a nucleophilic fluoride source were discussed in detail.
It seems that the tetrahedral BF4− moiety possesses a stable electronic and consequently stable energetic structure. The boron centre in the planar trigonal BF3 molecule is characterised by hypovalency [9] and the vacant p-orbital perpendicular to the plane of the molecule is responsible for the Lewis acid properties of BF3 species and for its interactions with Lewis bases [10,11,12]. In a case of strong nucleophiles such as the F− anion, it leads to the formation of the stable tetrahedral structures [13]. It is worth mentioning that in the Cambridge Structural Database [14,15] mostly tetravalent BF4− species are observed among the BF3 complexes with Lewis bases [13] and that, in general, the boron moieties are characterised mainly by tetravalency. The BF4− species is a conjugate base of the HBF4 superacid. However, the BF4− anion is unstable to H+ with respect to the HF elimination and this acid exists only in forms such as the H(H2O)n+BF4− or any another species where the proton is strongly coordinated by solvent molecules [16].
It has been described in various studies that the BF4− anion participates in hydrogen bond interactions since fluorine atoms may act as the Lewis base centres, especially as there are numerous examples of crystal structures where such interactions occur. One can mention the structure of S-amino thiodithiazyl salt, S3N2NH2+BF4− [17], where the hydrogen bonds are formed between the hydrogen atoms of the amine group and fluorine atoms of two related by symmetry BF4− anions; the F…H intermolecular distances are equal to 1.55(5) Å, and 2.14(6) Å. The crystal structure of the (CH3)3NHBF4 complex has been analysed by the X-ray diffraction methods in three phases [18]. For the room temperature phase III, the (CH3)3NH+ cations are attached to the BF4− anion by the N-H…F hydrogen bonds. These interactions are characterised by the following geometries: the N…F distance of 2.963(5) Å, the H…F distance amounting 2.07 Å, and the N-H…F angle is equal to 154.6°; for the second hydrogen bond; N…F - 2.932(2) Å, H…F - 2.03 Å, and N-H…F angle - 159.2°. It is worth noting that both N-H…F angles are not very close to linearity, below 160°.
In another study, interactions between imidazolium-based ionic liquid 1-butyl-3-methylimidazolium tetrafluoroborate and dimethyl sulfoxide were investigated by attenuated total reflection infrared spectroscopy (ATR-IR) and density functional theory calculations [19]. The similar experimental ATR-IR studies supported by the hydrogen nuclear magnetic resonance (1H NMR) and density functional theory calculations were performed for interactions between 1-butyl-3-methylimidazolium tetrafluoroborate and acetonitrile [20]. In both cases of latter studies [19,20], the Lewis base properties of fluorine centres of BF4− anion were analysed.
The angular (+)C-H…F(−) arrangements in complexes of the PF6− and BF4− species were analysed [21], and searches through the Cambridge Structural Database [14,15] were performed. The similar tendencies for these types of hydrogen bond as for the stronger O-H…O links were detected there. It means that for longer H…F distances, for both PF6− and BF4− complexes, the broad range of C-H…F angles it is observed that it is narrower for shorter distances, i.e., for stronger interactions. Thus, with the increase of the strength of the C-H…F hydrogen bond, this angle is closer to linearity.
The aim of this study is to check in detail the occurrence of hydrogen bonded arrangements with the BF4− anion acting as the Lewis base unit; this is based on the CSD searches, including the recent updates of this base. Two simple complexes and one cluster are analysed theoretically in detail to deepen the understanding of the nature of C-H…F hydrogen bonds.
2. Computational Methods
The calculations were performed on the BF4−…HCN complex and the BF4−…(HCN)4 cluster with the Gaussian16 set of codes [22] using the second-order Møller–Plesset perturbation theory (MP2) [23], and the aug-cc-pVTZ basis set [24]. Frequency calculations have been carried out at the same computational level to confirm that the obtained structures correspond to energetic minima or to transition states.
The Quantum Theory of ‘Atoms in Molecules’ (QTAIM) [25,26] was applied to analyse characteristics of bond critical points (BCPs). The QTAIM calculations were performed with the use of the AIMAll program [27]. The Natural Bond Orbital (NBO) method [28] was also applied to analyse electron charge density shifts being the result of complexation, particularly the orbital–orbital interactions. For example, the nB → σAH* overlap is often considered as the characteristic interaction of the A-H…B hydrogen bond [9,28]. nB designates the lone electron pair of the B proton acceptor (the Lewis base) and σAH* is an antibonding orbital of the proton donating bond (the Lewis acid). The nB → σAH* interaction is calculated as the second-order perturbation theory energy. For the complex and cluster considered here the hydrogen cyanide species play the role of the Lewis acid units and the BF4− anion acts as the Lewis base, hence for those species the nF → σHC* orbital–orbital interactions occur. The NBO orbital–orbital energies were calculated at HF/aug-cc-pVTZ level for the previously optimised geometries at the MP2/aug-cc-pVTZ level.
The BP86 functional [29,30] was applied and uncontracted Slater-type orbitals (STOs) with triple-ζ quality (ADF TZ2P basis set) as basis functions for all elements. The BP86/TZ2P decomposition energy calculations were performed with the ADF2017 program package [31] for two configurations of the BF4−…HCN complex using their geometries optimized previously at the MP2/aug-cc-pVTZ level. The ADF decomposition [31] applied follows the energy partition of Morokuma [32] and it is based on the instantaneous interaction energy, ΔEint, within, for example, the AB complex between two fragments (A and B), in the particular electronic reference state and in the frozen geometry of AB. This interaction energy is divided into three main components and the additional dispersion term, ΔEdisp, according to the equation given below.
ΔEint = ΔEelstat + ΔEPauli + ΔEorb + ΔEdisp
The term ΔEelstat corresponds to the quasi-classical electrostatic interaction between the unperturbed charge distributions of the prepared atoms and it is usually attractive. The Pauli repulsion, ΔEPauli, is the energy change associated with the transformation from the superposition of the unperturbed electron densities of the isolated fragments to the wave function that properly obeys the Pauli principle through explicit antisymmetrisation and renormalization of the product wave function. This term comprises the destabilizing interactions between electrons of the same spin on either fragment. The orbital interaction, ΔEorb, accounts for charge transfer and polarization effects.
3. Results and Discussion
3.1. Crystal Structures with BF4− Anion as Proton Acceptor in Hydrogen Bonds
It seems the BF4− anion is a very stable structure since its boron centre containing eight electrons in the valence shell obeys the octet rule. Besides, according to recent resources of crystal structures of the Cambridge Structural Database (CSD updates up to March 2020), the number of structures containing the BF4− anion is 16,088. This large number indicates that it is stable species not affected rather by the intermolecular interactions in crystals. However, it was mentioned earlier here that it may participate in hydrogen bond interactions and a few examples were specified.
Three CSD searches for the A-H…F (A = C, N and O) arrangements were performed here with the F-centre belonging to the BF4− anion. The following geometrical criteria of searches were applied: the A-H…F angle within the range of 120°–180°; H…F intermolecular distances up to 2.75 Å (distance longer by 0.4 Å than the corresponding sum of van der Waals radii according to the Pauling scale [33]), the A-H proton-donating bonds are normalized. The other search criteria related to the accuracy of structures determined were chosen; R ≤ 7.5%, e.s.d’s < 0.005 Å, additionally disorder, polymeric and powder structures as well as those with unresolved errors were excluded. Two thousand and eighty-six structures containing C-H…F arrangements were found—452 and 226 structures containing N-H…F and O-H…F systems, respectively. If the same search criteria are applied for only neutron diffraction results, four and one crystal structures are found with the C-H…F and N-H…F hydrogen bonds, respectively, but none for the O-H…F hydrogen bond.
Figure 1 presents two examples of crystal structures where the A-H…F links are observed; these are the neutron diffraction structures where the normalization of bonds containing hydrogen atoms was not performed. In the structure of bis(tetramethyl-tetraselenafulvalenium) tetrafluoroborate (Figure 1a, BIXBIT03 refcode) [34], each of B-F bonds of the BF4− anion is involved in the C-H…F hydrogen bond. Only the shortest H…F contact of 2.38 Å is below the corresponding sum of van der Waals radii, the remaining H…F links are longer than this sum, in the range of 2.55–2.69 Å. It indicates that, in spite, the C-H…F interactions may be classified as the charge-assisted hydrogen bond, since the BF4− anion plays a role of the Lewis base unit these are rather weak interactions, steered mainly by the dispersion forces.
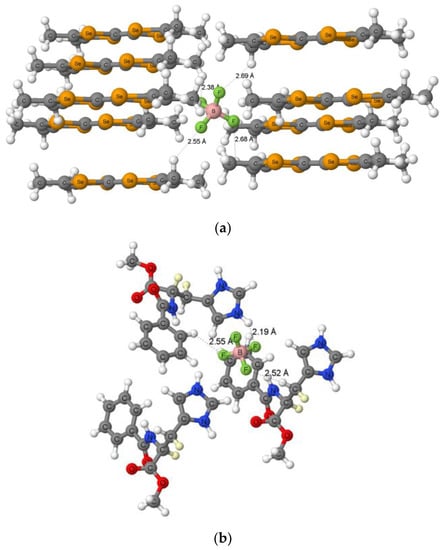
Figure 1.
The fragment of the crystal structure of (a) bis(tetramethyl-tetraselenafulvalenium) tetrafluoroborate, BIXBIT03 refcode, reference [34], and (b) (R)-dideutero methyl N-benzoyl -3-(1H-imidazol-3-ium-5-yl)alaninate tetrafluoroborate, SUXHID01 refcode, reference [35], H…F distances are shown.
The crystal structure of (R)-dideutero methyl N-benzoyl-3-(1H-imidazol-3-ium-5-yl)alaninate tetrafluoroborate (Figure 1b, SUXHID01 refcode) [35] is the only one neutron diffraction fulfilling the search criteria described above where the N-H…F(BF4−) hydrogen bonds exist. The corresponding H…F distance amounts 2.19 Å (Figure 1b) that is shorter than the corresponding sum of van der Waals radii; two additional C-H…F contacts are observed for the same Lewis base unit (BF4−) with the H…F distances overwhelming the above sum. The fourth B-F bond of the proton acceptor is not involved in any close contact.
For the three samples of complexes found in CSD (X-ray and neutron diffraction results), that are characterised by the occurrence of the C-H…F, N-H…F and O-H…F hydrogen bonds, the histograms of the H…F distances are presented in Figure 2. One should consider them as the approximate distribution of such distances since the C-H, N-H and O-H proton-donating bonds for X-ray structures were normalized here; it is a rather crude estimation since it does not take into account the complexation and, in general, the influence of crystal environment on these bonds. The maximum number of H…F contacts for the C-H…F hydrogen bonds occurs for the distance amounting to approximately 2.45 Å. This corresponds to the sum of van der Waals radii of fluorine and hydrogen atoms being in contact and it indicates the weak dispersion forces mainly steering the C-H…F arrangements. For the N-H…F and O-H…F hydrogen bonds, the maximum number of H…F contacts occur for 1.95 Å and 1.75–1.80 Å distances, respectively, that are shorter than the distance corresponding to the van der Waals sum. It may indicate the other forces, not dispersion ones, are more important to steer the N-H…F and O-H…F arrangements. These may be electrostatic and charge transfer/polarization forces, which is to be discussed here. The histograms presented in Figure 2 show the increasing strength of the hydrogen bonds in the following order: C-H…F < N-H…F < O-H…F, since the maximum number of contacts in three samples considered here corresponds to the decreasing H…F distance, respectively. It corresponds also to the increase of the proton-donating properties of A-H bonds that follow the electronegativity increase of the A-centre.
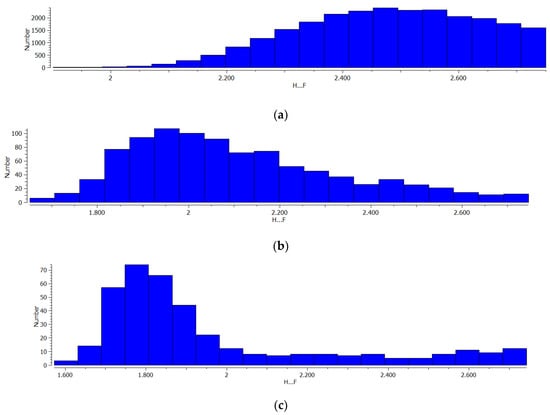
Figure 2.
The histograms of the H…F distances for (a) C-H…F, (b) N-H…F and (c) O-H…F systems taken from CSD and considered in this study.
Figure 3 presents histograms of the A-H…F angles. In the case of C-H…F systems (Figure 3a), the strictly defined maximum is not observed, and a similar number of systems occur in the range of 120°–155° angles. For the greater angles, a decrease of the number of systems occurs. In the case of N-H…F and O-H…F hydrogen bonds, the greatest number of systems are observed for the 156°–164° angles. This may mean the hydrogen bonds with fluorine centres of the BF4− anion are not strictly linear systems and any additional factors may disturb the expected linear arrangement. Figure 4 presents the scatter plots for the dependencies between H…F distance and the corresponding A-H…F angle for three samples of hydrogen bonds discussed here. One can see that for all samples the range of the A-H…F angle decreases with the decrease of the H…F distance being narrow and close to 180° for shorter H…F contacts. It is in line with the observations of the previous studies [21]. However, one can see (Figure 4) that this narrow range of angles is situated below the linearity, for 160°–170° angles rather, for C-H…F and N-H…F hydrogen bonds. This range is closer to 180° for the O-H…F systems but not exactly. It confirms the former conclusions concerning Figure 3.
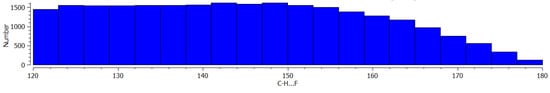
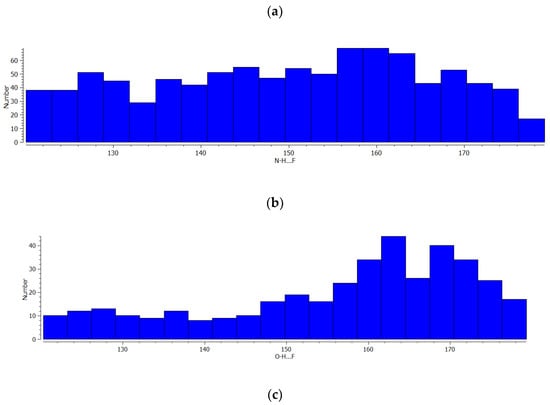
Figure 3.
The histograms of the A-H…F angles for systems of three samples taken from CSD and considered in this study (a) A = C, (b) A = N and (c) A = O.
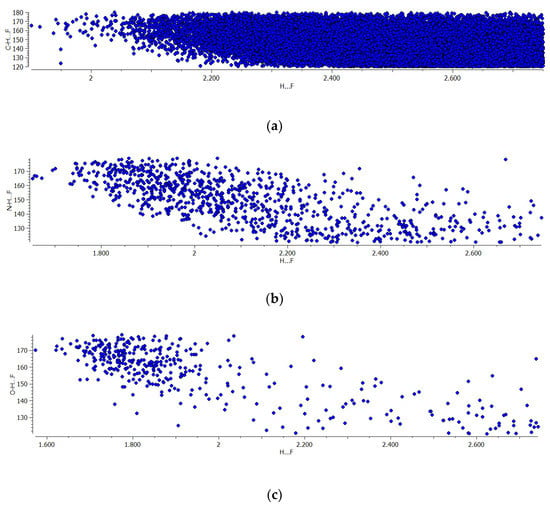
Figure 4.
The scatterplots H…F distance vs. A-H…F angle for systems of three samples taken from CSD and considered in this study (a) A = C, (b) A = N and (c) A = O.
3.2. Theoretical Analysis of the Lewis Base Properties of the BF4− Anion
It was noted earlier here that the A-H…F hydrogen bonds are slightly disturbed from linearity even for short H…F distances. This may result from external factors, like the intermolecular forces in crystals and with the internal factors related to properties of the BF4− anion. There are numerous studies where it is pointed out that the electrostatic potential (EP) at molecular surfaces of interacting species is responsible for their mutual arrangement in the complex formed [36,37,38,39,40]. Consequently, contacts between the most positive EP areas and the most negative EP sites are often observed in numerous complexes and clusters. This is only the rough description and numerous exceptions are observed. Especially, significant changes in the geometries of interacting species are observed for strong interactions characterized by meaningful electron charge density shifts being a result of complexation as well as by the contribution of dispersion forces in the establishment of the geometry of a complex considered [39,40].
The σ-hole concept [39,40] explains the existence of areas of the positive EP for numerous atomic centres that consequently may act as Lewis acids interacting with nucleophiles. The BF4− anion as negatively charged species reveals the Lewis base properties. Figure 5 presents the EP map for the BF4− anion calculated at the 0.001 au electron density surface. The whole surface is characterized by the negative EP; the maximum EP values of −0.188 au are observed for F-atoms while the EP minima of −0.208 au occur at the boron centre in directions being bisectors of the F-B-F angles, four such EP minima are observed (Figure 5). The EP map presented here results from the MP2/aug-c-pVTZ calculations that were also applied to optimise geometries of the BF4−…HCN complex and the BF4−…(HCN)4 cluster. The EP map of the BF4− anion may suggest that in the BF4−…HCN complex the C-H proton-donating bond of hydrogen cyanide is directed to B-centre that is characterised by the EP minimum. However, the B-centre is obscured by the fluorine atoms. The full optimization of the BF4−…HCN complex led to the configuration presented in Figure 6a that corresponds to the energetic minimum (named hereafter a nonlinear configuration). On the other hand, the configuration with the fixed linear B-F…H-C≡N arrangement was optimized (C3v symmetry, Figure 6b); it corresponded to the transition state; it is the second order saddle point, since two imaginary frequencies are observed here.
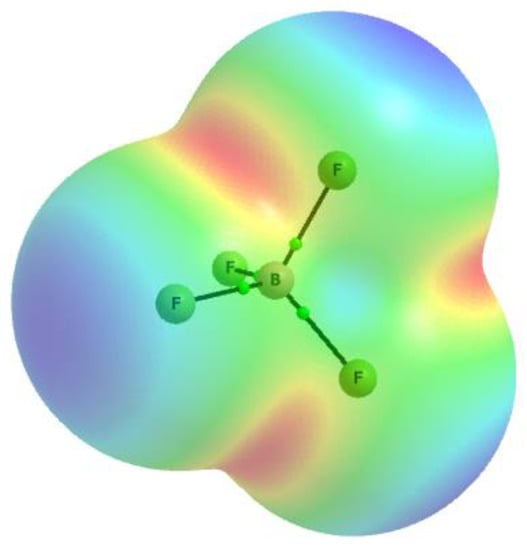
Figure 5.
The electrostatic potential (EP) map calculated at the 0.001 au molecular surface for the BF4− anion; from the EP minimum (red) to maximum (blue) values, there are four EP minima for the anion situated at bisectors of F-B-F angles (three are noticeable in the figure).
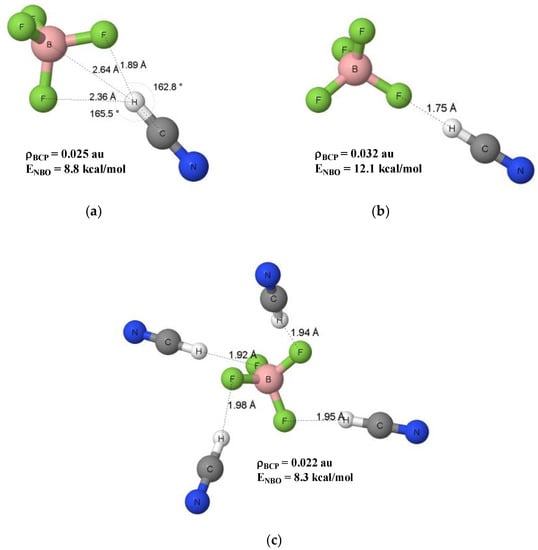
Figure 6.
The nonlinear (a) and linear (b) configurations of the BF4−…HCN complex, the BF4−…(HCN)4 cluster (c) is also presented; a few parameters are shown in this figure, such as distances, angles, electron densities at BCPs, ρBCP’s, and orbital–orbital overlap energies, ENBO’s.
For the nonlinear configuration, the H…F intermolecular distance equal to 1.89 Å is observed with the corresponding C-H…F angle of 162.8°. The H…B intermolecular distance is equal to 2.64 Å that corresponds to the C-H…B angle amounting 165.5°. There is another intermolecular contact characterised by the H…F distance of 2.36 Å (Figure 6a). In the case of linear configuration, the H…F distance of 1.75 Å is observed (Figure 6b). Table 1 presents energetic characteristics of both configurations as well as of the BF4−…(HCN)4 cluster. The MP2 binding energy that includes the correction for basis set superposition error (BSSE) [41], designated as ΔEbin(MP2,BSSE), shows that the nonlinear configuration is slightly more stable than the linear configuration since this energy is equal to −16.2 kcal/mol for the former species while it amounts −15.3 kcal/mol for the latter one. The BSSE correction amounts 0.6 and 0.4 kcal/mol, respectively.

Table 1.
The energetic characteristics (in kcal/mol) of the linear and nonlinear configurations of the BF4−…HCN complex.
Similarly, the interaction energy is “more negative’ for the nonlinear configuration than for the linear one. The deformation energy, ΔEdef [42], is not important for both configurations; it amounts to 0.4 and 0.5 kcal/mol, indicating that complexation does not affect the geometry of interacting species (BF4− and HCN) significantly. It is worth noting that the interaction energy does not take into account the deformation resulting from complexation but the binding energy does [42] (Equation (2)).
ΔEbin = ΔEint + ΔEdef
The Hartree–Fock interaction energies are presented in Table 1, ΔEint(HF); one may assume that correlation energy, ΔEcorr, may be calculated from the following equation (Equation (3)).
ΔEcorr = ΔEint(MP2) − ΔEint(HF)
However, it is an approximate evaluation since, according to the Löwdin definition [43] that is commonly accepted, “the correlation energy for a certain state with respect to a specified Hamiltonian is the difference between the exact eigenvalue of the Hamiltonian and its expectation value in the HF approximation for the state under consideration.” The MP2 method takes into account correlation but it is still an approximate approach. Table 1 shows that the correlation energy is more important for the nonlinear configuration than for the linear one; the dispersion energy is the most important, attractive term of the correlation energy. One may speculate that the dispersion forces are those which cause the nonlinear configuration to be more stable than the linear one since the difference between the HF interaction energies for both configurations amounts only 0.3 kcal/mol while it is more than three times greater for MP2 results.
The other important conclusion is that the more stable configuration is characterised by the C-H…F angle of 162.8° that corresponds to the CSD searches presented earlier here, since the maximum number of C-H…F systems are not linear ones. The nonlinearity of the BF4−…HCN complex is connected with another attractive H…F contact, and with the H…B interaction (the EP for boron is more negative than for F-centres).
It is worth mentioning that, for linear and nonlinear configurations, only one H…F bond path connects the BF4− and HCN species; in the nonlinear configuration it corresponds to the shorter H…F distance (Figure 6a). The electron density at the H…F BCP, ρBCP, amounts to 0.025 and 0.032 au for the nonlinear and linear configurations, respectively. The greater ρBCP value for the latter species than for the former one is observed since the H…F contact for the linear configuration is shorter than for the nonlinear configuration. In both cases, the positive laplacian values, ∇2ρBCP, are observed, while both total electron energy density values at BCPs are negligible, very close to zero.
It was pointed out in various studies that the electron density at BCP of the intermolecular bond path correlates with the interaction and/or binding energy [44]. It is not a case for configurations considered here where more negative ΔEin and ΔEbin values are observed for the smaller ρBCP value related to the nonlinear configuration. However, in this case, ρBCP corresponds to the single H…F contact, but the BF4− anion and the hydrogen cyanide species are connected also by another longer H…F contact and by the H…B link. However, the latter additional connections do not have the corresponding bond paths.
The energy corresponding to the nF → σCH* orbital–orbital interaction (ENBO) is presented in Figure 6. It corresponds to the shorter contact for the nonlinear configuration and it is equal to 8.8 kcal/mol, and this energy for the longer (C)H…F contact of 2.36 Å amounts 0.8 kcal/mol. For the linear configuration, only for the shortest H…F distance the nF → σCH* overlap is observed with the energy of interaction (ENBO) amounting 12.1 kcal/mol. These overlaps do not correspond to the HOMO-LUMO (highest occupied molecular orbital - lowest unoccupied molecular orbital) gap, however. The ADF-NBO calculations [31,45] were performed here for the abovementioned BP86/TZ2P results that are related to previously optimised MP2/aug-cc-pVTZ structures. The HOMO-LUMO energy gap for the isolated BF4− anion is equal to 0.693 hartree, showing this is the stable structure; this gap corresponds to nF (lone electron pair–HOMO) and σBF* (LUMO) orbitals. In a case of the linear BF4−…HCN complex, this energy gap amounts to 0.287 hartree that is related to nF (HOMO, BF4− unit) and πCN* (LUMO, HCN unit) orbitals. The abovementioned nF → σCH* overlap is energetically related to the HOMO(−1)-LUMO(+1) pair of orbitals. For the nonlinear BF4−…HCN complex, the HOMO-LUMO gap is equal to 0.152 hartree and it corresponds to the same pair of orbitals as in the linear configuration. The nF → σCH* overlap for this nonlinear configuration corresponds to the HOMO(−5)-LUMO(+3) pair of orbitals. However, the HOMO’s (from −1 to −5) are very close energetically; similarly, the LUMO’s (from +1 to +3) are energetically close.
Figure 6c shows the BF4−…(HCN)4 cluster corresponding to the energetic minimum. Each C-F bond of the anion is involved in the C-H…F hydrogen bond with the H…F distances of 1.92-1.98 Å, the C-H…F angles in the range of 169.0°–169.4° are observed for these contacts. The mean electron density, ρBCP for the bond critical point of the H…F bond path is equal to 0.022 au while the mean value of the energy corresponding to the nF → σCH* overlap amounts 8.3 kcal/mol. One can see that four (C)H…F contacts in the cluster are similar to that occurring for the nonlinear configuration of the BF4−…HCN complex. It is worth to mention that the mean ΔEbin(MP2) energy for the cluster is equal to −13.7 kcal/mol. It is calculated as the difference between the energy of the cluster and four times the energy of hydrogen cyanide, and this difference is divided by four. This energy for the nonlinear complex is equal to −16.8 kcal/mol (Table 1, values not corrected for BSSE are compared). It means that the interactions between HCN molecules and the anion are weaker than the interaction in the nonlinear complex.
Table 2 presents the results of the decomposition of the energy of interaction for the nonlinear and linear configurations of the BF4−…HCN complex. One can see that the DFT (Density Functional Theory) interaction energies, ΔEint’s, with the use of the uncontracted Slater-type orbitals (STOs) as basis functions being equal to −17.4 kcal/mol and −16.0 kcal/mol for nonlinear and linear configurations, respectively, are in good agreement with the MP2/aug-cc-pVTZ results, −17.2 kcal/mol and −16.2 kcal/mol (Table 1).

Table 2.
The BP86/TZ2P interaction energy for two configurations of the BF4−…HCN complex, the terms resulting from the decomposition are given (all energies in kcal/mol) according to Equation (1).
Table 2 shows that for both configurations the electrostatic interaction is the most important attractive term, almost the same electrostatic energies are observed for these configurations. The orbital–orbital interactions are more important for linear configuration that is in line with the NBO results which show the interaction corresponding to nF → σCH* overlap is more important for the linear configuration. However, for the latter configuration, the repulsive interaction, ΔEPauli, is greater than for the nonlinear system. This is the reason the nonlinear configuration is more stable than the linear one, less important Pauli repulsion and ¨more negative¨ dispersion energy. It was pointed out earlier here, on the basis of the HF and MP2 results, that the dispersion forces are responsible the nonlinear configuration is more stable than the linear one.
4. Summary
In contrast to earlier studies stating that the tetrafluoroborate BF4− species is a non-coordinating anion, it is shown here that it interacts with electrophiles very often. There are numerous examples of crystal structures where this anion participates in hydrogen bonds. However, the structural results show that the linearity of the hydrogen bonds with the BF4− anion acting as the proton acceptor is slightly disturbed. This is the internal reason of such a situation connected with the structure of this anion and it is confirmed by the theoretical calculations. The MP2/aug-cc-pVTZ results for the BF4−…HCN complex and for the BF4−…(HCN)4 cluster show that the non-linear C-H…F arrangements are more stable than the linear one. The non-linear systems are partly stabilised by the dispersion forces.
The CSD searches performed here show that the slight nonlinearity of the hydrogen bonds with the BF4− species as the proton acceptor is their common characteristic. The question is if this is also the common feature of other tetrahedral ions. For example the NH4+…NCH and NH4+…N2 complexes, as well as the NH4+…(NCH)n and NH4+…(N2)n clusters (n up to 8), have been analysed theoretically. It has been found that these systems are linked by the N-H…N hydrogen bonds and the N…N pnicogen bonds. The latter interactions occur only for clusters containing five and more NCH or N2 ligands. All N-H…N hydrogen bonds observed for the above species are linear [46]. Similarly, the NF4+…NCH complex and the NF4+…(NCH)n clusters have been analysed theoretically and it was found the N-F…N links are linear or nearly so [47].
Maybe the angle distortion observed for the crystal structures of the BF4− anion is a common characteristic of hydrogen bonds of other structures of tetrahalogenoborates and of 13th group analogues (triel elements). It seems probable since such distortion is often observed in crystal structures. For example, the structures of ammonia boranes were analysed; in one of these structures, AlCl4− anions occur that are proton acceptors in the N-H…Cl hydrogen bonds [48]; the latter systems are not linear. However, such nonlinearity of hydrogen bonds in crystal structures containing TX4− anions (T is the triel centre while X designates halogen) needs additional investigations.
Funding
This research was funded by the Spanish Government MINECO/FEDER, grant number CTQ2016-80955-P and by the Basque Government-Eusko Jaurlaritza, grant number IT1254-19.
Acknowledgments
Technical and human support provided by Informatikako Zerbitzu Orokora-Servicio General de Informática de la Universidad del País Vasco (SGI/IZO-SGIker UPV/EHU), Ministerio de Ciencia e Innovación (MICINN), Gobierno Vasco Eusko Jaurlanitza (GV/EJ), European Social Fund (ESF) is gratefully acknowledged.
Conflicts of Interest
The author declares no conflict of interest.
References
- Beck, W.; Sünkel, K. Metal Complexes of Weakly Coordinating Anions. Precursors of Strong Cationic Organometallic Lewis Acids. Chem. Rev. 1988, 88, 1405–1421. [Google Scholar] [CrossRef]
- Garrison, J.C.; Youngs, W.J. Ag(I) N-Heterocyclic Carbene Complexes: Synthesis, Structure, and Application. Chem. Rev. 2005, 105, 3978–4008. [Google Scholar] [CrossRef] [PubMed]
- Pérez, J.; Riera, L. Organometallic complexes as anion hosts. Chem. Commun. 2008, 5, 533–543. [Google Scholar] [CrossRef] [PubMed]
- Krossing, I.; Raabe, I. Noncoordinating Anions–fact or Fiction? A Survay of Likely Candidates. Angew. Chem. Int. Ed. 2004, 43, 2066–2090. [Google Scholar] [CrossRef] [PubMed]
- Phipps, R.J.; Hamilton, G.L.; Toste, F.D. The progression of chiral anions from concepts to applications in asymmetric catalysis. Nat. Chem. 2012, 4, 603–614. [Google Scholar] [CrossRef]
- Rosenthal, M.R. The Myth of the Non-Coordinating Anion. J. Chem. Educ. 1973, 50, 331–335. [Google Scholar] [CrossRef]
- Farooq, O.; Tiers, G.V.D. Alkali metal salts of perfluorinated complex anions. Effective reagents for nucleophilic fluorination. J. Org. Chem. 1994, 59, 2122–2124. [Google Scholar] [CrossRef]
- Cresswell, A.J.; Davies, S.G.; Roberts, P.M.; Thomson, J.E. Beyond the Balz-Schiemann Reaction: The Utility of Tetrafluoroborates and Boron Trifluoride as Nucleophilic Fluoride Sources. Chem. Rev. 2015, 115, 566–611. [Google Scholar] [CrossRef]
- Weinhold, F.; Landis, C. Valency and Bonding, A Natural Bond Orbital Donor—Acceptor Perspective; Cambridge University Press: Cambridge, UK, 2005; pp. 306–351, 593–660. [Google Scholar]
- Hirao, H.; Omoto, K.; Fujimoto, H. Lewis Acidity of Boron Trihalides. J. Phys. Chem. A 1999, 103, 5807–5811. [Google Scholar] [CrossRef]
- Jacobsen, H. Hypovalency—A kinetic energy density description of 4c-2e bond. Dalton Trans. 2009, 21, 4252–4258. [Google Scholar] [CrossRef]
- Grabowski, S.J. Boron and other Triel Lewis Acid Centers: From Hypovalency to Hypervalency. ChemPhysChem 2014, 15, 2985–2993. [Google Scholar] [CrossRef] [PubMed]
- Grabowski, S.J. π-Hole Bonds: Boron and Aluminium Lewis Acid Centers. ChemPhysChem 2015, 18, 1470–1479. [Google Scholar] [CrossRef] [PubMed]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge structural database. Acta Cryst. 2016, B72, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.; Allen, F.H.; Willett, P. The scientific impact of the Cambridge Structural Database: A citation-based study. J. Appl. Cryst. 2010, 43, 811–824. [Google Scholar] [CrossRef]
- Reed, C.A. The Strongest Acid. Chem. N. Z. 2011, 174–179. [Google Scholar]
- Marcellus, C.G.; Oakley, R.T.; Cordes, A.W.; Pennington, W.T. The preparation and the crystal and molecular structure of S3N2NH2+BF4−; a molecular orbital study of the protonation of the trisulphur trinitride anion. Can. J. Chem. 1984, 62, 1822–1827. [Google Scholar] [CrossRef]
- Ishida, H.; Kashino, S.; Ikeda, R. Crystal Structure of Trimethylammonium Tetrafluoroborate in Three Solid Phases. Z. Naturforsch. A Phys. Sci. 2000, 55, 765–768. [Google Scholar] [CrossRef]
- Wang, N.-N.; Zhang, Q.-G.; Wu, F.-G.; Li, Q.-Z.; Yu, Z.-W. Hydrogen Bonding Interactions between a Representative Pyridinium-Based Ionic Liquid [BuPy][BF4] and Water/Dimethyl Sulfoxide. J. Phys. Chem. B 2010, 114, 8689–8700. [Google Scholar] [CrossRef]
- Zheng, Y.-Z.; Wang, N.-N.; Luo, J.-J.; Zhou, Y.; Yu, Z.-W. Hydrogen-bonding interactions between [BMIM][BF4] and acetonitrile. Phys. Chem. Chem. Phys. 2013, 15, 18055–18064. [Google Scholar] [CrossRef]
- Braga, D.; Maini, L.; Polito, M.; Grepioni, F. Hydrogen Bonding Interactions Between Ions: A Powerful Tool in Molecular Crystal Engineering. In Supramolecular Assembly via Hydrogen Bonds II; Springer: Berlin/Heidelberg, Germany, 2004; Volume 111, pp. 1–32. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 16, Revision A.03; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Møller, C.; Plesset, M.S. Note on an Approximation Treatment for Many-Electron Systems. Phys. Rev. 1934, 46, 618–622. [Google Scholar] [CrossRef]
- Kendall, R.A.; Dunning, T.H., Jr.; Harrison, R.J. Electron Affinities of the First-Row Atoms Revisited. Systematic Basis Sets and Wave Functions. J. Chem. Phys. 1992, 96, 6796–6806. [Google Scholar] [CrossRef]
- Bader, R.F.W. Atoms in Molecules, A Quantum Theory; Oxford University Press: Oxford, UK, 1990. [Google Scholar]
- Matta, C.; Boyd, R.J. Quantum Theory of Atoms in Molecules: Recent Progress in Theory and Application; Wiley-VCH: Weinheim, Germany, 2007. [Google Scholar]
- Keith, T.A. AIMAll, Version 13.05.06; TK Gristmill Software: Overland Park, KS, USA, 2013. [Google Scholar]
- Reed, E.; Curtiss, L.A.; Weinhold, F. Intermolecular Interactions from a Natural Bond Orbital, Donor-Acceptor Viewpoint. Chem. Rev. 1988, 88, 899–926. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P. Density-functional approximation for the correlation energy of the inhomogeneous electron gas. Phys. Rev. B 1986, 33, 8822–8824. [Google Scholar] [CrossRef] [PubMed]
- ADF2017, SCM, Theoretical Chemistry, Vrije Universiteit, Amsterdam, The Netherlands. Available online: http://www.scm.com (accessed on 18 May 2020).
- Morokuma, K.J. Molecular Orbital Studies of Hydrogen Bonds. III. C=O···H–O Hydrogen Bond in H2CO···H2O and H2CO···2 H2O. J. Chem. Phys. 1971, 55, 1236. [Google Scholar] [CrossRef]
- Pauling, L. The Nature of the Chemical Bond, 3rd ed.; Cornell University Press: New York, NY, USA, 1960; pp. 88–102. [Google Scholar]
- Emge, T.J.; Wang, H.H.; Beno, M.A.; Williams, J.M.; Whangbo, M.-H.; Evain, M. Effect of anion ordering on the H-anion interactions and band electronic structure of (TMTSF)2BF4 at 20 K. J. Am. Chem. Soc. 1986, 108, 8215–8223. [Google Scholar] [CrossRef]
- Albinati, A.; Cesarotti, E.; Mason, S.A.; Rimoldi, I.; Rizzato, S.; Zerla, D. Histidine and deuterium-labelled histidine by asymmetric catalytic reduction and assignment of the absolute stereochemistry by neutron diffraction. Tetrahedron Assym. 2010, 21, 1162–1165. [Google Scholar] [CrossRef]
- Politzer, P.; Truhlar, D.G. Chemical Applications od Atomic and Molecular Electrostatic Potentials; Plenum; Springer Science & Business Media: New York, NY, USA, 1981. [Google Scholar]
- Pingale, S.S.; Gadre, S.R.; Bartolotti, L.J. Electrostatic insights into molecular hydration process: A case study of crown ethers. J. Phys. Chem. A 1998, 102, 9987–9992. [Google Scholar] [CrossRef]
- Murray, J.S.; Lane, P.; Politzer, P. Expansion of the σ-hole concept. J. Mol. Model. 2009, 15, 723–729. [Google Scholar] [CrossRef]
- Politzer, P.; Murray, J.S.; Clark, T. Halogen bonding: An electrostatically-driven highly directional noncovalent interaction. Phys. Chem. Chem. Phys. 2010, 12, 7748–7758. [Google Scholar] [CrossRef]
- Politzer, P.; Murray, J.S.; Clark, T. Halogen bonding and other σ-hole interactions: A perspective. Phys. Chem. Chem. Phys. 2013, 15, 11178–11189. [Google Scholar] [CrossRef] [PubMed]
- Boys, S.F.; Bernardi, F. The calculation of small molecular interactions by the differences of separate total energies. Some procedures with reduced errors. Mol. Phys. 1970, 19, 553–561. [Google Scholar] [CrossRef]
- Grabowski, S.J.; Sokalski, W.A. Diffrent types of hydrogen bonds: Correlation analysis of interaction energy components. J. Phys. Org. Chem. 2005, 18, 779–784. [Google Scholar] [CrossRef]
- Löwdin, P.-O. Correlation Problem in Many-Electron Quantum Mechanics I. Review of Different Approaches and Discussion of Some Current Ideas. Adv. Chem. Phys. 1959, 2, 207–322. [Google Scholar]
- Grabowski, S.J. What is the Covalency of Hydrogen Bonding? Chem. Rev. 2011, 11, 2597–2625. [Google Scholar] [CrossRef] [PubMed]
- Glendening, E.D.; Badenhoop, J.K.; Reed, A.E.; Carpenter, J.E.; Bohmann, J.A.; Morales, C.M.; Landis, C.R.; Weinhold, F. NBO, 6.0; Theoretical Chemistry Institute, University of Wisconsin: Madison, WI, USA, 2013; Available online: http://nbo6.chem.wisc.edu/ (accessed on 18 May 2020)(this web-side refers nowadays to NBO 7.0 version, all pre-NBO7 versions are cease).
- Grabowski, S.J. Clusters of Ammonium Cation–Hydrogen Bond versus σ-Hole Bond. ChemPhysChem 2014, 15, 289–297. [Google Scholar] [CrossRef]
- Grabowski, S.J. Lewis acid–Lewis base interactions: From NFH3+...NCH and NF4+…NCH complexes to NFH3+...(NCH)n and NF4+…(NCH)n clusters. Comput. Theor. Chem. 2015, 1053, 876–884. [Google Scholar] [CrossRef]
- Dovgaliuk, I.; Møller, K.T.; Robeyns, K.; Louppe, V.; Jensen, T.R.; Filinchuk, Y. Complexation of Ammonia Boranes with Al3+. Inorg. Chem. 2019, 58, 4753–4760. [Google Scholar] [CrossRef]
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).