Computer Simulation of the Incorporation of V2+, V3+, V4+, V5+ and Mo3+, Mo4+, Mo5+, Mo6+ Dopants in LiNbO3
Abstract
1. Introduction
2. Materials and Methods
2.1. Interatomic Potentials
2.2. Defect Formation Energies
3. Results and Discussion
3.1. Derivation of Interatomic Potential Parameters
3.2. Defect Calculations
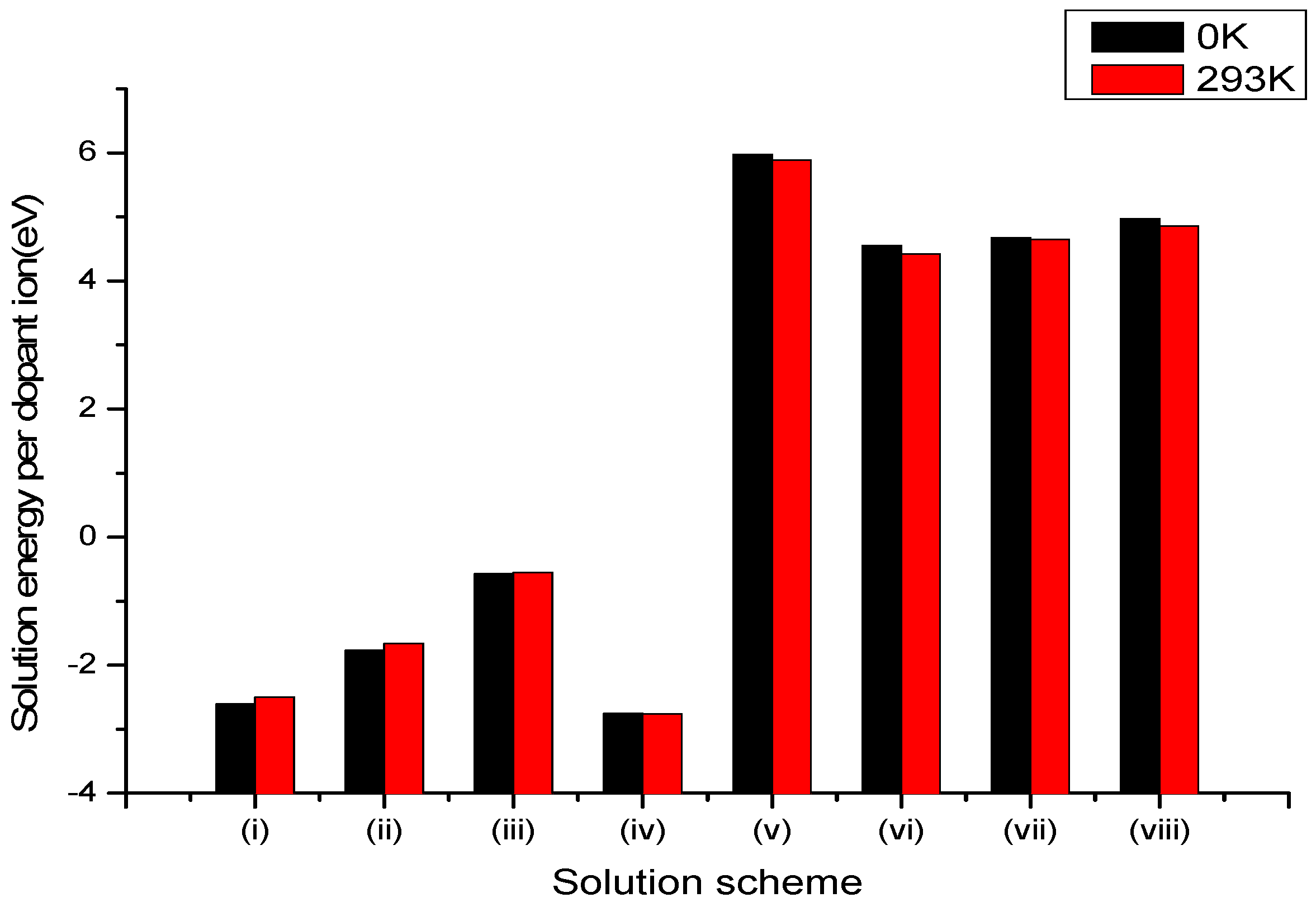
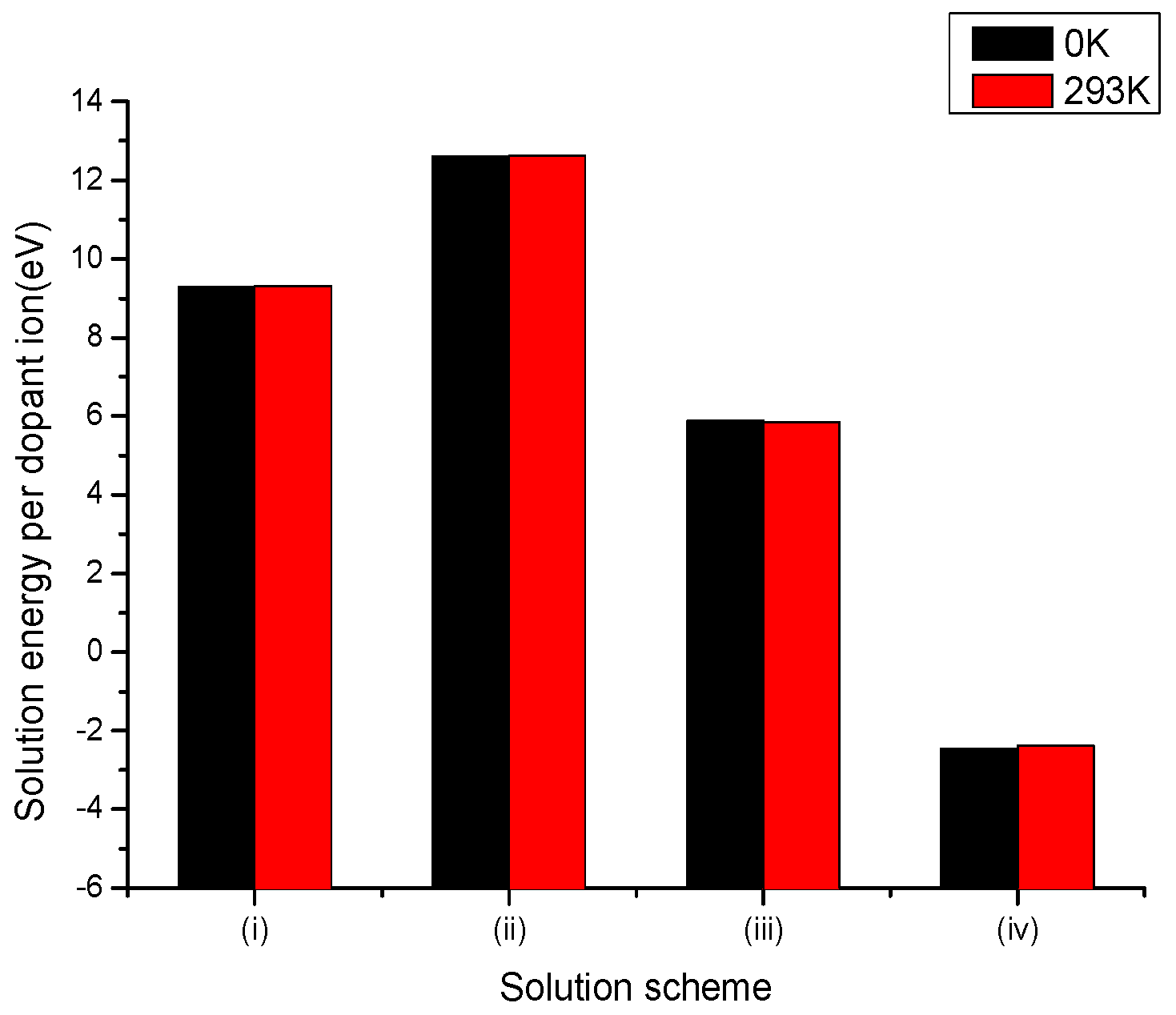
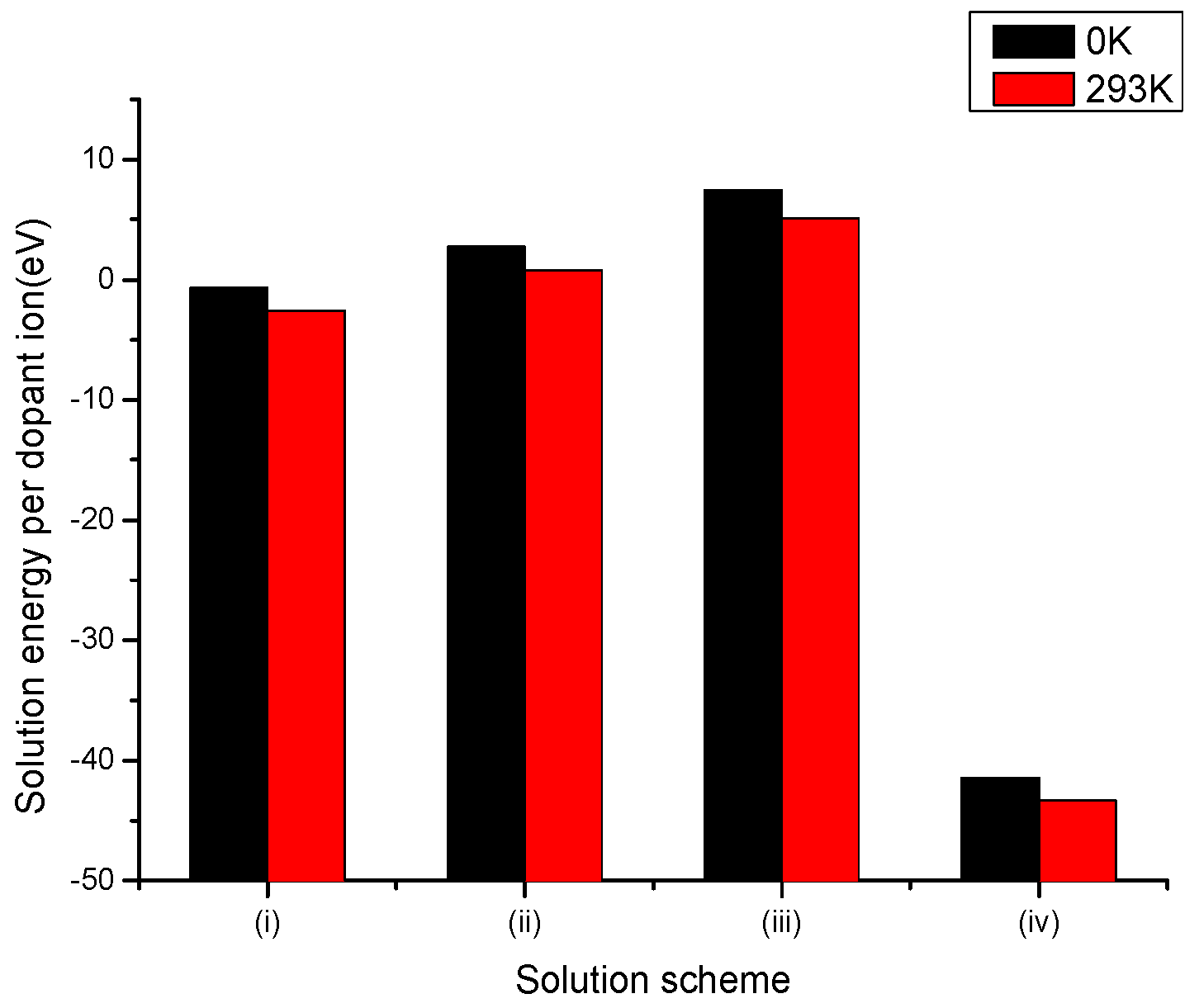
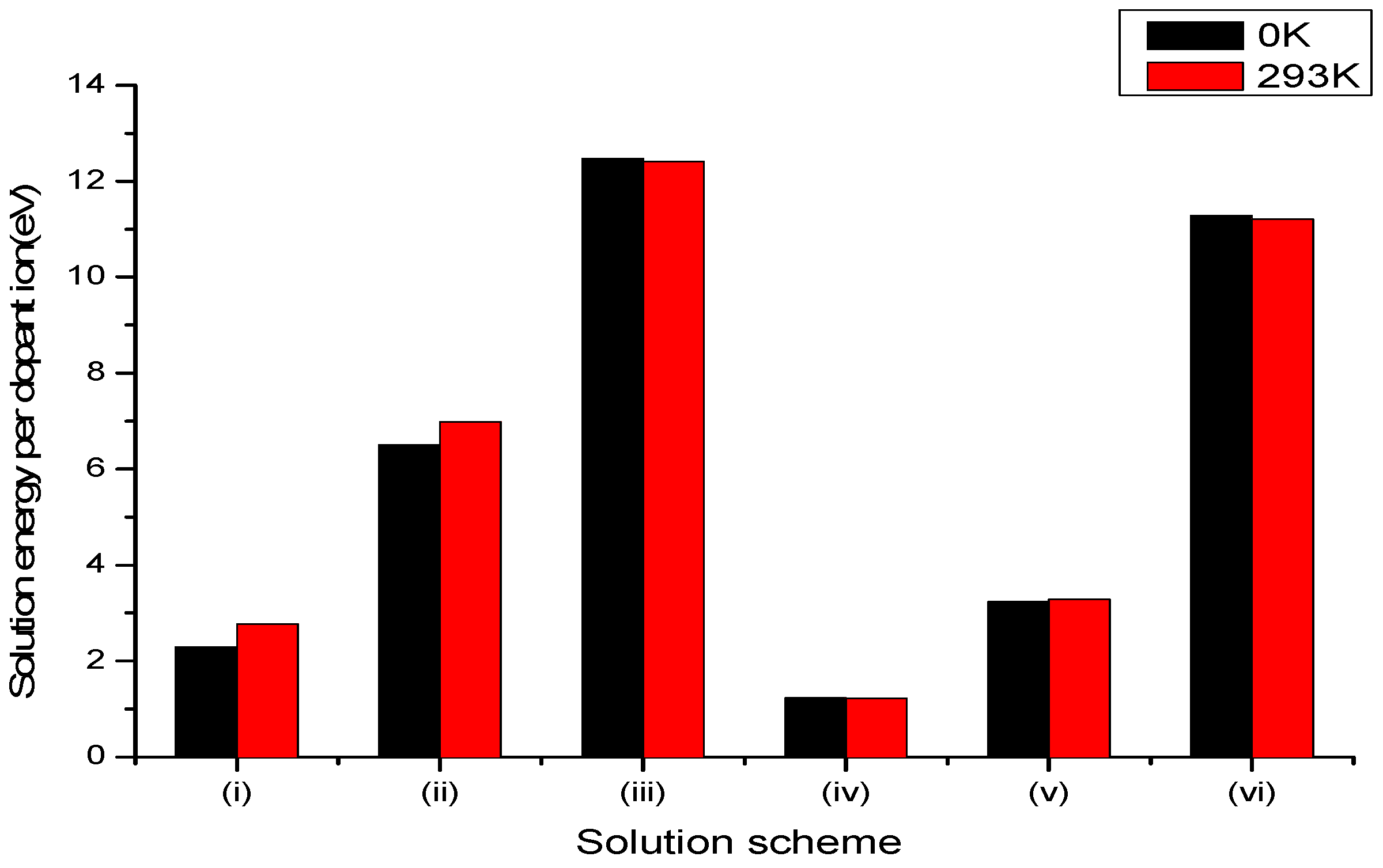
3.2.1. Divalent Dopants
3.2.2. Trivalent Dopants
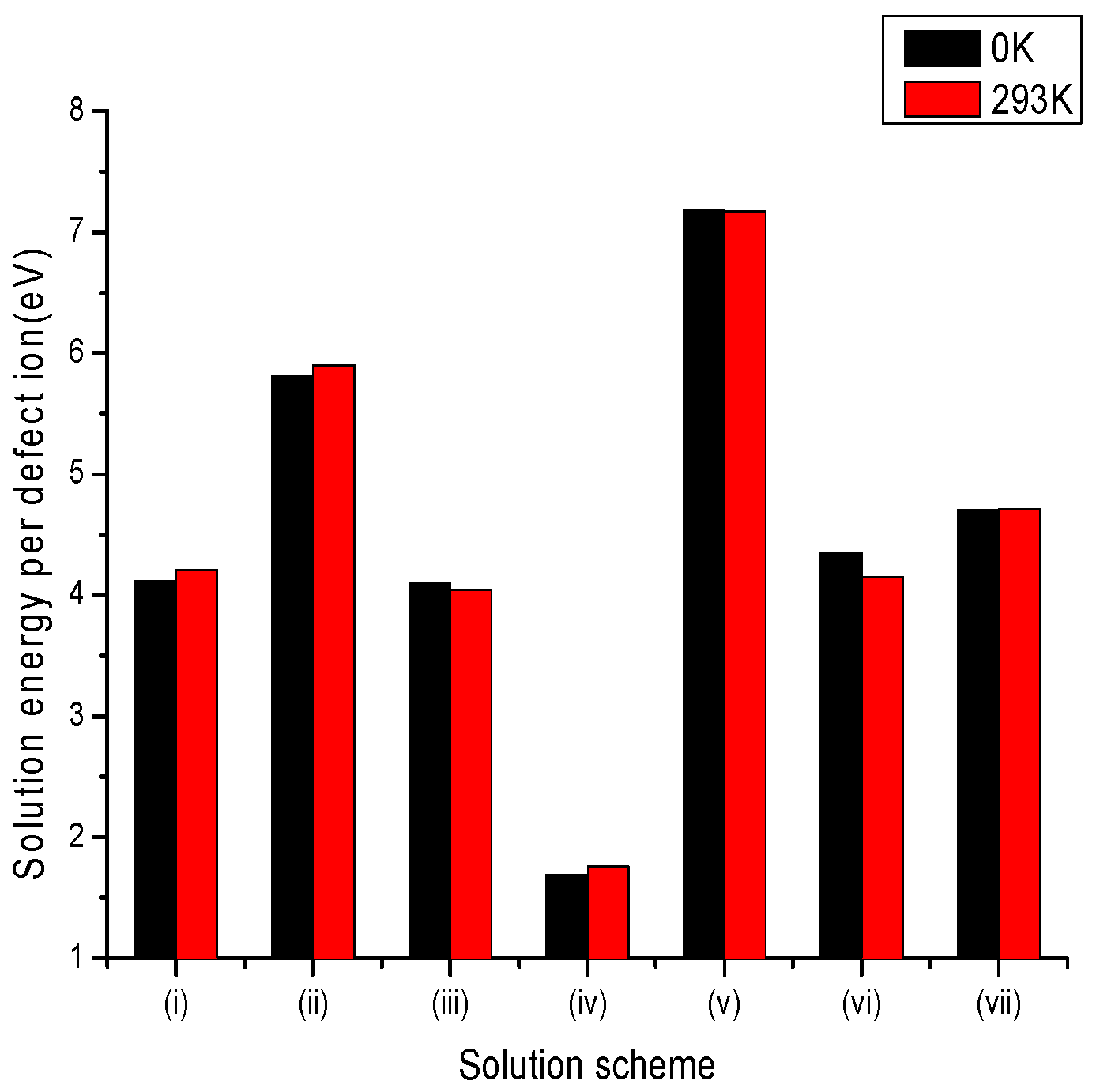
3.2.3. Tetravalent Dopants
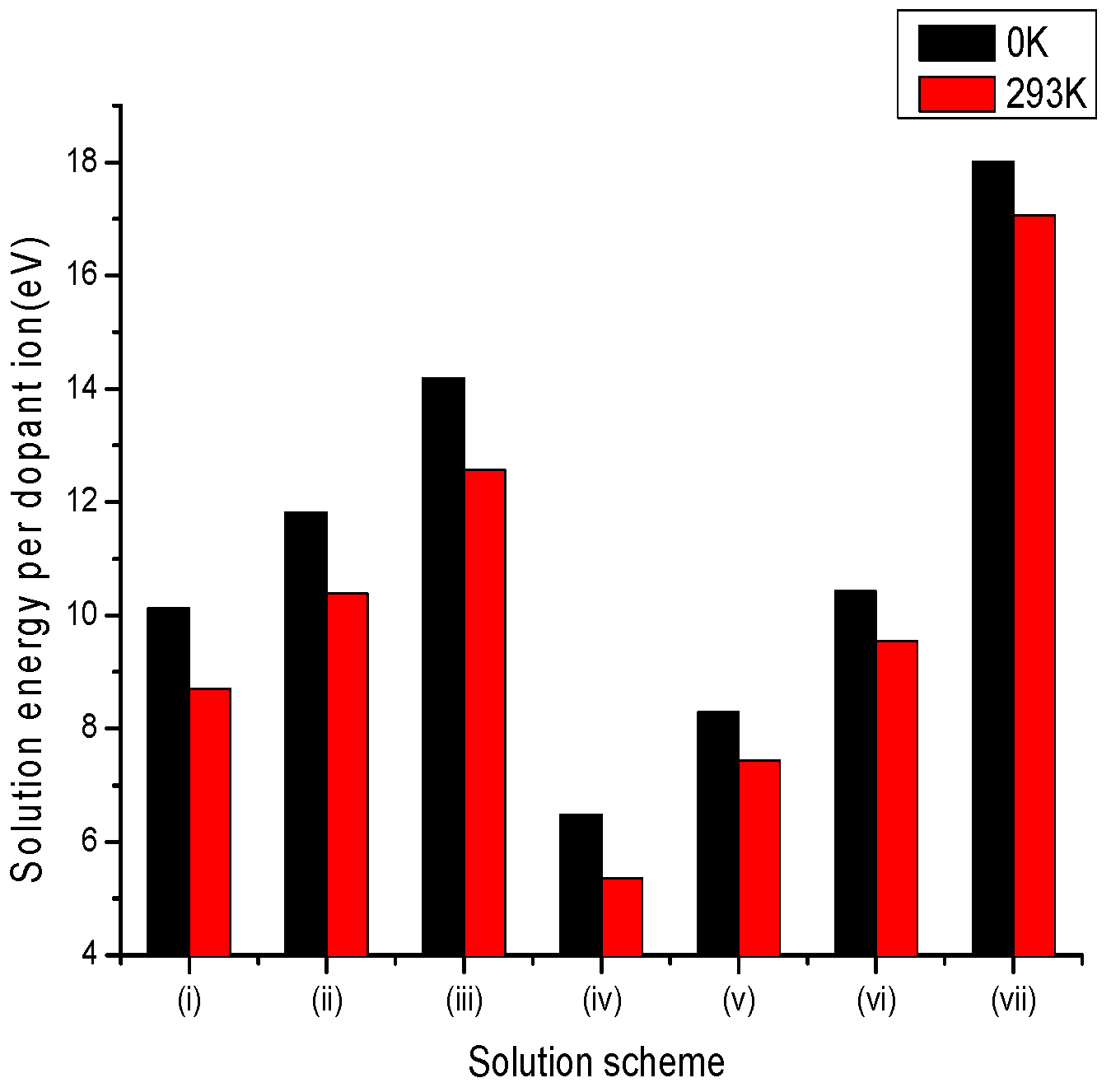
3.2.4. Pentavalent Dopants
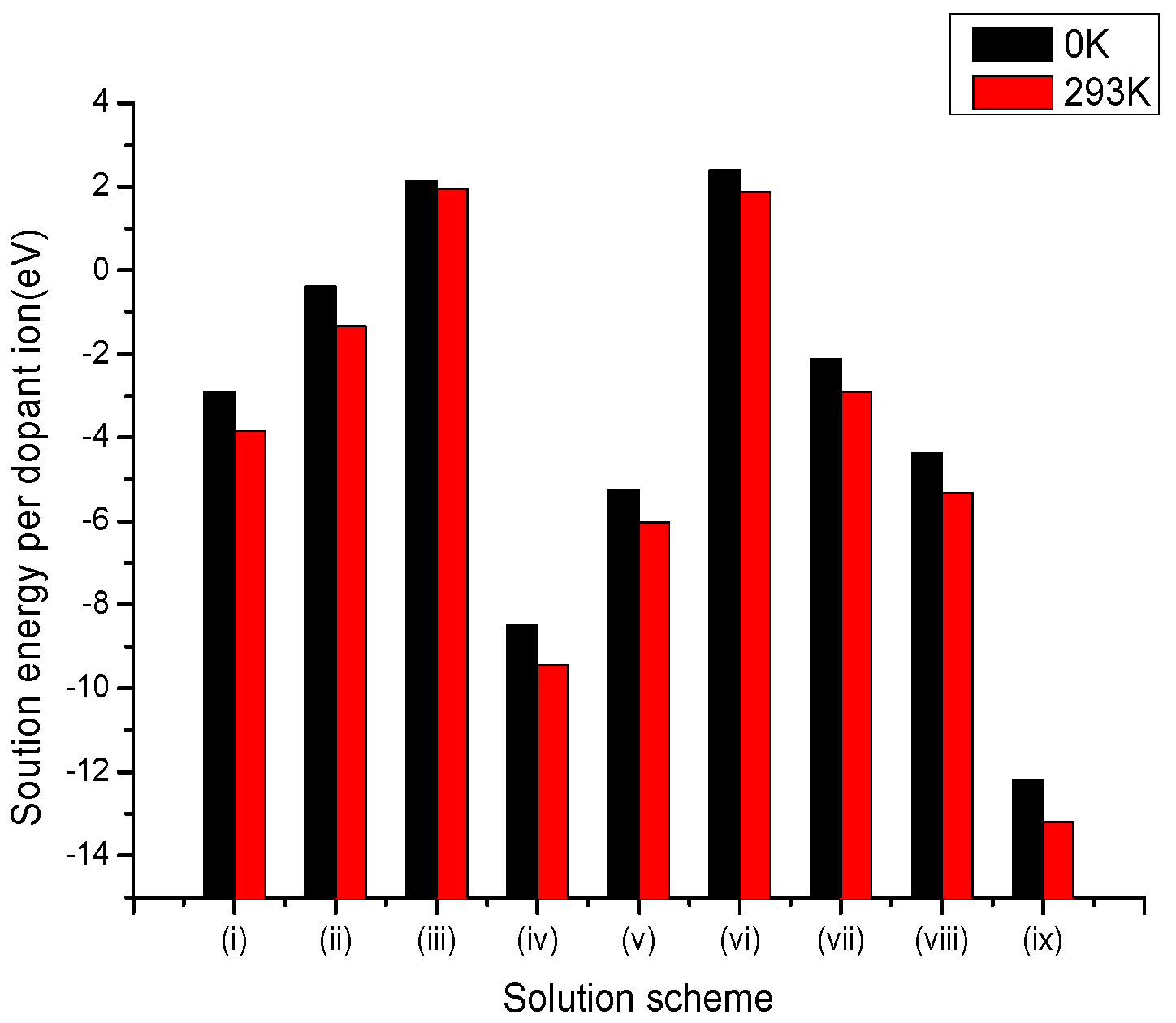
3.2.5. Hexavalent Dopants
3.2.6. Summary of Results for Vanadium and Molybdenum Dopants in LiNbO3
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mandula, G.; Rupp, R.A.; Balaskó, M.; Kovács, L. Decay of photorefractive gratings in LiNbO3:Fe by neutron irradiation. Appl. Phys. Lett. 2005, 86, 141107. [Google Scholar] [CrossRef]
- Ionita, I.; Jaque, F. Photoconductivity and electron mobility in LiNbO3 co-doped with Cr3+ and MgO. Opt. Mater. 1998, 10, 171–173. [Google Scholar] [CrossRef]
- Kaczmarec, S.M.; Bodziony, T. Low symmetry centers in LiNbO3 with Yb and Er. J. Non-Cryst. Solids 2008, 354, 4202–4210. [Google Scholar] [CrossRef]
- Kokanyan, E.P.; Razzari, L.; Cristiani, I.; Degiorgio, V.; Gruber, J.B. Reduced photorefraction in hafnium-doped single-domain and periodically poled lithium niobate crystals. Appl. Phys. Lett. 2004, 84, 1880. [Google Scholar] [CrossRef]
- Corradi, G.; Meyer, M.; Kovács, L.; Polgár, K. Gap levels of Ti3+ on Nb or Li sites in LiNbO3:(Mg):Ti crystals and their effect on charge transfer processes. Appl. Phys. B 2004, 78, 607–614. [Google Scholar] [CrossRef]
- Cantelar, E.; Quintanilla, M.; Pernas, P.L.; Torchia, G.A.; Lifante, G.; Cussó, F. Polarized emission and absorption cross-section calculation in LiNbO3:Tm3+. J. Lumin. 2008, 128, 988–991. [Google Scholar] [CrossRef]
- Shura, J.W.; Shina, T.I.; Leea, S.M.; Baekb, S.W.; Yoon, D.H. Photoluminescence properties of Nd: LiNbO3 co-doped with ZnO fiber single crystals grown by micro-pulling-down method. Mater. Sci. Eng. B 2003, 105, 16–19. [Google Scholar] [CrossRef]
- Li, S.; Liu, S.; Kong, Y.; Deng, D.; Gao, G.; Li, Y.; Gao, H.; Zhang, L.; Hang, Z.; Chen, S.; et al. The optical damage resistance and absorption spectra of LiNbO3:Hf crystals. J. Phys. Condens. Matter 2006, 18, 3527–3534. [Google Scholar] [CrossRef]
- Hesselink, L.; Orlov, S.S.; Liu, A.; Akella, A.; Lande, D.; Neurgaonkar, R.R. Photorefractive materials for nonvolatile volume holographic data storage. Science 1998, 282, 1089–1094. [Google Scholar] [CrossRef]
- Camarillo, E.; Murrieta, H.; Hernandez, J.M.; Zoilo, R.; Flores, M.C.; Han, T.P.J.; Jaque, F. Optical properties of LiNbO3:Cr crystals co-doped with germanium oxide. J. Lumin. 2008, 128, 747–750. [Google Scholar] [CrossRef]
- Luo, S.; Meng, Q.; Wang, J.; Sun, X. Effect of In3+ concentration on the photorefraction and scattering properties in In: Fe:Cu:LiNbO3 crystals at 532 nm wavelength. Opt. Commun. 2016, 358, 198–201. [Google Scholar] [CrossRef]
- Nie, Y.; Wang, R.; Wang, B. Growth and holographic storage properties of In:Ce:Cu:LiNbO3 crystal. Mater. Chem. Phys. 2007, 102, 281–283. [Google Scholar] [CrossRef]
- Zhen, X.H.; Li, H.T.; Sun, Z.J.; Ye, S.J.; Zhao, L.C.; Xu, Y.H. Holographic properties of double-doped Zn:Fe:LiNbO3 crystals. Mater. Lett. 2004, 58, 1000–1002. [Google Scholar] [CrossRef]
- Wei, Z.; Naidong, Z.; Qingquan, L. Growth and Holographic Storage Properties of Sc, Fe Co-Doped Lithium Niobate Crystals. J. Rare Earth 2007, 25, 775–778. [Google Scholar] [CrossRef]
- Xu, C.; Leng, X.; Xu, L.; Wen, A.; Xu, Y. Enhanced nonvolatile holographic properties in Zn, Ru and Fe co-doped LiNbO3 crystals. Opt. Commun. 2012, 285, 3868–3871. [Google Scholar] [CrossRef]
- Tian, T.; Kong, Y.; Liu, S.; Li, W.; Wu, L.; Chen, S.; Xu, J. The photorefraction of molybdenum-doped lithium niobate crystals. Opt. Lett. 2012, 37, 2679–2681. [Google Scholar] [CrossRef]
- Kong, Y.; Liu, S.; Xu, J. Recent Advances in the Photorefraction of Doped Lithium Niobate Crystals. Materials 2012, 5, 1954–1971. [Google Scholar] [CrossRef]
- Saeed, S.; Zheng, D.; Liu, H.; Xue, L.; Wang, W.; Zhu, L.; Hu, M.; Liu, S.; Chen, S.; Zhang, L.; et al. Rapid response of photorefraction in vanadium and magnesium co-doped lithium niobate. J. Phys. D Appl. Phys. 2019, 52, 405303. [Google Scholar] [CrossRef]
- Xue, L.; Liu, H.; Zheng, D.; Saeed, S.; Wang, X.; Tian, T.; Zhu, L.; Kong, Y.; Liu, S.; Chen, S.; et al. The Photorefractive Response of Zn and Mo Codoped LiNbO3 in the Visible Region. Crystals 2019, 9, 228. [Google Scholar] [CrossRef]
- Fan, Y.; Li, L.; Li, Y.; Sun, X.; Zhao, X. Hybrid density functional theory study of vanadium doping in stoichiometric and congruent LiNbO3. Phys. Rev. B 2019, 99, 035147. [Google Scholar] [CrossRef]
- Wang, W.; Liu, H.; Zheng, D.; Kong, Y.; Zhang, L.; Xu, J. Interaction between Mo and intrinsic or extrinsic defects of Mo doped LiNbO3 from first-principles calculations. J. Phys. Condens. Matter 2020, 32, 255701. [Google Scholar] [CrossRef] [PubMed]
- Jackson, R.A.; Valerio, M.E.G. A new interatomic potential for the ferroelectric and paraelectric phases of LiNbO3. J. Phys. Condens. Matter 2005, 17, 837. [Google Scholar] [CrossRef]
- Araujo, R.M.; Lengyel, K.; Jackson, R.A.; Valerio, M.E.G.; Kovacs, L. Computer modelling of intrinsic and substitutional defects in LiNbO3. Phys. Status Solidi 2007, 4, 1201–1204.22. [Google Scholar] [CrossRef]
- Araujo, R.M.; Lengyel, K.; Jackson, R.A.; Kovacs, L.; Valerio, M.E.G. A computational study of intrinsic and extrinsic defects in LiNbO3. J. Phys. Condens. Matter 2007, 19, 046211. [Google Scholar] [CrossRef]
- Araujo, R.M.; Valerio, M.E.G.; Jackson, R.A. Computer modelling of trivalent metal dopants in lithium niobite. J. Phys. Condens. Matter 2008, 20, 035201. [Google Scholar] [CrossRef]
- Araujo, R.M.; Valerio, M.E.G.; Jackson, R.A. Computer simulation of metal co-doping in lithium niobate. Proc. R. Soc. A 2014, 470, 0406. [Google Scholar] [CrossRef]
- Araujo, R.M.; Valerio, M.E.G.; Jackson, R.A. Computer Modelling of Hafnium Doping in Lithium Niobate. Crystals 2018, 8, 123. [Google Scholar] [CrossRef]
- Mott, N.F.; Littleton, M.J. Conduction in polar crystals. Electrolytic conduction in solid salts. Trans. Faraday Soc. 1938, 34, 485–499. [Google Scholar] [CrossRef]
- Sanders, M.J.; Leslie, M.; Catlow, C.R.A. Interatomic potentials for SiO2. J. Chem. Soc. Chem. Commun. 1984, 1271–1273. [Google Scholar] [CrossRef]
- Dick, B.J.; Overhauser, A.W. Theory of the dielectric constants of alkali halide crystals. Phys. Rev. 1958, 112, 90. [Google Scholar] [CrossRef]
- Taylor, D. Thermal expansion data. I: Binary oxides with the sodium chloride and wurtzite structures, MO. Trans. J. Br. Ceram. Soc. 1984, 83, 5–9. [Google Scholar]
- Luedtke, T.; Weber, D.; Schmidt, A.; Mueller, A.; Reimann, C.; Becker, N.; Bredow, T.; Dronskowski, R.; Ressler, T.; Lerch, M. Synthesis and characterization of metastable transition metal oxides and oxide nitrides. Z. Fuer Krist. Cryst. Mater. 2017, 232, 3–14. [Google Scholar] [CrossRef]
- McWhan, D.B.; Marezio, M.; Remeika, J.P.; Dernier, P.D. X-ray diffraction study of metallic VO2. Phys. Rev. B Solid State 1974, 10, 490–495. [Google Scholar] [CrossRef]
- Balog, P.; Orosel, D.; Cancarevic, Z.; Schoen, C.; Jansen, M. V2O5 phase diagram revisited at high pressures and high temperatures. J. Alloy. Compd. 2007, 429, 87–98. [Google Scholar] [CrossRef]
- Aleandri, L.E.; McCarley, R.E. Hexagonal lithium molybdate, LiMoO2: A close-packed layered structure with infinite molybdenum-molybdenum-bonded sheets. Inorg. Chem. 1988, 27, 1041–1044. [Google Scholar] [CrossRef]
- Hibble, S.J.; Fawcett, I.D.; Hannon, A.C. Structure of Two Disordered Molybdates, Li2MoIVO3 and Li4Mo3IVO8, from Total Neutron Scattering. Acta Crystallogr. Sect. B Struct. Sci. 1997, 53, 604–612. [Google Scholar] [CrossRef]
- Mikhailova, D.; Voss, A.; Oswald, S.; Tsirlin, A.A.; Schmidt, M.; Senyshyn, A.; Eckert, J.; Ehrenberg, H. Lithium Insertion into Li2MoO4: Reversible Formation of (Li3Mo)O4 with a Disordered Rock-Salt Structure. Chem. Mater. 2015, 27, 4485–4492. [Google Scholar] [CrossRef]
- Kolitsch, U. The crystal structures of phenacite-type Li2(MoO4), and scheelite-type LiY(MoO4)2 and LiNd(MoO4)2. Z. Fuer Krist. 2001, 216, 449–454. [Google Scholar] [CrossRef]
- Kröger, F.A.; Vink, H.J. The origin of the fluorescence in self-activated ZnS, CdS, and ZnO. J. Chem. Phys. 1954, 22, 250. [Google Scholar] [CrossRef]
- Shannon, R.D.; Prewitt, C.T. Revised values of effective ionic radii. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1969, 26, 1046–1048. [Google Scholar] [CrossRef]
- Zhu, L.; Zheng, D.; Saeed, S.; Wang, S.; Liu, H.; Kong, Y.; Liu, S.; Chen, S.; Zhang, L.; Xu, J. Photorefractive Properties of Molybdenum and Hafnium Co-Doped LiNbO3. Crystals 2018, 8, 322. [Google Scholar] [CrossRef]

| Interaction | Aij(eV) | ρij(Å) | Cij(Å6 eV) |
|---|---|---|---|
| Licore-Oshell | 950.0 | 0.2610 | 0.0 |
| Vcore-Oshell | 293.240087 | 0.475181 | 0.0 |
| Mocore-Licore | 573.532325 | 0.369602 | 0.0 |
| Mocore-O2−shell | 3003.79 | 0.3474 | 0.0 |
| Mocore-Ocore | 600.263736 | 0.328558 | 0.0 |
| O2−shell-O2−shell | 22764.0 | 0.1490 | 27.88 |
| Harmonic | k(eV Å2) | ro(Å) | |
| Vcore-Ocore | 46.997833 | 1.942956 | |
| Mocore-Ocore | 385.638986 | 2.073074 | |
| Species | Y(e) | ||
| Mocore | 3.0 4.0 5.0 6.0 | ||
| Vcore | 2.0 3.0 4.0 5.0 | ||
| Ocore | 0.9 | ||
| Oshell | −2.9 | ||
| Spring | k(Å−2 eV) | ||
| Ocore-Ooore | 70.0 |
| Oxide | Lattice Parameter | Exp. | Calc. (0 K) | Δ% | Calc. (293 K) | Δ% |
| VO | a(Å) = b(Å) = c(Å) | 4.067800 | 4.108237 | 0.99 | 4.10683 | 0.98 |
| V2O3 | a(Å) = b(Å) = c(Å) | 9.393000 | 9.304757 | 0.90 | 9.346331 | 0.94 |
| VO2 | a (Å) = b(Å) | 4.556100 | 4.569483 | 0.20 | 4.566212 | 0.22 |
| c(Å) | 2.859800 | 2.866421 | 0.23 | 2.857861 | 0.07 | |
| V2O5 | a(Å) | 11.971900 | 11.99652 | 0.20 | 12.01247 | 0.33 |
| b(Å) | 4.701700 | 4.722561 | 0.44 | 4.660343 | 0.88 | |
| c(Å) | 5.325300 | 5.355671 | 0.57 | 5.371149 | 0.86 | |
| Lithium Molybdates | Lattice Parameter | Exp. | Calc. (0 K) | Δ% | Calc. (293 K) | Δ% |
| LiMoO2 | a(Å) = b(Å) | 2.866300 | 2.880528 | 0.50 | 2.887246 | 0.73 |
| c(Å) | 15.474300 | 15.409390 | 0.42 | 15.595024 | 0.78 | |
| Li2MoO3 | a(Å) = b(Å) | 2.878000 | 2.854443 | 0.82 | 2.859809 | 0.63 |
| c(Å) | 14.91190 | 15.002886 | 0.61 | 15.04632 | 0.90 | |
| Li3MoO4 | a(Å) = b(Å) = c(Å) | 4.1389 | 4.107762 | 0.75 | 4.106941 | 0.77 |
| Li2MoO4 | a(Å) = b(Å) | 14.330000 | 14.301305 | 0.20 | 14.384501 | 0.38 |
| c(Å) | 9.584 | 9.492067 | 0.96 | 9.632413 | 0.96 |
| Site | Charge Compensation | Reaction |
|---|---|---|
| Li+ | Lithium Vacancies | (i) |
| Niobium Vacancies | (ii) | |
| Oxygen Interstitial | (iii) | |
| Li+ and Nb5+ | Self-Compensation | (iv) |
| Nb5+ | Lithium Vacancies and Anti-site () | (v) |
| Anti-site () | (vi) | |
| (vii) | ||
| Oxygen Vacancies | (viii) |
| Site | Charge Compensation | Reaction |
|---|---|---|
| Li+ | Lithium Vacancies | (i) |
| Niobium Vacancies | (ii) | |
| Oxygen Interstitial | (iii) | |
| Li+ and Nb5+ | Self-Compensation | (iv) |
| Nb5+ | Oxygen Vacancies | (v) |
| Anti-site () | (vi) | |
| Lithium Vacancies and Anti-site () | (vii) |
| Site | Charge Compensation | Reaction |
|---|---|---|
| Li+ | Lithium Vacancies | (i) |
| Niobium Vacancies | (ii) | |
| Oxygen Interstitial | (iii) | |
| Li+ and Nb5+ | Self-Compensation | (iv) |
| Nb5+ | Oxygen Vacancies | (v) |
| Nb5+ | Anti-site () | (vi) |
| Nb5+ | Lithium Vacancies and Anti-site () | (vii) |
| Site | Charge Compensation | Reaction |
|---|---|---|
| Li+ | Lithium Vacancies | (i) |
| Niobium Vacancies | (ii) | |
| Oxygen Interstitial | (iii) | |
| Li+ and Nb5+ | Self-Compensation | (iv) |
| Nb5+ | Anti-site () | (v) |
| Lithium Vacancies and Anti-site () | (vi) | |
| (vii) | ||
| (viii) | ||
| Oxygen Vacancies | (ix) |
| Site | Charge Compensation | Reaction |
|---|---|---|
| Li+ | Lithium Vacancies | (i) |
| Niobium Vacancies | (ii) | |
| Oxygen Interstitial | (iii) | |
| Li+ and Nb5+ | Self-Compensation | (iv) |
| Nb5+ | Anti-site () | (v) |
| Lithium Vacancies and Anti-site () | (vi) | |
| (vii) | ||
| (viii) | ||
| Oxygen Vacancies | (ix) |
| Site | Charge Compensation | Reaction |
|---|---|---|
| Li+ | Lithium Vacancies | (i) |
| Niobium Vacancies | (ii) | |
| Oxygen Interstitial | (iii) | |
| Nb5+ | No Charge Compensation | (iv) |
| Site | Charge Compensation | Reaction |
|---|---|---|
| Li+ | Lithium Vacancies | (i) |
| Niobium Vacancies | (ii) | |
| Oxygen Interstitial | (iii) | |
| Nb5+ | No Charge Compensation | (iv) |
| Site | Charge Compensation | Reaction |
|---|---|---|
| Li+ | Lithium Vacancies | (i) |
| Niobium Vacancies | (ii) | |
| Oxygen Interstitial | (iii) | |
| Nb5+ | Lithium Vacancies | (iv) |
| Niobium Vacancies | (v) | |
| Oxygen Interstitial | (vi) |
| Compound | Lattice Energy | Lattice Energy |
|---|---|---|
| 0 K | 293 K | |
| LiNbO3 | −174.45 | −174.66 |
| Li2O | −33.16 | −32.92 |
| Nb2O5 | −314.37 | −313.39 |
| VO | −22.06 | −22.07 |
| V2O3 | −124.37 | −124.39 |
| VO2 | −111.54 | −111.57 |
| V2O5 | −315.65 | −274.18 |
| LiMoO2 | −98.07 | −97.09 |
| Li2MoO3 | −150.38 | −149.10 |
| Li3MoO4 | −181.28 | −178.88 |
| Li2MoO4 | −234.06 | −234.12 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Araujo, R.M.; dos Santos Mattos, E.F.; Valerio, M.E.G.; Jackson, R.A. Computer Simulation of the Incorporation of V2+, V3+, V4+, V5+ and Mo3+, Mo4+, Mo5+, Mo6+ Dopants in LiNbO3. Crystals 2020, 10, 457. https://doi.org/10.3390/cryst10060457
Araujo RM, dos Santos Mattos EF, Valerio MEG, Jackson RA. Computer Simulation of the Incorporation of V2+, V3+, V4+, V5+ and Mo3+, Mo4+, Mo5+, Mo6+ Dopants in LiNbO3. Crystals. 2020; 10(6):457. https://doi.org/10.3390/cryst10060457
Chicago/Turabian StyleAraujo, Romel Menezes, Emanuel Felipe dos Santos Mattos, Mário Ernesto Giroldo Valerio, and Robert A. Jackson. 2020. "Computer Simulation of the Incorporation of V2+, V3+, V4+, V5+ and Mo3+, Mo4+, Mo5+, Mo6+ Dopants in LiNbO3" Crystals 10, no. 6: 457. https://doi.org/10.3390/cryst10060457
APA StyleAraujo, R. M., dos Santos Mattos, E. F., Valerio, M. E. G., & Jackson, R. A. (2020). Computer Simulation of the Incorporation of V2+, V3+, V4+, V5+ and Mo3+, Mo4+, Mo5+, Mo6+ Dopants in LiNbO3. Crystals, 10(6), 457. https://doi.org/10.3390/cryst10060457






