Crystal Structure and Theoretical Investigation of Thiobarbituric Acid Derivatives as Nonlinear Optical (NLO) Materials
Abstract
1. Introduction
2. Materials and Methods
2.1. General
2.2. Structure Determination
2.3. Theoretical Calculations
3. Results
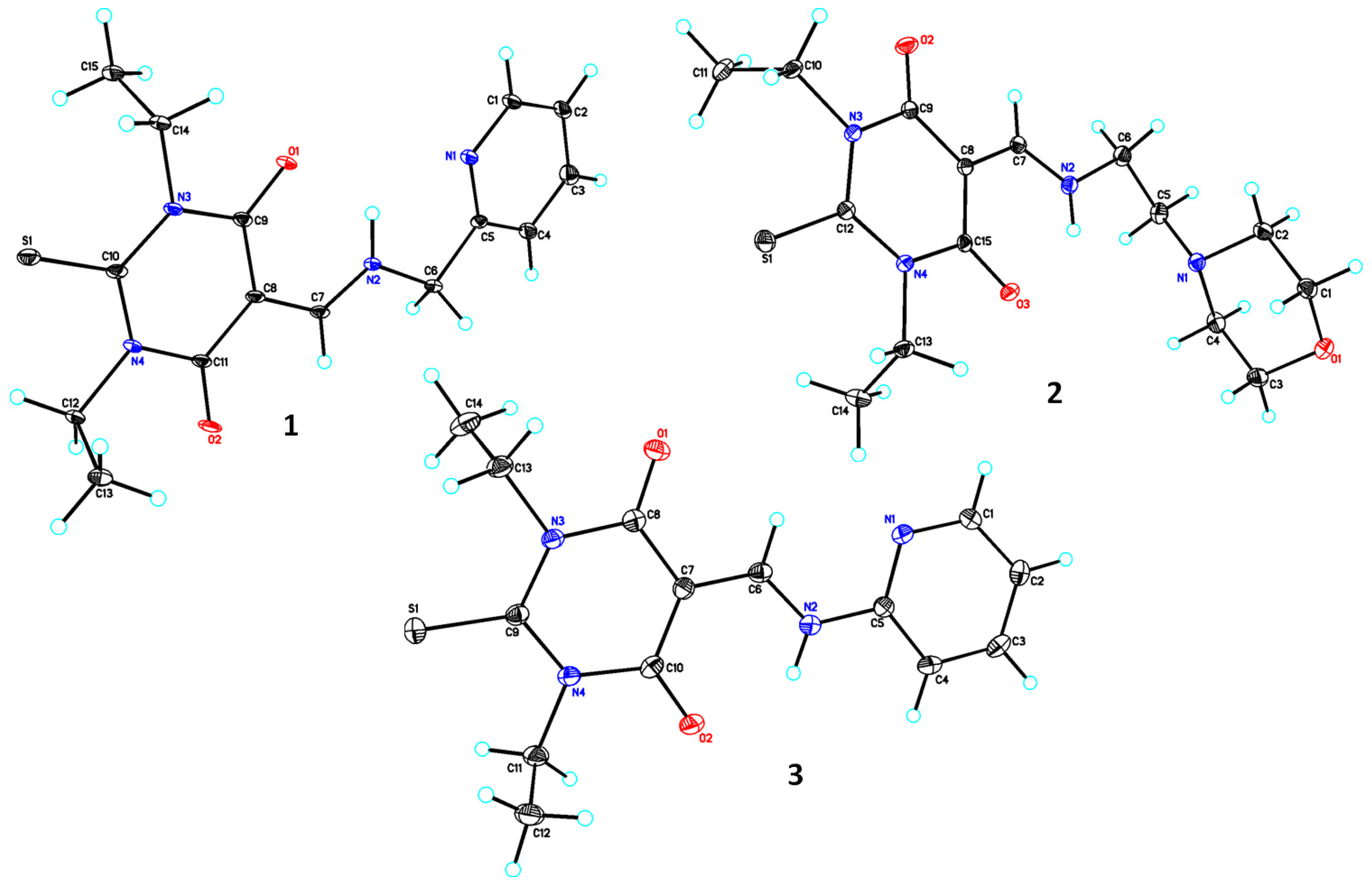
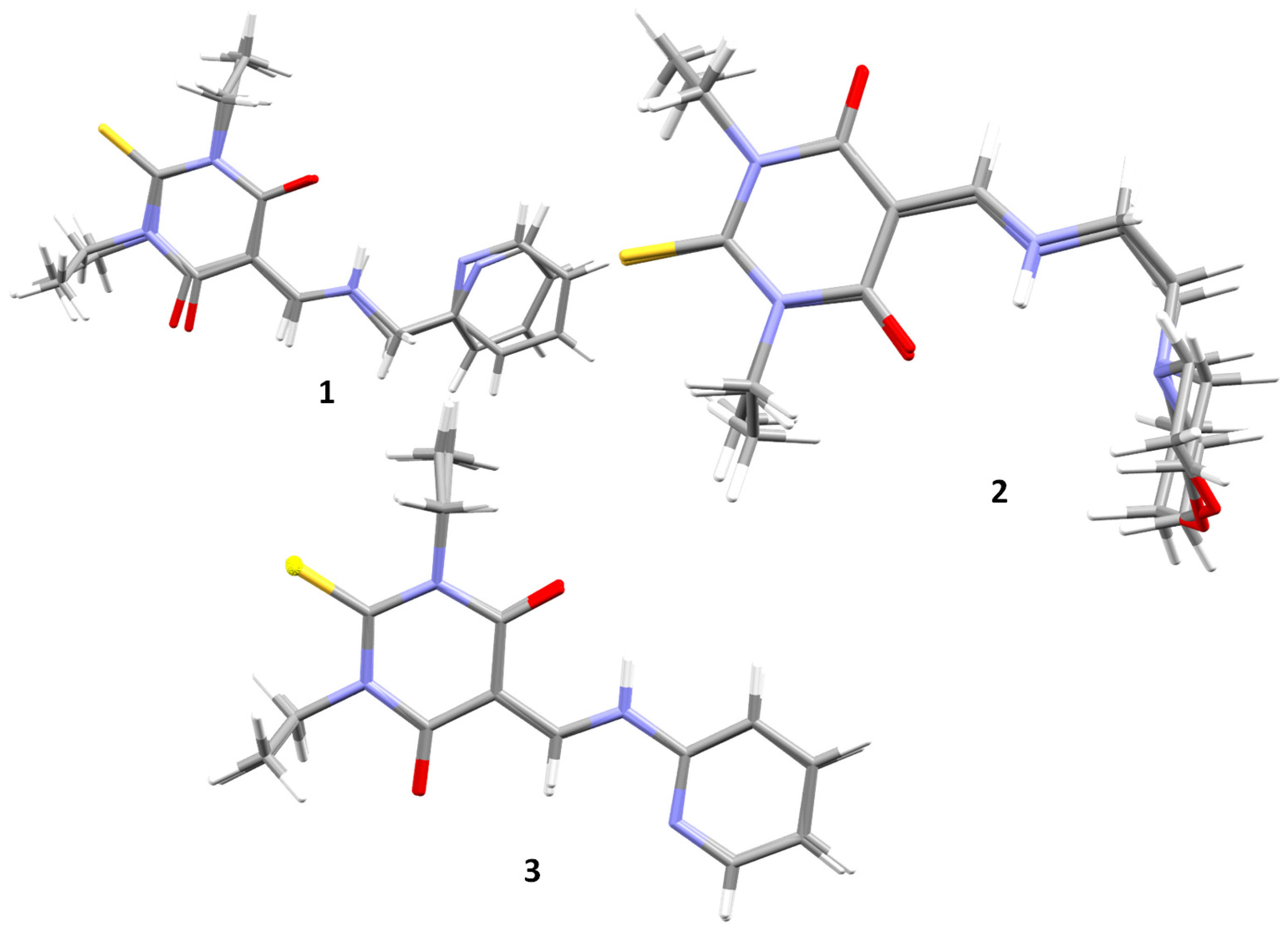
3.1. Single Crystal X-ray Analysis
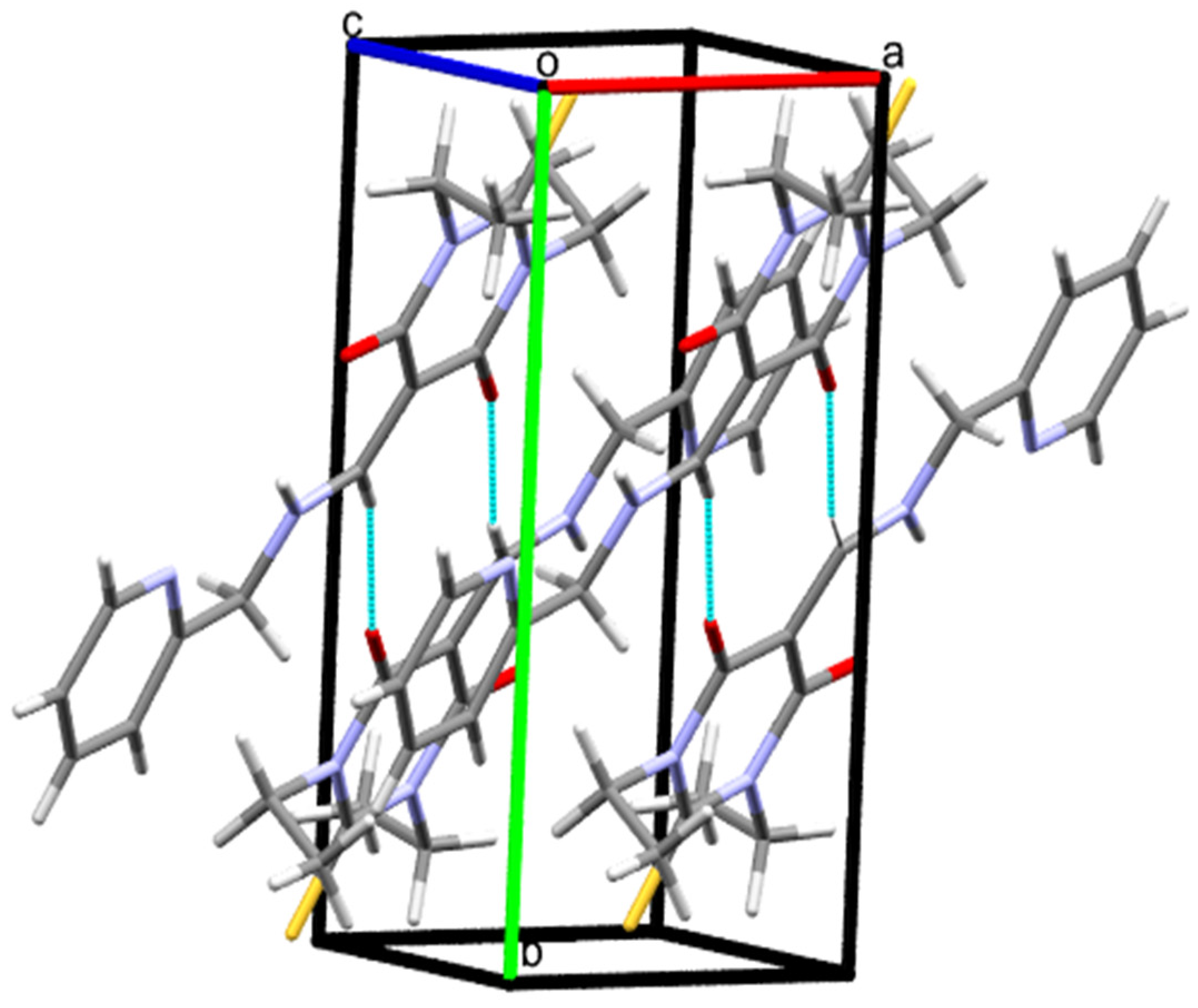
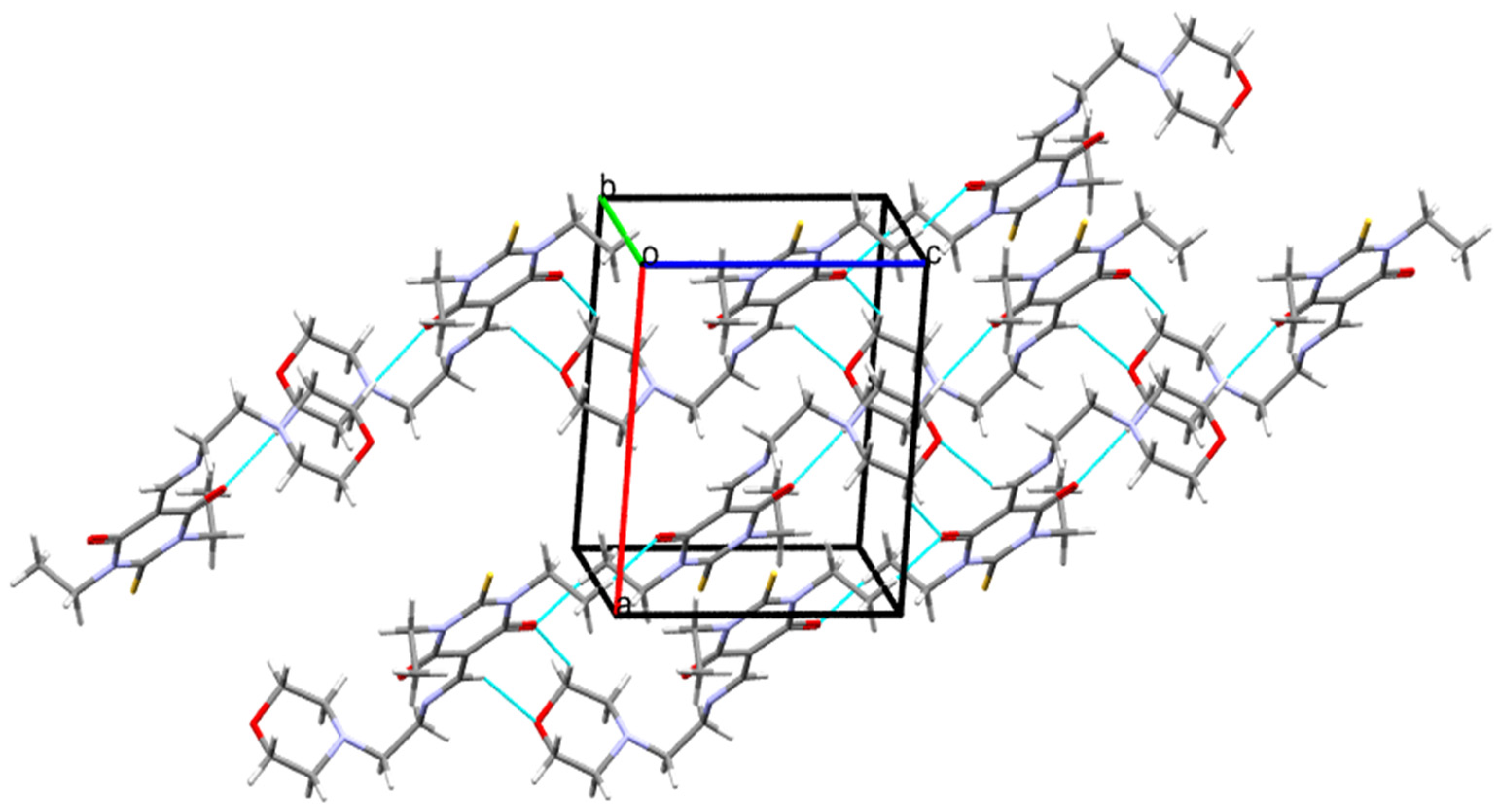
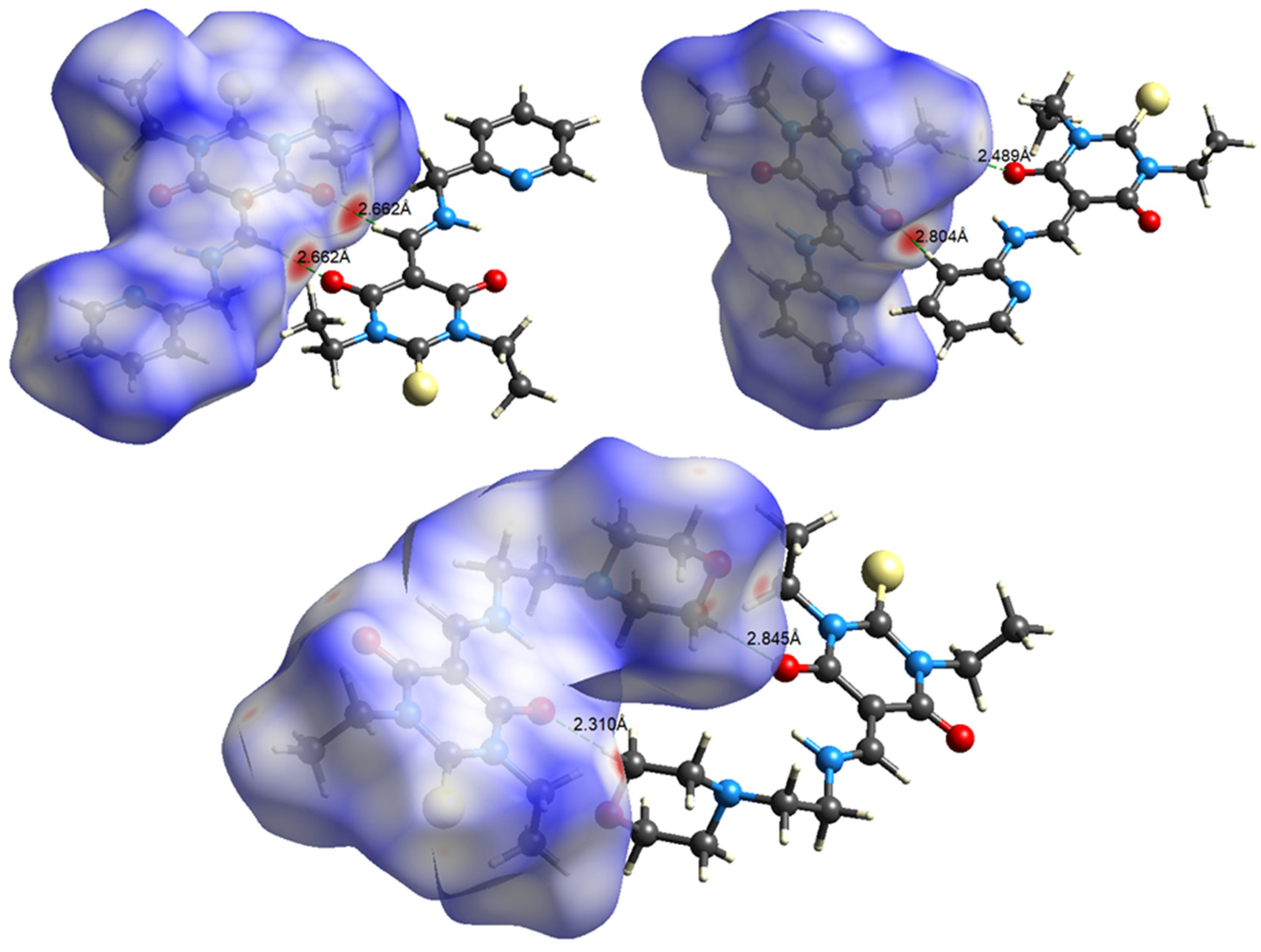
3.2. Crystal Packing
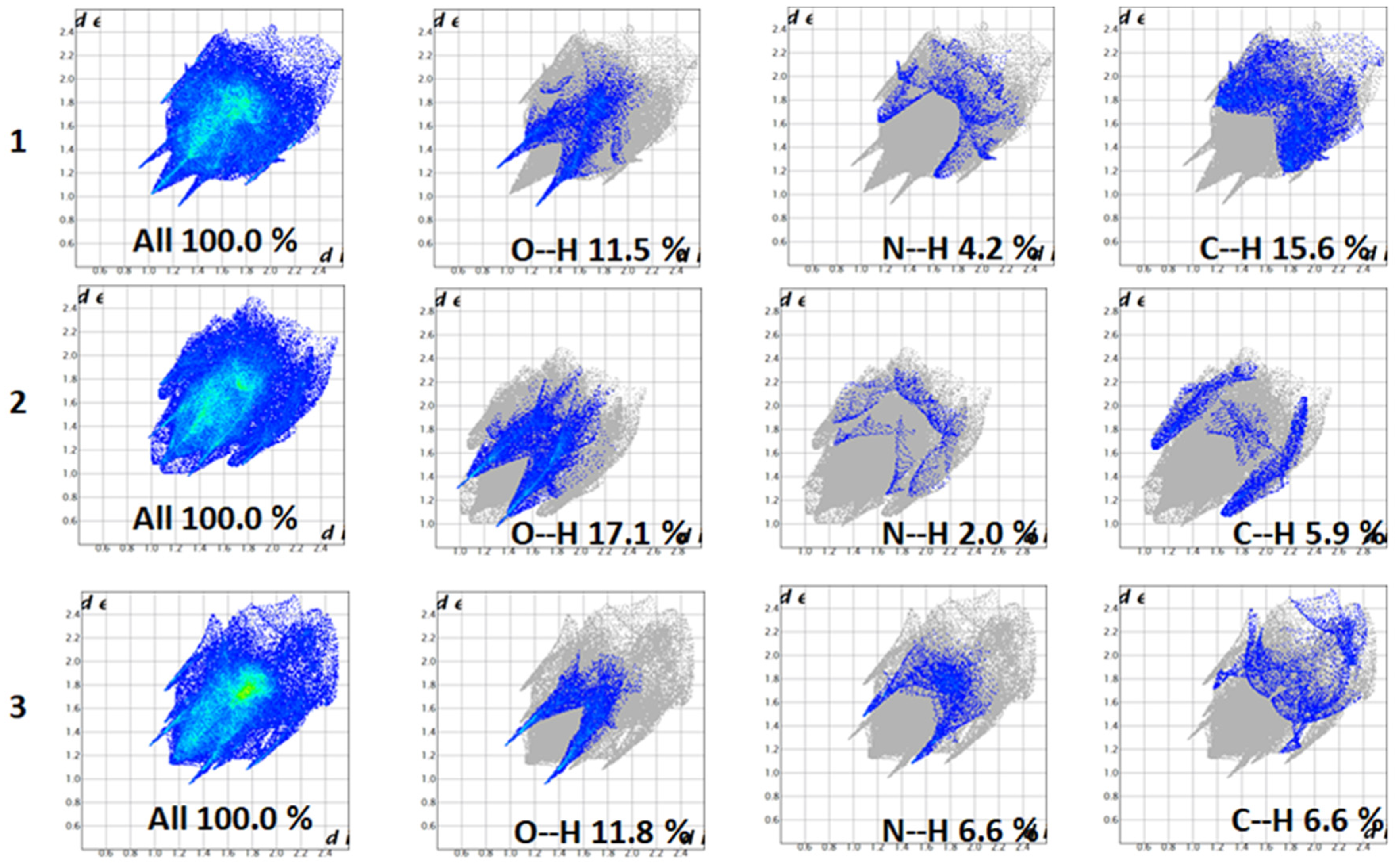
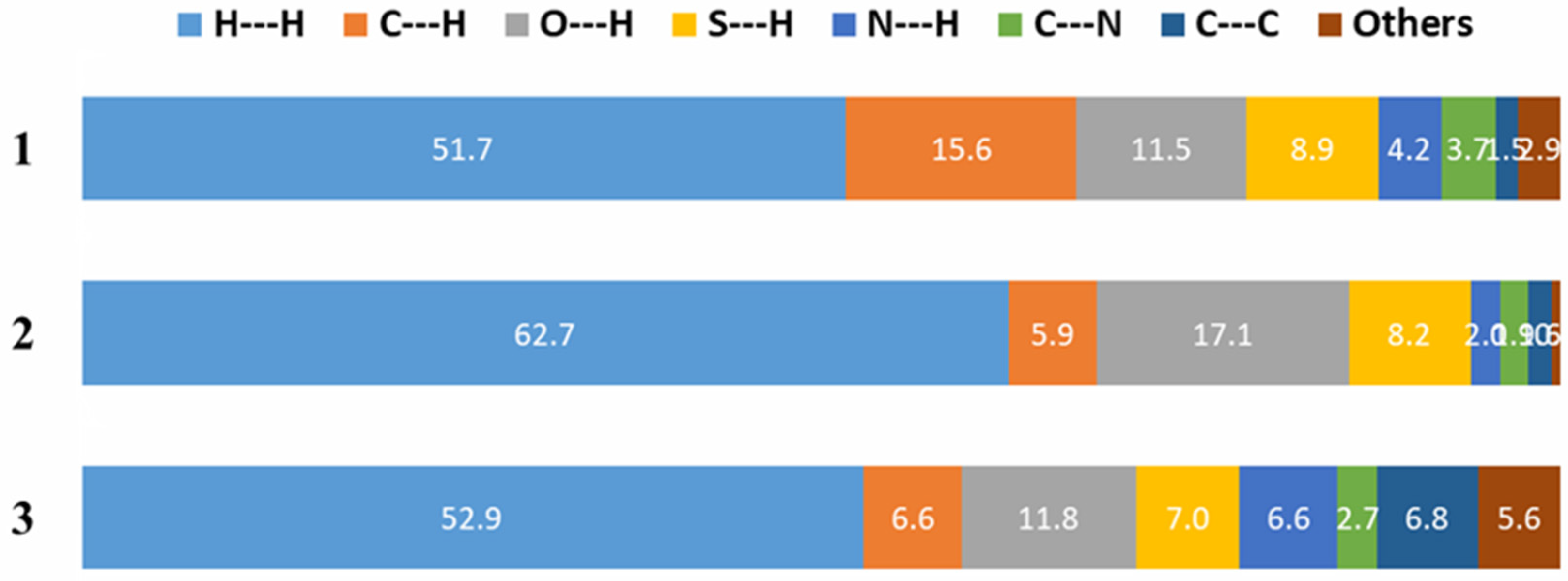
3.3. Hirshfeld Surface Analysis
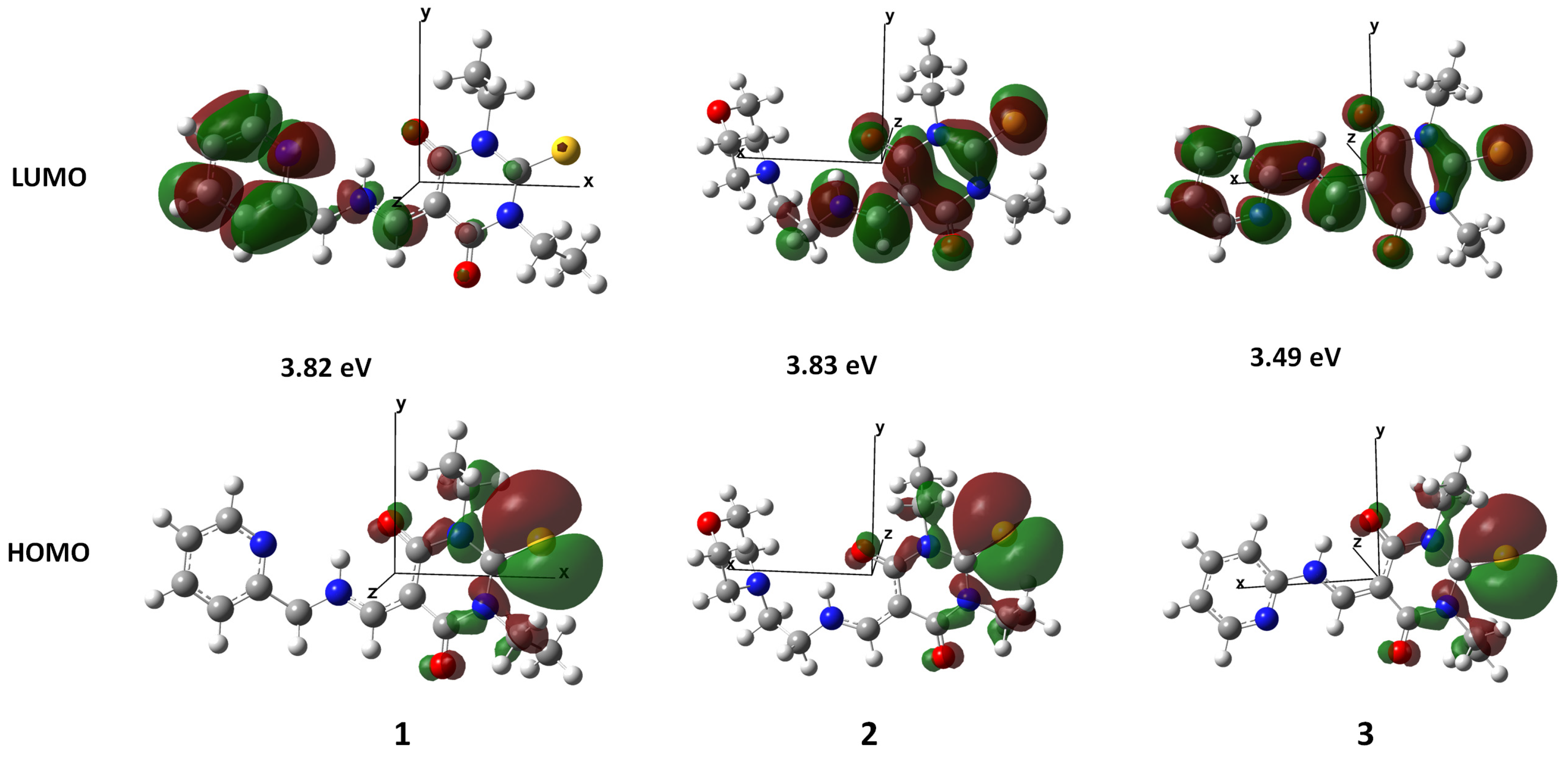
3.4. Theoretical Calculations
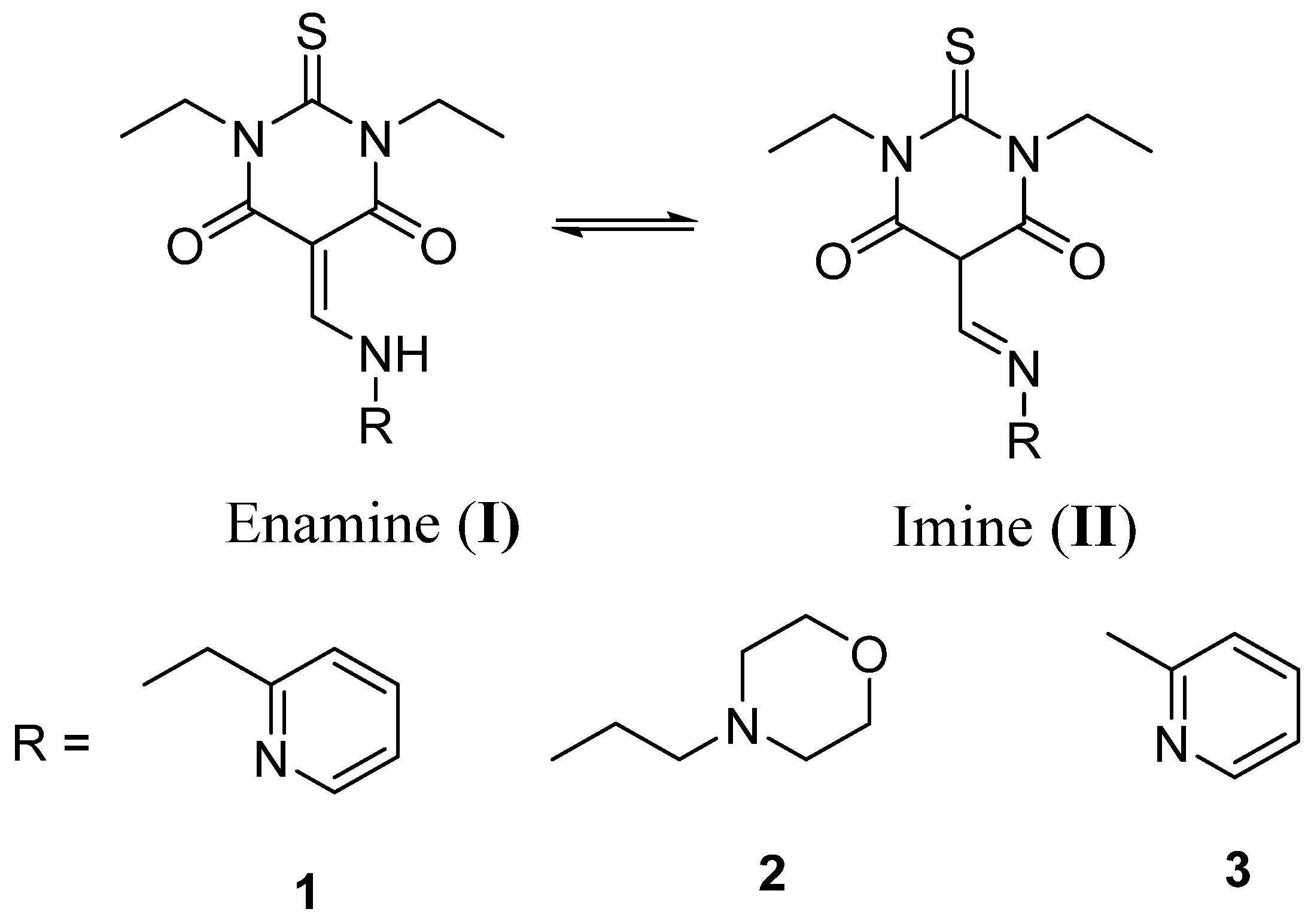
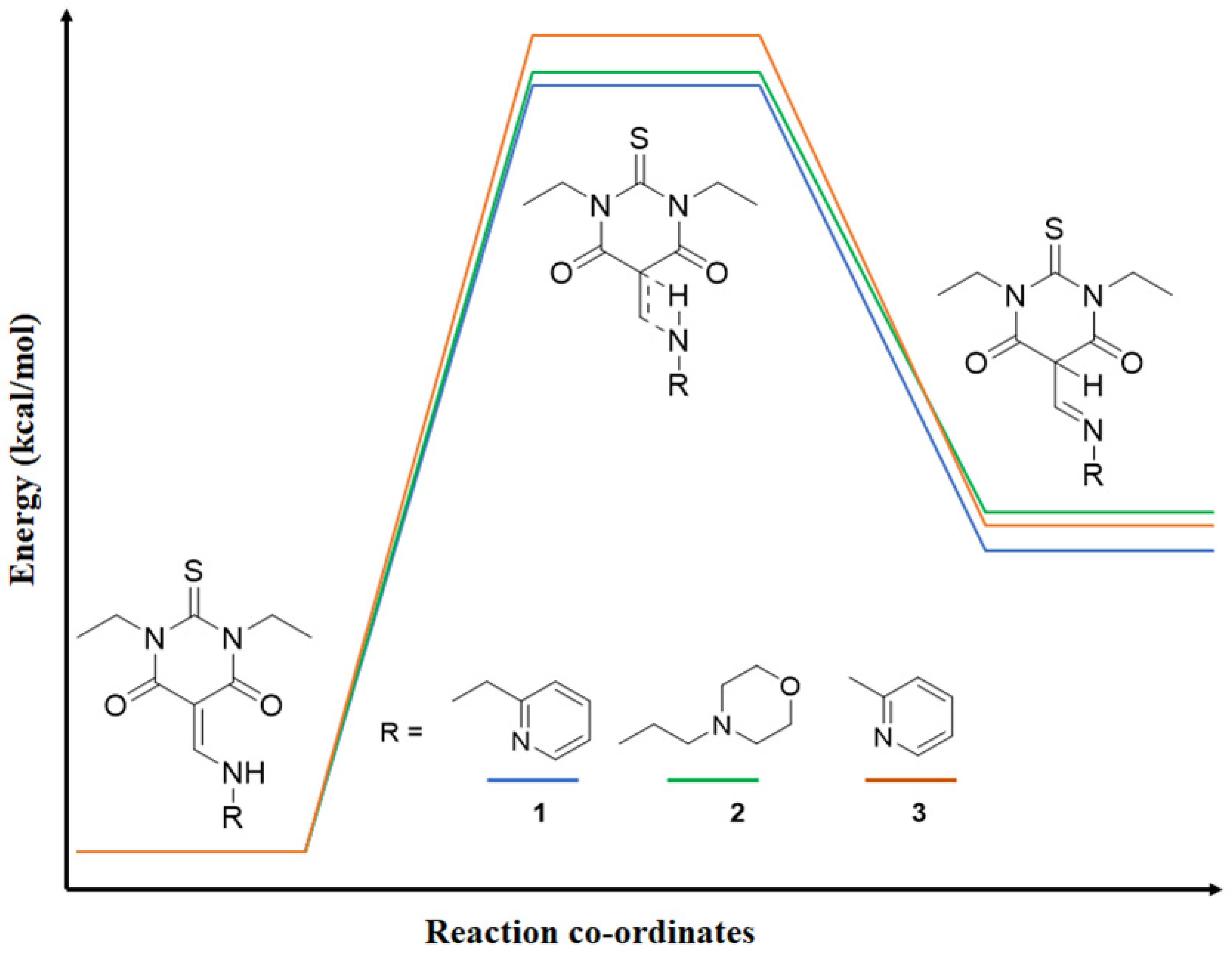
3.5. Transition State Calculations
3.6. NLO Calculations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Shell, J.W. X-ray and Crystallographic Applications in Pharmaceutical Research II. J. Pharm. Sci. 1963, 52, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Deschamps, J. The role of crystallography in drug design. AAPS J. 2005, 7, E813–E819. [Google Scholar] [CrossRef] [PubMed]
- Aitipamula, S.; Vangala, V.R. X-Ray Crystallography and its Role in Understanding the Physicochemical Properties of Pharmaceutical Cocrystals. J. Indian Inst. Sci. 2017, 97, 227–243. [Google Scholar] [CrossRef]
- Zheng, H.; Hou, J.; Zimmerman, M.; Wlodawer, A.; Minor, W. The future of crystallography in drug discovery. Expert Opin. Drug Deliv. 2013, 9, 125–137. [Google Scholar] [CrossRef]
- Mason, S. Molecular structure and biological activity. Trends Biochem. Sci. 1983, 8, 422–423. [Google Scholar] [CrossRef]
- Jursic, B.S.; Neumann, N.M.; Bowdy, K.L.; Stevens, E.D. Simple, efficient, high yield syntheses of substituted and unsubstituted 5-benzoylbarbituric acids, and their corresponding schiff base phenylhydrazones. J. Heterocycl. Chem. 2004, 41, 233–246. [Google Scholar] [CrossRef]
- Kondo, K.; Fukutome, M.; Ohnishi, N.; Aso, H.; Kitaoka, Y.; Sasaki, T. Laser Properties of Nonlinear Optical Benzal Barbituric Acid Crystals. Jpn. J. Appl. Phys. 1991, 30, 3419–3420. [Google Scholar] [CrossRef]
- Knill, E.; Laflamme, R.; Milburn, G.J. A scheme for efficient quantum computation with linear optics. Nature 2001, 409, 46–52. [Google Scholar] [CrossRef]
- Feng, J.-D.; Yan, L.-K.; Su, Z.; Kan, Y.; Lan, Y.-Q.; Liao, Y.; Zhu, Y.-L. Theoretical Studies on Electronic Spectra and Second-order Nonlinear Optical Properties of Glucosyl Substituted Barbituric Acid Derivatives. Chin. J. Chem. 2006, 24, 119–123. [Google Scholar] [CrossRef]
- Smith, S.D. Lasers, nonlinear optics and optical computers. Nature 1985, 316, 319–324. [Google Scholar] [CrossRef]
- Cammi, R.; Mennucci, B.; Tomasi, J. Solvent Effects on Linear and Nonlinear Optical Properties of Donor–Acceptor Polyenes: Investigation of Electronic and Vibrational Components in Terms of Structure and Charge Distribution Changes. J. Am. Chem. Soc. 1998, 120, 8834–8847. [Google Scholar] [CrossRef]
- Ivanova, B.B.; Spiteller, M. Possible Application of the Organic Barbiturates as NLO Materials. Cryst. Growth Des. 2010, 10, 2470–2474. [Google Scholar] [CrossRef]
- Amudha, M.; Rajkumar, R.; Thayanithi, V.; Kumar, P.P. Growth and Characterization of Benzimidazolium Salicylate: NLO Property from a Centrosymmetric Crystal. Adv. Opt. Technol. 2015, 2015, 1–9. [Google Scholar] [CrossRef]
- Arulmozhi, S.; Madhavan, J. Molecular structure, First-order hyperpolarizability and HOMO-LUMO studies of L- Histidinium Dinitrate. IOP Conf. Ser.: Mater. Sci. Eng. 2015, 73, 012035. [Google Scholar] [CrossRef]
- Nalla, V.; Medishetty, R.; Wang, Y.; Bai, Z.; Sun, H.; Wei, J.; Vittal, J.J. Second harmonic generation from the ‘centrosymmetric’ crystals. IUCrJ 2015, 2, 317–321. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.-W.; Chai, X.-D.; Chen, S.-G.; Jiang, Y.-S.; Yang, W.-S.; Lu, R.; Ren, Y.-Z.; Blanchard-Desce, M.; Li, T.-J.; Lehn, J.-M. A new series of nonlinear optical organic materials with molecular receptor: Design and synthesis. Synth. Met. 1995, 71, 1733–1734. [Google Scholar] [CrossRef]
- Gryl, M.; Kozieł, M.; Stadnicka, K.; Matulková, I.; Němec, I.; Mrkyvkova, N.; Němec, P. Lidocaine barbiturate: A promising material for second harmonic generation. CrystEngComm 2013, 15, 3275. [Google Scholar] [CrossRef]
- Moylan, C.R.; Twieg, R.J.; Lee, V.Y.; Swanson, S.A.; Betterton, K.M.; Miller, R.D. Nonlinear optical chromophores with large hyperpolarizabilities and enhanced thermal stabilities. J. Am. Chem. Soc. 1993, 115, 12599–12600. [Google Scholar] [CrossRef]
- Sun, G.; Qiu, Y.-Q.; Sun, H.-Z.; Su, Z.; Feng, J.-D.; Zhu, Y.-L. Theoretical studies on electronic spectra and second-order nonlinear optical properties of barbituric acid derivatives substituted with schiff base. Chin. J. Chem. 2010, 22, 425–429. [Google Scholar] [CrossRef]
- Tron, A.; Rocher, M.; Thornton, P.J.; Tucker, J.H.R.; McClenaghan, N.D. Supramolecular Architectures Incorporating Hydrogen-Bonding Barbiturate Receptors. Asian J. Org. Chem. 2015, 4, 192–202. [Google Scholar] [CrossRef]
- Sharma, A.; Noki, S.; Zamisa, S.J.; Hazzah, H.A.; Almarhoon, Z.; El-Faham, A.; De La Torre, B.G.; Albericio, F.; Noki, S. Exploiting the Thiobarbituric Acid Scaffold for Antibacterial Activity. ChemMedChem 2018, 13, 1923–1930. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Zamisa, S.J.; Noki, S.; Almarhoon, Z.; El-Faham, A.; De La Torre, B.G.; Albericio, F. Crystal structure, spectroscopic studies and theoretical studies of thiobarbituric acid derivatives: Understanding the hydrogen-bonding patterns. Acta Crystallogr. C Struct. Chem. 2018, 74, 1703–1714. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Barakat, A.; El-Faham, A.; Al-Rasheed, H.H.; Dahlous, K.; Al-Majid, A.M.; Sharma, A.; Yousuf, S.; Sanam, M.; Ul-Haq, Z.; et al. Synthesis and characterisation of thiobarbituric acid enamine derivatives, and evaluation of their α-glucosidase inhibitory and anti-glycation activity. J. Enzym. Inhib. Med. Chem. 2020, 35, 692–701. [Google Scholar] [CrossRef] [PubMed]
- Bruker, A. SAINT Software Reference Manual; Bruker AXS, Inc.: Madison, WI, USA, 1998. [Google Scholar]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.; Schlegel, H.; Scuseria, G.; Robb, M.; Cheeseman, J.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G. Gaussian 09, Revision D. 01; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Gonzalez, C.; Schlegel, H.B. Reaction path following in mass-weighted internal coordinates. J. Phys. Chem. 1990, 94, 5523–5527. [Google Scholar] [CrossRef]
- Spek, A.L. Single-crystal structure validation with the programPLATON. J. Appl. Crystallogr. 2003, 36, 7–13. [Google Scholar] [CrossRef]
- Aihara, J.-I. Correlation found between the HOMO–LUMO energy separation and the chemical reactivity at the most reactive site for isolated-pentagon isomers of fullerenes. Phys. Chem. Chem. Phys. 2000, 2, 3121–3125. [Google Scholar] [CrossRef]
- Lewars, E.G. (Ed.) The Concept of the Potential Energy Surface. In Computational Chemistry: Introduction to the Theory and Applications of Molecular and Quantum Mechanics; Springer International Publishing: Cham, Germany, 2016; pp. 9–49. [Google Scholar] [CrossRef]
- Machado, D.F.S.; Lopes, T.; Lima, I.T.; Filho, D.A.D.S.; De Oliveira, H.C.B. Strong Solvent Effects on the Nonlinear Optical Properties of Z and E Isomers from Azo-Enaminone Derivatives. J. Phys. Chem. C 2016, 120, 17660–17669. [Google Scholar] [CrossRef]
- Pegu, D. Solvent effects on nonlinear optical properties of novel para-nitroaniline derivatives: A density functional approach. Int. J. Sci. Res. 2014, 3, 469–474. [Google Scholar]
- Rajkumar, R.; Kamaraj, A.; Bharanidharan, S.; Saleem, H.; Krishnasamy, K. Synthesis, spectral characterization, single crystal X-ray diffraction and DFT studies of 4-((2,4,5-triphenyl-1H-imidazol-1-yl)methyl)pyridine derivatives. J. Mol. Struct. 2015, 1084, 74–81. [Google Scholar] [CrossRef]


| # | 1 | 2 | 3 |
|---|---|---|---|
| Empirical formula | C15H18N4O2S | C15H24N4O3S | C14H16N4O2S |
| Formula weight | 318.39 | 340.44 | 304.37 |
| Temperature | 100(2) K | 100(2) K | 173(2) K |
| Wavelength | 1.54178 Å | 1.54178 Å | 1.54178 Å |
| Crystal system | Triclinic | Monoclinic | Monoclinic |
| Space group | P − 1 | P 21/c | P 21/n |
| Unit cell dimensions | a = 4.8848(2) Å | a = 9.7627(3) Å | a = 11.3925(3) Å |
| α = 93.6450(10)° | α = 90° | α = 90° | |
| b = 11.8126(4) Å | b = 19.5503(5) Å | b = 8.9642(3) Å | |
| β = 100.1890(10)° | β = 94.6850(10)° | β = 108.7440(10)° | |
| c = 13.2589(4) Å | c = 8.9301(3) Å | c = 14.6437(3) Å | |
| γ = 93.9350(10)° | γ = 90° | γ = 90° | |
| Volume | 748.99(5) Å3 | 1698.74(9) Å3 | 1416.17(7) Å3 |
| Z | 2 | 4 | 4 |
| Density (calculated) | 1.412 Mg/m3 | 1.327 Mg/m3 | 1.428 Mg/m3 |
| Absorption coefficient | 2.037 mm−1 | 1.839 mm−1 | 2.129 mm−1 |
| F(000) | 336 | 728 | 640 |
| Crystal size (mm3) | 0.170 × 0.040 × 0.020 | 0.170 × 0.120 × 0.070 | 0.160 × 0.140 × 0.090 |
| Theta range for data collection | 3.397 to 68.333°. | 4.523 to 68.224°. | 5.877 to 68.226°. |
| Index ranges | −5 ≤ h ≤ 5 | −11 ≤ h ≤ 11 | −13 ≤ h ≤ 13 |
| −14 ≤ k ≤ 14 | −23 ≤ k ≤ 23 | −10 ≤ k ≤ 10 | |
| −15 ≤ l ≤ 15 | −10 ≤ l ≤ 10 | −17 ≤ l ≤ 17 | |
| Reflections collected | 19056 | 22428 | 40991 |
| Independent reflections | 2729 [R(int) = 0.0636] | 3114 [R(int) = 0.0316] | 2593 [R(int) = 0.0381] |
| Completeness to theta = 67.679° | 100.0% | 99.9% | 99.6% |
| Refinement method | Full-matrix least-squares on F2 | Full-matrix least-squares on F2 | Full-matrix least-squares on F2 |
| Data/restraints/parameters | 2729/0/203 | 3114/0/212 | 2593/0/196 |
| Goodness-of-fit on F2 | 1.067 | 1.054 | 1.089 |
| Final R indices [I > 2sigma(I)] | R1 = 0.0598, wR2 = 0.1802 | R1 = 0.0323, wR2 = 0.0846 | R1 = 0.0353, wR2 = 0.0881 |
| R indices (all data) | R1 = 0.0652, wR2 = 0.1868 | R1 = 0.0347, wR2 = 0.0866 | R1 = 0.0359, wR2 = 0.0885 |
| Largest diff. peak and hole | 0.622 and −0.672 e.Å-3 | 0.737 and −0.240 e.Å-3 | 0.396 and −0.241 e.Å-3 |
| D | H | A | D-H | H···A | D···A | D-H···A |
|---|---|---|---|---|---|---|
| C1 | H25 | O2 | 0.99 | 2.58 | 3.4464(18) | 146 |
| C5 | H6 | O1 | 0.95 | 2.60 | 3.4395(17) | 148 |
| C9 | H9 | O2 | 0.98 | 2.57 | 3.3370(18) | 135 |
| C15 | H20 | O3 | 0.99 | 2.40 | 3.3831(19) | 171 |
| D. | H | A | D-H | H···A | D···A | D-H···A |
|---|---|---|---|---|---|---|
| C7 | H7A | O2 | 0.95 | 2.28 | 3.174(3) | 156 |
| D | H | A | D-H | H···A | D···A | D-H···A |
|---|---|---|---|---|---|---|
| C4 | H4 | O1 | 0.95 | 2.40 | 3.3420(19) | 172 |
| C12 | H12A | O1 | 0.98 | 2.58 | 3.531(2) | 163 |
| C14 | H14C | O2 | 0.98 | 2.58 | 3.442(2) | 147 |
| - | 1 | 2 | 3 |
|---|---|---|---|
| Etotal in Hartree | −1350.42096974 | −1429.26867147 | −1311.10670189 |
| EHOMO in Hartree | −0.21654 | −0.21799 | −0.22352 |
| ELUMO in Hartree | −0.07593 | −0.07714 | −0.09533 |
| ΔE * in Hartree (eV) | 0.14061 (3.82) | 0.14085 (3.83) | 0.12819 (3.49) |
| Properties | Compound | ΔH° (kcal/mol) | ΔG°# (kcal/mol/K) | ΔS° (cal/mol) |
|---|---|---|---|---|
| Thermodynamics | 1 | 24.2 | 23.4 | 3.0 |
| 2 | 27.2 | 26.5 | 2.5 | |
| 3 | 26.8 | 25.5 | 4.5 | |
| Kinetics | 1 | 60.8 | 60.5 | 1.1 |
| 2 | 61.8 | 61.1 | 2.3 | |
| 3 | 64.3 | 63.7 | 2.1 |
| Parameter | Gaseous | Chloroform | Methanol | Acetonitrile | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | |
| μx | −7.40 | 5.22 | 5.78 | −9.82 | 6.72 | 7.67 | −11.29 | 7.34 | 8.43 | −10.72 | 7.34 | 8.44 |
| μy | −1.35 | −2.70 | 1.92 | −2.10 | −3.81 | 2.55 | −2.71 | −4.42 | 2.88 | −2.58 | −4.42 | 2.88 |
| μz | 0.41 | 0.48 | 0.34 | 0.24 | 0.37 | 0.41 | 0.01 | 0.34 | 0.47 | 0.08 | 0.34 | 0.47 |
| total (μ) | 7.53 | 5.90 | 6.10 | 10.04 | 7.73 | 10.01 | 11.62 | 8.57 | 8.92 | 11.02 | 8.57 | 8.93 |
| αxx | −123.63 | −163.28 | −112.49 | −119.27 | −169.93 | −109.52 | −115.29 | −173.27 | −109.15 | −118.15 | −173.27 | −109.15 |
| αyy | −138.94 | −150.12 | −132.80 | −137.75 | −150.10 | −132.86 | −136.70 | −150.14 | −133.00 | −137.42 | −150.14 | −133.01 |
| αzz | −136.63 | −142.71 | −133.62 | −137.91 | −141.68 | −133.67 | −139.64 | −141.15 | −133.65 | −138.18 | −141.15 | −133.65 |
| αxy | 3.23 | −10.22 | 5.78 | 4.12 | −12.19 | 7.48 | 4.58 | −12.84 | 8.35 | 4.93 | −12.84 | 8.37 |
| αxz | −9.15 | −1.02 | 1.15 | −8.78 | −1.89 | 1.34 | −5.05 | −1.92 | 1.45 | −8.13 | −1.92 | 1.46 |
| αyz | 2.38 | 1.94 | 0.13 | 2.72 | 2.36 | −0.04 | 2.18 | 2.44 | −0.04 | 3.00 | 2.44 | −0.04 |
| α (esu) × 10−23 | 1.97 | 2.25 | 1.87 | 1.95 | 2.28 | 1.86 | 1.93 | 2.29 | 1.86 | 1.95 | 2.29 | 1.86 |
| βxxx | −237.81 | 21.77 | 282.13 | −339.44 | 38.91 | 357.94 | −416.28 | 47.41 | 388.35 | −381.27 | 47.41 | 388.86 |
| βyyy | 31.96 | 17.08 | 23.85 | 34.02 | 13.10 | 29.10 | 32.53 | 9.98 | 31.57 | 33.25 | 9.98 | 31.62 |
| βzzz | −5.15 | −1.91 | −0.88 | −6.16 | −0.53 | −0.68 | −5.15 | −0.21 | −0.54 | −6.84 | −0.21 | −0.54 |
| βxyy | −26.81 | 44.25 | −2.89 | −43.30 | 52.91 | −1.12 | −59.00 | 57.40 | −0.50 | −48.58 | 57.40 | −0.48 |
| βxxy | −38.97 | −100.23 | −5.76 | −58.43 | −127.04 | −1.98 | −73.38 | −139.33 | 0.21 | −69.70 | −139.33 | 0.25 |
| βxxz | 53.09 | 0.11 | 1.00 | 53.85 | 0.29 | 1.15 | 36.45 | 2.03 | 1.82 | 51.44 | 2.03 | 1.84 |
| βxzz | −11.70 | 34.96 | −13.90 | −5.64 | 41.18 | −12.51 | 5.96 | 44.08 | −11.84 | −3.45 | 44.08 | −11.83 |
| βyzz | −8.76 | 1.32 | −0.53 | −8.00 | 0.23 | 0.01 | −5.88 | −0.44 | 0.29 | −7.60 | −0.44 | 0.29 |
| βyyz | −1.97 | 1.47 | 4.43 | −5.84 | −0.31 | 5.05 | −8.22 | −0.64 | 5.38 | −6.85 | −0.64 | 5.39 |
| βxyz | −14.66 | 6.14 | 1.46 | −12.94 | 6.02 | 0.86 | −8.65 | 6.35 | 0.95 | −13.71 | 6.35 | 0.95 |
| β (esu) × 10−31 | 2.42 | 1.12 | 2.30 | 3.39 | 1.51 | 2.98 | 4.08 | 1.71 | 3.26 | 3.78 | 1.71 | 3.27 |
| μ (×10−30 esu) * | β (×10−30 esu) * | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 1 | 2 | 3 | |
| Gaseous | 7.53 | 5.90 | 6.10 | 2.96 | 1.38 | 2.40 |
| Acetonitrile (ACN) | 11.02 | 8.57 | 8.93 | 4.10 | 2.07 | 3.36 |
| Chloroform (CHCl3) | 10.04 | 7.73 | 10.01 | 3.76 | 1.84 | 3.09 |
| Methanol (MeOH) | 11.62 | 8.57 | 8.92 | 4.09 | 2.07 | 3.36 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, A.; Barakat, A.; Al-Rasheed, H.H.; Al-Majid, A.M.; Yousuf, S.; Choudhary, M.I.; El-Faham, A.; de la Torre, B.G.; Albericio, F. Crystal Structure and Theoretical Investigation of Thiobarbituric Acid Derivatives as Nonlinear Optical (NLO) Materials. Crystals 2020, 10, 442. https://doi.org/10.3390/cryst10060442
Sharma A, Barakat A, Al-Rasheed HH, Al-Majid AM, Yousuf S, Choudhary MI, El-Faham A, de la Torre BG, Albericio F. Crystal Structure and Theoretical Investigation of Thiobarbituric Acid Derivatives as Nonlinear Optical (NLO) Materials. Crystals. 2020; 10(6):442. https://doi.org/10.3390/cryst10060442
Chicago/Turabian StyleSharma, Anamika, Assem Barakat, Hessa H. Al-Rasheed, Abdullah Mohammed Al-Majid, Sammer Yousuf, M. Iqbal Choudhary, Ayman El-Faham, Beatriz G. de la Torre, and Fernando Albericio. 2020. "Crystal Structure and Theoretical Investigation of Thiobarbituric Acid Derivatives as Nonlinear Optical (NLO) Materials" Crystals 10, no. 6: 442. https://doi.org/10.3390/cryst10060442
APA StyleSharma, A., Barakat, A., Al-Rasheed, H. H., Al-Majid, A. M., Yousuf, S., Choudhary, M. I., El-Faham, A., de la Torre, B. G., & Albericio, F. (2020). Crystal Structure and Theoretical Investigation of Thiobarbituric Acid Derivatives as Nonlinear Optical (NLO) Materials. Crystals, 10(6), 442. https://doi.org/10.3390/cryst10060442









