Synthesis Dependent Structural Magnetic and Electrical Properties of CR-Doped Lead-Free Multiferroic AlFeO3
Abstract
1. Introduction
2. Experimental Section
3. Results and Discussion
3.1. Structural Characterization
3.2. Magnetic Properties
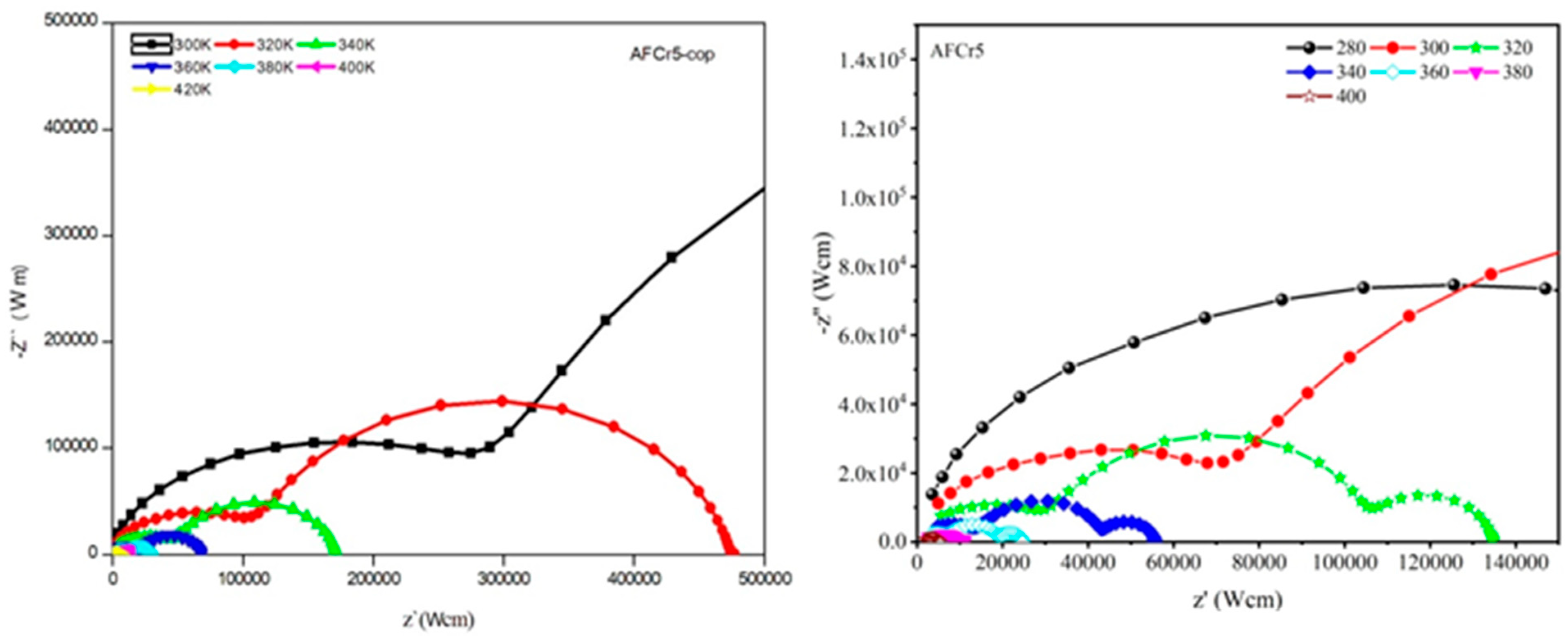
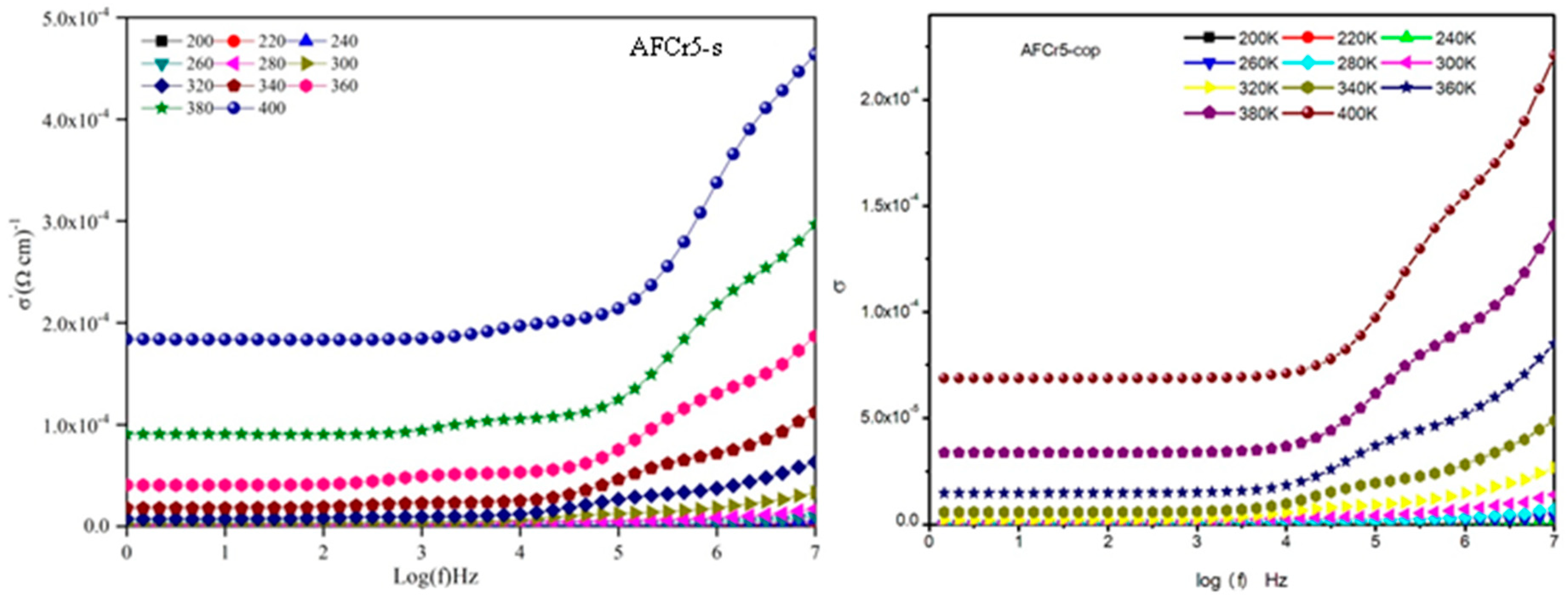
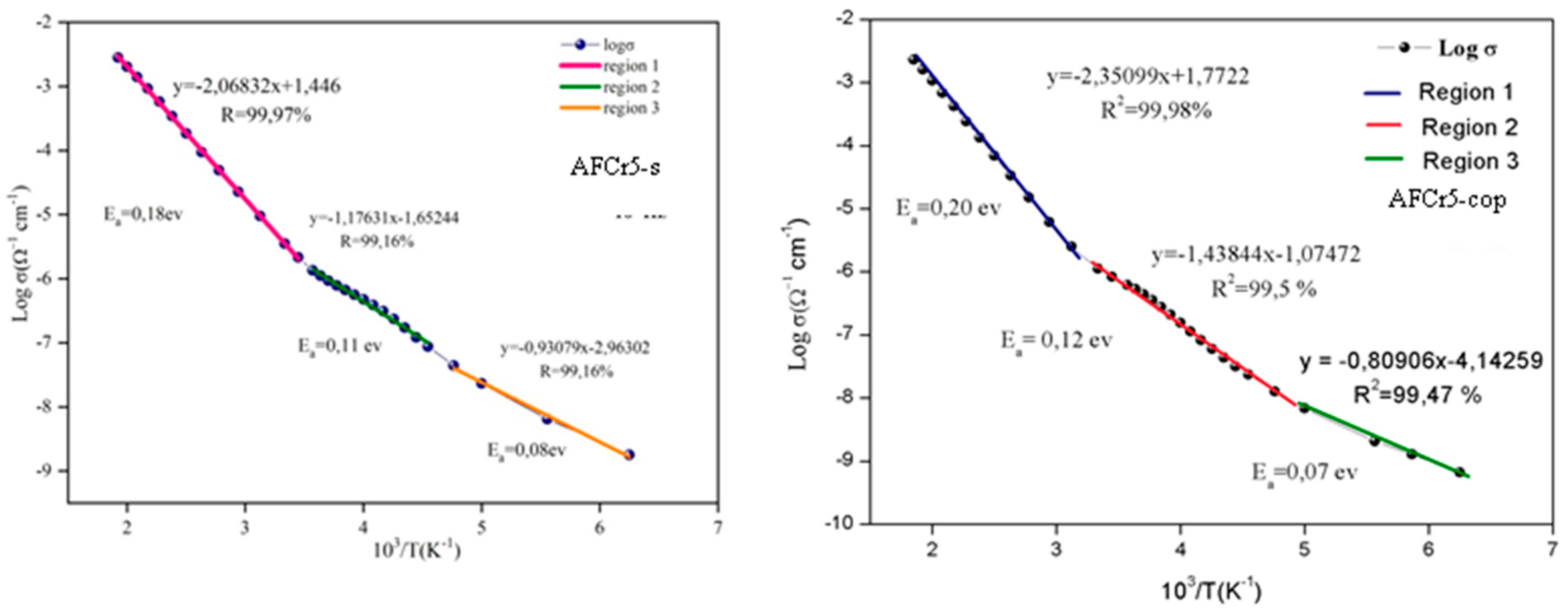
3.3. Dielectric Measurements
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Catalan, G.; Scott, J.F. Physics and applications of bismuth ferrite. Adv. Mater. 2009, 21, 2463–2485. [Google Scholar] [CrossRef]
- Hill, N.A. Why are there so few magnetic ferroelectrics? J. Phys. Chem. B 2000, 104, 6694–6709. [Google Scholar] [CrossRef]
- Ma, J.; Hu, J.; Li, Z.; Nan, C.W. Recent progress in multiferroic magnetoelectric composites: From bulk to thin films. Adv. Mater. 2011, 23, 1062–1087. [Google Scholar] [CrossRef] [PubMed]
- Shirolkar, M.M.; Hao, C.; Dong, X.; Guo, T.; Zhang, L.; Li, M.; Wang, H. Tunable multiferroic and bistable/complementary resistive switching properties of dilutely Li-doped BiFeO3 nanoparticles: An effect of aliovalent substitution. Nanoscale 2014, 6, 4735–4744. [Google Scholar] [CrossRef]
- Rao, C.N.; Sundaresan, A.; Saha, R. Multiferroic and magnetoelectric oxides: The emerging scenario. J. Phys. Chem. Lett. 2012, 3, 2237–2246. [Google Scholar] [CrossRef]
- Tokura, Y.; Seki, S. Multiferroics with spiral spin orders. Adv. Mater. 2010, 22, 1554–1565. [Google Scholar] [CrossRef]
- Edri, E.; Kirmayer, S.; Cahen, D.; Hodes, G. High open-circuit voltage solar cells based on organic–inorganic lead bromide perovskite. J. Phys. Chem. Lett. 2013, 4, 897–902. [Google Scholar] [CrossRef]
- Oishi, Y.; Tsukamoto, E.; Shimoda, M.; Takamuku, T.; Narita, T.; Era, M. A novel preparation method of lead-based layered perovskite Langmuir films with a negligible amount of PbBr2. New J. Chem. 2013, 37, 568. [Google Scholar] [CrossRef]
- Saha, R.; Shireen, A.; Shirodkar, S.N.; Singh, M.S.; Waghmare, U.V.; Waghmare, A.; Rao, C.N. Phase Transitions of AlFeO3 and GaFeO3 from the Chiral Orthorhombic (Pna 21) Structure to the Rhombohedral (R c). Struct. Inorg. Chem. 2011, 50, 9527–9532. [Google Scholar]
- Saha, R.; Shireen, A.; Shirodkar, S.N.; Waghmare, U.V.; Sundaresan, A.; Rao, C.N.R. Multiferroic and magnetoelectric nature of GaFeO3, AlFeO3 and related oxides. Solid State Commun. 2012, 152, 1964–1968. [Google Scholar] [CrossRef]
- Shireen, A.; Saha, R.; Mandal, P.; Sundaresan, A.; Rao, C.N.R. Multiferroic and magnetodielectric properties of the Al1−xGaxFeO3 family of oxides. J. Mater. Chem. 2011, 21, 57–59. [Google Scholar] [CrossRef]
- Saha, R.; Sundaresan, A.; Rao, C.N.R. Novel features of multiferroic and magnetoelectric ferrites and chromites exhibiting magnetically driven ferroelectricity. Mater. Horiz. 2014, 1, 20–31. [Google Scholar] [CrossRef]
- Hamasaki, Y.; Shimizu, T.; Taniguchi, H.; Taniyama, T.; Yasui, S.; Itoh, M. Epitaxial growth of metastable multiferroic AlFeO3 film on SrTiO3 (111) substrate. Appl. Phys. 2014, 104, 082906. [Google Scholar] [CrossRef]
- Pirc, R.; Blinc, R. Nonlinear magnetoelectric effect in magnetically disordered relaxor ferroelectrics. Ferroelectrics 2010, 400, 387–394. [Google Scholar] [CrossRef]
- Pirc, R.; Blinc, R.; Scott, J.F. Mesoscopic model of a system possessing both relaxor ferroelectric and relaxor ferromagnetic properties. Phys. Rev. B 2009, 79, 214114. [Google Scholar] [CrossRef]
- Liao, L.; An, R.; Shi, S.; Xu, Y.; Luo, Y.; Liao, W. One New Species of the Genus Dryomys (Rodentia, Glirgae) from Xinjiang China, Dryomys Yarkandensis sp. nov. Magn 2020, 33, 795–808. [Google Scholar] [CrossRef]
- Kumar, A.; Mishra, V.; Warshi, M.K.; Sati, A.; Sagdeo, A.; Kumar, R.; Sagdeo, P.R. Strain induced disordered phonon modes in Cr doped PrFeO3. J. Phys. Condens. Matter 2019, 31, 275602. [Google Scholar] [CrossRef]
- Sharma, N.; Mall, A.K.; Gupta, R.; Garg, A.; Singh, A.K.; Kumar, S. Temperature dependent structural and electrical analysis of Cr-doped multiferroic GaFeO3 ceramics. Mater. Res. Express 2019, 6, 115704. [Google Scholar] [CrossRef]
- Mohamed, M.B.; Senyshyn, A.; Ehrenberg, H.; Fuess, H. Structural, magnetic, dielectric properties of multiferroic GaFeO3 prepared by solid state reaction and sol–gel methods. J. Alloys Compd. 2010, 492, L20–L27. [Google Scholar] [CrossRef]
- Sharma, K.; Reddy, V.R.; Kothari, D.; Gupta, A.; Banerjee, A.; Sathe, V.G. Low temperature Raman and high field 57Fe Mossbauer study of polycrystalline GaFeO3. Phys. Condens. Matter 2010, 22, 146005. [Google Scholar] [CrossRef]
- Goodenough, J.B. Theory of the Role of Covalence in the Perovskite-Type Manganites[La, M(II)]MnO3. Phys. Rev. 1955, 100, 564–573. [Google Scholar] [CrossRef]
- Bora, T.; Ravi, S. Study of magnetization reversal in LaCr1−xFexO3compounds. J. Appl. Phys. 2013, 114, 033906. [Google Scholar] [CrossRef]
- Selvadurai, A.P.B.; Pazhanivelu, V.; Jagadeeshwaran, C.; Murugaraj, R.; Muthuselvam, I.P.; Chou, F.C. Influence of Cr substitution on structural, magnetic and electrical conductivity spectra of LaFeO3. J. Alloys Compd. 2015, 646, 924–931. [Google Scholar] [CrossRef]
- Bakr, M.; Mohamed; Wang, H.; Fuess, H. Dielectric relaxation and magnetic properties of Cr doped GaFeO3. J. Phys. D Appl. Phys. 2010, 43, 43455409. [Google Scholar]
- Lunkenheimer, P.; Bobnar, V.; Pronin, A.V.; Ritus, A.I.; Volkov, A.A.; Loidl, A. Origin of apparent colossal dielectric constants. Phys. Rev. B 2002, 66, 052105. [Google Scholar] [CrossRef]
- Cohen, M.H.; Neaton, J.B.; He, L.; Vanderbilt, D.J. Extrinsic models for the dielectric response of CaCu3Ti4O12. Appl. Phys. 2003, 94, 3299. [Google Scholar] [CrossRef]
- Catalan, G. Magnetocapacitance without magnetoelectric coupling. Appl. Phys. Lett. 2006, 88, 102902. [Google Scholar] [CrossRef]
- Santos, G.M.; Silva, D.M.; Freitas, V.F.; Dias, G.S.; Coelho, A.A.; Pál, M.; Santos, I.; Cotica, L.; Guo, R.; Bhalla, A.S. Multiferroic Behavior of Lead-free AlFeO3 and Mn, Nb Doped Compositions. Ferroelectrics 2014, 460, 108–116. [Google Scholar] [CrossRef]
- Khan, A.A.; Satapathy, S.; AnjuAhlawat; Deshmukh, P.; Karnal, A.K. Magneto-dielectric coupling in SmFeO3: A study on anomalous dielectric, conductivity, impedance at spin reorientation temperature. Ceram. Int. 2018, 44, 12401–12413. [Google Scholar] [CrossRef]
- Penn, S.J.; Alford, N.M.; Templeton, A.; Wang, X.; Xu, M.; Reece, M.; Schrapel, K. Effect of porosity and grain size on the microwave dielectric properties of sintered alumina. J. Am. Ceram. Soc. 1997, 80, 1885. [Google Scholar] [CrossRef]
- Ortega, N.; Kumar, A.; Bhattacharya, P.; Majumder, S.B.; Katiyar, R.S. Impedance spectroscopy of multiferroic PbZrxTi1−xO3/CoFe2O4 layered thin films. Phys. Rev. B 2008, 77, 014111. [Google Scholar] [CrossRef]
- Pike, G.E. AC conductivity of scandium oxide and a new hopping model for conductivity. Phys. Rev. B 1972, 6, 1572. [Google Scholar] [CrossRef]
- Elliot, S.R. Frequency-dependent conductivity in ionic glasses: A possible model. Solid State Ion. 1988, 27, 131–149. [Google Scholar] [CrossRef]
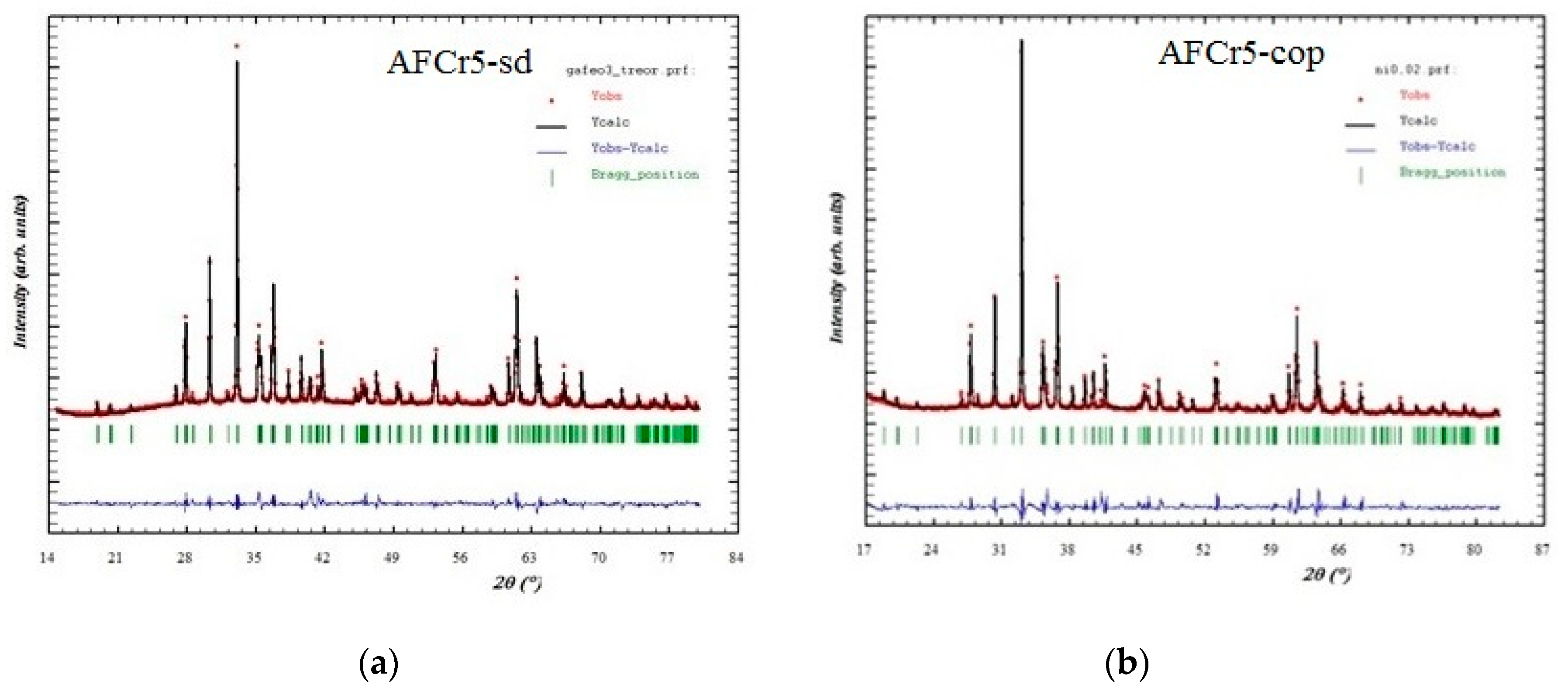
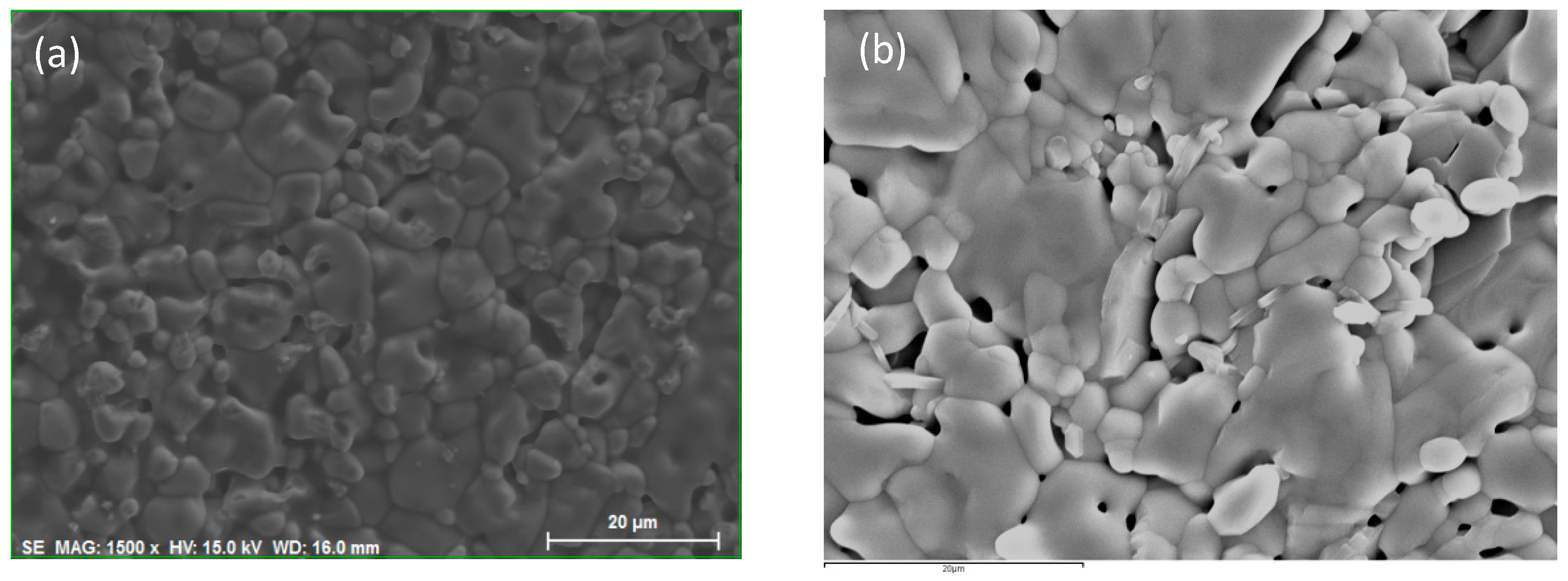

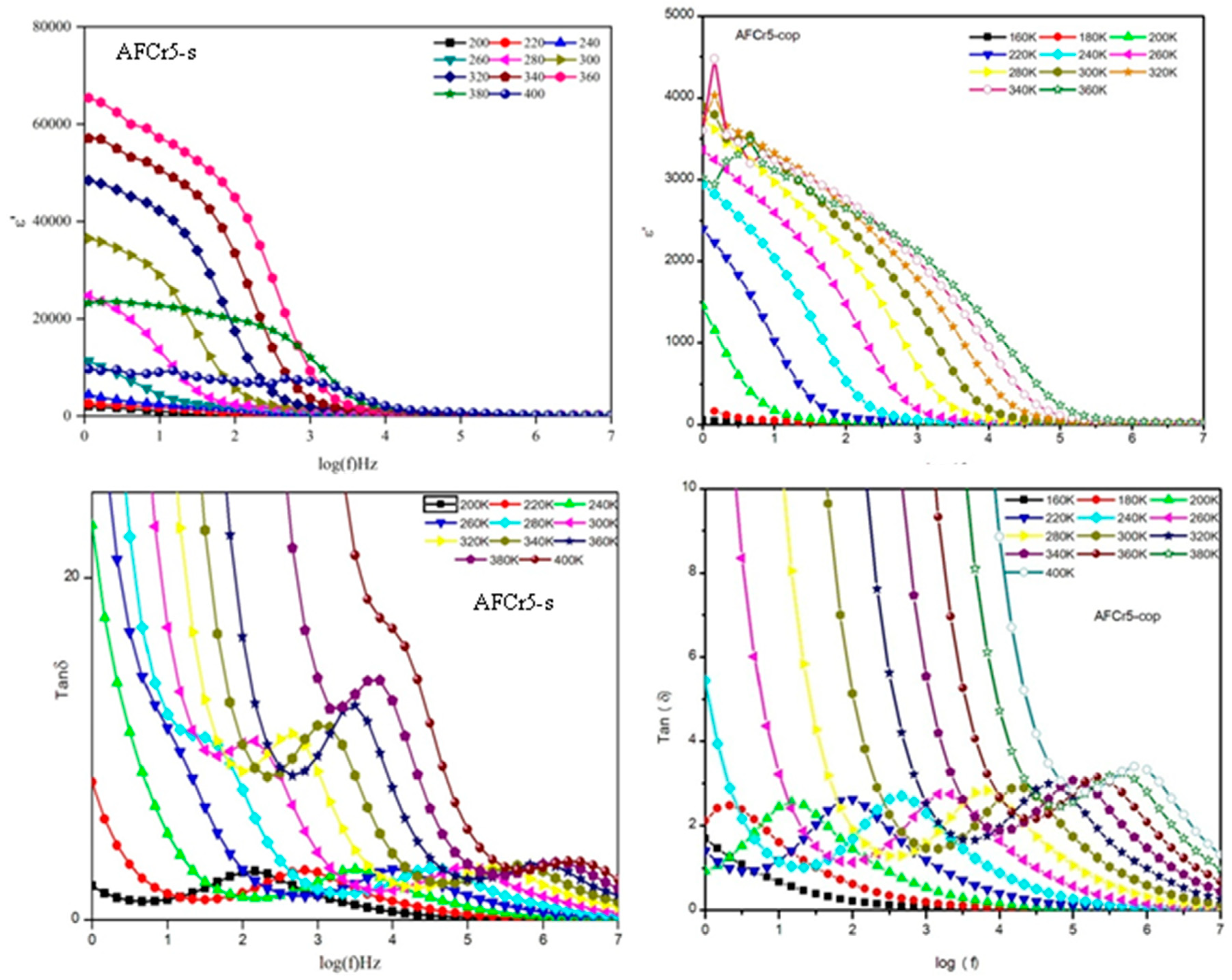
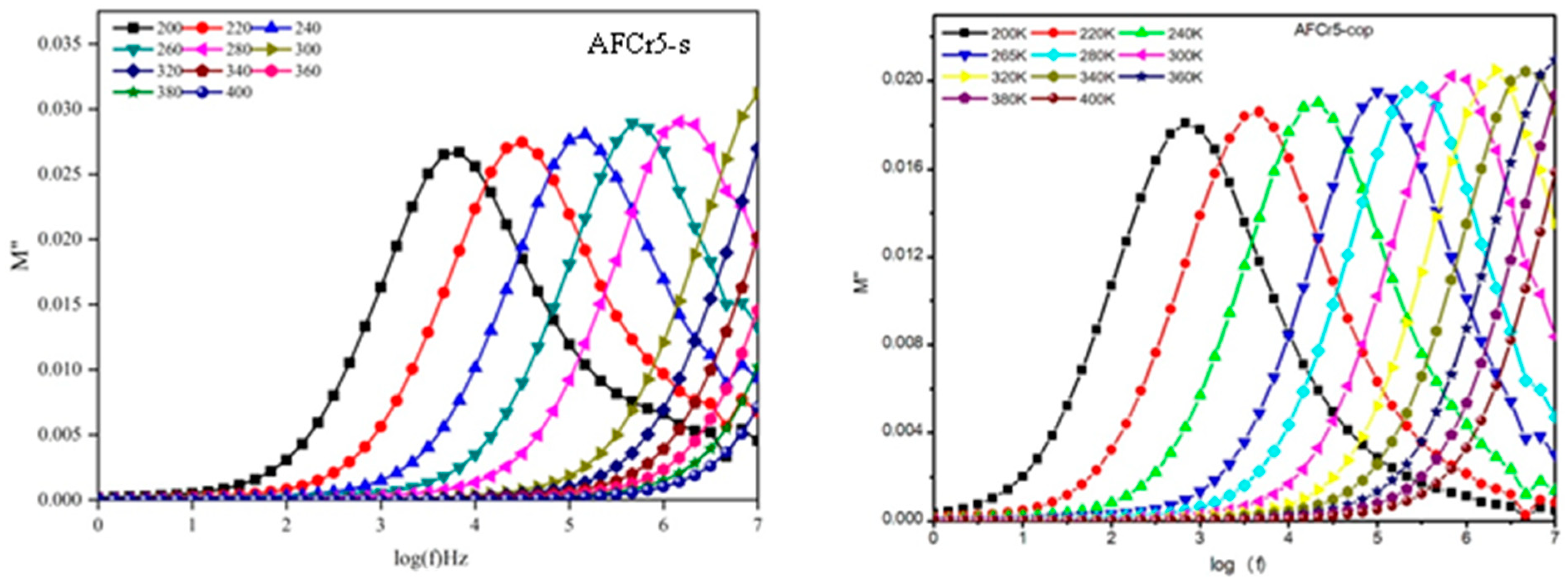


| a | b | c | V | |
|---|---|---|---|---|
| AFCr5-s | 8.7427(7) | 9.3875(5) | 5.0803(7) | 416.96(2) |
| AFCr5-cop | 8.7577(3) | 9.4104(6) | 5.0853(5) | 419.10(5) |
| Ms(emu/Fe) | Hc(Oe) | Mr(emu/Fe) | |
|---|---|---|---|
| AFCr5-s | 0.77 | 6435 | 0.34 |
| AFCr5-cop | 0.89 | 4035 | 0.49 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aldulmani, S.A.A.; Raies, I.; Amami, M.; Ben Farhat, L. Synthesis Dependent Structural Magnetic and Electrical Properties of CR-Doped Lead-Free Multiferroic AlFeO3. Crystals 2020, 10, 440. https://doi.org/10.3390/cryst10060440
Aldulmani SAA, Raies I, Amami M, Ben Farhat L. Synthesis Dependent Structural Magnetic and Electrical Properties of CR-Doped Lead-Free Multiferroic AlFeO3. Crystals. 2020; 10(6):440. https://doi.org/10.3390/cryst10060440
Chicago/Turabian StyleAldulmani, Sharah Ali A., Imen Raies, Mongi Amami, and Lamia Ben Farhat. 2020. "Synthesis Dependent Structural Magnetic and Electrical Properties of CR-Doped Lead-Free Multiferroic AlFeO3" Crystals 10, no. 6: 440. https://doi.org/10.3390/cryst10060440
APA StyleAldulmani, S. A. A., Raies, I., Amami, M., & Ben Farhat, L. (2020). Synthesis Dependent Structural Magnetic and Electrical Properties of CR-Doped Lead-Free Multiferroic AlFeO3. Crystals, 10(6), 440. https://doi.org/10.3390/cryst10060440




