Novel Cd (II) Coordination Polymers Afforded with EDTA or Trans-1,2-Cdta Chelators and Imidazole, Adenine, or 9-(2-Hydroxyethyl) Adenine Coligands
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Crystallography
2.3. Other Physical Measurements
2.4. Synthesis
2.4.1. {[Cd(µ3-EDTA)(Him)·Cd(Him)(H2O)2]·H2O}n (1)
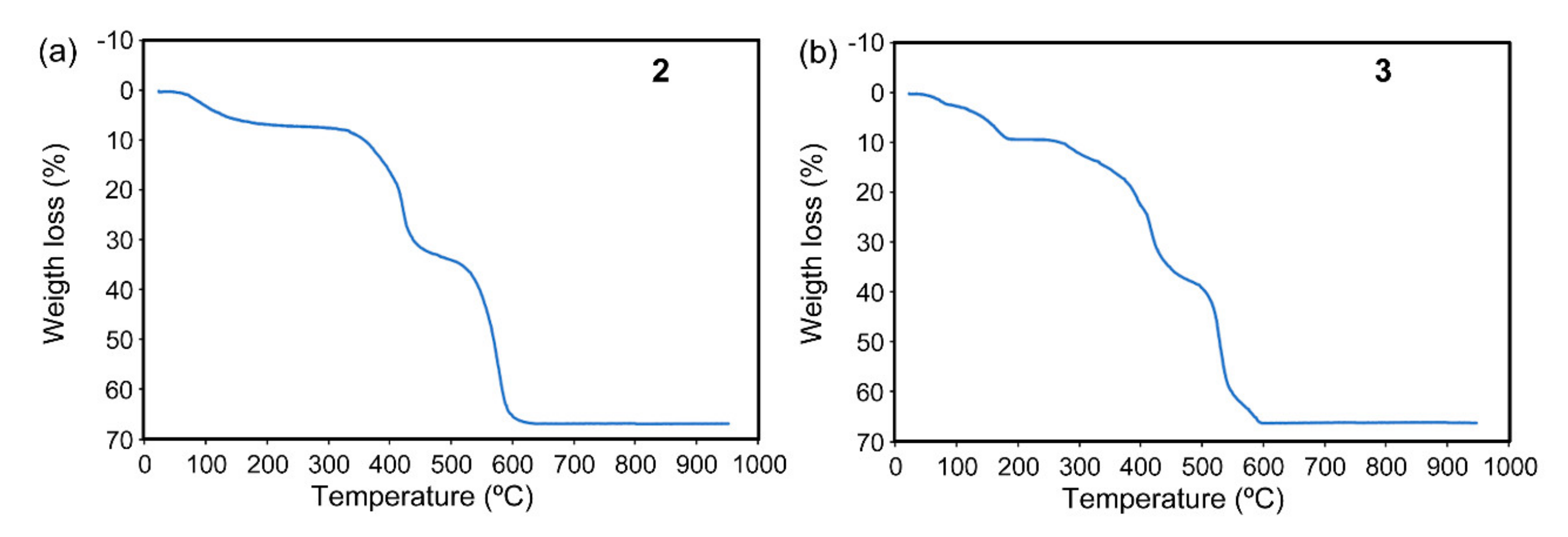
2.4.2. {[Cd(µ4-CDTA)(Hade)·Cd(Hade)2]}n (2)
2.4.3. {[Cd(µ3-EDTA)(H2O)·Cd(H9heade)(H2O)]·4H2O}n (3)
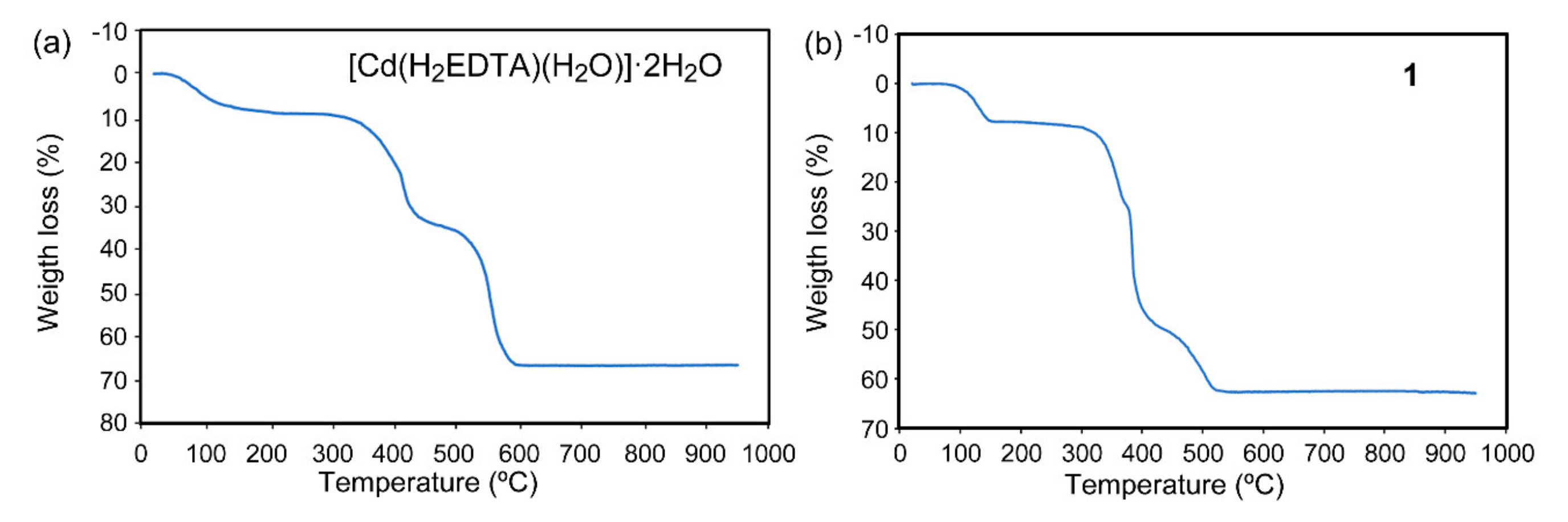
2.4.4. {[Cd(H2EDTA(H2O)]·2H2O}n
2.5. Theoretical Methods
3. Results and Discussion
3.1. Synthetic Considerations
3.2. Thermal Stability of [Cd(H2EDTA)(H2O)]·2H2O and the Polymeric Compounds 1 to 3
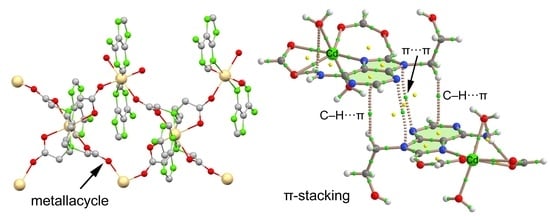
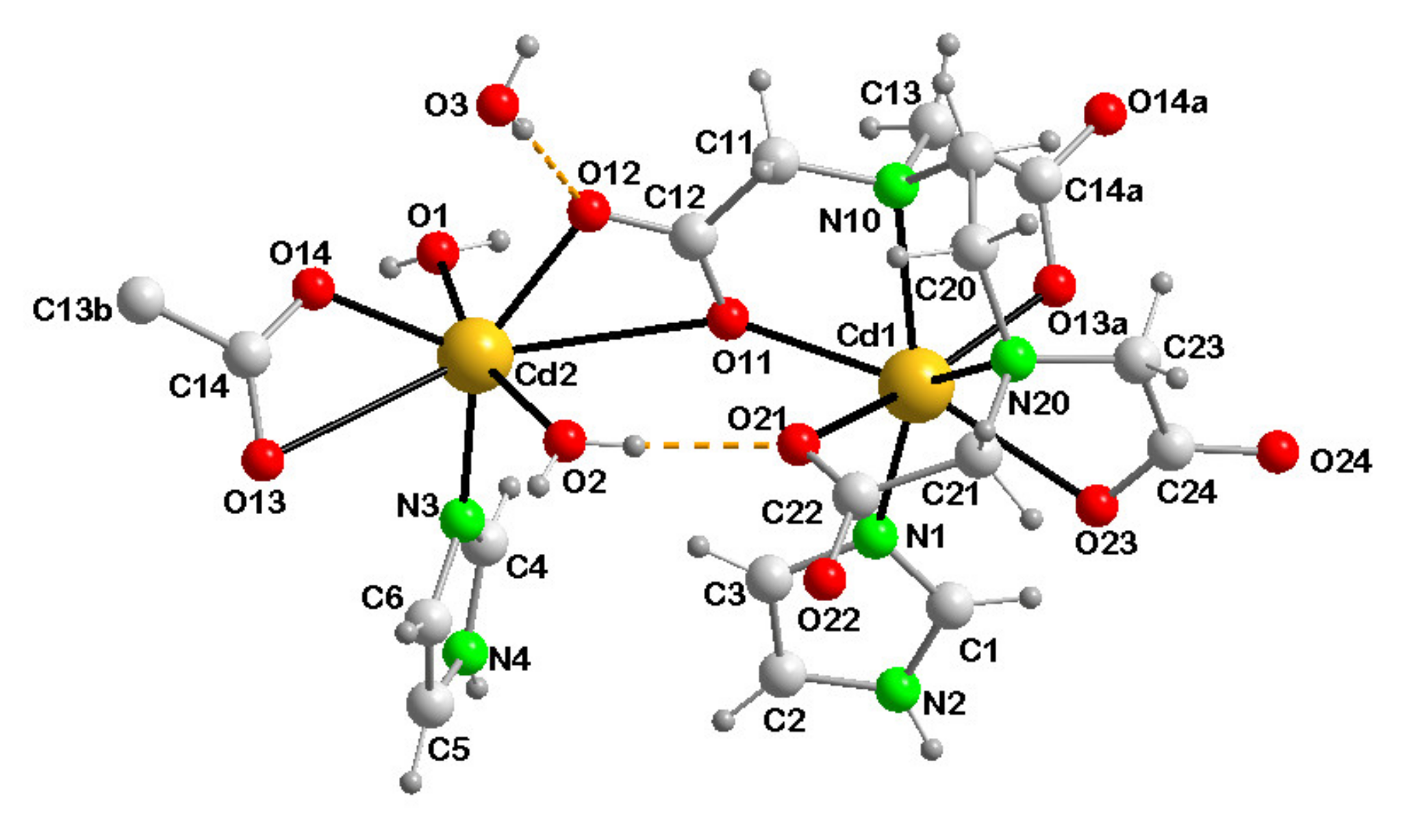
3.3. Structural Description
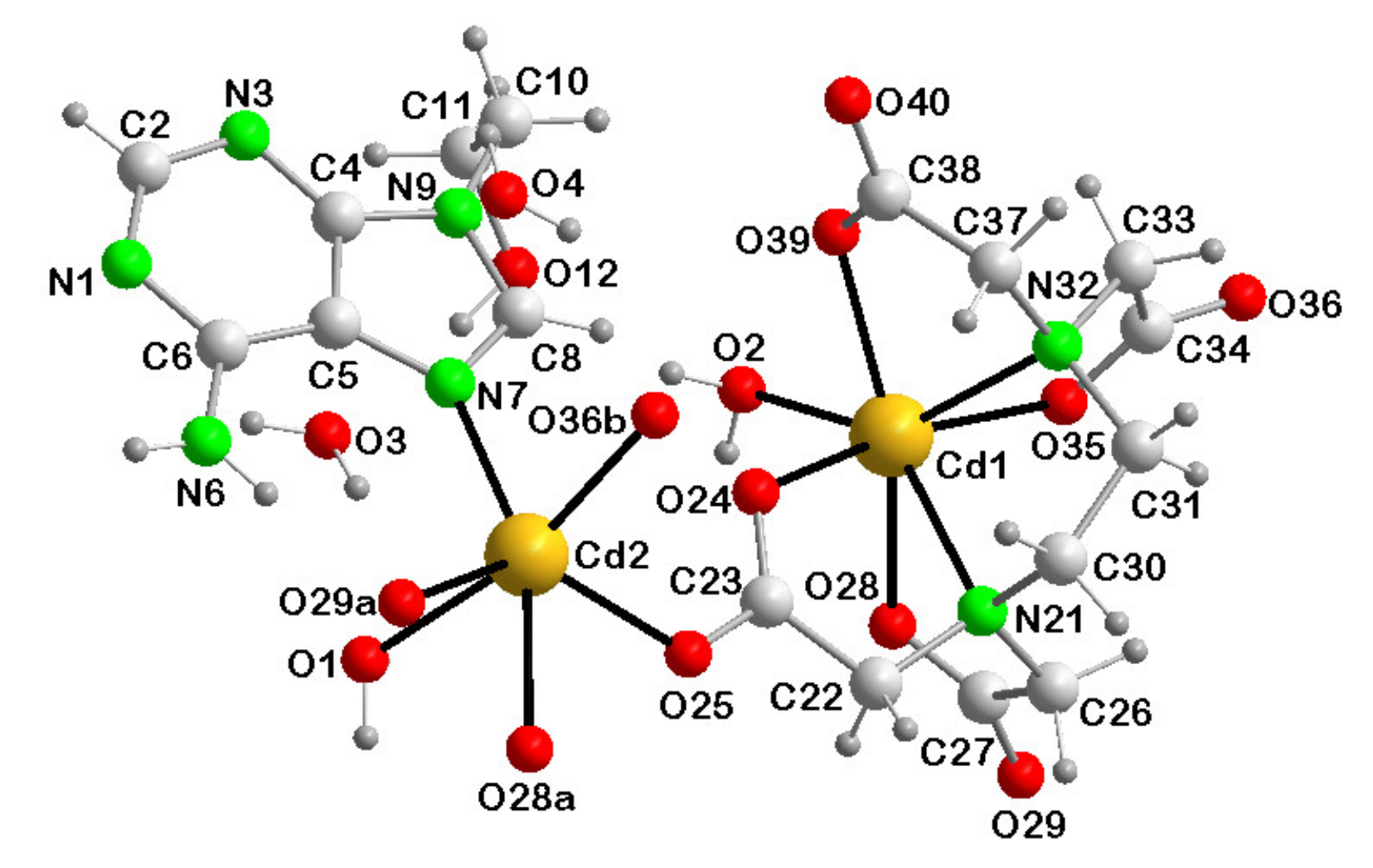
3.3.1. {[Cd(µ3-EDTA)(Him)·Cd(Him)(H2O)2]·H2O}n (1)
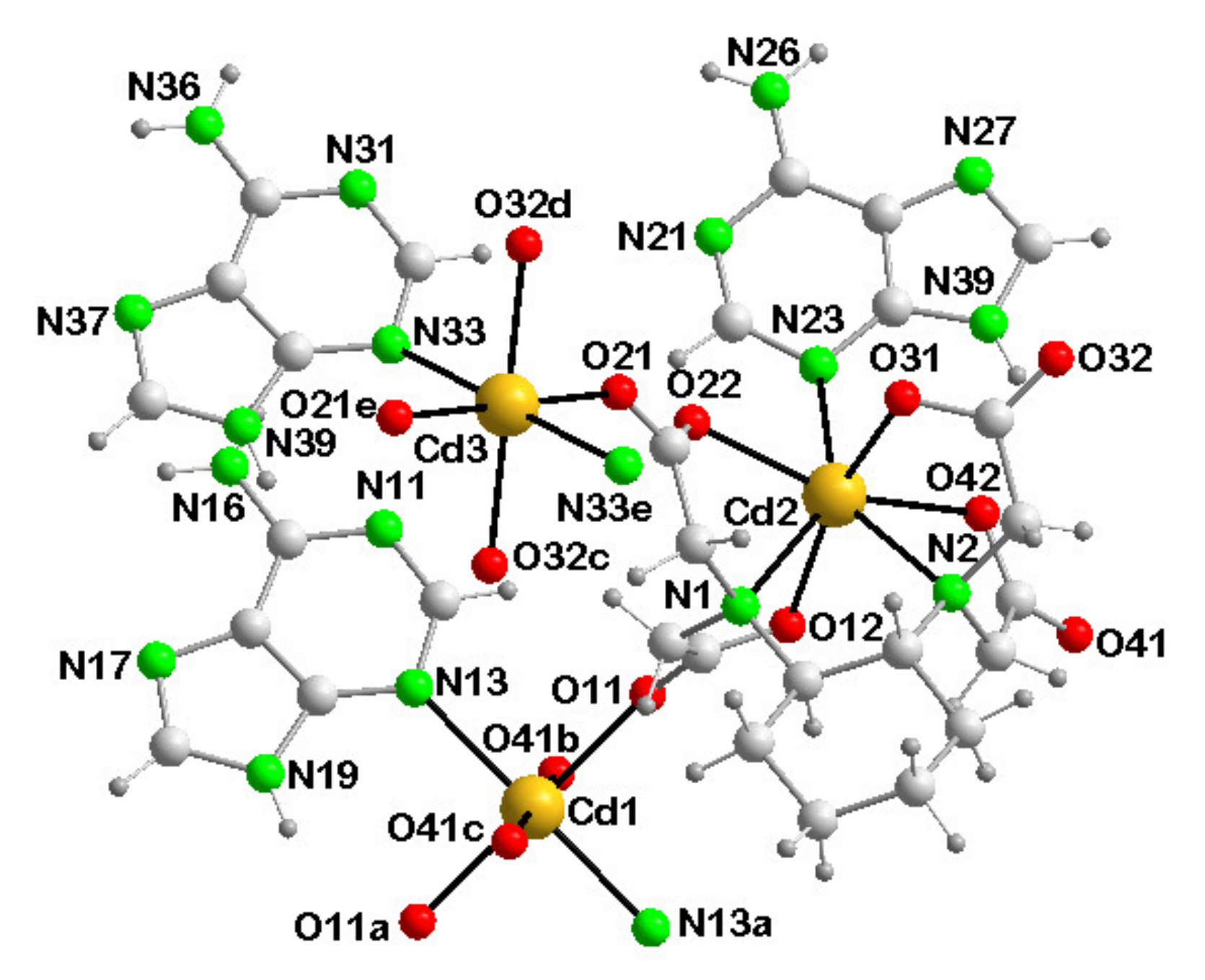
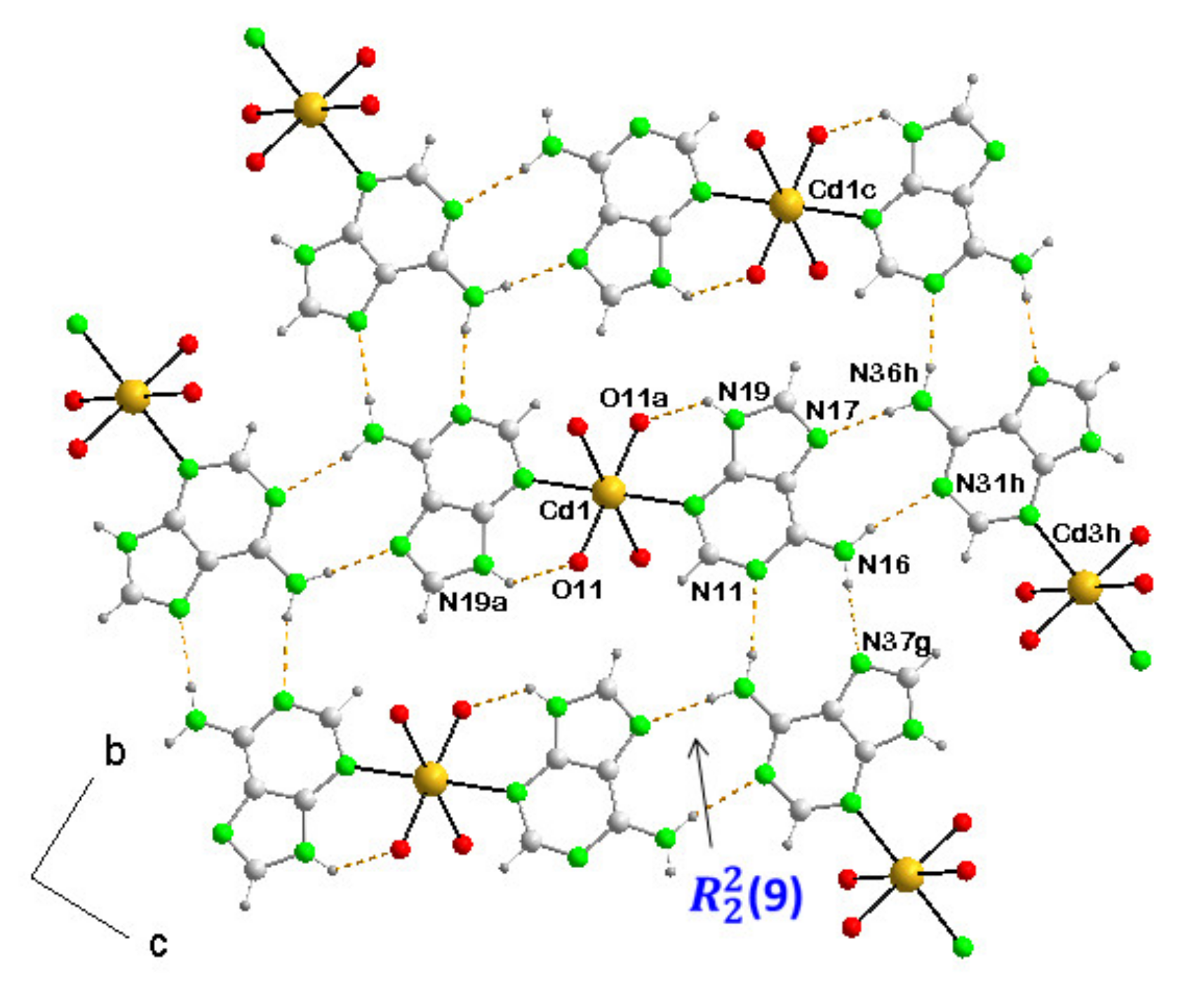
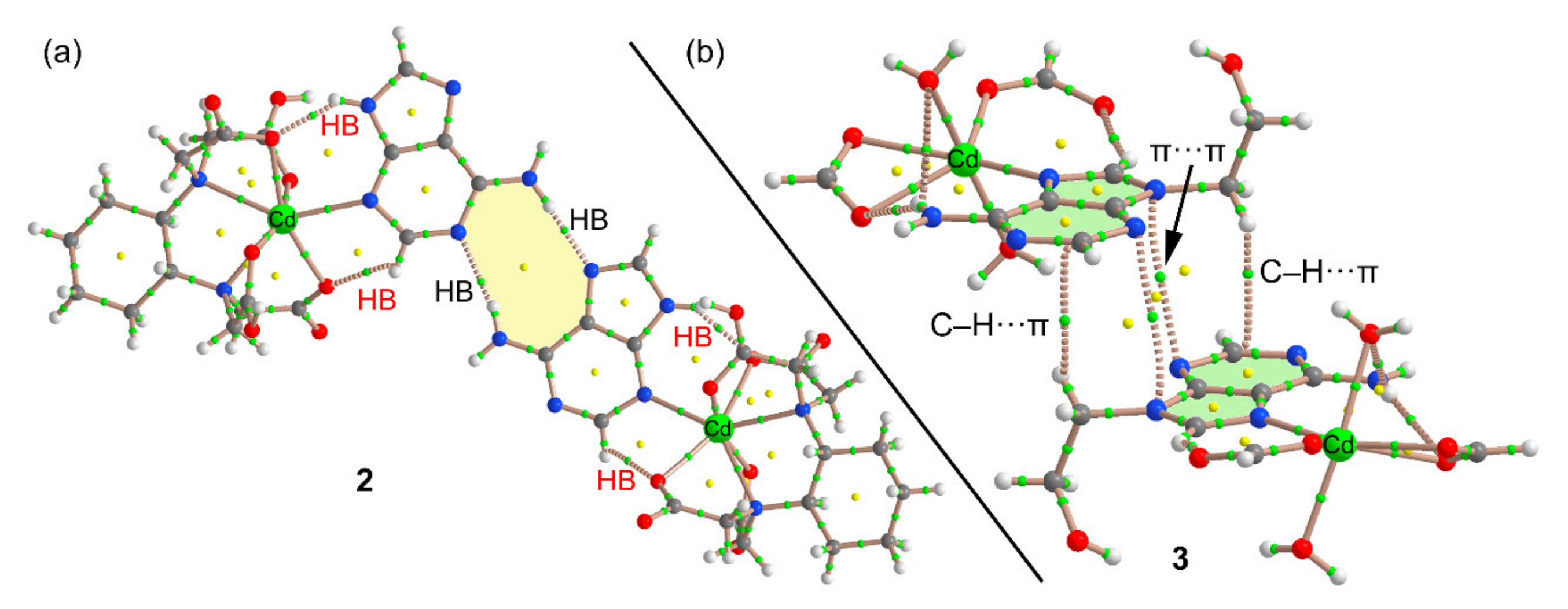
3.3.2. [Cd(µ4-CDTA)(Hade)·Cd(Hade)2]n (2)
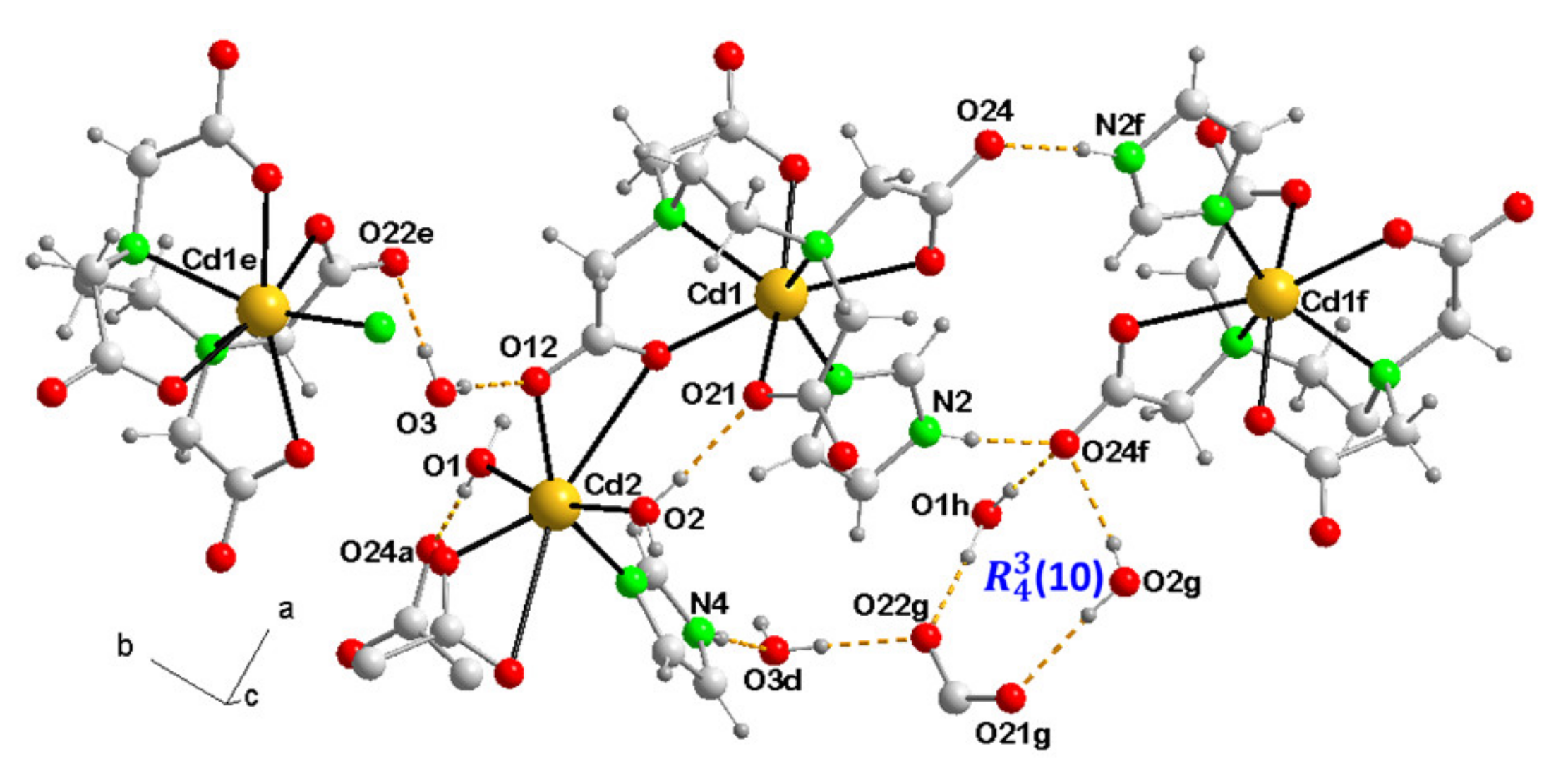
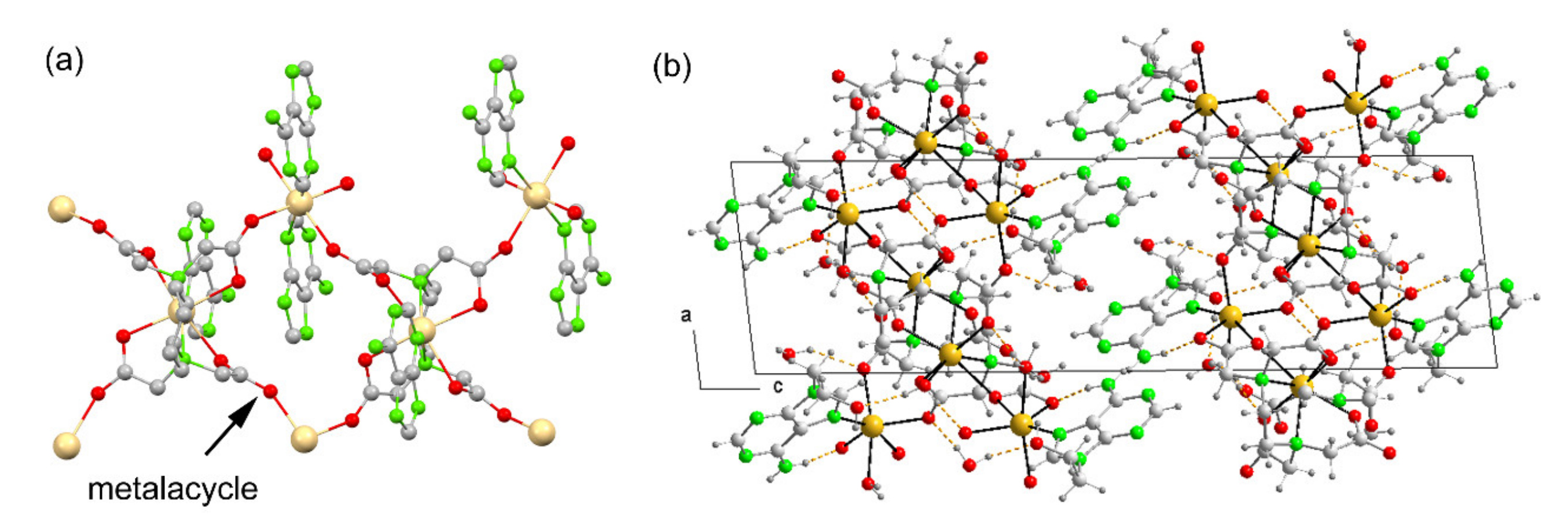
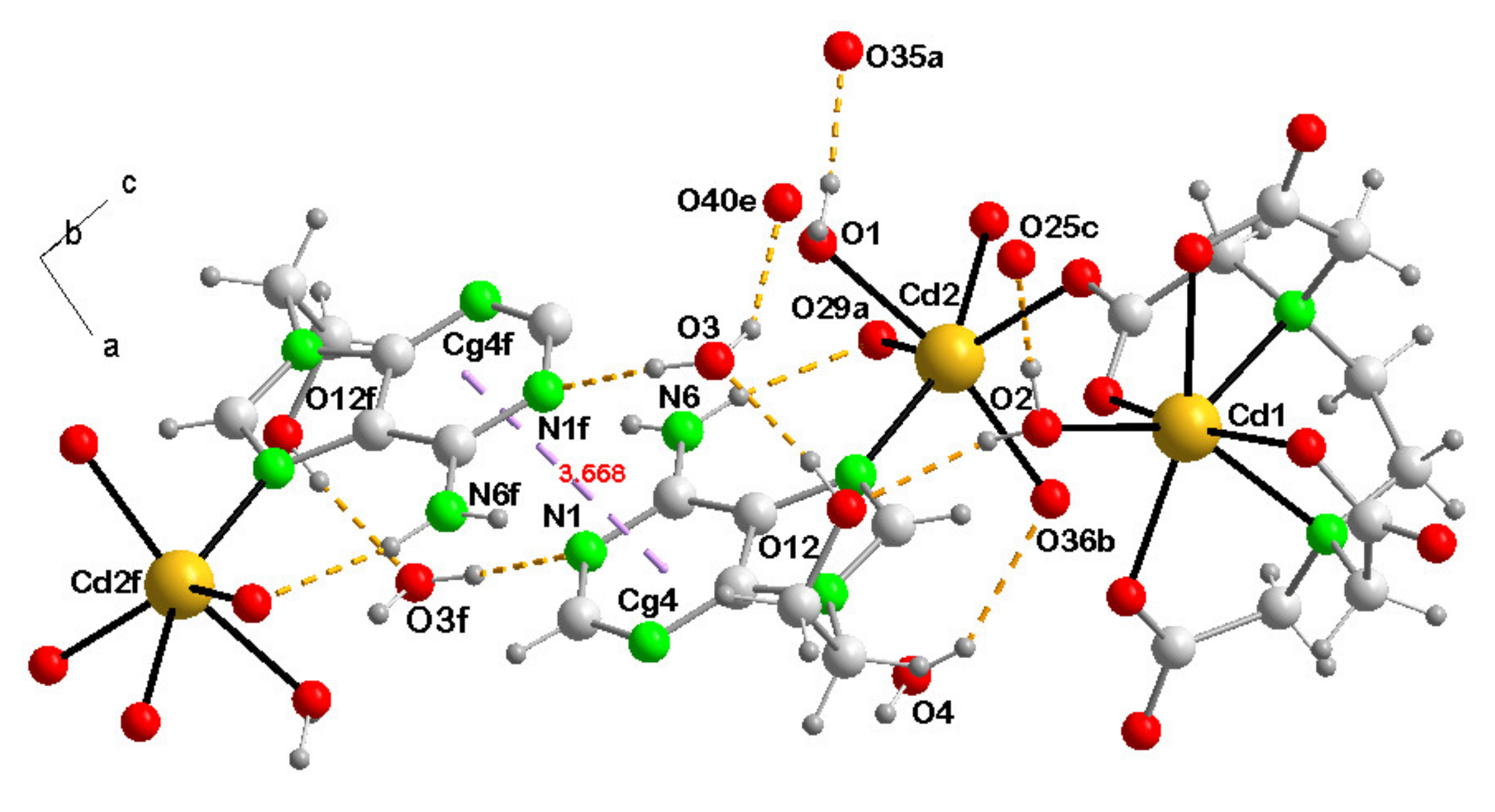
3.3.3. {[Cd(µ3-EDTA)(H2O)·Cd(H9heade)(H2O)]·4H2O}n (3)
3.3.4. CSD Search
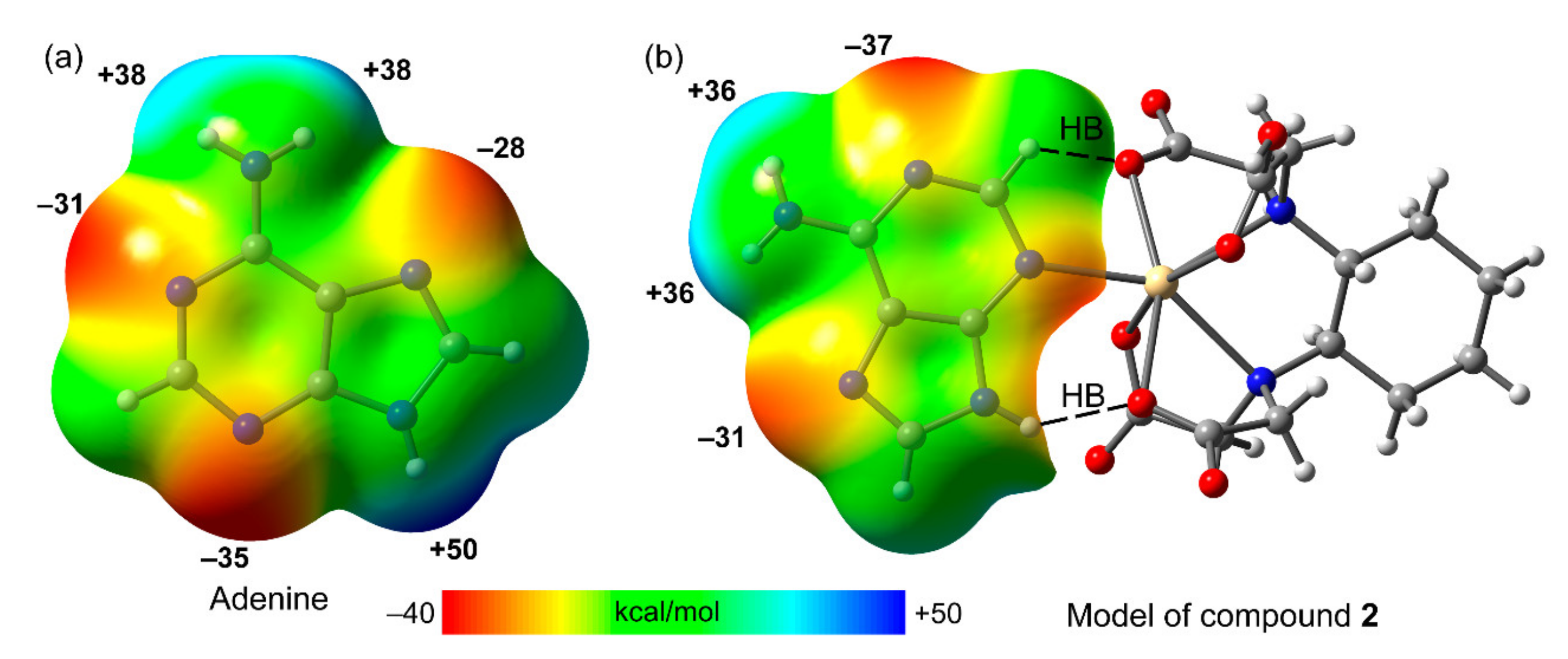
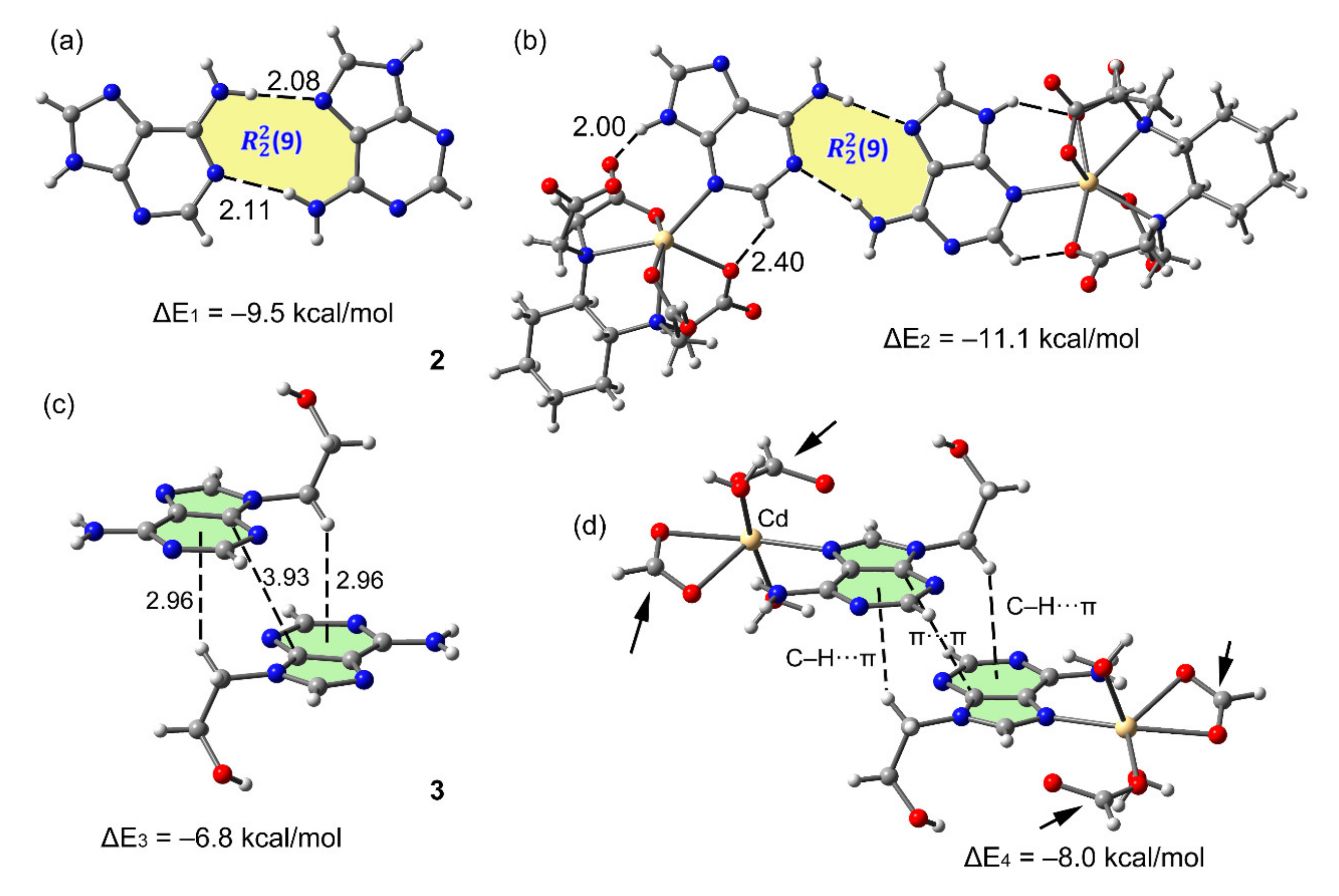
3.4. DFT Calculations
4. Concluding Remarks
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Antsyshkina, A.S.; Sadikov, G.G.; Poznyak, A.L.; Sergienko, V.S. Crystal Structure of [Cu(Edta)(Py)2(H2O)2]·2H2O and [Cu(Im)6]{[Cu(Im)4][Cu(Edta)(Im)]2}·6H2O, Products of the Interaction of (Ethylenediaminotetraacetato)diaquadicopper(II) with Pyridine and Imidazole. Russ. J. Inorg. Chem. 2006, 51, 241–252. [Google Scholar] [CrossRef]
- Liu, H.; Xu, L.; Gao, G.-G.; Li, F.-Y.; Yang, Y.-Y.; Li, Z.-K.; Sun, Y. Two-dimensional layer architecture assembled by Keggin polyoxotungstate, Cu(II) EDTA complex and sodium linker: Synthesis, crystal structures, and magnetic properties. J. Solid State Chem. 2007, 180, 1664–1671. [Google Scholar] [CrossRef]
- Domínguez-Martín, A.; Choquesillo-Lazarte, D.; Dobado, J.A.; Vidal, I.; González-Pérez, J.M.; Castiñeiras, A.; Niclós-Gutiérrez, J. From 7-azaindole to adenine: Molecular recognition aspects on mixed-ligand Cu(II) complexes with deaza-adenine ligands. Dalton Trans. 2013, 42, 6119–6130. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Martín, A.; Choquesillo-Lazarte, D.; Dobado, J.A.; Martínez-García, H.; Lezama, L.; González-Pérez, J.M.; Castiñeiras, A.; Niclós-Gutiérrez, J. Structural consequences of the N7 and C8 translocation on the metal binding behavior of adenine. Inorg. Chem. 2013, 52, 1916–1925. [Google Scholar] [CrossRef] [PubMed]
- Domínguez Martín, A.; Brandi-Blanco, M.P.; Matilla-Hernández, A.; El Bakkali, H.; Nurchi, V.M.; González-Pérez, J.M.; Castiñeiras, A.; Niclós-Gutiérrez, J. Unravelling the versatile metal binding modes of adenine: Looking at the molecular recognition patterns of deaza- and aza-adenines in mixed-ligand metal complexes. Coord. Chem. Rev. 2013, 257, 2814–2818. [Google Scholar] [CrossRef]
- Domínguez-Martín, A.; García-Raso, A.; Cabot, C.; Choquesillo-Lazarte, D.; Pérez-Toro, I.; Matilla-Hernández, A.; Castiñeiras, A.; Niclós-Gutiérrez, J. Structural insights on the molecular recognition patterns between N6-substituted adenines and N-(aryl-methyl)iminodiacetate copper(II) chelates. J. Inorg. Biochem. 2013, 127, 141–149. [Google Scholar] [CrossRef]
- El Bakkali, H.; Castiñeiras, A.; García-Santos, I.; González-Pérez, J.M.; Niclós-Gutiérrez, J. Metallo-supramolecular structures by self-assembly through weak interactions in mixed ligand metal complexes of adenine and malonate. Cryst. Growth Des. 2014, 14, 249–260. [Google Scholar] [CrossRef]
- Pérez-Toro, I.; Domínguez-Martín, A.; Choquesillo-Lazarte, D.; Vílchez-Rodríguez, E.; González-Pérez, J.M.; Castiñeiras, A.; Niclós-Gutiérrez, J. Lights and shadows in the challenge of binding acyclovir, a synthetic purine-like nucleoside with antiviral activity, at an apical-distal coordination site in copper(II)-polyamine chelates. J. Inorg. Biochem. 2015, 148, 84–92. [Google Scholar] [CrossRef]
- González-Pérez, J.M.; Choquesillo-Lazarte, D.; Domínguez-Martín, A.; El Bakkali, H.; García-Rubiño, M.E.; Pérez-Toro, I.; Vílchez-Rodríguez, E.; Castiñeiras, A.; Nurchi, V.M.; Niclós-Gutiérrez, J. Molecular recognition between adenine or 2,6-diaminopurine and copper(II) chelates with N,O2,S-tripodal tetradentate chelators having thioether or disulfide donor groups. J. Inorg. Biochem. 2015, 151, 75–86. [Google Scholar] [CrossRef]
- González-Pérez, J.M.; Choquesillo-Lazarte, D.; Domínguez-Martín, A.; Vílchez-Rodríguez, E.; Pérez-Toro, I.; Castiñeiras, A.; Arriortua, O.K.; García-Rubiño, M.E.; Matilla-Hernández, A.; Niclós-Gutiérrez, J. The metal binding pattern of acyclovir in ternary copper(II) complexes also having an S-thioether or S-disulfide NO2S-tripodal tetradentate chelator. Inorg. Chim. Acta 2016, 452, 258–267. [Google Scholar] [CrossRef]
- Pérez-Toro, I.; Domínguez-Martín, A.; Choquesillo-Lazarte, D.; García-Rubiño, M.E.; González-Pérez, J.M.; Castiñeiras, A.; Bauzá, A.; Frontera, A.; Niclós-Gutiérrez, J. Copper(II) polyamine chelates as efficient receptors for acyclovir: Synthesis, crystal structures and DFT study. Polyhedron 2018, 145, 218–226. [Google Scholar] [CrossRef]
- Pérez-Toro, I.; Domínguez-Martín, A.; Choquesillo-Lazarte, D.; González-Pérez, J.M.; Castiñeiras, A.; Niclós-Gutiérrez, J. The highest reported denticity of a synthetic nucleoside in the unprecedented tetradentate mode of acyclovir. Cryst. Growth Des. 2018, 18, 4282–4286. [Google Scholar] [CrossRef]
- Ruiz-González, N.; García-Rubiño, M.E.; Domínguez-Martín, A.; Choquesillo-Lazarte, D.; Franconetti, A.; Frontera, A.; Castiñeiras, A.; González-Pérez, J.M.; Niclós-Gutiérrez, J. Molecular and supra-molecular recognition patterns in ternary copper(II) or zinc(II) complexes with selected rigid-planar chelators and a synthetic adenine-nucleoside. J. Inorg. Biochem. 2020, 203, 110920. [Google Scholar] [CrossRef] [PubMed]
- Polyakova, I.N.; Poznyak, A.L.; Segienko, V.S.; Stopolyanskaya, L.V. Crystal Structures of Acid Ethylenediaminotetracetares [Cd(H2Edta)(H2O)]·2H2O and [Mn(H2O)4][Mn(HEdta)(H2O)]2·4H2O. Crystallogr. Rep. 2001, 41, 40–45. [Google Scholar] [CrossRef]
- Fecher, R.; Boswell, K.H.; Wittick, J.J.; Shen, T.Y. Nucleosides VI: The Synthesis and Optical Properties of the 5’-Adenin-9yl)-5’-Deoxy Derivatives of the Thymidine and 2‘-Deoxyadenosine. Carbohydr. Res. 1970, 13, 105–111. [Google Scholar] [CrossRef]
- Takenaka, A.; Shibata, M.; Sasada, Y. Three Crystalline Forms of 9-(2-Hydroxyethyl)adenine resulting from the Different Stacking of Hydrogen-Bonded Layers. Acta Crystallogr. 1986, C42, 1336–1340. [Google Scholar] [CrossRef]
- BRUKER. APEX3 Software; v2018.7-2; Bruker AXS Inc.: Madison, WI, USA, 2018. [Google Scholar]
- Sheldrick, G.M. SADABS. In Program for Empirical Absorption Correction of Area Detector Data; University of Goettingen: Goettingen, Germany, 1997. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. 2008, A64, 112–122. [Google Scholar] [CrossRef]
- Wilson, A.J.C. International Tables of Crystallography; Vol. C; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1995. [Google Scholar]
- Spek, A.L. PLATON. A multipurpose Crystallographic tool. Acta Crystallogr. 2009, D65, 148–155. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision A.01; Gaussian, Inc.: Wallingford, UK, 2016. [Google Scholar]
- Bader, R.F.W. A quantum theory of molecular structure and its applications. Chem Rev. 1991, 91, 893–928. [Google Scholar] [CrossRef]
- Keith, T.A. AIMAll (Version 13.05.06); TK Gristmill Software: Overland Park, KS, USA, 2013. [Google Scholar]
- Bugella-Altamirano, E.; Choquesillo-Lazarte, D.; González-Pérez, J.M.; Sánchez-Moreno, M.J.; Martín-Ramos, R.; Covelo, B.; Carballo, R.; Castiñeiras, A.; Niclós-Gutiérrez, J. Three new modes of adenine-copper(II) coordination: Interligand interactions controlling the selective N3-, N7- and bridging μ-N3,N7–metal-bonding of adenine to different N-substituted iminodiacetato-copper(II) chelates. Inorg. Chim. Acta 2002, 339, 160–170. [Google Scholar] [CrossRef]
- Bader, R.F.W. A Bond Path: A Universal Indicator of Bonded Interactions. J. Phys. Chem. A 1998, 102, 7314–7323. [Google Scholar] [CrossRef]
- Belmont-Sánchez, J.C.; Ruiz-González, N.; Frontera, A.; Matilla-Hernández, M.; Castiñeiras, A.; Niclós-Gutiérrez, J. Anion–Cation Recognition Pattern, Thermal Stability and DFT-Calculations in the Crystal Structure of H2dap[Cd(HEDTA)(H2O)] Salt (H2dap = H2(N3,N7)-2,6-Diaminopurinium Cation). Crystals 2020, 10, 304. [Google Scholar] [CrossRef]
- Roselló, Y.; Benito, M.; Bagués, N.; Martínez, N.; Moradell, A.; Mata, I.; Galcerà, J.; Barceló-Oliver, M.; Frontera, A.; Molins, E. 9-Ethyladenine: Mechanochemical Synthesis, Characterization, and DFT Calculations of Novel Cocrystals and Salts. Cryst. Growth Des. 2020. [Google Scholar] [CrossRef]
- García-Raso, A.; Terrón, A.; Ortega-Castro, J.; Barceló-Oliver, M.; Lorenzo, J.; Rodríguez-Calado, S.; Franconetti, A.; Frontera, A.; Vázquez-López, E.M.; Fiol, J.J. Iridium (III) coordination of N(6) modified adenine derivatives with aminoacid chains. J. Inog. Biochem. 2020, 205, 111000. [Google Scholar] [CrossRef]
- Martínez, D.; Pérez, A.; Cañellas, S.; Silió, I.; Lancho, A.; García-Raso, A.; Fiol, J.J.; Terrón, A.; Barceló-Oliver, M.; Ortega-Castro, J.; et al. Synthesis, reactivity, X-ray characterization and docking studies of N7/N9-(2-pyrimidyl)-adenine derivatives. J. Inorg. Biochem. 2020, 203, 110879. [Google Scholar] [CrossRef]
- Pons, R.; Ibáñez, C.; Buades, A.B.; Franconetti, A.; Garcia-Raso, A.; Juan J Fiol, J.J.; Angel Terrón, A.; Molins, E.; Frontera, A. Synthesis, X-ray characterization and density functional theory studies of N6-benzyl-N6-methyladenine-M(II) complexes (M = Zn, Cd): The prominent role of π–π, C–H···π and anion–π interactions. Appl. Organomet. Chem. 2019, 33, e4906. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belmont-Sánchez, J.C.; García-Rubiño, M.E.; Frontera, A.; Matilla-Hernández, A.; Castiñeiras, A.; Niclós-Gutiérrez, J. Novel Cd (II) Coordination Polymers Afforded with EDTA or Trans-1,2-Cdta Chelators and Imidazole, Adenine, or 9-(2-Hydroxyethyl) Adenine Coligands. Crystals 2020, 10, 391. https://doi.org/10.3390/cryst10050391
Belmont-Sánchez JC, García-Rubiño ME, Frontera A, Matilla-Hernández A, Castiñeiras A, Niclós-Gutiérrez J. Novel Cd (II) Coordination Polymers Afforded with EDTA or Trans-1,2-Cdta Chelators and Imidazole, Adenine, or 9-(2-Hydroxyethyl) Adenine Coligands. Crystals. 2020; 10(5):391. https://doi.org/10.3390/cryst10050391
Chicago/Turabian StyleBelmont-Sánchez, Jeannette Carolina, María Eugenia García-Rubiño, Antonio Frontera, Antonio Matilla-Hernández, Alfonso Castiñeiras, and Juan Niclós-Gutiérrez. 2020. "Novel Cd (II) Coordination Polymers Afforded with EDTA or Trans-1,2-Cdta Chelators and Imidazole, Adenine, or 9-(2-Hydroxyethyl) Adenine Coligands" Crystals 10, no. 5: 391. https://doi.org/10.3390/cryst10050391
APA StyleBelmont-Sánchez, J. C., García-Rubiño, M. E., Frontera, A., Matilla-Hernández, A., Castiñeiras, A., & Niclós-Gutiérrez, J. (2020). Novel Cd (II) Coordination Polymers Afforded with EDTA or Trans-1,2-Cdta Chelators and Imidazole, Adenine, or 9-(2-Hydroxyethyl) Adenine Coligands. Crystals, 10(5), 391. https://doi.org/10.3390/cryst10050391