Abstract
Single crystals of BaRuO were grown by the floating zone method. The crystal structure is formed by an alternating stacking of pseudo-hexagonal Ru single layers and double layers. The Ru ions within the double layers are dimerized (RuO) whereas the Ru ions within the single layers arrange in a distorted Kagome lattice of trigonal bipyramidally coordinated RuO polyhedra. Additionally, this Kagome lattice is “decorated” with RuO octahedra that are situated in the central free spaces within this Kagome lattice. According to the composition, the oxidation state of most of the Ru ions should be formally close to 5+.
1. Introduction
Ruthenates exhibit a broad scope of interesting physical properties ranging from itinerant ferromagnetism in SrRuO[1] and metamagnetim in SrRuO[2,3] to unconventional superconductivity in SrRuO [4] (see also the reviews [5,6]). Recently, it was tried to dope Ba ions into SrRuO[7,8,9]. For the compound SrBaRuO the Ba-doping was still possible but for distinctly higher Ba-doping levels no single crystals could be grown anymore by the floating zone technique. It seems possible that a miscibility gap might appear above these Ba-doping levels within the SrBaRuO phase diagram (for ambient pressure, high temperature synthesis conditions). Therefore, we investigated the other side of the phase diagram around BaRuO.
Within the ternary Ba-Ru-O system, BaRuO is known to exist and crystalizes in different polytypes depending on the synthesis pressure (9R, 4H, 6H and possibly 3C) [10]. The crystal structure at ambient pressure (nine-layer rhombohedral 9R) BaRuO consists of Ru trimers with Ru ions sitting in face sharing Ru-oxygen octahedra [11]. For increasing Ba to Ru ratio also the compounds with the Ba to Ru ratio 4:3, 5:3 and 5:2 are known to exist. For BaRuO corner-shared RuO trimers are formed [12] and the material is antiferromagnetic with a magnetic ordering temperature of T ∼ 105 K [13]. For BaRuO Ru trimers are formed [14] whereas the compound BaRuO is crystallizing in a structure formed of Ru-Ru dimers [15]. However, for these two compounds the dimers are not interconnected with other dimers (or trimers) like in the aforementioned Ba ruthenates with lower Ba to Ru ratio. Instead, the planes with the Ru-Ru dimers are separated by Ba oxide planes. For such high levels of the Ba to Ru ratio the Ru ions (also) appear formally in a higher 5+ oxidation state. For the Ba to Ru ratio of 2:1 (which would be the end point in the SrBaRuO phase diagram) no compound in single crystalline form has been reported to exist so far. However, for this Ba to Ru ratio studies on thin films [16] or high pressure phases [17]—both with layered perovskite structure-exist.
2. Results and Discussion
The BaRuO single crystal was grown in a mirror furnace (HKZ,Scidre). Therefore, the starting materials were prepared by thorough grinding of BaCO and RuO with a Ba to Ru ratio of 2:1 and sintering this mixture at 1000 C for one day in air. The crystal was grown with a speed of 6 mm/h under 16 bar of an Argon/Oxygen atmosphere (with a ratio of 1:6). Thus, black, shiny single crystals of BaRuO could be finally obtained, see Figure 1. As known for other ruthenates, also during the growth of BaRuO a larger evaporation of Ru oxide could be observed during the crystal growth which explains the finally obtained Ba:Ru ratio in the grown single crystal. Also other Ba:Ru ratios of 2:1.1 and 2:1.05 have been tried for a compensation of the Ru loss, but only the ratio of 2:1 results in the growth of large impurity-free single crystals of BaRuO. Its composition has been confirmed with EDX (energy dispersive X-ray spectroscopy) measurements in a scanning electron microscope yielding a molar Ba:Ru ratio of 2.07(8):1.00(5) which is within the error bars in agreement with the composition of BaRuO.

Figure 1.
Photo of the as-grown BaRuO single crystal in the left (with a ruler below that is showing large ticks in units of 1 cm).
Afterwards, we have also synthesized BaRuO powder samples by conventional solid state reaction in order to confirm that our BaRuO single crystals crystallized in the ambient pressure phase. Therefore, stoichiometric amounts of BaCO and RuO (molar ratio of 26:12) were ground for 1/2 h and pressed into pellets which were placed in a corundum crucible and sintered at 1000 C for 12 h in air. After an intermediate re-grinding the sample was sintered again for 48 h under same conditions at ambient pressure.
For powder X-ray diffraction (XRD) measurements parts of the grown single crystals have been ground into fine powders. The XRD measurements have been performed using Cu radiation on a Bruker D8 Discover A25 powder X-ray diffractometer. The FullProf program package [18] was used for Rietveld refinements, see Figure 2.
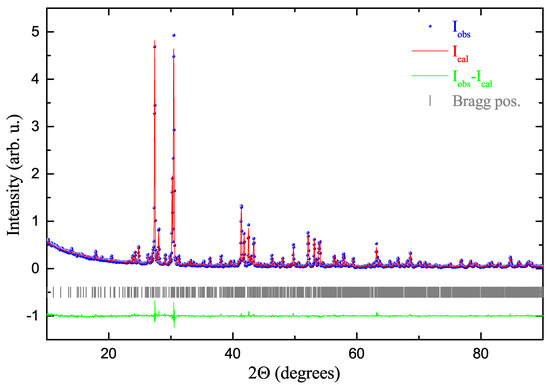
Figure 2.
Powder X-ray diffraction pattern (Cu-K) of the grown BaRuO single crystal collected at room temperature. The red solid line represents a Rietveld fit based on the structure model obtained by single crystal X-ray diffraction (using the FullProf software package [18]). The calculated Bragg peak positions (according to space group Fdd2) are indicated by vertical bars and the difference between the experimental and calculated intensities are shown by the green solid line at the bottom. The obtained lattice parameters (space group Fdd2) amount to a = 20.4536(4) Å, b = 51.1908(8) Å and c = 11.7732(2) Å.
Using Mo radiation single crystal X-ray diffraction measurements have been performed on a Bruker D8 VENTURE single crystal X-ray diffractometer equipped with a bent graphite monochromator and a Photon III detector. A crystal of roughly 10 m size has been measured, see Figure 3. Due to the small size of the sample we were able to find an almost untwined single crystal with regard to the possible orthorhombic twin domains. Nevertheless, the inversion twin could not be avoided. (For distinctly larger sample sizes we could always observe the appearance of all six possible twin domains that might appear for this structure.) The Jana2006 program suite [19] was used for the crystal structure refinement. The refinement was based on F(obs) with an instability factor of 0.01. For this tiny sample no extinction correction was necessary. The volume fractions of the main domain (id) and the corresponding twin domain obtained by inversion (-id) amount to 49(3)% and 40(1)% whereas the remaining four twin domains related to rotations (plus inversions) are very small (i.e., 1.6(1.6)%, 2.4(1.6)%, 2.5(1.6)% and 4.1%) with a volume fraction almost comparable to their error bars. The reduced amount (size) of twin domains in this sample with pseudo-hexagonal crystal structure underlines the reliability of the refinement results. Only for the heavier atoms Ba and Ru the anisotropic displacement parameters have been refined. For the oxygen atoms has been refined. Goodness of fit, R- and weighted R-values and the obtained structural parameters and bond lengths are listed in Table 1, Table 2, Table 3 and Table 4.
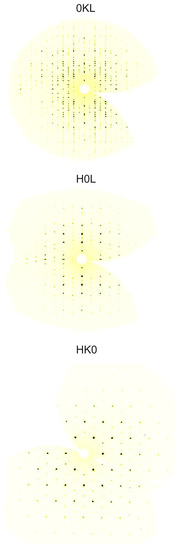
Figure 3.
Single crystal X-ray diffraction intensities of BaRuO within the , and planes of reciprocal space (space group I4/mmm).

Table 1.
Crystallographic & structural refinement data.

Table 2.
Refinement results of single crystal X-ray diffraction measurements of BaRuO of the refinement with space group Fdd2 (a = 20.4638(12) Å, b = 51.191(3) Å, c = 11.7698(7) Å).

Table 3.
Refinement results of single crystal X-ray diffraction measurements of BaRuO.

Table 4.
Ru-oxygen distances in BaRuO.
Structure
The crystal structure of BaRuO alternatingly consists of layers of single Ru ions and of layers of dimerized Ru-ions located in face sharing octahedra which are denoted in Figure 4 as layers ’A#’ (Ru single layers) and ’B#’ (Ru double layers) respectively. Within these Ru-layers the Ru ions arrange in a pseudohexagonal manner, see Figure 5. Two third of the Ru ions within the ’A#’ layers have a trigonal bipyramidal oxygen coordination—see Figure 5a—with an enhanced oxidation state compared to the other Ru ions, see Table 5. These Ru ions form a distorted Kagome lattice. The other Ru ions are octahedrally coordinated by the oxygen ions, see Figure 5a and are situated in the free space of the Kagome lattice. This difference in the Ru sites becomes also apparent in their Ba coordination which can be seen in Figure 5b. The RuO octahedra are located in quite compact RuBa octahedra (red) wheras the RuO bipyramids are situated in much larger RuBa icosahedra (yellow). The dimerized Ru ions within the ’B#’ layers all form RuO polyhedra consisting of two face sharing RuO octahedra. The corresponding Ru-Ba polyhedra (orange) exhibit more uniform sizes compared to the ones within the ‘A#’ (single-)layers, see Figure 5d. It is the different stacking (‘A1-B1-A2-B2-A3-B1-A4-B2-A1’) of these layers that is responsible for the large value of the lattice constant in b-direction, compare Figure 4. Note, that also the heavy Ba-ions follow this stacking.
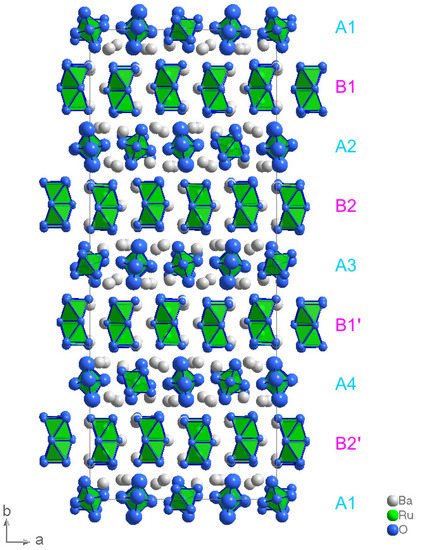
Figure 4.
Representation of the crystal structure of BaRuO as obtained from refinements with space group Fdd2. Green/white spheres: Ru-/Ba-ions, blue spheres: 99.9% probability ellipsoids for the oxygen ions.
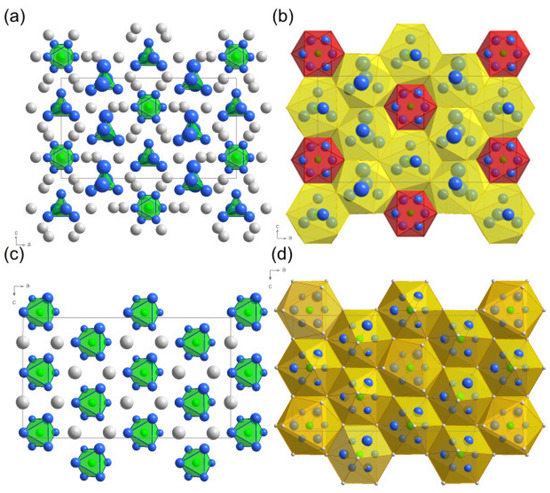
Figure 5.
(a,b) represent the Ru oxygen layer ’A3’ shown in Figure 4. Accordingly, (c,d) represent the Ru oxygen doublelayer of type ’B2’. Green/white spheres: Ru-/Ba-ions, blue spheres: 99.9% probability ellipsoids for the oxygen ions. In (a,c) the RuO and RuO polyhedra are shown wheras the Ru-Ba-polyhedra are shown in (b,d).

Table 5.
Bond valence sums (BVS) for Ba and Ru ions together with their oxygen coordination. Parameters were taken from Ref. [24]. The values in the brackets indicate the number of the of oxygen ions that are (i) distinctly below, (ii) roughly within and (iii) clearly above the plane of the Ru ions (that is perpendicular to the b-axis) for a certain layer.
For many Ba-containing compounds the Ba ions are twelve-fold coordinated by anions as is observed for Ba7, Ba10 and Ba13 in BaRuO. An example in literature is BaNiO[20]. But also a plethora of other oxygen coordinations have been reported for Ba in literature. The Ba1 ions in our new crystal structure exhibit an 8-fold oxygen coordination that can be also found e.g., in BaOSiO[21]. A different 8-fold oxygen coordination in our new crystal structure can be observed for the Ba4, Ba5 and Ba6 ions. This latter oxygen coordination was reported for Ba(BO)[22]. Also an octahedral anionic coordination is known for Ba (although an octahedral oxygen coordination is much more typical for somewhat smaller but still very large Sr ions) and has been reported for BaCuOCl [23]. The oxygen coordination of the Ba ions is also summarized in Table 5. The Ba-O polyhedra are characterized by three values given in brackets. The central values denotes the amount of oxygen ions that are situated very roughly in the plane of the Ba-ion (perpendicular to the pseudohexagonal axis). The first and the last values denote the number of oxygen ions far above and below this plane. [e.g., an octahedral coordination would be abbreviated by (3-0-3).] A cif-file is provided as a Supplementary Materials File for a detailed visualization.
The nominal Ru oxidation state should be slightly higher than 5+ according to the composition of BaRuO. Note, that the absolute values of the bond valence sums (listed in Table 5) are not necessarily equal (or even close) to the real oxidation states, especially also because of the huge complexity of the crystal structure of BaRuO. Note that there is a scattering in the BVS values of the Ba ions (oxygen ions) of () around 2.085+ (1.888−) which provides an indication for the reliability of the BVS in this very complex structure. A similar scattering range of of the BVS values around 4.305+ can be observed for the Ru ions. If one would take the values serious, the bond valence sum (BVS) formalism would indicate that the oxidation state of the Ru ions with trigonal bipyramidal oxygen coordination is higher than that of the other all octahedrally oxygen coordinated Ru ions. Such a higher oxidation state of the Ru ions with trigonal bipyramidal oxygen coordination would be in agreement with the total oxygen composition. Note, that a trigonal bipyramidal oxygen coordination is known to exist for Ru ions e.g., in KRu(OH)O [25]. Nevertheless, the scattering of all the BVS values is somewhat enhanced (see above) and future X-ray absorption spectroscopy measurements are required to study the real Ru valencies in BaRuO.
Besides floating zone grown single crystals also conventional solid state reaction under ambient pressure conditions (air) was successful. We conclude that BaRuO is the ambient pressure phase within the phase diagram. Note, that high pressures of several GPa are needed to stabilize Ba ruthenates with layered perovskite (KNiF) structure for Ba to Ru ratios of 2:1 [17]. For smaller Ba:Ru ratios (like 2:1.1), we observed the formation of a BaRuO impurity phase that is growing under the same growth conditions during our floating zone growth.
3. Conclusions
In summary, we synthesized the new Ba ruthenate compound BaRuO. Within the ternary system Ba-Ru-O this compound is stable at ambient pressure for Ba to Ru ratios close to 2:1. Its pseudo-hexagonal crystal structure has been determined by means of single crystal X-ray diffraction and consists of alternating double and single layers of Ru. In the double layers the Ru ions are dimerized (RuO) whereas the Ru ions in the single layers with trigonal bipyramidal coordination (RuO) are forming a distorted Kagome lattice which is “decorated” by octahedrally oxygen coordinated Ru ions (RuO) in the central free space within the Kagome lattice.
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-4352/10/5/355/s1, Crystal structure of Cif file.
Author Contributions
Project management: A.C.K., chemical synthesis: J.-E.L., A.C.K., EDX measurements: U.B., X-ray measurements: A.C.K., J.-E.L., manuscript writing: all authors contributed. All authors have read and agreed to the published version of the manuscript.
Funding
The research in Dresden is (partially) supported by the Deutsche Forschungsgemeinschaft through Grant No. 320571839.
Acknowledgments
We thank D. I. Khomskii for helpful discussions. We acknowledge support from the Max Planck-POSTECH-Hsinchu Center for Complex Phase Materials.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Longo, J.M.; Raccah, P.M.; Goodenough, J.B. Magnetic Properties of SrRuO3 and CaRuO3. J. Appl. Phys. 1968, 39, 1327. [Google Scholar] [CrossRef]
- Grigera, S.A.; Perry, R.S.; Schofield, A.J.; Chiao, M.; Julian, S.R.; Lonzarich, G.G.; Ikeda, S.I.; Maeno, Y.; Millis, A.J.; Mackenzie, A.P. Magnetic field-tuned quantum criticality in the metallic ruthenate Sr3Ru2O7. Science 2001, 294, 329. [Google Scholar] [CrossRef] [PubMed]
- Perry, R.S.; Galvin, L.M.; Grigera, S.A.; Capogna, L.; Schofield, A.J.; Mackenzie, A.P.; Chiao, M.; Julian, S.R.; Ikeda, S.I.; Nakatsuji, S.; et al. Metamagnetism and critical fluctuations in high quality single crystals of the bilayer ruthenate Sr3Ru2O7. Phys. Rev. Lett. 2001, 86, 2661. [Google Scholar] [CrossRef] [PubMed]
- Maeno, Y.; Hashimoto, H.; Yoshida, K.; Nishizaki, S.; Fujita, T.; Bednorz, J.G.; Lichtenberg, F. Superconductivity in a layered perovskite without copper. Nature 1994, 372, 532. [Google Scholar] [CrossRef]
- Koster, G.; Klein, L.; Siemons, W.; Rijnders, G.; Dodge, J.S.; Eom, C.-B.; Blank, D.H.A.; Beasley, M.R. Structure, physical properties, and applications of SrRuO3 thin films. Rev. Mod. Phys. 2012, 84, 253. [Google Scholar] [CrossRef]
- Eremin, I.; Manske, D.; Ovchinnikov, S.G.; Annett, J.F. Unconventional superconductivity and magnetism in Sr2RuO4 and related materials. Ann. Phys. 2004, 13, 149–174. [Google Scholar] [CrossRef][Green Version]
- Li, Z.; Liu, C.-F.; Skoulatos, M.; Tjeng, L.; Komarek, A. Floating zone growth of Ba-substituted ruthenate Sr2-xBaxRuO4. J. Cryst. Growth 2015, 427, 94. [Google Scholar] [CrossRef]
- Li, Z.W.; Guo, H.; Liu, C.-F.; Bourdarot, F.; Schmidt, W.; Skoulatos, M.; Komarek, A.C. Spin fluctuations in Sr1.6Ba0.4RuO4: An inelastic neutron scattering study with polarization analysis. Phys. Rev. B 2017, 95, 045105. [Google Scholar] [CrossRef]
- Chandrasekaran, K.; Vijayaraghavan, R.; Varadaraju, U.V. Effects of oxygen non-stoichiometry and cationic substitutions on the properties of Sr2RuO4+x. Materials chemistry and physics. Mater. Chem. Phys. 1998, 56, 63–69. [Google Scholar] [CrossRef]
- Hong, S.-T.; Sleight, A.W.J. Scanning transmission electron microscopy (STEM) and X-ray absorption spectroscopy (XAS) investigations of catalytic systems. Solid State Chem. 1997, 128, 251–255. [Google Scholar] [CrossRef]
- Donohue, P.C.; Katz, L.; Ward, R. The crystal structure of barium ruthenium oxide and related compounds. Inorg. Chem. 1965, 4, 306–310. [Google Scholar] [CrossRef]
- Igarashi, T.; Nogami, Y.; Klein, Y.; Rousse, G.; Okazaki, R.; Taniguchi, H.; Yasui, Y.; Terasaki, I. X-ray Crystal Structure Analysis and Ru Valence of Ba4Ru3O10 Single Crystals. J. Phys. Soc. Jpn. 2013, 82, 104603. [Google Scholar] [CrossRef]
- Klein, Y.; Rousse, G.; Damay, F.; Porcher, F.; André, G.; Terasaki, I. Antiferromagnetic order and consequences on the transport properties of Ba4Ru3O10. Phys. Rev. B 2011, 84, 054439. [Google Scholar] [CrossRef]
- Dussarra, C.; Grasse, F.; Bontchev, R.; Darriet, J. Crystal structures and magnetic properties of Ba4Ru3O10 and Ba5Ru3O12. J. Alloys Compd. 1996, 233, 15–22. [Google Scholar] [CrossRef]
- Grasset, F.; Zakhour, M.; Darriet, J. Synthesis, crystal structure and magnetic properties of Ba5Ru2O9 (O2), Ba5Nb2O9 (O2) and Ba5Ru2O10 related to the perovskite-type structure, and structural relationships with corresponding sulfides. J. Alloys Compd. 1999, 287, 25–31. [Google Scholar] [CrossRef]
- Jia, Y.; Zurbuchen, M.A.; Wozniak, S.; Carim, A.H.; Schlom, D.G. Epitaxial growth of metastable Ba2RuO4 films with the K2NiF4 structure. Appl. Phys. Lett. 1999, 74, 3830. [Google Scholar] [CrossRef]
- Kafalas, J.A.; Longo, J.M. High pressure synthesis of (ABX3)(AX) n compounds. J. Solid State Chem. 1972, 4, 55. [Google Scholar] [CrossRef]
- Rodrguez-Carvajal, J. Recent advances in magnetic structure determination by neutron powder diffraction. Physics B 1993, 192, 55–69. [Google Scholar] [CrossRef]
- Petricek, V.; Dusek, M.; Palatinus, L. Crystallographic computing system JANA2006: General features. Z. Kristallogr. 2014, 229, 345. [Google Scholar]
- Takeda, Y.; Kanamura, F.; Shimada, M.; Koizumi, M. The crystal structure of BaNiO3. Acta Cryst. B 1976, 32, 2464–2466. [Google Scholar] [CrossRef]
- Tillmanns, E.; Grosse, H.-P. Refinement of tribarium silicate. Acta Cryst. B 1978, 34, 649–651. [Google Scholar] [CrossRef]
- Bekker, T.B.; Rashchenko, S.V.; Seryotkin, Y.V.; Kokh, A.E.; Davydov, A.V.; Fedorov, P.P. BaO-B2O3 system and its mysterious member Ba3B2O6. J. Am. Ceram. Soc. 2018, 101, 450–457. [Google Scholar] [CrossRef]
- Kipka, R.; Mueller-Buschbaum, H. Uber Oxocuprate. XIX. Ein Oxohalogenocuprat (I): Ba2CuO2Cl. Anorg. Allg. Chem. 1977, 430, 250–254. [Google Scholar] [CrossRef]
- Brese, N.E.; O’Keeffe, M. Bond-valence parameters for solids. Acta Cryst. B 1991, 47, 192–197. [Google Scholar] [CrossRef]
- Fischer, D.; Hoppe, R.Z. Zur Konstitution von Alkaliruthenaten (VI).2. Uber den Aufbau von K2[RuO3(OH)2]. Anorg. Allg. Chem. 1991, 601, 41–46. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).