Curcumin Incorporation into Zn3Al Layered Double Hydroxides—Preparation, Characterization and Curcumin Release
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Characterization of the Curcumin Containing Zn3Al-LDHs
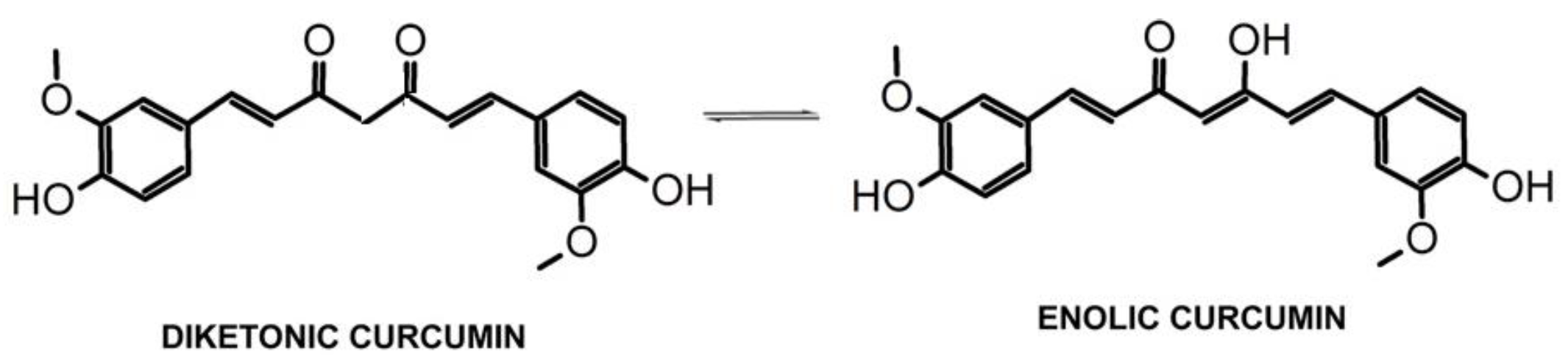
3.1.1. Chemical Composition
3.1.2. XRD Characterization of CR Functionalized Zn3Al-LDH Samples
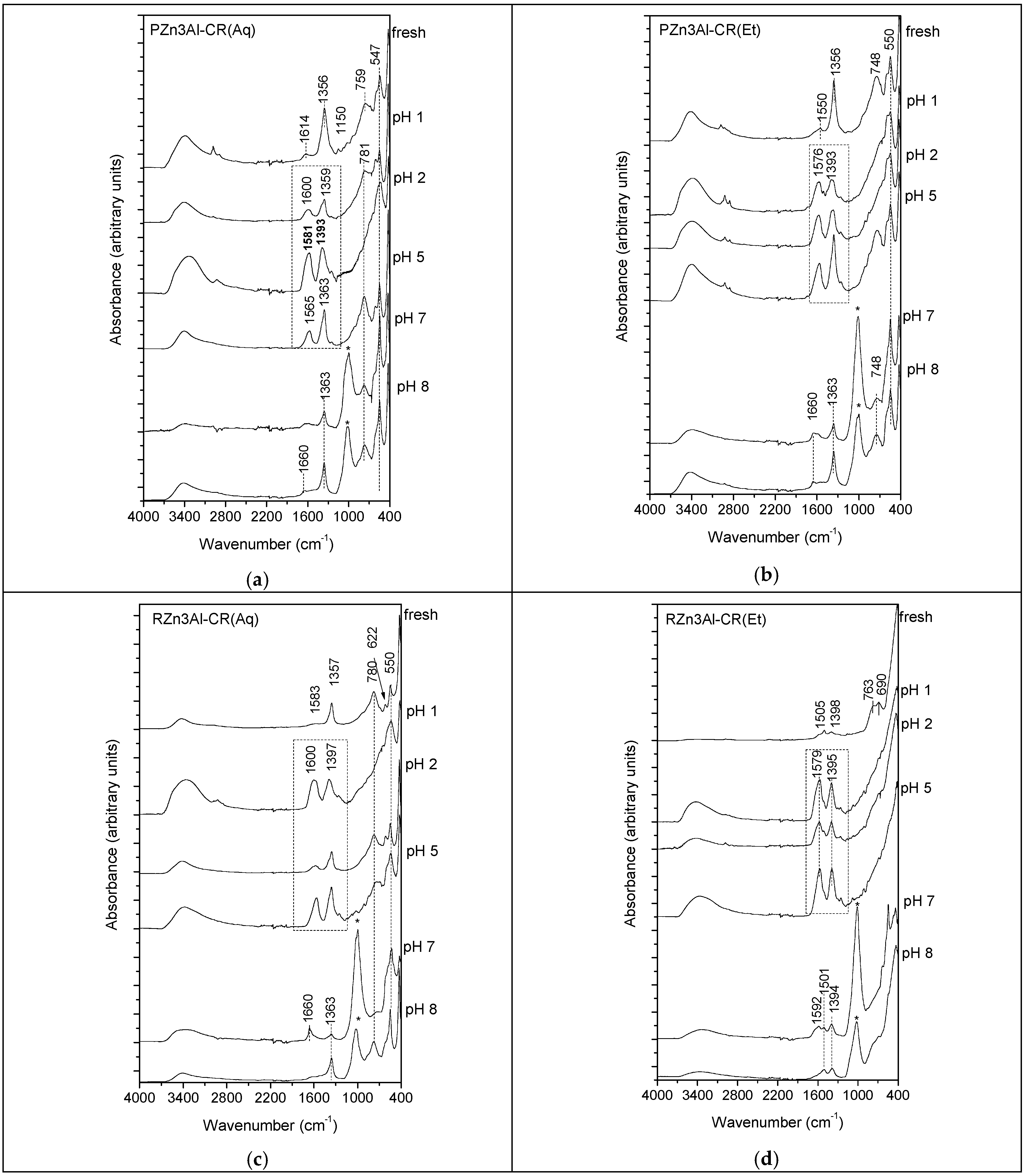
3.1.3. ATR-FTIR Characterization
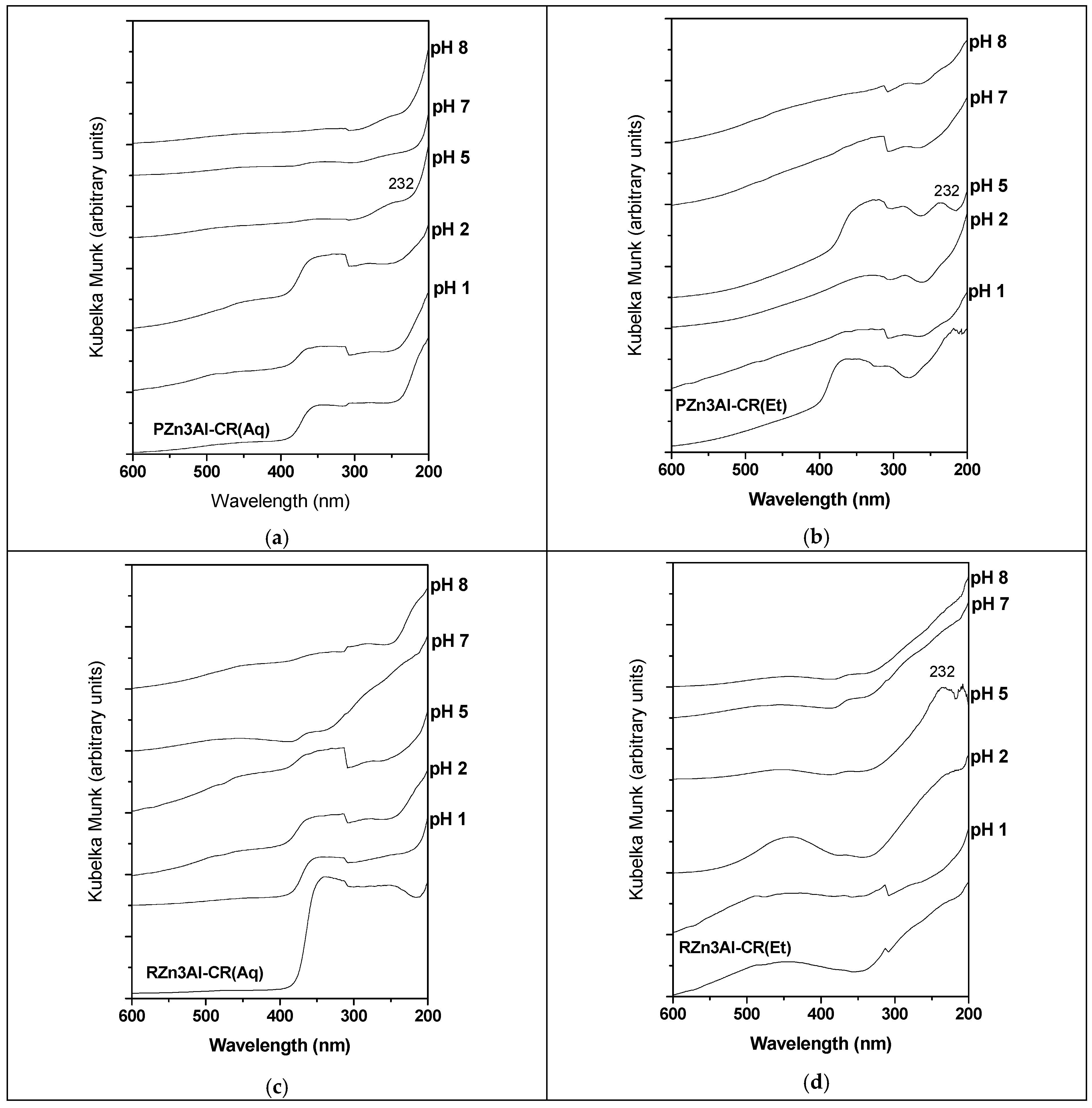
3.1.4. DR-UV–Vis Characterization
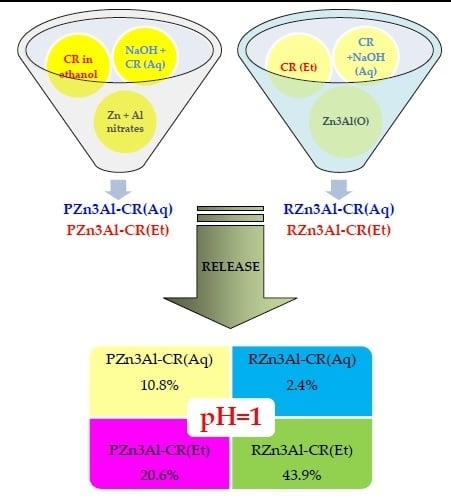
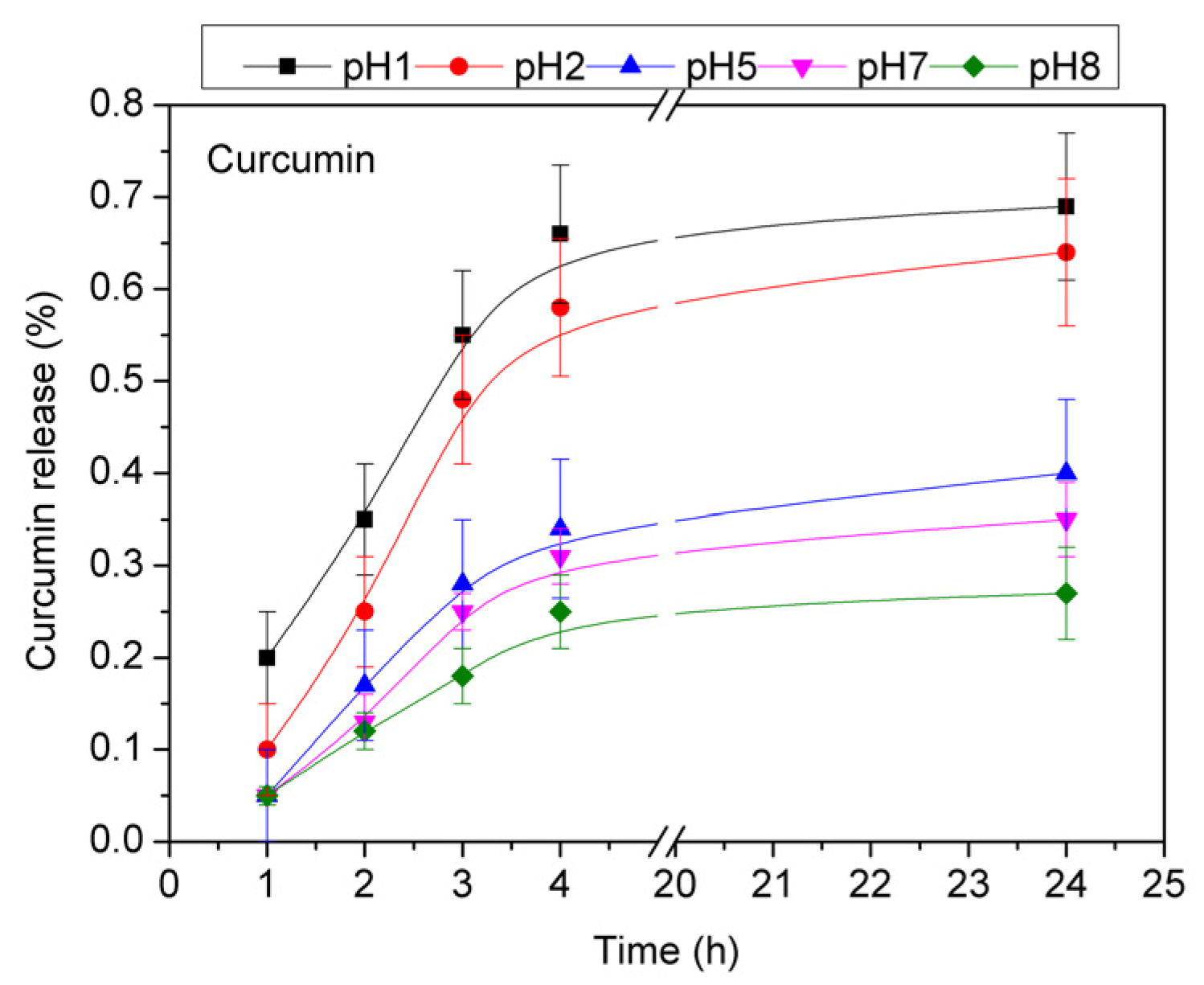
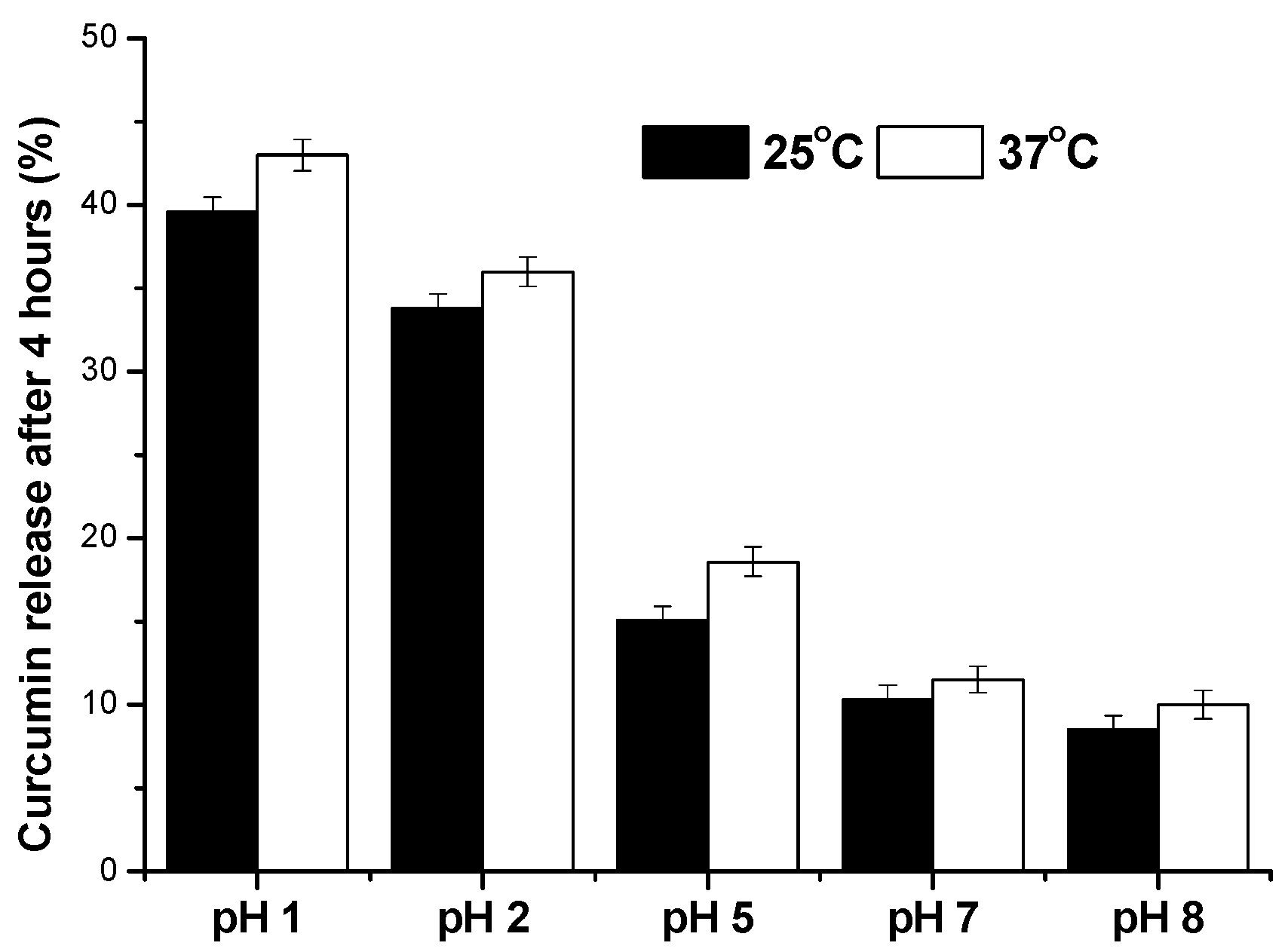
3.2. Curcumin Release Studies
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Aggarwal, S.; Ichikawa, H.; Takada, Y.; Sandur, S.K.; Shishodia, S.; Aggarwal, B.B. Curcumin (diferuloylmethane) down-regulates expression of cell proliferation and antiapoptotic and metastatic gene products through suppression of IkappaBalpha kinase and Akt activation. Mol. Pharmacol. 2006, 69, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Heger, M.; van Golen, R.F.; Broekgaarden, M.; Michel, M.C. The molecular basis for the pharmacokinetics and pharmacodynamics of curcumin and its metabolites in relation to cancer. Pharmacol. Rev. 2014, 66, 222–307. [Google Scholar] [CrossRef] [PubMed]
- Pulido-Moran, M.; Moreno-Fernandez, J.; Ramirez-Tortosa, C.; Ramirez-Tortosa, M.C. Curcumin and Health. Molecules 2016, 21, 264. [Google Scholar] [CrossRef] [PubMed]
- Priyadarsini, K.I.; Maity, D.K.; Naik, G.H.; Kumar, M.S.; Unnikrishnan, M.K.; Satav, J.G.; Mohan, H. Role of phenolic O-H and methylene hydrogen on the free radical reactions and antioxidant activity of curcumin. Free Radic. Biol. Med. 2003, 35, 475–484. [Google Scholar] [CrossRef]
- Kolev, T.M.; Velcheva, E.A.; Stamboliyska, B.A.; Spiteller, M. DFT and Experimental Studies of the Structure and Vibrational Spectra of Curcumin. Int. J. Quantum Chem. 2005, 102, 1069–1079. [Google Scholar] [CrossRef]
- Jovanovic, S.V.; Steenken, S.; Boone, C.W.; Simic, M.G. H-Atom Transfer is a Preferred Antioxidant Mechanism of Curcumin. J. Am. Chem. Soc. 1999, 121, 9677–9681. [Google Scholar] [CrossRef]
- Jagannathan, R.; Abraham, P.M.; Poddar, P. Temperature-Dependent Spectroscopic Evidences of Curcumin in Aqueous Medium: A Mechanistic Study of Its Solubility and Stability. J. Phys. Chem. B 2012, 116, 14533–14540. [Google Scholar] [CrossRef]
- Tønnesen, H.H.; Másson, M.; Loftsson, T. Studies of curcumin and curcuminoids. XXVII. Cyclodextrin complexation: Solubility, chemical and photochemical stability. Int. J. Pharm. 2002, 244, 127–135. [Google Scholar] [CrossRef]
- Parvathy, K.S.; Negi, P.S.; Srinivas, P. Antioxidant, antimutagenic and antibacterial activities of curcumin-β-diglucoside. Food Chem. 2009, 115, 265–271. [Google Scholar] [CrossRef]
- Sneharani, A.H.; Singh, S.A.; Appu Rao, A.G. Interaction of αS1-Casein with Curcumin and Its Biological Implications. J. Agric. Food Chem. 2009, 57, 10386–10391. [Google Scholar] [CrossRef]
- Sneharani, A.H.; Karakkat, J.V.; Singh, S.A.; Appu Rao, A.G. Interaction of Curcumin with β-Lactoglobulin—Stability, Spectroscopic Analysis, and Molecular Modeling of the Complex. J. Agric. Food Chem. 2010, 58, 11130–11139. [Google Scholar] [CrossRef] [PubMed]
- Bisht, S.; Feldmann, G.; Soni, S.; Ravi, R.; Karikar, C.; Maitra, A.M.; Maitra, A.N. Polymeric nanoparticle encapsulated curcumin (“nanocurcumin”): A novel strategy for human cancer therapy. J. Nanobiotechnol. 2007, 5, 3. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.K.; Agarwal, S.; Seth, B.; Yadav, A.; Nair, S.; Bhatnagar, P.; Karmakar, M.; Kumari, M.; Chauhan, L.K.; Patel, D.K.; et al. Curcumin-loaded nanoparticles potently induce adult neurogenesis and reverse cognitive deficits in Alzheimer’s disease model via canonical Wnt/b-catenin pathway. ACS Nano 2014, 8, 76–103. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Zhuang, X.; Xiang, X.; Liu, Y.; Zhang, S.; Liu, C.; Barnes, S.; Grizzle, W.; Miller, D.; Zhang, H.G. A novel nanoparticle drug delivery system: The anti-inflammatory activity of curcumin is enhanced when encapsulated in exosomes. Mol. Ther. 2010, 18, 1606–1614. [Google Scholar] [CrossRef]
- Wang, W.; Zhu, R.; Xie, Q.; Li, A.; Xiao, Y.; Li, K.; Liu, H.; Cui, D.; Chen, Y.; Wang, S. Enhanced bioavailability and efficiency of curcumin for the treatment of asthma by its formulation in solid lipid nanoparticles. Int. J. NanoMed. 2012, 7, 3667–3677. [Google Scholar] [CrossRef]
- Gonçalves, C.; Pereira, P.; Schellenberg, P.; Coutinho, P.J.; Gama, F.M. Self-assembled dextrin nanogel as curcumin delivery system. J. Biomater. Nanobiotechnol. 2012, 3, 178–184. [Google Scholar] [CrossRef]
- Falconieri, M.C.; Adamo, M.; Monasterolo, C.; Bergonzi, M.C.; Coronnello, M.; Bilia, A.R. New dendrimer based nanoparticles enhance curcumin solubility. Planta Med. 2017, 83, 420–425. [Google Scholar] [CrossRef]
- Shome, S.; Talukdar, A.D.; Choudhury, M.D.; Bhattacharya, M.K.; Upadhyaya, H. Curcumin as potential therapeutic natural product: A nanobiotechnological perspective. J. Pharm. Pharmacol. 2016, 68, 1481–1500. [Google Scholar] [CrossRef]
- Zebib, B.; Mouloungui, Z.; Noirot, V. Stabilization of Curcumin by Complexation with Divalent Cations in Glycerol/Water System. Bioinorg. Chem. Appl. 2010, 2010, 292760. [Google Scholar] [CrossRef]
- Jiang, T.; Wang, L.; Zhang, S.; Sun, P.C.; Ding, C.F.; Chu, Y.Q.; Zhou, P. Interaction of curcumin with Al (III) and its complex structures based on experiments and theoretical calculations. J. Mol. Struct. 2011, 1004, 163–173. [Google Scholar] [CrossRef]
- Cavani, F.; Trifirò, F.; Vaccari, A. Hydrotalcite-type anionic clays: Preparation, properties and applications. Catal. Today 1991, 11, 173–301. [Google Scholar] [CrossRef]
- Trifiro, F.; Vaccari, A. Hydrotalcite-like Anionic Clays (Layered Double Hydroxides). In Comprehensive Supramolecular Chemistry; Atwood, J.L., Davies, J.E.D., MacNicol, D.D., Vögtle, F., Eds.; Pergamon: Oxford, UK, 1996; Volume 7, pp. 251–291. [Google Scholar]
- Isupov, V.P.; Chupakhina, L.E.; Mitrofanova, R.P. Mechanochemical synthesis of double hydroxides. J. Mater. Synth. Process. 2000, 8, 251–253. [Google Scholar] [CrossRef]
- Richetta, M.; Medaglia, P.G.; Mattoccia, A.; Varone, A.; Pizzoferrato, R. Layered Double Hydroxides: Tailoring Interlamellar Nanospace for a Vast Field of Applications. J. Mater. Sci. Eng. 2017, 6, 360. [Google Scholar] [CrossRef]
- Choy, J.H.; Jung, J.S.; Oh, J.M.; Park, M.; Jeong, J.; Kang, Y.K.; Han, O.J. Layered double hydroxide as an efficient drug reservoir for folate derivatives. Biomaterials 2004, 25, 3059–3064. [Google Scholar] [CrossRef] [PubMed]
- Gordijo, C.R.; Barbosa, C.A.S.; Ferreira, A.M.D.C.; Constantino, V.R.L.; Silva, D.D. Immobilization of ibuprofen and copper-ibuprofen drugs on layered double hydroxides. J. Pharm. Sci. 2005, 94, 1135–1148. [Google Scholar] [CrossRef] [PubMed]
- Costantino, U.; Ambrogi, V.; Nocchetti, M.; Perioli, L. Hydrotalcite-like compounds: Versatile layered hosts of molecular anions with biological activity. Microporous Mesoporous Mater. 2008, 107, 149–160. [Google Scholar] [CrossRef]
- Oh, J.-M.; Park, M.; Kim, S.-T.; Jung, J.-Y.; Kang, Y.-G.; Choy, J.-H. Efficient delivery of anticancer drug MTX through MTX-LDH nanohybrid system. J. Phys. Chem. Solids 2006, 67, 1024–1027. [Google Scholar] [CrossRef]
- Bi, X.; Zhang, H.; Dou, L. Layered Double Hydroxide-Based Nanocarriers for Drug Delivery. Pharmaceutics 2014, 6, 298–332. [Google Scholar] [CrossRef]
- Muráth, S.; Szerlauth, A.; Sebók, D.; Szilágyi, I. Layered Double Hydroxide Nanoparticles to Overcome the Hydrophobicity of Ellagic Acid: An Antioxidant Hybrid Material. Antioxidants 2020, 9, 153. [Google Scholar] [CrossRef]
- Muráth, S.; Alsharif, N.B.; Sáringer, S.; Katana, B.; Somosi, Z.; Szilágyi, I. Antioxidant Materials Based on 2D Nanostructures: A Review on Recent Progresses. Crystals 2020, 10, 148. [Google Scholar] [CrossRef]
- Wang, Q.; O’Hare, D. Recent Advances in the Synthesis and Application of Layered Double Hydroxide (LDH) Nanosheets. Chem. Rev. 2012, 112, 4124–4155. [Google Scholar] [CrossRef]
- Supun Samindra, K.M.; Kottegoda, N. Encapsulation of curcumin into layered double hydroxides. Nanotechnol. Rev. 2014, 3, 579–589. [Google Scholar] [CrossRef]
- Megalathan, A.; Kumarage, S.; Dilhari, A.; Weerasekera, M.M.; Samarasinghe, S.; Kottegoda, N. Natural curcuminoids encapsulated in layered double hydroxides: A novel antimicrobial nanohybrid. Chem. Cent. J. 2016, 10, 35. [Google Scholar] [CrossRef]
- Gayani, B.; Dilhari, A.; Wijesinghe, G.K.; Kumarage, S.; Abayaweera, G.; Samarakoon, S.R.; Perera, I.C.; Kottegoda, N.; Weerasekera, M.M. Effect of natural curcuminoids-intercalated layered double hydroxide nanohybrid against Staphylococcus aureus, Pseudomonas aeruginosa, and Enterococcus faecalis: A bactericidal, antibiofilm, and mechanistic study. MicrobiologyOpen 2019, 8, e723. [Google Scholar] [CrossRef] [PubMed]
- Król, A.; Pomastowski, P.; Rafińska, K.; Railean-Plugaru, V.; Buszewski, B. Zinc oxide nanoparticles: Synthesis, antiseptic activity and toxicity mechanism. Adv. Colloid Interface Sci. 2017, 249, 37–52. [Google Scholar] [CrossRef]
- Zăvoianu, R.; Pavel, O.D.; Cruceanu, A.; Florea, M.; Bîrjega, R. Functional layered double hydroxides and their catalytic activity for 1,4-addition of n-octanol to 2-propenonitrile. Appl. Clay Sci. 2017, 146, 411–422. [Google Scholar] [CrossRef]
- Kooli, E.; Depège, C.; Ennaqadi, A.; De Roy, A.; Besse, J.E. Rehydration of Zn-Al layered double hydroxides. Clays Clay Miner. 1997, 45, 92–98. [Google Scholar] [CrossRef]
- Elhalil, A.; Farnane, M.; Machrouhi, A.; Mahjoubi, F.Z.; Elmoubarki, R.; Tounsadi, H.; Abdennouri, M.; Barka, N. Effects of molar ratio and calcination temperature on the adsorption performance of Zn/Al layered double hydroxide nanoparticles in the removal of pharmaceutical pollutants. J. Sci. Adv. Mater. Devices 2018, 3, 188–195. [Google Scholar] [CrossRef]
- Karami, Z.; Aghazadeh, M.; Jouyandeh, M.; Zarrintaj, P.; Vahabi, H.; Ganjalia, M.R.; Torre, L.; Puglia, D.; Sae, M.R. Epoxy/Zn-Al-CO3 LDH nanocomposites: Curability assessment. Prog. Org. Coat. 2020, 138, 105355. [Google Scholar] [CrossRef]
- George, G.; Saravanakumar, M.P. Facile synthesis of carbon-coated layered double hydroxide and its comparative characterisation with Zn–Al LDH: Application on crystal violet and malachite green dye adsorption—Isotherm, kinetics and Box-Behnken design. Environ. Sci. Pollut. Res. 2018, 25, 30236–30254. [Google Scholar] [CrossRef]
- Zhao, X.Z.; Jiang, T.; Wang, L.; Yang, H.; Zhang, S.; Zhou, P. Interaction of curcumin with Zn (II) and Cu (II) ions based on experiment and theoretical calculation. J. Mol. Struct. 2010, 984, 316–325. [Google Scholar] [CrossRef]
- Gordon, O.N.; Schneider, C. Vanillin and ferulic acid: Not the major degradation products of curcumin. Trends Mol. Med. 2012, 18, 361–363. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.M.N.; Kong, X.; Parkin, M.C.; Cammack, R.; Hider, R.C. Iron (III) citrate speciation in aqueous solution. Dalton Trans. 2009, 40, 8616–8625. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.J.; Pan, M.H.; Cheng, A.L.; Lin, L.I.; Ho, Y.S.; Hsieh, C.Y.; Lin, J.K. Stability of curcumin in buffer solutions and characterization of its degradation products. J. Pharm. BioMed. Anal. 1997, 15, 1867–1876. [Google Scholar] [CrossRef]
- Allegany Nutrition. The Enzyme Specialists. Available online: https://www.alleganynutrition.com/supporting-pages/the-human-digestive-tract-ph-range-diagram/ (accessed on 1 February 2020).
- Beasley, D.E.; Koltz, A.M.; Lambert, J.E.; Fierer, N.; Dunn, R.R. The evolution of stomach acidity and its relevance to the Human Microbiome. PLoS ONE 2015, 10, e0134116. [Google Scholar] [CrossRef] [PubMed]
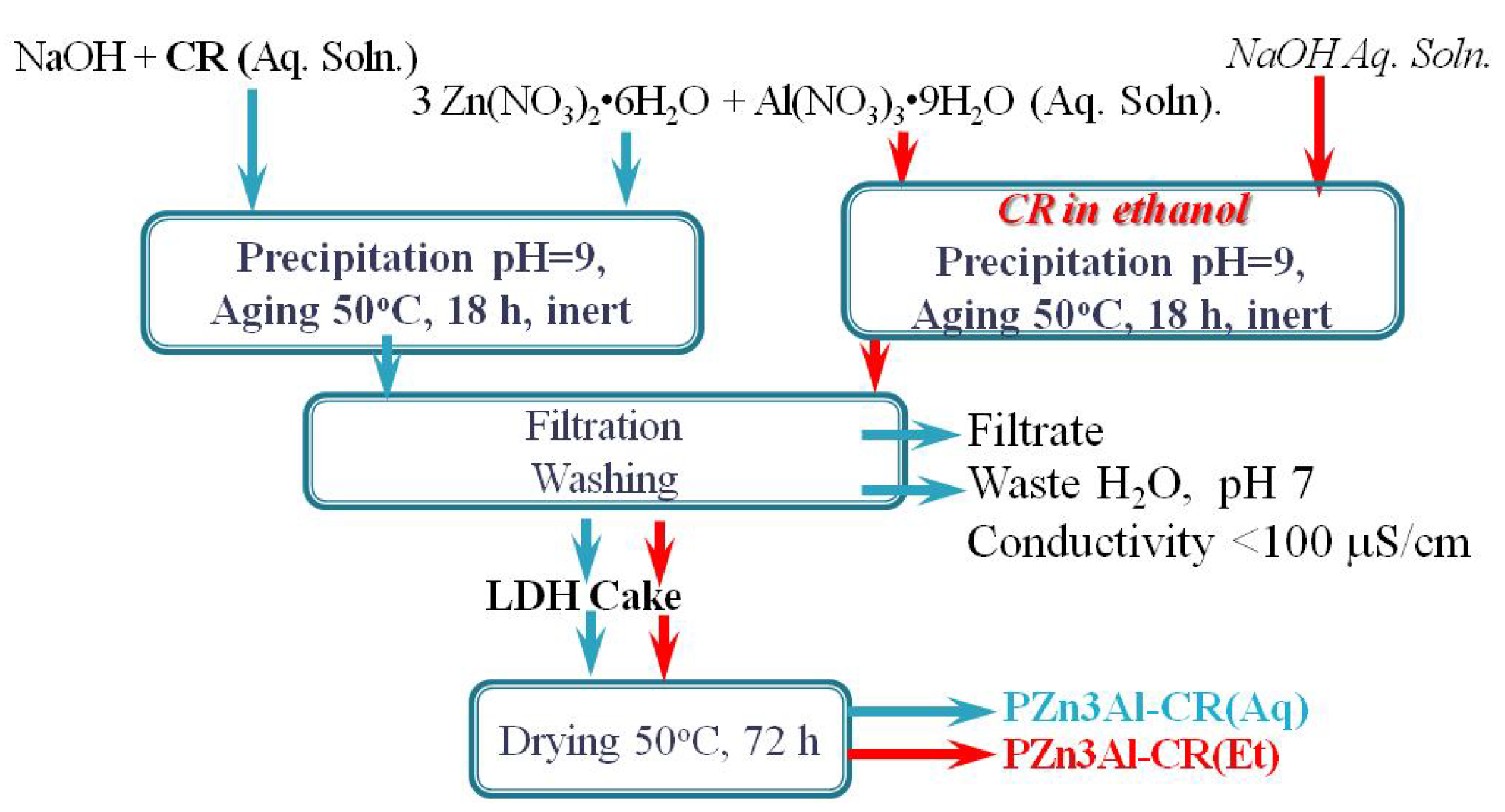
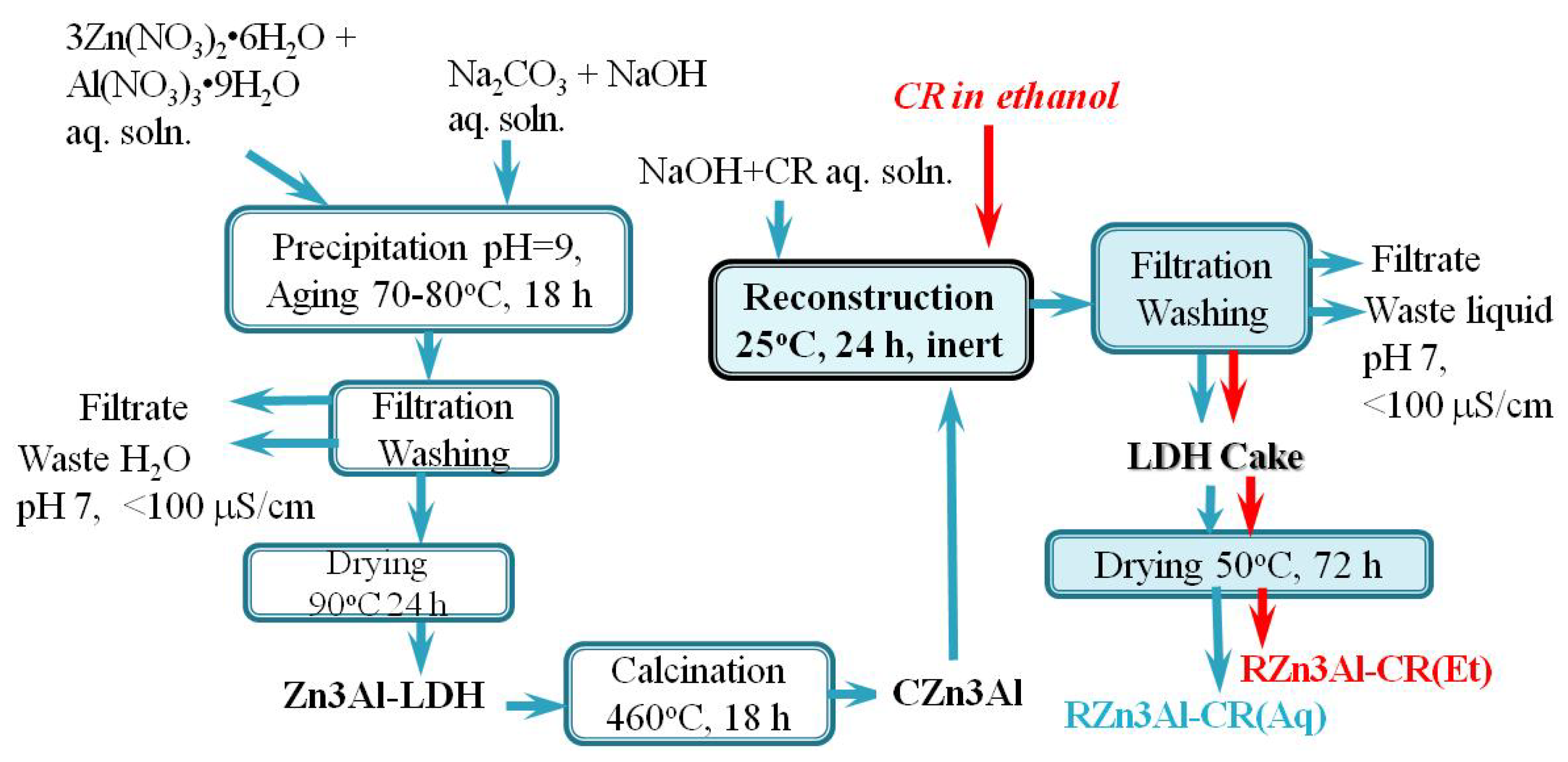
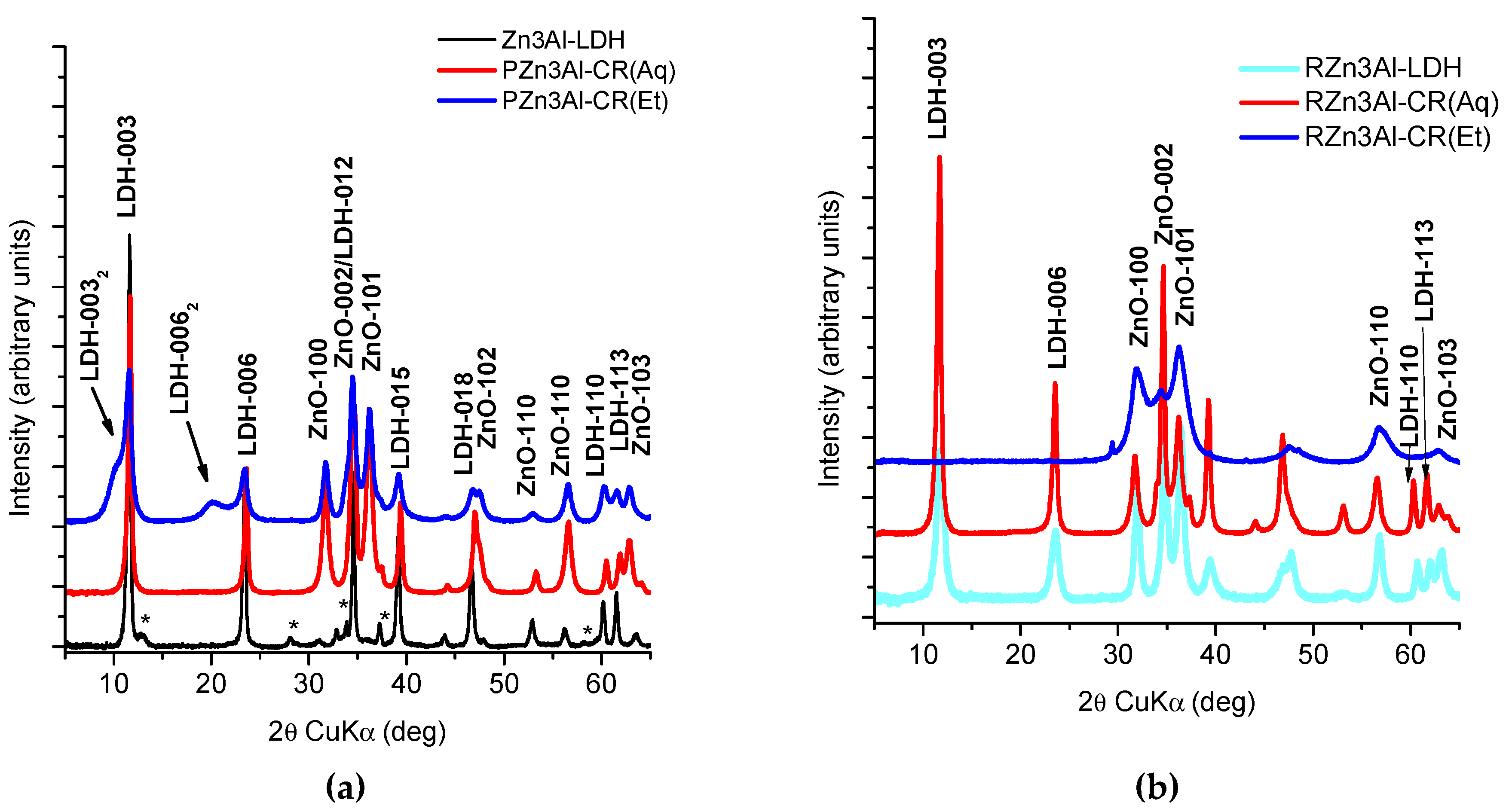

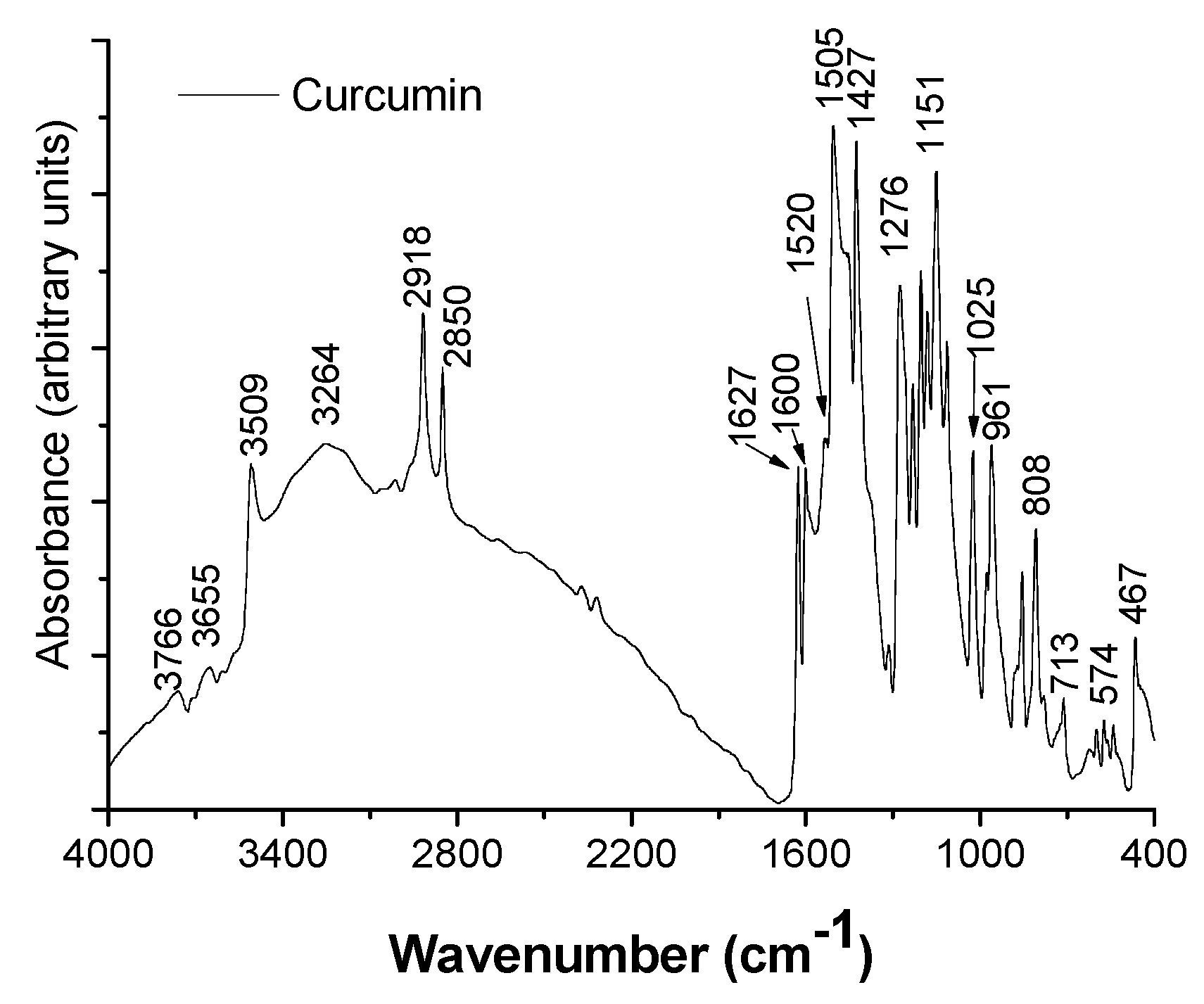
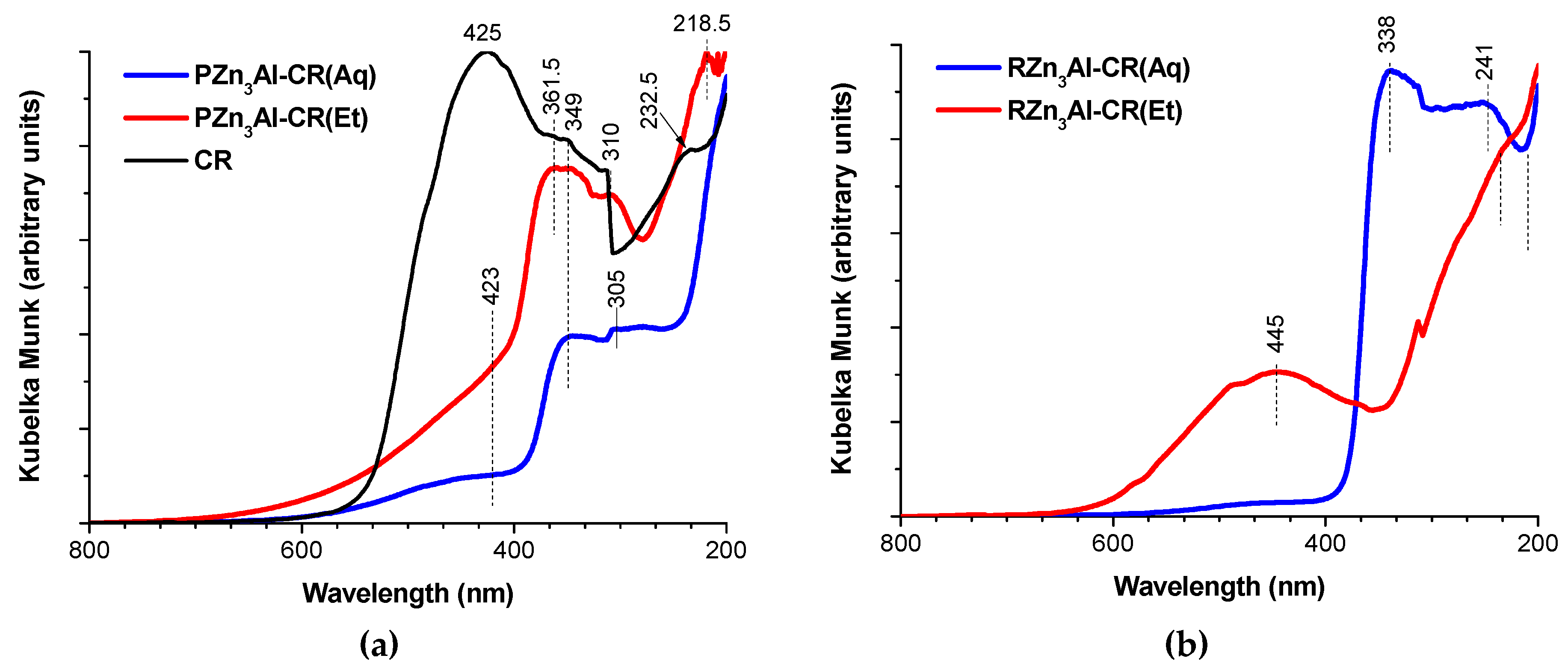
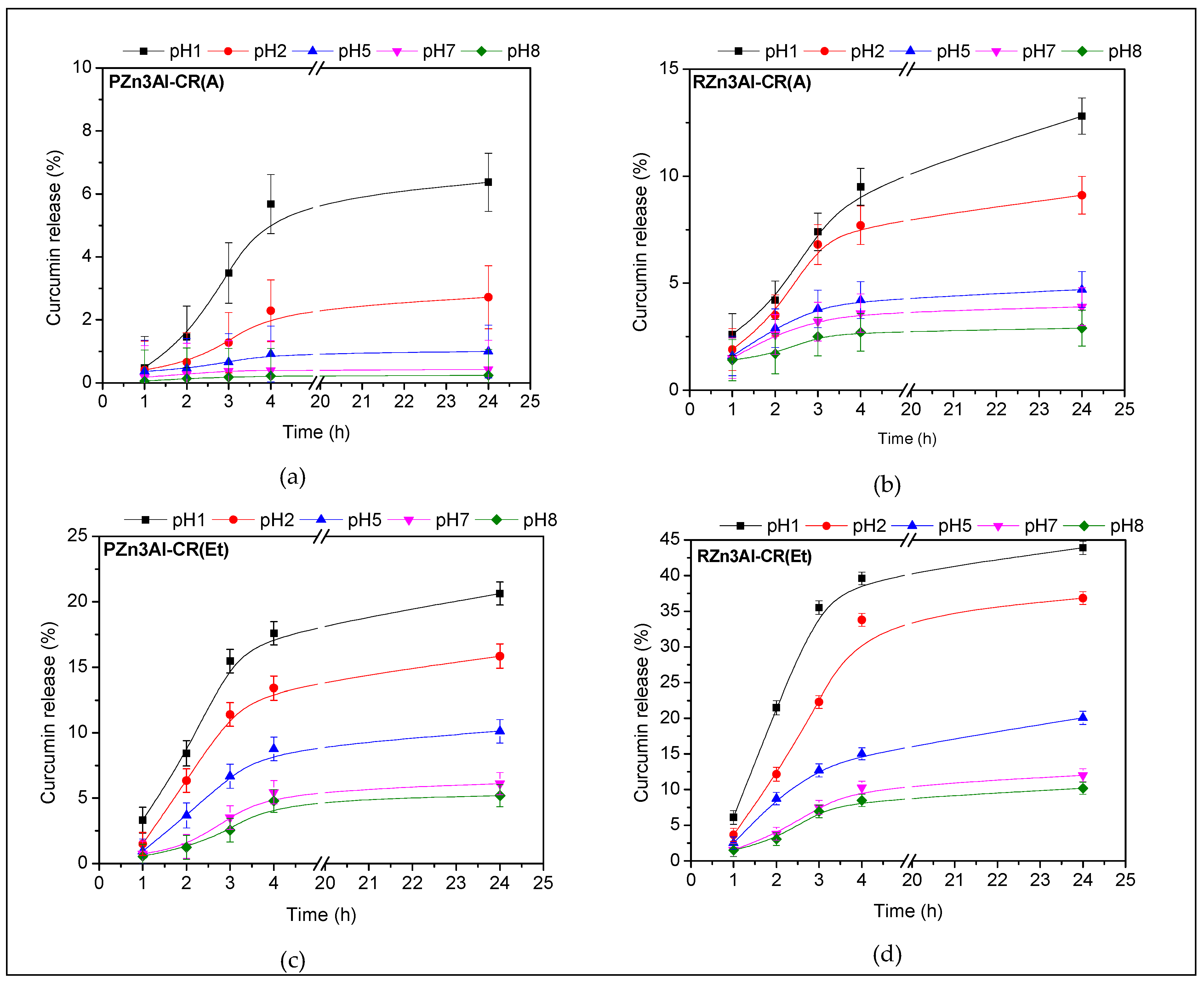
| Sample | Zn (wt. %) | Al (wt. %) | CO3 (wt. %) 1 | CR (wt. %) 2 | H2O (wt. %) 3 | Molar Ratio | |
|---|---|---|---|---|---|---|---|
| Zn/Al | CR/Al | ||||||
| Zn3Al-LDH | 46.2 | 6.4 | 7.1 | 0 | 8.5 | 2.97 | 0 |
| PZn3Al-CR(Aq) | 49.9 | 7.2 | 2.8 | 12.1 | 8.9 | 2.86 | 1/8.5 |
| PZn3Al-CR(Et) | 48.1 | 6.8 | 1.7 | 9.3 | 8.8 | 2.90 | 1/10 |
| RZn3Al-CR(Aq) | 51.1 | 7.2 | 1.2 | 10.0 | 5.6 | 2.91 | 1/9.9 |
| RZn3Al-CR(Et) | 60.3 | 8.4 | 0.2 | 11.5 | 2.9 | 2.97 | 1/9.9 |
| Samples | LDH Phase | ZnO Phase | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| a (Å) | c (Å) | I003/I110 | D110 (nm) | D003 (nm) | a (Å) | c (Å) | Vol (Å3) | D (nm) | ZnO (%) | |
| Zn3Al-LDH | 3.075(2) | 22.9026 | 7.66 | 35.6 | 23.4 | |||||
| RZn3Al-LDH 1 | 3.07(2) | 22.84(7) | 5.10 | 16.6 | 8.9 | 3.243(2) | 5.183(6) | 47.21 | 13.2 | 42 |
| PZn3Al-CR(Aq) | 3.061(5) | 22.56(4) | 7.71 | 24.4 | 20.2 | 3.251(6) | 5.21(1) | 47.69 | 12.0 | 45 |
| PZn3Al-CR(Et) | 3.071(6) | 22.99(7) | 3.37 | 21.9 | 11.9 | 3.262(4) | 5.20(1) | 47.92 | 11.6 | 22 |
| 25.476(11) | 1.34 | 3.9 | ||||||||
| RZn3Al-CR(Aq) | 3.071(4) | 22.62(5) | 5.88 | 26.7 | 16.5 | 3.263(9) | 5.21(3) | 48.04 | 11.3 | 41 |
| RZn3Al-CR(Et) | 3.234(2) | 5.238(3) | 47.44 | 5.3 | 100 | |||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pavel, O.D.; Şerban, A.; Zăvoianu, R.; Bacalum, E.; Bîrjega, R. Curcumin Incorporation into Zn3Al Layered Double Hydroxides—Preparation, Characterization and Curcumin Release. Crystals 2020, 10, 244. https://doi.org/10.3390/cryst10040244
Pavel OD, Şerban A, Zăvoianu R, Bacalum E, Bîrjega R. Curcumin Incorporation into Zn3Al Layered Double Hydroxides—Preparation, Characterization and Curcumin Release. Crystals. 2020; 10(4):244. https://doi.org/10.3390/cryst10040244
Chicago/Turabian StylePavel, Octavian D., Ariana Şerban, Rodica Zăvoianu, Elena Bacalum, and Ruxandra Bîrjega. 2020. "Curcumin Incorporation into Zn3Al Layered Double Hydroxides—Preparation, Characterization and Curcumin Release" Crystals 10, no. 4: 244. https://doi.org/10.3390/cryst10040244
APA StylePavel, O. D., Şerban, A., Zăvoianu, R., Bacalum, E., & Bîrjega, R. (2020). Curcumin Incorporation into Zn3Al Layered Double Hydroxides—Preparation, Characterization and Curcumin Release. Crystals, 10(4), 244. https://doi.org/10.3390/cryst10040244