Abstract
Three binary systems were prepared by mixing of two different mesogenic derivatives, homologues, the first is azo/ester, namely 4-alkoxyphenylazo-4′-phenyl-4″-alkoxybenzoates (IIn+m) and the second is Schiff base/ester, namely 4-(arylideneamino)phenyl-4″-alkoxy benzoates (In+m). The two corresponding analogues from both series in the binary mixtures investigated are of the same terminal alkoxy chain length. Mesomorphic properties were investigated by differential scanning calorimetry (DSC) and phases identified by polarized optical microscope (POM). Photophysical studies were investigated by UV spectroscopy connected to a hot stage. Results were discussed based on constructed binary phase diagrams. All mixtures were found to exhibit eutectic compositions, with linear or slightly linear nematic and smectic A stability/composition dependences. Geometrical parameters were predicted applying density functional theory (DFT) calculations. Twist angle (θ), aspect ratio, dipole moment and the polarizability of the individual compounds were discussed and correlated with the experimental results to illustrate the enhanced the mesophase stability and the mesophase range of the mixture at the eutectic composition compared with those of their individual components.
1. Introduction
Nowadays, mixtures of liquid crystalline (LC) materials are of an extensive area of interest [1,2,3,4,5,6,7,8]. For practical applications, LC materials exhibiting transition at room temperature and retain their mesomorphic character over a wide range of temperatures are preferred. Mesomorphic criteria of mesogenic cores are greatly modified upon the mixing of individual components. Many reports [9,10,11,12,13,14,15,16,17,18,19,20] have been used to investigate the mesophase behavior upon the mixing of two or more compounds. On the other hand, dimeric LCs have been studied [7,8,21,22,23,24] for the same applications. Binary or ternary mixtures may be symmetric, with the same mesogenic moieties but of different terminal groups, or nonsymmetrical, with different mesogenic units. In both types of binary mixtures, the intermolecular interactions between the two mesogenic cores lead to a significant variation in the optical activity [21,22,23,24]. In previous work [25], it was observed that the mesophase stability is enhanced by an increment of the polarity and/or polarizability of the mesogenic core of the molecule. In general, the linking groups and the terminal polar substituents play important roles in the mesophase formation, type, thermal stability, and temperature ranges of the LCs compounds [26,27,28,29,30,31,32].
Calamitic molecules are linear, with two terminals; these groups may be similar or not. Many-wing units have been designed in the generation of LCs, but the most general route [33], is to use either an alkyl/alkoxy chain or a small compact polar substituent [34,35,36,37]. The role of these groups is to act as dipolar parts to introduce anisotropy in physical characteristics. Azobenzene moiety is considered to be a good fragment to develop and design a new molecular shape of thermally stable phase transitions, often with very interesting polymorphisms [5,36,37,38,39,40,41]. Liquid crystals containing an azo group in the mesogenic core are attractive for studying their electro-optical properties [39]. Ester linkages are also commonly used, as they offer good advantages such as thermal and wide range of stability. Liquid-crystal-based Schiff base linkages have been synthesized and investigated in many interesting studies [42,43,44,45,46,47]. The development and investigation of new advanced functional thermotropic liquid crystals require the proper selection of a mesogenic core, linking group, and terminal functional substituents. [48] The photochromic phenomena for such spacers (-N=N, -N=CH-, and -COO-) are used to control the mesophase behavior and optical activities of several LCs [42,44,46,47,49,50,51,52,53,54,55,56,57,58]. The reversible trans-cis isomerization, induced by thermal and ultraviolet irradiation, brings about a molecular ordering in the trans isomer that stabilizes the mesophase structure of the LC, while the disordering of cis isomers will destroy the mesophase formation.
Our present work aims to investigate the mesomorphic and optical activity of binary mixtures made from two systems with different mesogens, the Schiff base/ester (In+m) and the azo/ester (IIn+m) compounds (Scheme 1). We also conducted a brief study of the thermal cis-trans isomerization of pure components and their binary mixtures at their eutectic compositions. We intended to measure the thermal back cis-to-trans interconversion in a pure compound using UV–Vis spectroscopy technique.
Scheme 1.
Investigated Schiff base/ester (In+m) and azo/ester (IIn+m) compounds.
2. Experimental
2.1. Materials and Preparation
Compounds investigated in this study were synthesized and characterized according to supplementary methods.
2.2. Physical Characterization
Calorimetric measurements were carried out using a TA Instrument Co. Q20 differential scanning calorimeter (DSC; Waltham, MA, USA). The DSC was calibrated using the melting temperature and enthalpy of indium and lead. DSC investigations were conducted using small samples (2–3 mg) placed in aluminum pans. All measurements were made at a heating rate of 10 °C/min in an inert atmosphere of nitrogen gas (30 mL/min) and all transitions were recorded from the second heating scan.
Transition temperatures were checked and the types of the mesophases identified for all compounds. Molar ratios of both components were melted and mixed to form intimate blends, then cooled. The mixture was put between two glass plates and heated to obtain a very thin layer. The texture was observed under a polarized optical microscope POM (POM, Wild, Germany). Temperature was recorded by an attached thermocouple with a Brookfield temperature controller, England (Mettler FP82HT hot stage).
2.3. Computational Method and Calculations
The theoretical calculations were carried out by Gaussian 09 software (2009, Gaussian. Inc.: Wallingford, CT, USA) [59]. DFT/B3LYP method and a 6–31G (d,p) basis set was selected for calculations. The structures of the optimized geometries were drawn with Gauss View [60].
3. Results and Discussion
3.1. Binary Mesophase Behavior of Prepared Mixtures
Figure 1 shows the DSC heating curve of 20 mol % I6+8/II6+8 as an example. As for pure I6 and I8, the binary mixtures exhibited different endothermic peaks, crystalline (Cr)–smectic A (SmA) or smectic C (SmC)–nematic (N)–isotropic (I), Representative POM textures are depicted in Figure 2. Transition temperatures and their corresponding enthalpy of transitions for three homologues binary mixture systems (n = 6 and m= 8, 10 and 12 carbons) are tabulated in Table 1. Individual compounds were previously reported in our investigations [40,41]. The thermal transitions of the pure compounds, as well as their mixtures, are graphically illustrated in Figure 3a–c. As can be seen from the Table 1 and Figure 1, both homologues bearing similar alkoxy chain length upon mixing showed to be enantiotropic dimorphic, covering all compositions accompanied by a decrease in the melting temperatures compared with their components.
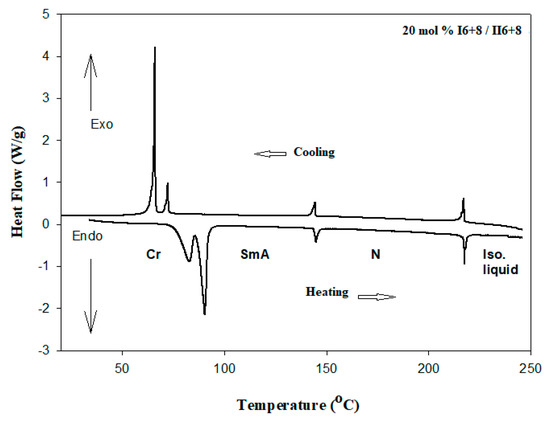
Figure 1.
Differential scanning calorimetry (DSC) curves obtained during heating/cooling scans of 20 mol % I6+8 in the I6+8/II6+8 binary system with heating rate 10 °C/min.

Figure 2.
Polarized optical microscope (POM) textures upon heating of 40 mol % of the I6+12 in the binary mixture II6+12/I6+12: (a) N phase at 120.0 °C; (b) SmA phase at 190.0 °C.

Table 1.
Phase transition temperatures (°C), (enthalpy of transition ΔH, kJ/g), for binary mixtures/In+m.
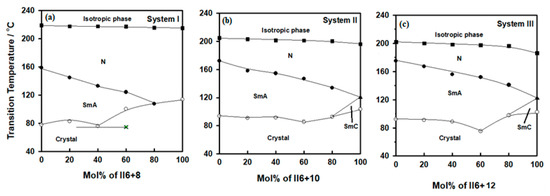
Figure 3.
Binary phase diagrams for (a) system I (I6+8/II6+8); (b) system II (I6+10/II6+10); and (c) system III (I6+12/II6+12).
In the first system, (I6+8/II6+8), the Schiff base I6+8 was dimorphic, exhibiting SmA and nematic mesophases, whereas the azo analogue II6+8, was purely nematogenic (N). The addition of I6+8 to II6+8 resulted in a heterogeneous eutectic mixture around 40 mol% of II6+8. The eutectic mixture of this system possessed a broad mesophase temperature range, 141.6 °C. The nematic stability varied regularly with composition. This suggests that the molecular interactions between the mixed components were comparable to the interactions between pure components. On the other hand, the addition of I6+8 to II6+8 disrupted somewhat the formation of the SmA phase of the analogue I6+8. The thermal stability of the SmA decreased with increasing of the II6+8 component until it vanished at about 80 mol % (Figure 3a).
In the case of system II (I6+10/II6+10), similar behavior of the nematic phase was observed, Figure 3b. It was previously reported that the II6+10 analogue is dimorphic, exhibiting N and smectic C (SmC) phases [41]. Mixtures of the two compounds resulted in a dimorphic SmA and N phases in the whole composition range. With the addition of about 10% of the I6+10 the SmC phase of II6+10 derivative disappeared and was replaced by the SmA phase as the I6+10 content further increased. The stability of the SmA diversity increased with increasing the I6+10. The eutectic mixture appeared around 60 mol % of II6+10.
Similarly, the binary system of the higher chain length system III (I6+12/II6+12), Figure 3c, exhibited dimorphic mesophases. The concentrations of the mixture from 10% to 80% of II6+12 possessed SmA and N, but a binary mixture with a higher concentration of II6+12 (>80%) possessed SmC and N. As can be seen from Figure 3c, both the TSm-I and TN-I decreased gradually with increased content of the II6+12. Further, the eutectic mixture was observed around 60 mol % of II6+12.
Generally, the mesophase behavior of rod-like mesogen was affected by many parameters such as the polarizability, dipole moment, aspect ratio, and the competitive interaction between the lateral and terminal aggregation. Moreover, the molecular shape that was affected by stereo and/or mesomeric configurations also affected molecular-molecular interactions. In the present study, the molecular aggregations of calamitic molecules played a principal role in mesophase stability due to the lateral attraction of planar molecules will reinforced by longer alkoxy-chains (n). Another factor that also contributed to mesophase stability was end-to-end aggregation of terminal alkoxy chains that differed according to mesomeric effects. These factors, with different ratios, affect the stability (Tc), range, and type of the mesophase for pure components as well as their binary mixtures.
3.2. DFT Calculations
Theoretical DFT calculations for the individual compounds I6+8 and II6+8 were performed in the gas phase by DFT/B3LYP method at 6−311G basis set. All optimum compounds proved to be stable, due to the absence of the imaginary frequency. The results of the theoretical DFT calculations of the investigated compounds I6+8 and II6+8 before mixing showed a planar geometry with little bent shape. The three phenyl rings of the individual analogues were completely planar, regardless of the type of the mesogenic core. The planarity of the liquid crystalline compounds before mixing impacted their observed mesophase. Recently, it was reported [33,47,52,54,61] that the planar structures permit a strong lateral interaction in addition to the end to end aggregation of the alkoxy chains. The strong terminal interaction enhanced the nematic stability while the lateral aggregation enhances the smectic phase as observed for all mixture compositions, Figure 4.
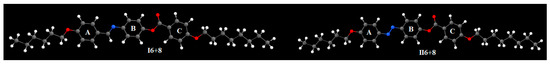
Figure 4.
Optimized geometrical structures of analogues I6+8 and II6+8.
The coplanarity of the aromatic rings of such types of compounds was investigated from dihedral angles, due to the presence of the twist angle (θ) between the rings A, B, and C. The twist angle between the aromatic rings of the Schiff base derivatives was lower than that of the azo ones. The twist angles (θ) were found to be 21.5, 37.0, and 16.2° between A-B, B-C, and A-C, respectively, for compound I6+8, however, the twist angle θ = 8.0, 0.8 and 8.8° for compound II6+8. The dihedral angle showed a higher degree of twisting of the rings A and B in compound I. The azo derivative, II6+8, showed a slightly higher twisting angle between rings B and C. The incorporation of non-coplanar compound I in the high-backed more coplanar azo derivative II, improved the mesomorphic properties of both mixed compounds. Such a mixture between two compounds of different degrees of coplanarity may enhance a certain type of interaction (perhaps π-π stacking interaction) that cannot appear in the components, and consequently may improve mesophase stability and its ranges.
The estimated DFT calculations for dipole moment and the polarizability of the individual compounds are summarized in Table 2. The polarizability of the compounds was highly affected by the dimension, as well as the electronic nature of the mesogenic core. The length and the width of both compounds under investigation were almost the same, aspect ratio = 8.45 for both. The equal aspect ratios of both compounds may be explained in terms of different coplanarity of these compounds as previously mentioned. The little difference of the polarizability of the azo compound (α = 487 Bohr3) compared to that of the Schiff base (α = 470 Bohr3) may be explained in terms of the higher polarity of imine group, compared to the azo group. The dipole moment of the Schiff base/ester I6+8 was higher than that of the azo ester II6+8. However, the high dipole moment of the individual compounds may permit a high degree of specific interactions (perhaps quadrupolar) and π-π stacking interactions in the mixture that enhance mesophase stabilities and their temperature ranges of the mixture concerning their components.

Table 2.
Parameters (Hartree/Particle) and dipole moment (Debye) of I6+8 and II6+8.
The charge distribution map for the Schiff base/ester I6+8 and the azo ester II6+8 was calculated under the same basis sets according to molecular electrostatic potential (MEP) (Figure 5). The red region (negatively charged atomic sites) was distributed on the aromatic moiety and the maximum was carbonyl oxygen of the H-bonded carboxylic group, while alkoxy chains showed the least negatively charged atomic sites (blue regions). As shown in Figure 5, there was no significant effect of the mesogenic core on the mapping of the charge distribution. Thus, we can conclude that an excellent choice of compounds for mixtures to modify the mesomorphic behavior. Moreover, the similar charge distribution explains the enhancement of the mesophase stability and the mesophase range of the mixture at the eutectic ratios. Recently, we also reported [33,47,52,54,56,61] that mesophase temperature range is highly affected by competitive interactions between end-to-end and side-to-side interactions by increasing chain length, and that these factors are highly impacted by the map of the charge distribution.

Figure 5.
Molecular Electrostatic potentials (MEP) of I6+8 and II6+8.
3.3. Photophysical Study
The study of individual pure I6+8 and II6+8 derivatives, as well as their binary mixtures at their eutectic compositions, nearly around 40%, in DMF (C = 1.8 × 10−6 mol/L) at temperature range 298–398 K was carried out by measuring UV-visible spectra. Figure 6, Figure 7 and Figure 8 display the influence of temperature on the shape and the intensity of the absorption bands for I6+8, II6+8 and their eutectic mixture I6+8/II6+8, respectively. As shown from these figures, all the compounds investigated exhibited an absorption maximum in the range 335–356 nm and weak absorption band at 445 nm, which were related to π–π* and n–π* transitions of the azobenzene trans–cis configuration, respectively [62,63,64]. The Schiff base derivative I6+8 showed a maximum absorption band at ~335 nm, and that absorbance slightly increased with increasing the temperature. A highly delocalized electronic system was observed and π–π*, as well as transitions, due to the presence of conjugated imine linking parts and high molar absorption. The UV spectra of the corresponding azo compound II6+8 displayed a maximum absorption band at ~356 nm in which the intensity of absorption increased with the rise of temperature (Figure 6). The binary eutectic mixture of azo and Schiff base components, Figure 8, showed that this mixture’s absorption maximum band of the π–π* was at ~350 nm. This shift from ~356 nm to ~350 nm was attributed to the electronic transitions from the highest occupied molecular orbital (HOMO) to the lowest unoccupied molecular orbital (LUMO). As an example, the weak absorption maximum at 445 nm is presented for the eutectic mixture in the inset in Figure 8.
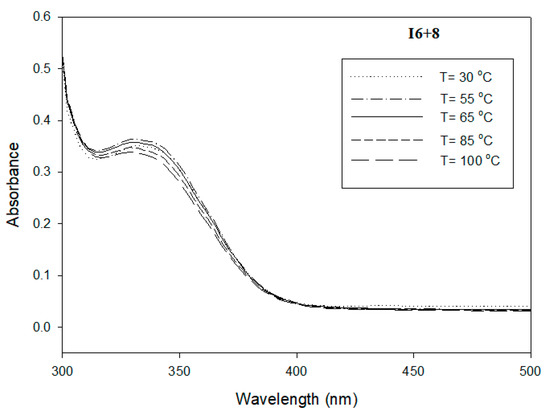
Figure 6.
UV absorption spectrum of I6+8 in DMF (C = 1.8 × 10−6 mol/L) at temperature range 298–398 K.
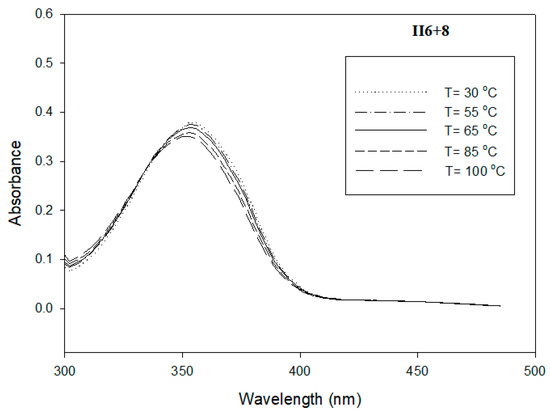
Figure 7.
UV absorption spectrum of II6+8 in DMF (C = 1.8 × 10−6 mol/L) at temperature range 298–398 K.
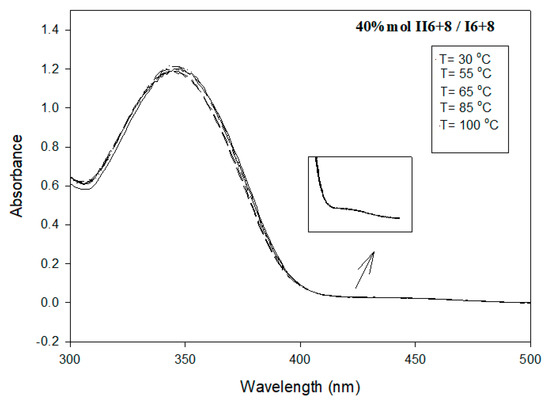
Figure 8.
UV absorption spectrum of binary eutectic mixture of II6+8/I6+8 in DMF (C = 1.8 × 10−6 mol/L) at temperature range 298–398 K.
4. Conclusions
The present study was undertaken to investigate how the mixing of LC molecules with different mesogenic cores and variable terminal alkoxy chain lengths affect the mesomorphic and photophysical properties of the resulted mixtures. The first group of compounds was 4-alkoxyphenylazo-4′-phenyl 4″-alkoxybenzoates (In+m), and the second group was a Schiff base/ester, 4-(arylideneamino) phenyl 4″-alkoxy benzoates (IIn+m). The two groups of compounds investigated differ in their mesogenic cores, azo/ester, and Schiff/ester. The length of the two terminal alkoxy chains was chosen as examples. Theoretical calculations for individual components were carried out by DFT. The study revealed that:
- The constructed diagrams showed enantiotropic mesomorphic behavior for all compositions.
- All systems showed nearly linear nematic-to-isotropic composition dependencies.
- The binary phase diagrams exhibited eutectic composition with high mesophase stability.
- The polarizability was highly affected by the dimensions and the type of the mesogenic core.
- The dipole moment of the Schiff base ester I6+8 was higher than that of the azo ester II6+8.
- The π-π stacking interactions in mixture enhanced the mesophase stabilities as well as their temperature ranges.
- Finally, the similar charge distribution observed in both series rationalized the enhanced mesophase stability and the mesophase range of the mixture at its eutectic compositions.
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-4352/10/3/223/s1.
Author Contributions
H.A.A., M.H., O.A.A., and K.A.A.A.-O. designed the experiment and carried out the laboratory work; M.H., H.A.A., G.R.S., O.A.A., and K.A.A.A.-O. accomplished the data analysis and drafted the manuscript; H.A.A., M.H., and M.M.N. gave final approval for publication. All authors have read and agree to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Thangavel, V.; Venkataraman, B.; Prakasan, S.; Ramasamy, J.; Nallagounder, V.V. Experimental and DFT Studies on Thermochromism Induced Binary HBLC Mixture. Braz. J. Phys. 2019, 50, 39–51. [Google Scholar] [CrossRef]
- Mohamady, S.Z.; Nessim, R.I.; Shehab, O.R.; Naoum, M.M. Effect of steric factor on mesomorphic stability, II: Binary mixtures of homologues of 4-(4′-substituted phenylazo)-1-naphthyl-4″-alkoxybenzoates. Mol. Cryst. Liq. Cryst. 2006, 451, 53–64. [Google Scholar] [CrossRef]
- Naoum, M.; Mohammady, S.; Ahmed, H. Lateral protrusion and mesophase behaviour in pure and mixed states of model compounds of the type 4-(4′-substituted phenylazo)-2-(or 3-) methyl phenyl-4′-alkoxy benzoates. Liq. Cryst. 2010, 37, 1245–1257. [Google Scholar] [CrossRef]
- Naoum, M.; Fahmi, A.; Alaasar, M.; Abdel-Aziz, M. Effect of lateral substitution of different polarity on the mesophase behaviour in pure and mixed states of 4-(4′-substituted phenylazo)-2-substituted phenyl-4″-alkoxy benzoates. Liq. Cryst. 2011, 38, 391–405. [Google Scholar] [CrossRef]
- Ahmed, H.; Naoum, M.; Saad, G. Effect of alkoxy-chain length proportionation on the mesophase behaviour of terminally di-substituted phenylazo phenyl benzoates. Liq. Cryst. 2013, 40, 914–921. [Google Scholar] [CrossRef]
- Ahmed, H.; Naoum, M. Mesophase behavior of binary and ternary mixtures of benzoic acids bearing terminal substituents of different polarity and chain-lengths. Thermochim. Acta 2014, 575, 122–128. [Google Scholar] [CrossRef]
- Ahmed, H.; Naoum, M.; Saad, G. Mesophase behaviour of 1: 1 mixtures of 4-n-alkoxyphenylazo benzoic acids bearing terminal alkoxy groups of different chain lengths. Liq. Cryst. 2016, 43, 1259–1267. [Google Scholar] [CrossRef]
- Ahmed, H. Mesophase stability in binary mixtures of monotropic nematogens. Liq. Cryst. 2015, 42, 70–80. [Google Scholar] [CrossRef]
- Dave, J.; Vasanth, K. Influence of molecular structure on liquid crystalline properties and phase transitions in mixed liquid crystals. Mol. Cryst. Liq. Cryst. 1966, 2, 125–134. [Google Scholar] [CrossRef]
- Dave, J.S.; Menon, M.R.; Patel, P.R. Chiral phases induced by doping nonmesogenic component into mesogenic esters. Mol. Cryst. Liq. Cryst. 2003, 392, 83–95. [Google Scholar] [CrossRef]
- Vora, R.; Gupta, R.; Patel, K. Exhibition of induced mesophases in the binary systems where one or both the components are non-mesogenic. Mol. Cryst. Liq. Cryst. 1991, 209, 251–263. [Google Scholar] [CrossRef]
- Fujimura, S.; Yamamura, Y.; Hishida, M.; Nagatomo, S.; Saito, K. Reentrant nematic phase in 4-alkyl-4′-cyanobiphenyl (n CB) binary mixtures. Liq. Cryst. 2014, 41, 927–932. [Google Scholar] [CrossRef]
- Prasad, A.; Das, M.K. Determination of elastic constants of a binary system (7CPB+9.CN) showing nematic, induced smectic Ad and re-entrant nematic phases. Liq. Cryst. 2014, 41, 1261–1268. [Google Scholar] [CrossRef]
- Cvetinov, M.; Obadović, D.; Stojanović, M.; Lazar, D.; Vajda, A.; Éber, N.; Fodor-Csorba, K.; Ristić, I. Mesophase behaviour of binary mixtures of bent-core and calamitic compounds. Liq. Cryst. 2013, 40, 1512–1519. [Google Scholar] [CrossRef][Green Version]
- Salud, J.; Lopez, D.; Diez-Berart, S.; de la Fuente, M. Tests of the tricritical point in the SmA-to-N phase transition of binary mixtures of butyloxybenzylidene octylaniline and hexyloxybenzylidene octylaniline. Liq. Cryst. 2013, 40, 293–304. [Google Scholar] [CrossRef]
- Prajapati, A.K.; Patel, N.S.; Lad, V.G. Induction of chirality by doping mesogens with non-mesogenic chiral dopant. Mol. Cryst. Liq. Cryst. Sci. Technol. Sect. A. Mol. Cryst. Liq. Cryst. 2000, 348, 41–51. [Google Scholar] [CrossRef]
- Vora, R.; Rajput, S. Binary mesogenic systems comprised of ester mesogens and non-mesogens. Mol. Cryst. Liq. Cryst. 1991, 209, 265–277. [Google Scholar] [CrossRef]
- Govindaiah, T.; Nagappa; Sathyanarayana, P.; Mahadeva, J.; Sreepad, H. Induced chiral smectic phase in mixtures of mesogenic and non-mesogenic compounds. Mol. Cryst. Liq. Cryst. 2011, 548, 55–60. [Google Scholar] [CrossRef]
- Lohar, J.; Dave, J.S., Jr. Emergence of smectic mesophase in binary mixtures of pure nematogens. Mol. Cryst. Liq. Cryst. 1983, 103, 181–192. [Google Scholar] [CrossRef]
- Lu, Z.; Henderson, P.A.; Paterson, B.J.; Imrie, C.T. Liquid crystal dimers and the twist-bend nematic phase. The preparation and characterisation of the α, ω-bis (4-cyanobiphenyl-4′-yl) alkanedioates. Liq. Cryst. 2014, 41, 471–483. [Google Scholar] [CrossRef]
- Chan, T.-N.; Lu, Z.; Yam, W.-S.; Yeap, G.-Y.; Imrie, C.T. Non-symmetric liquid crystal dimers containing an isoflavone moiety. Liq. Cryst. 2012, 39, 393–402. [Google Scholar] [CrossRef]
- Yeap, G.-Y.; Osman, F.; Imrie, C.T. Non-symmetric dimers: Effects of varying the mesogenic linking unit and terminal substituent. Liq. Cryst. 2015, 42, 543–554. [Google Scholar] [CrossRef]
- Imrie, C.T.; Henderson, P.A.; Yeap, G.-Y. Liquid crystal oligomers: Going beyond dimers. Liq. Cryst. 2009, 36, 755–777. [Google Scholar] [CrossRef]
- Naoum, M.; Fahmi, A.; Ahmed, H. Liquid crystalline behaviour of model compounds di-laterally substituted with different polar groups. Liq. Cryst. 2011, 38, 511–519. [Google Scholar] [CrossRef]
- Niezgoda, I.; Jaworska, J.; Pociecha, D.; Galewski, Z. The kinetics of the EZE isomerisation and liquid-crystalline properties of selected azobenzene derivatives investigated by the prism of the ester group inversion. Liq. Cryst. 2015, 42, 1148–1158. [Google Scholar] [CrossRef]
- Hegde, G.; Shanker, G.; Gan, S.; Yuvaraj, A.; Mahmood, S.; Mandal, U.K. Synthesis and liquid crystalline behaviour of substituted (E)-phenyl-4-(phenyldiazenyl) benzoate derivatives and their photo switching ability. Liq. Cryst. 2016, 43, 1578–1588. [Google Scholar] [CrossRef]
- Paterson, D.A.; Xiang, J.; Singh, G.; Walker, R.; Agra-Kooijman, D.A.M.; Martínez-Felipe, A.; Gao, M.; Storey, J.M.; Kumar, S.; Lavrentovich, O.D. Reversible isothermal twist–bend nematic–nematic phase transition driven by the photoisomerization of an azobenzene-based nonsymmetric liquid crystal dimer. J. Am. Chem. Soc. 2016, 138, 5283–5289. [Google Scholar] [CrossRef]
- Romero-Hasler, P.; Fierro-Armijo, A.; Soto-Bustamante, E.; Meneses-Franco, A. Synthesis and characterisation of two homologous series of LC acrylic monomers based on phenolic and resorcinic azobenzene groups. Liq. Cryst. 2016, 43, 1804–1812. [Google Scholar] [CrossRef]
- Henderson, P.; Cook, A.; Imrie, C. Oligomeric liquid crystals: From monomers to trimers. Liq. Cryst. 2004, 31, 1427–1434. [Google Scholar] [CrossRef]
- Osman, F.; Yeap, G.-Y.; Nagashima, A.; Ito, M.M. Structure property and mesomorphic behaviour of S-shaped liquid crystal oligomers possessing two azobenzene moieties. Liq. Cryst. 2016, 43, 1283–1292. [Google Scholar] [CrossRef]
- Srinivasa, H. New symmetric azobenzene molecules of varied central cores: Synthesis and characterisation for liquid crystalline properties. Liq. Cryst. 2017, 44, 1384–1393. [Google Scholar] [CrossRef]
- Collings, P.J.; Hird, M. Introduction to Liquid Crystals: Chemistry and Physics; Taylor and Francis Group: London, UK; CRC Press: NewYork, NY, USA, 2017. [Google Scholar]
- Hagar, M.; Ahmed, H.; Saad, G. Synthesis and mesophase behaviour of Schiff base/ester 4-(arylideneamino) phenyl-4″-alkoxy benzoates and their binary mixtures. J. Mol. Liq. 2019, 273, 266–273. [Google Scholar] [CrossRef]
- Ahmed, H.A.; Hagar, M.; Aljuhani, A. Mesophase behavior of new linear supramolecular hydrogen-bonding complexes. RSC Adv. 2018, 8, 34937–34946. [Google Scholar]
- Ahmed, H.A.; Hagar, M.; Alhaddad, O.A. Phase behavior and DFT calculations of laterally methyl supramolecular hydrogen-bonding complexes. Crystals 2019, 9, 133. [Google Scholar] [CrossRef]
- Hagar, M.; Ahmed, H.A.; El-Sayed, T.H.; Alnoman, R. Mesophase behavior and DFT conformational analysis of new symmetrical diester chalcone liquid crystals. J. Mol. Liq. 2019, 285, 96–105. [Google Scholar] [CrossRef]
- Ahmed, H.; Hagar, M.; Alaasar, M.; Naoum, M. Wide nematic phases induced by hydrogen-bonding. Liq. Cryst. 2019, 46, 550–559. [Google Scholar] [CrossRef]
- Zaki, A.A.; Ahmed, H.; Hagar, M. Impact of fluorine orientation on the optical properties of difluorophenylazophenyl benzoates liquid crystal. Mater. Chem. Phys. 2018, 216, 316–324. [Google Scholar] [CrossRef]
- Hagar, M.; Ahmed, H.; Alhaddadd, O. DFT calculations and mesophase study of coumarin esters and its azoesters. Crystals 2018, 8, 359. [Google Scholar] [CrossRef]
- Naoum, M.M.; Metwally, N.H.; Abd Eltawab, M.M.; Ahmed, H.A. Polarity and steric effect of the lateral substituent on the mesophase behaviour of some newly prepared liquid crystals. Liq. Cryst. 2015, 42, 1351–1369. [Google Scholar] [CrossRef]
- Hagar, M.; Ahmed, H.; Saad, G. Mesophase stability of new Schiff base ester liquid crystals with different polar substituents. Liq. Cryst. 2018, 45, 1324–1332. [Google Scholar] [CrossRef]
- Ahmed, H.; Hagar, M.; Alhaddad, O. Mesomorphic and geometrical orientation study of the relative position of fluorine atom in some thermotropic liquid crystal systems. Liq. Cryst. 2019, 1–10. [Google Scholar] [CrossRef]
- Ahmed, H.; Hagar, M.; El-Sayed, T.; Alnoman, R.B. Schiff base/ester liquid crystals with different lateral substituents: Mesophase behaviour and DFT calculations. Liq. Cryst. 2019, 46, 1–11. [Google Scholar] [CrossRef]
- Nafee, S.S.; Hagar, M.; Ahmed, H.A.; El-Shishtawy, R.M.; Raffah, B.M. The synthesis of new thermal stable schiff base/ester liquid crystals: A computational, mesomorphic, and optical study. Molecules 2019, 24, 3032. [Google Scholar] [CrossRef]
- Ahmed, H.; Hagar, M.; Alhaddad, O. New chair shaped supramolecular complexes-based aryl nicotinate derivative; mesomorphic properties and DFT molecular geometry. RSC Adv. 2019, 9, 16366–16374. [Google Scholar] [CrossRef]
- Alnoman, R.; Ahmed, H.A.; Hagar, M. Synthesis, optical, and geometrical approaches of new natural fatty acids’ esters/Schiff base liquid crystals. Molecules 2019, 24, 4293. [Google Scholar] [CrossRef]
- Nafee, S.S.; Ahmed, H.; Hagar, M. Theoretical, experimental and optical study of new thiophene-based liquid crystals and their positional isomers. Liq. Cryst. 2020, 1–12. [Google Scholar] [CrossRef]
- O’Neill, M.; Kelly, S.M. Liquid crystals for charge transport, luminescence, and photonics. Adv. Mater. 2003, 15, 1135–1146. [Google Scholar] [CrossRef]
- Ikeda, T. Photomodulation of liquid crystal orientations for photonic applications. J. Mater. Chem. 2003, 13, 2037–2057. [Google Scholar] [CrossRef]
- Prasad, S.K.; Nair, G.G.; Sandhya, K.; Rao, D.S. Photoinduced phase transitions in liquid crystalline systems. Curr. Sci. 2004, 86, 815–823. [Google Scholar]
- Selvarasu, C.; Kannan, P. Effect of azo and ester linkages on rod shaped Schiff base liquid crystals and their photophysical investigations. J. Mol. Struct. 2016, 1125, 234–240. [Google Scholar] [CrossRef]
- Hagar, M.; Ahmed, H.; Saad, G. New calamitic thermotropic liquid crystals of 2-hydroxypyridine ester mesogenic core: Mesophase behaviour and DFT calculations. Liq. Cryst. 2020, 47, 114–124. [Google Scholar] [CrossRef]
- Nafee, S.S.; Hagar, M.; Ahmed, H.A.; Alhaddad, O.; El-Shishtawy, R.M.; Raffah, B.M. New two rings Schiff base liquid crystals; ball mill synthesis, mesomorphic, Hammett and DFT studies. J. Mol. Liq. 2020, 299, 112161. [Google Scholar] [CrossRef]
- Alhaddad, O.; Ahmed, H.; Hagar, M. Experimental and Theoretical Approaches of New Nematogenic Chair Architectures of Supramolecular H-Bonded Liquid Crystals. Molecules 2020, 25, 365. [Google Scholar] [CrossRef]
- Hagar, M.; Ahmed, H.; Alhaddad, O. Experimental and theoretical approaches of molecular geometry and mesophase behaviour relationship of laterally substituted azopyridines. Liq. Cryst. 2019, 46, 1440–1451. [Google Scholar] [CrossRef]
- Hagar, M.; Ahmed, H.; Alhaddad, O. New azobenzene-based natural fatty acid liquid crystals with low melting point: Synthesis, DFT calculations and binary mixtures. Liq. Cryst. 2019, 46, 2223–2234. [Google Scholar] [CrossRef]
- Sherif, S.; Nafee, H.A.A.; Hagar, M. New architectures of supramolecular H-bonded liquid crystal complexes based on dipyridine Derivatives. Liq. Cryst. 2020, 1–12. [Google Scholar] [CrossRef]
- Ahmed, N.H.S.; Saad, G.R.; Ahmed, H.A.; Hagar, M. New wide-stability four-ring azo/ester/Schiff base liquid crystals: Synthesis, mesomorphic, photophysical, and DFT approaches. RSC Adv. 2020, 10, 9643–9656. [Google Scholar] [CrossRef]
- Frisch, M.; Trucks, G.; Schlegel, H.B.; Scuseria, G.; Robb, M.; Cheeseman, J.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G. Gaussian 09, Revision a. 02; Gaussian. Inc.: Wallingford, CT, USA, 2009; p. 200. [Google Scholar]
- Dennington, R.; Keith, T.; Millam, J. GaussView, Version 5; Semichem Inc.: Shawnee Mission, KS, USA, 2009. [Google Scholar]
- Ahmed, H.; Hagar, M.; Saad, G. Impact of the proportionation of dialkoxy chain length on the mesophase behaviour of Schiff base/ester liquid crystals; experimental and theoretical study. Liq. Cryst. 2019, 46, 1611–1620. [Google Scholar] [CrossRef]
- Lyons, C.; Rathi, N.; Dev, P.; Byrne, O.; Surolia, P.K.; Maji, P.; MacElroy, J.; Yella, A.; Grätzel, M.; Magner, E. Organic Dyes Containing Coplanar Dihexyl-Substituted Dithienosilole Groups for Efficient Dye-Sensitised Solar Cells. Int. J. Photoenergy 2017, 2017, 7594869. [Google Scholar] [CrossRef]
- Sui, M.Y.; Pan, Q.Q.; Yin, H.; Sun, G.Y.; Geng, Y.; Su, Z.M. Design of Hexabenzocoronene Derivatives as Non-Fullerene Acceptors in Organic Photovoltaics by Bridging Dimers and Modulating Structural Twists. Sol. Rrl 2017, 1, 1700060. [Google Scholar] [CrossRef]
- Yamamoto, R.; Yamamoto, T.; Ohara, K.; Naito, T. Dye-Sensitized Molecular Charge Transfer Complexes: Magnetic and Conduction Properties in the Photoexcited States of Ni (dmit) 2 Salts Containing Photosensitive Dyes. Magnetochemistry 2017, 3, 20. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).