Enhanced Thermoelectric Properties of WS2/Single-Walled Carbon Nanohorn Nanocomposites
Abstract
1. Introduction
2. Materials and Methods
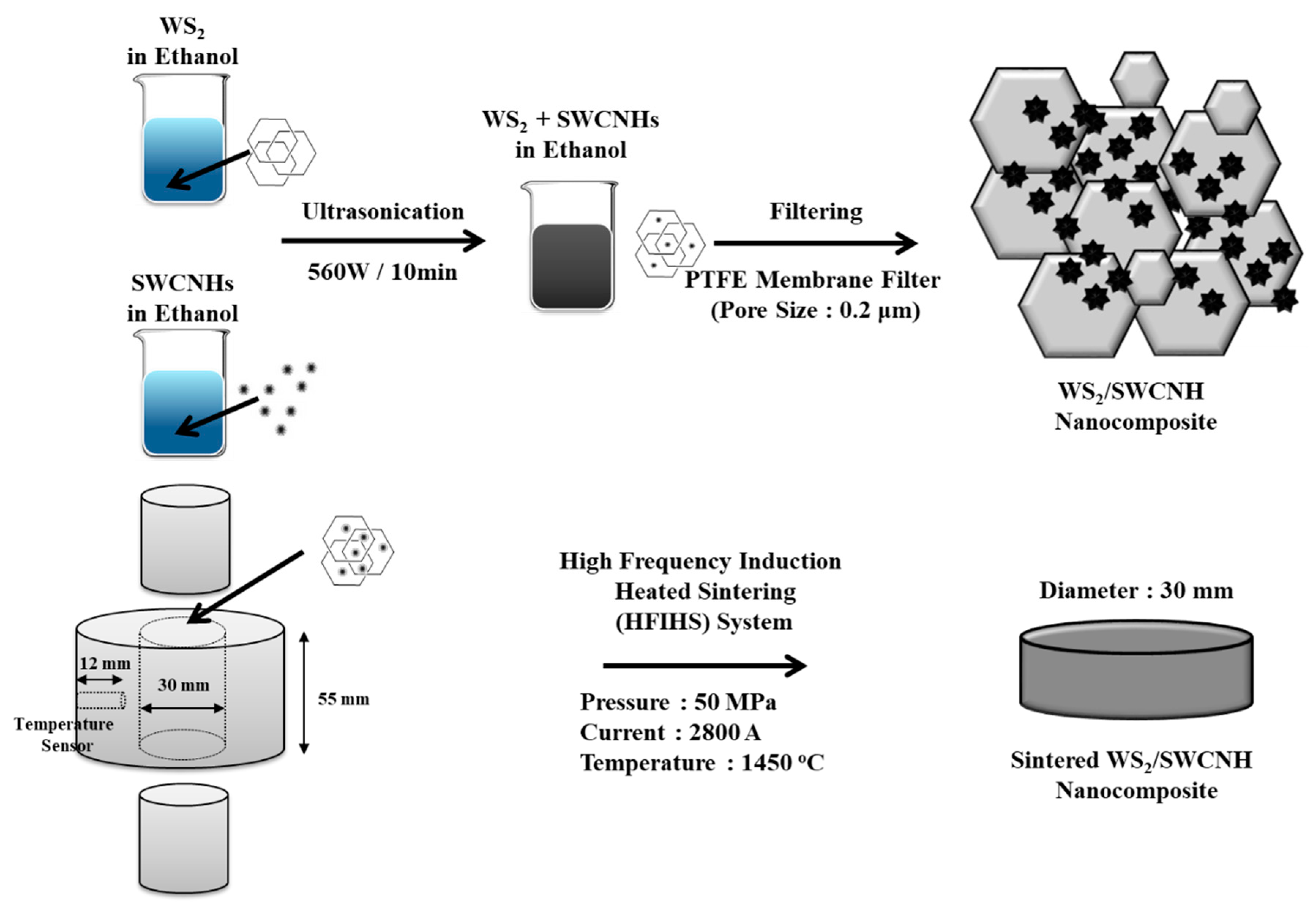
2.1. Preparation of Tungsten Disulfide (WS2)/Single-Walled Carbon Nanohorn (SWCNH) Nanocomposites via High-Frequency Induction Heated Sintering (HFIHS)
2.2. Characterizations
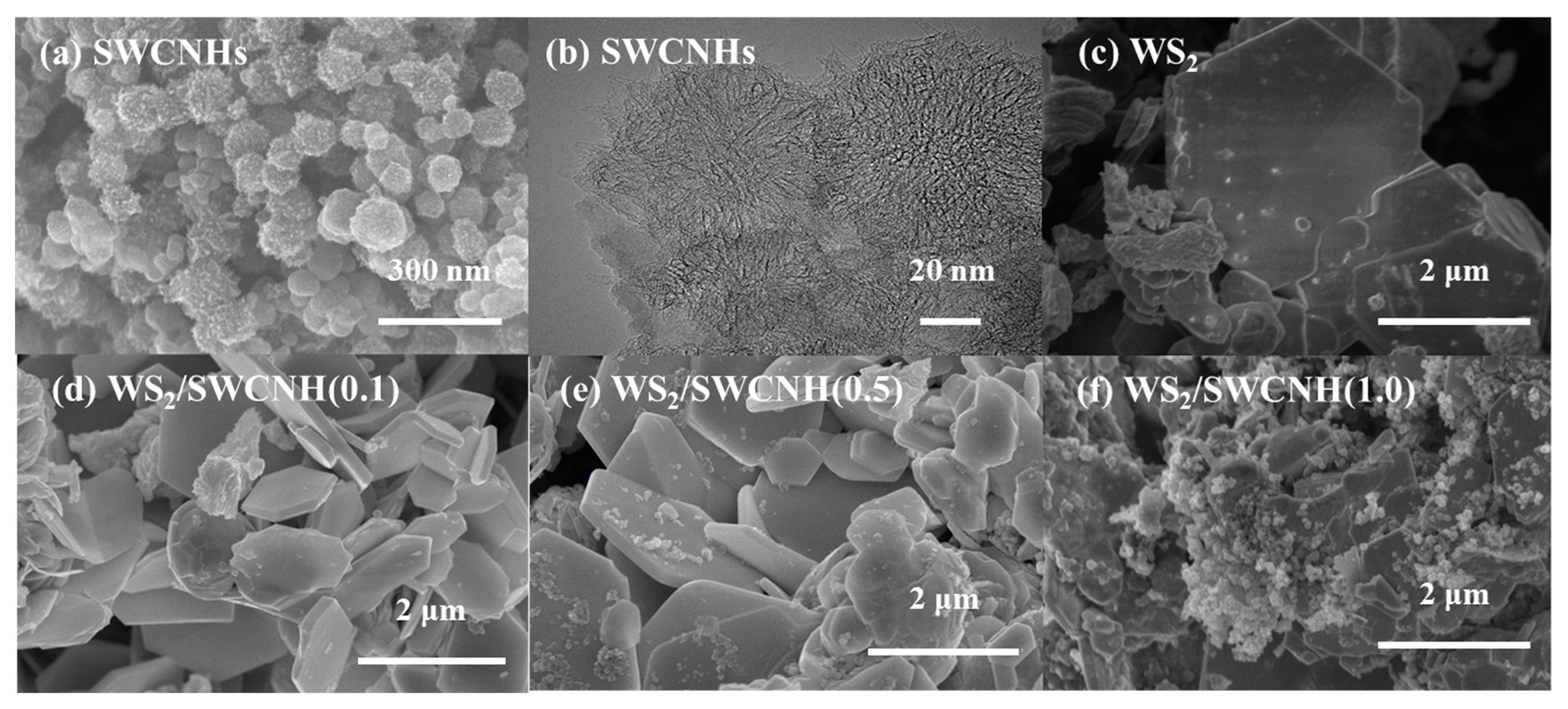
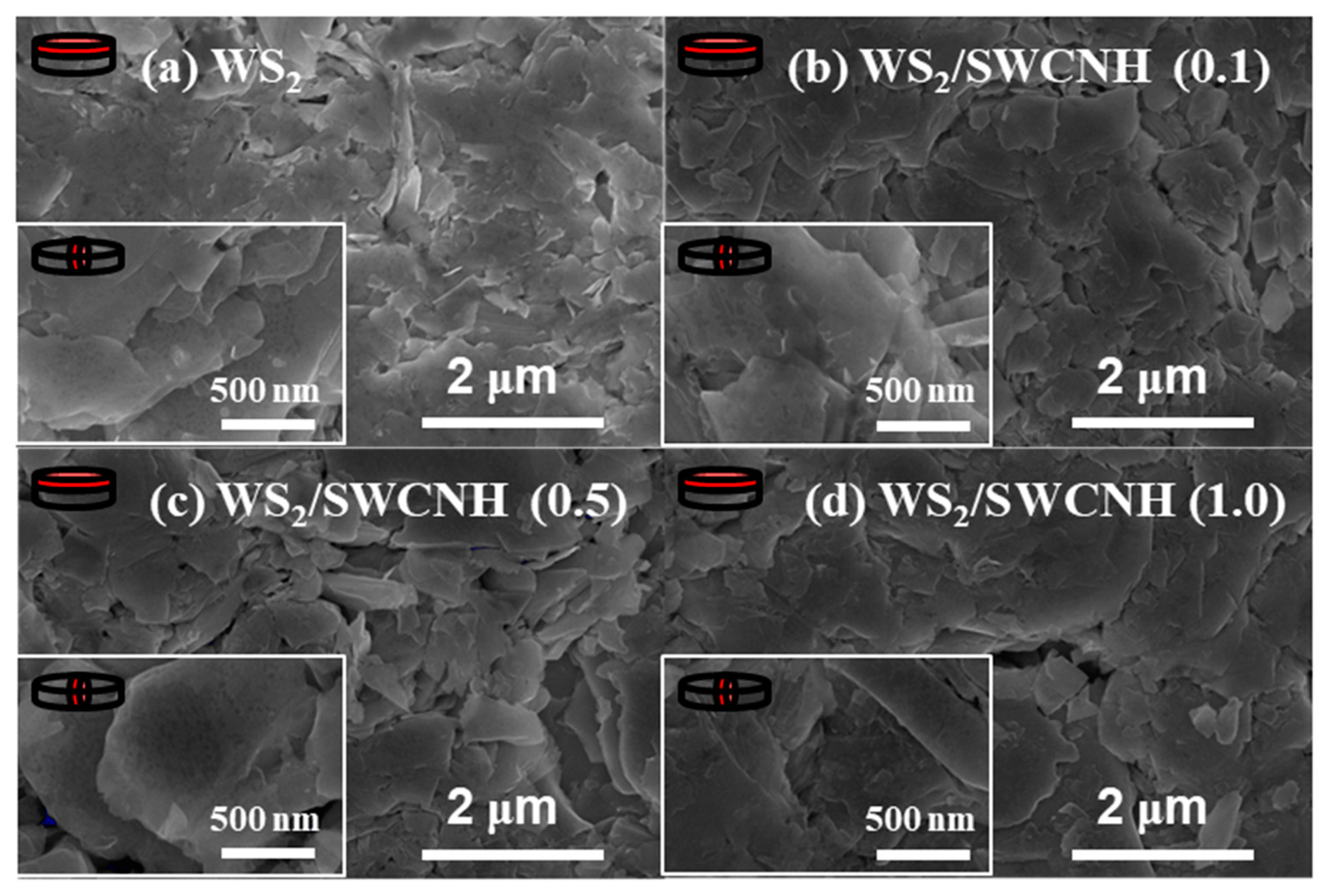
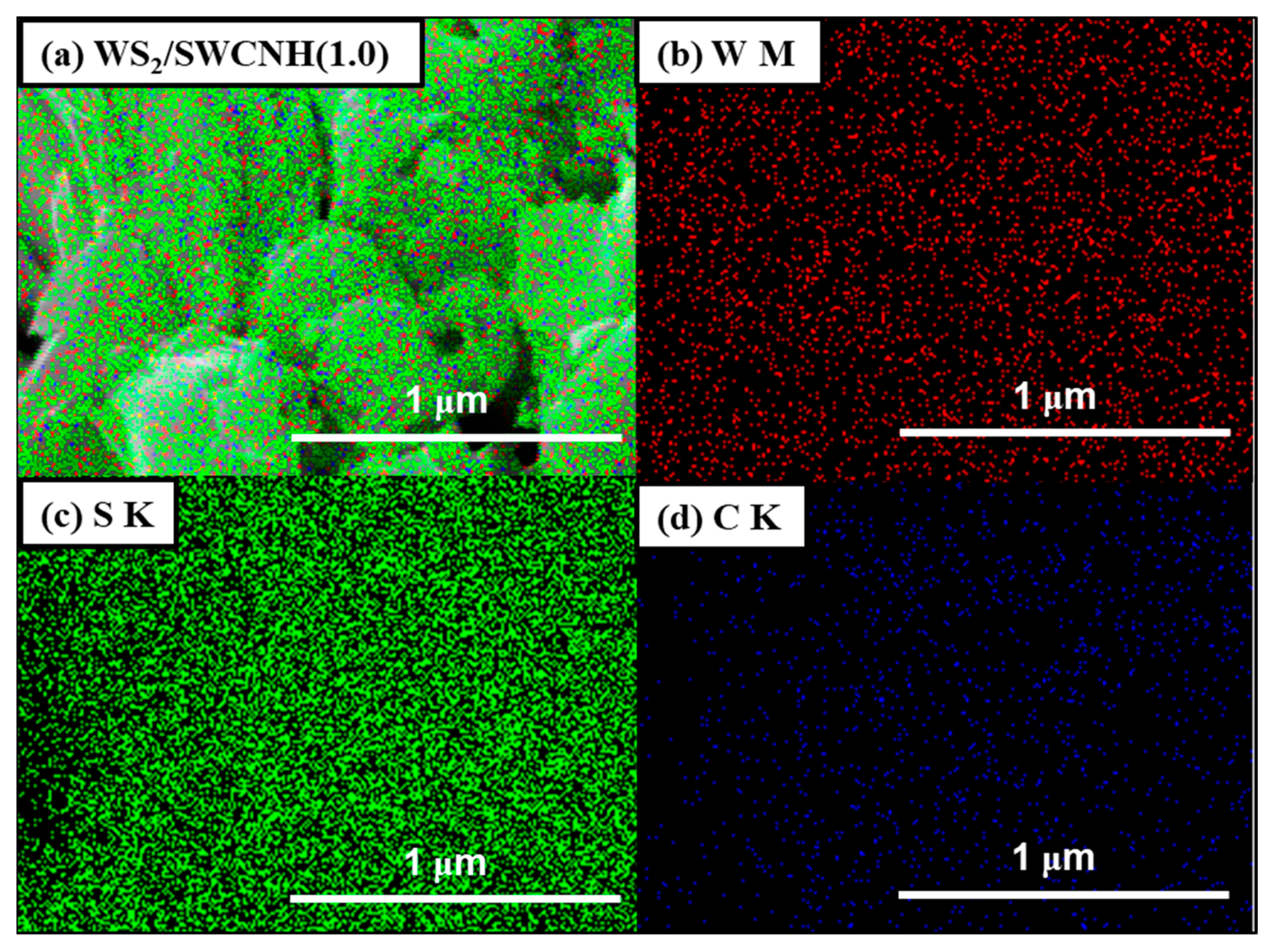
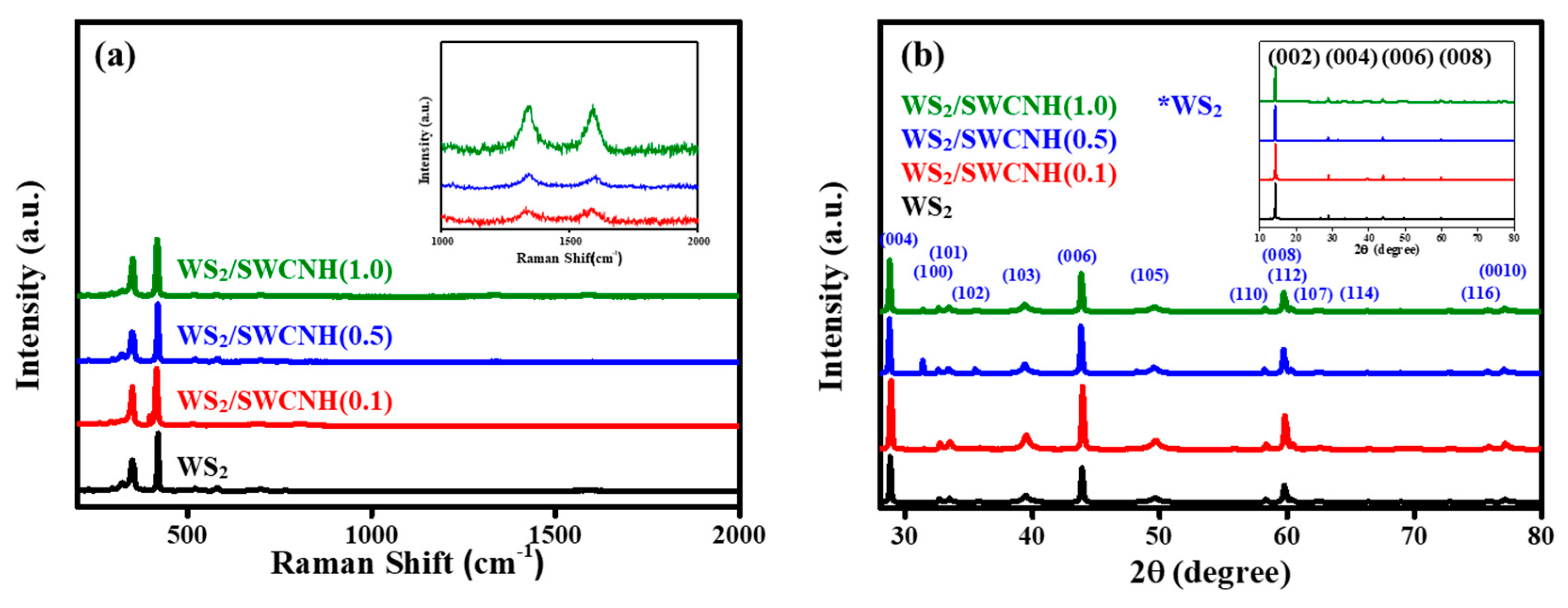
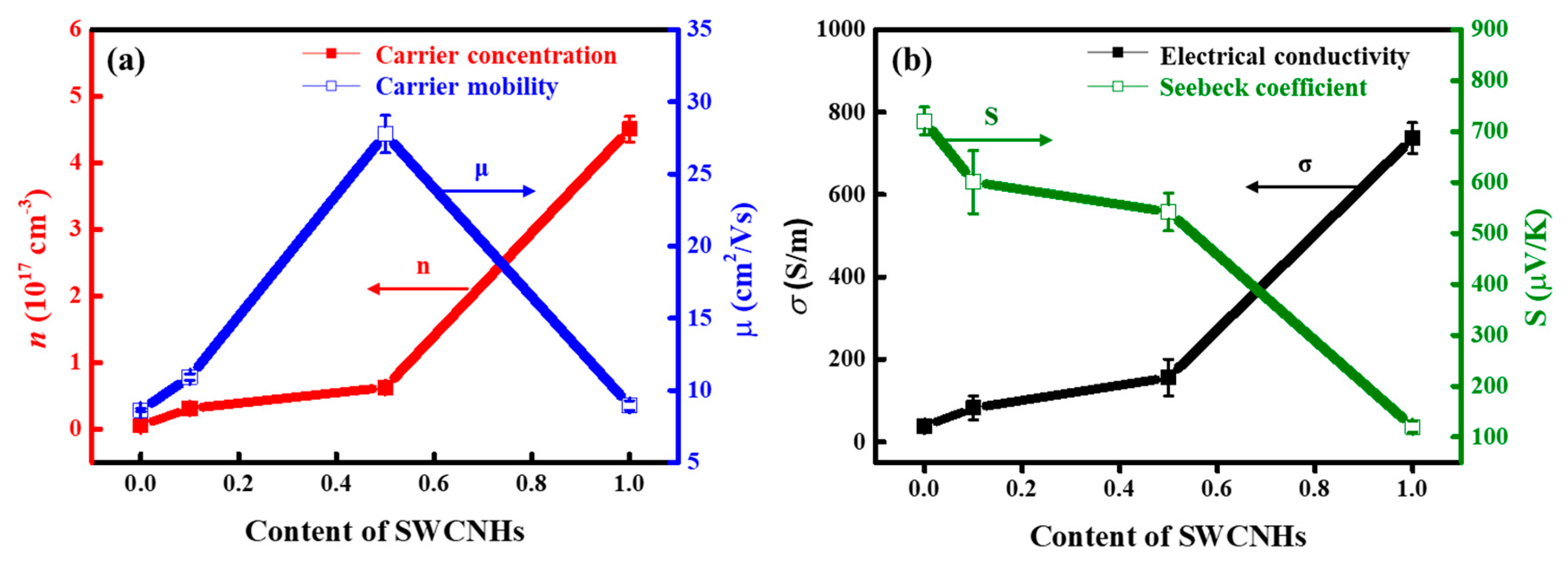
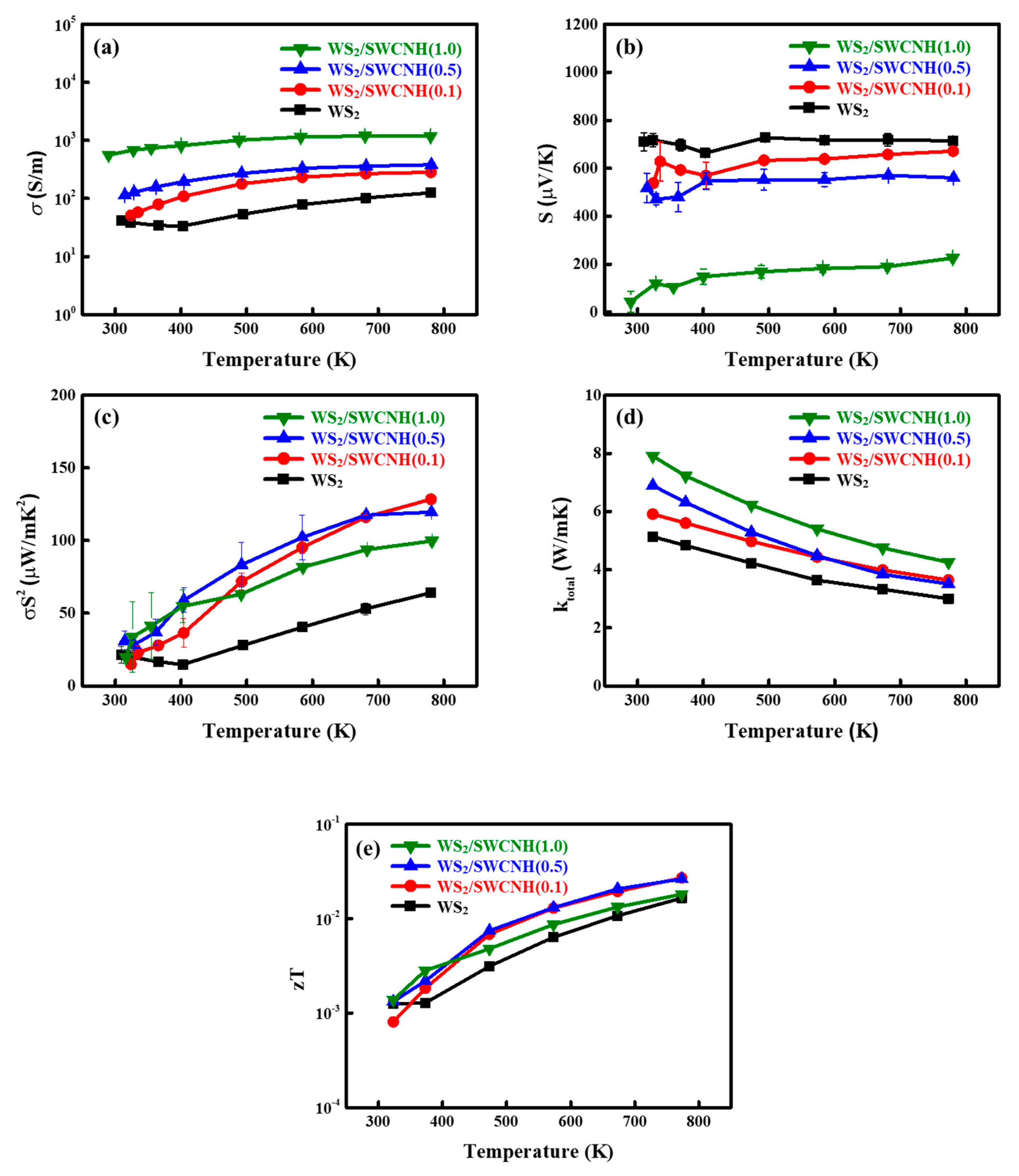
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bell, L.E. Cooling, Heating, generating power, and recovering waste heat with thermoelectric systems. Science 2008, 321, 1457–1461. [Google Scholar] [CrossRef] [PubMed]
- Omer, A.M. Energy, environment and sustainable development. Renew. Sustain. Energy Rev. 2008, 12, 2265–2300. [Google Scholar] [CrossRef]
- Glenk, G.; Stefan, R. Economics of converting renewable power to hydrogen. Nat. Energy 2019, 4, 216–222. [Google Scholar] [CrossRef]
- Harman, T.C.; Taylar, P.J.; Walsh, M.P.; LaForge, B.E. Quantum dot superlattice thermoelectric materials and devices. Science 2002, 297, 2229–2232. [Google Scholar] [CrossRef]
- Zheng, G.; Su, X.L.; Li, X.R.; Liang, T.; Xie, H.Y.; She, X.Y.; Yan, Y.G.; Uher, C.; Kanatzidis, M.G.; Tang, X.F. Toward high-thermoelectric-performance large-size nanostructured BiSbTe alloys via optimization of sintering-temperature distribution. Adv. Energy Mater. 2016, 6, 1600595. [Google Scholar] [CrossRef]
- Pei, Y.Z.; LaLonde, A.; Iwanaga, S.; Snyder, G.J. High thermoelectric figure of merit in heavy hole dominated PbTe. Energy Environ. Sci. 2011, 4, 2085–2089. [Google Scholar] [CrossRef]
- Heremans, J.P.; Jovovic, V.; Toberer, E.S.; Saramat, A.; Kurosaki, K.; Charoenphakdee, A.; Yamanaka, S.; Snyder, G.J. Enhancement of thermoelectric efficiency in PbTe by distortion of the electronic density of states. Science 2008, 321, 554–557. [Google Scholar] [CrossRef]
- Coleman, J.N.; Lotya, M.; O’Neill, A.; Bergin, S.D.; King, P.J.; Khan, U.; Young, K.; Gaucher, A.; De, S.; Smith, R.J.; et al. Two-dimensional nanosheets produced by liquid exfoliation of layered materials. Science 2011, 331, 568–571. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Choi, S.-M.; Seo, W.-S.; Cho, W.-S. Thermal and electronic properties of exfoliated metal chalcogenides. Bull. Korean Chem. Soc. 2010, 31, 3225–3227. [Google Scholar] [CrossRef]
- Solanki, G.K.; Gujarathi, D.N.; Deshpande, M.P.; Lakshminarayana, D.; Agarwal, M.K. Transport property measurements in tungsten sulphoselenide single crystals grown by a CVT technique. Cryst. Res. Technol. 2008, 43, 179–185. [Google Scholar] [CrossRef]
- Huang, Z.; Wu, T.; Kong, S.; Meng, Q.-L.; Zhuang, W.; Jiang, P.; Bao, X. Enhancement of anisotropic thermoelectric performance of tungsten disulfide by titanium doping. J. Mater. Chem. A 2016, 4, 10159–10165. [Google Scholar] [CrossRef]
- Georgiou, T.; Jalil, R.; Belle, B.D.; Britnell, L.; Gorbachev, R.V.; Morozov, S.V.; Kim, Y.J.; Gholinia, A.; Haigh, S.J.; Makarovsky, O.; et al. Vertical field-effect transistor based on graphene-WS2 heterostructures for flexible and transparent electronics. Nat. Nanotechnol. 2013, 8, 100–103. [Google Scholar] [CrossRef]
- Iijima, S.; Yudasaka, M.; Yamada, R.; Bandow, S.; Suenaga, K.; Kokai, F.; Takahashi, K. Nano-aggreagtes of single-walled graphitic carbon nano-horns. Chem. Phys. Lett. 1999, 309, 165–170. [Google Scholar] [CrossRef]
- Hwang, H.; Kim, C.H.; Wee, J.-H.; Han, J.H.; Yang, C.-M. High-density graphene/single-walled carbon nanohorn composite supercapacitor electrode with high volumetric capacitance. Appl. Surf. Sci. 2019, 489, 708–716. [Google Scholar] [CrossRef]
- Jung, H.J.; Kim, Y.-J.; Han, J.H.; Yudasaka, M.; Iijima, S.; Kanoh, H.; Kim, Y.A.; Kaneko, K.; Yang, C.-M. Thermal-treatment-induced enhancement in effective surface area of single-walled carbon nanohorns for supercapacitor application. J. Phys. Chem. C 2013, 117, 25877–25883. [Google Scholar] [CrossRef]
- Yang, C.-M.; Kim, Y.-J.; Endo, M.; Kanoh, H.; Yudasaka, M.; Iijima, S.; Kaneko, K. Nanowindow-regulated specific capacitance of supercapacitor electrodes of single-wall carbon nanohorns. J. Am. Chem. Soc. 2007, 129, 20–21. [Google Scholar] [CrossRef]
- Yang, C.-M.; Noguchi, H.; Murata, K.; Yudasaka, M.; Hashimoto, A.; Iijima, S.; Kaneko, K. Highly ultramicroporous single-walled carbon nanohorn assemblies. Adv. Mater. 2005, 17, 866–870. [Google Scholar] [CrossRef]
- Khasimsaheb, B.; Singh, N.K.; Bathula, S.; Gahtori, B.; Haranath, D.; Neeleshwar, S. The effect of carbon nanotubes (CNT) on thermoelectric properties of lead telluride (PbTe) nanocubes. Curr. Appl. Phys. 2017, 17, 306–313. [Google Scholar] [CrossRef]
- Ren, F.; Wang, H.; Menchhofer, P.A.; Kiggans, J.O. Thermoelectric and mechanical properties of multi-walled carbon nanotube doped Bi0.4Sb1.6Te3 thermoelectric material. Appl. Phys. Lett. 2013, 103, 221907. [Google Scholar] [CrossRef]
- Zhou, H.; Yu, F.; Sun, J.; He, R.; Wang, Y.; Guo, C.F.; Wang, F.; Lan, Y.; Ren, Z.; Chen, S. Highly active and durable self-standing WS2/graphene hybrid catalysts for hydrogen evolution reaction. J. Mater. Chem. A 2016, 4, 9472–9476. [Google Scholar] [CrossRef]
- Lim, Y.V.; Wang, Y.; Kong, D.; Guo, L.; Wong, J.I.; Ang, L.K.; Yang, H.Y. Cubic-shaped WS2 nanopetals on a Prussian blue derived nitrogen-doped carbon nanoporous framework for high performance sodium-ion batteries. J. Mater. Chem. A 2017, 5, 10406–10415. [Google Scholar] [CrossRef]
- Sharma, S.; Bhagat, S.; Singh, J.; Singh, R.C.; Sharma, S. Excitation-dependent photoluminescence from WS2 nanostructures synthesized via top-down approach. J. Mater. Sci. 2017, 52, 11326–11336. [Google Scholar] [CrossRef]
- Biswas, K.; He, J.; Zhang, Q.; Wang, G.; Uher, C.; Dravid, V.P.; Kanatzidis, M.G. Strained endotaxial nanostructures with high thermoelectric figure of merit. Nat. Chem. 2011, 3, 160–166. [Google Scholar] [CrossRef]
- Bera, R.; Karan, S.K.; Das, A.K.; Paria, S.; Khatua, B.B. Single wall carbon nanohorn (SWCNH)/graphene nanoplate/poly(methyl methacrylate) nanocomposites: a promising material for electromagnetic interference shielding applications. RSC Adv. 2015, 5, 70482–70493. [Google Scholar] [CrossRef]
- Zevalkink, A.; Toberer, E.S.; Zeier, W.G.; Flage-Larsen, E.; Snyder, G.J. Ca3AlSb3: an inexpensive, non-toxic thermoelectric material for waste heat recovery. Energy Environ. Sci. 2011, 4, 510–518. [Google Scholar] [CrossRef]
- Freedman, J.P.; Leach, J.H.; Preble, E.A.; Sitar, Z.; Davis, R.F.; Malen, J.A. Universal phonon mean free path spectra in crystalline semiconductors at high temperature. Sci. Rep. 2013, 3, 2963. [Google Scholar] [CrossRef]
- Selvam, C.; Harish, S.; Lal, D.M. Effective thermal conductivity and rheological characteristics of ethylene glycol-based nanofluids with single-walled carbon nanohorn inclusions. Fullerenes Nanotubes Carbon Nanostruct. 2017, 25, 86–93. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.H.; Yu, S.; Lee, S.W.; Lee, S.-Y.; Kim, K.S.; Kim, Y.A.; Yang, C.-M. Enhanced Thermoelectric Properties of WS2/Single-Walled Carbon Nanohorn Nanocomposites. Crystals 2020, 10, 140. https://doi.org/10.3390/cryst10020140
Kim JH, Yu S, Lee SW, Lee S-Y, Kim KS, Kim YA, Yang C-M. Enhanced Thermoelectric Properties of WS2/Single-Walled Carbon Nanohorn Nanocomposites. Crystals. 2020; 10(2):140. https://doi.org/10.3390/cryst10020140
Chicago/Turabian StyleKim, Ji Hoon, Seunggun Yu, Sang Won Lee, Seung-Yong Lee, Keun Soo Kim, Yoong Ahm Kim, and Cheol-Min Yang. 2020. "Enhanced Thermoelectric Properties of WS2/Single-Walled Carbon Nanohorn Nanocomposites" Crystals 10, no. 2: 140. https://doi.org/10.3390/cryst10020140
APA StyleKim, J. H., Yu, S., Lee, S. W., Lee, S.-Y., Kim, K. S., Kim, Y. A., & Yang, C.-M. (2020). Enhanced Thermoelectric Properties of WS2/Single-Walled Carbon Nanohorn Nanocomposites. Crystals, 10(2), 140. https://doi.org/10.3390/cryst10020140





