A Thermal and Nanomechanical Study of Molecular Crystals as Versatile Mocks for Pentaerythritol Tetranitrate
Abstract
:1. Introduction
2. Materials and Methods
3. Results
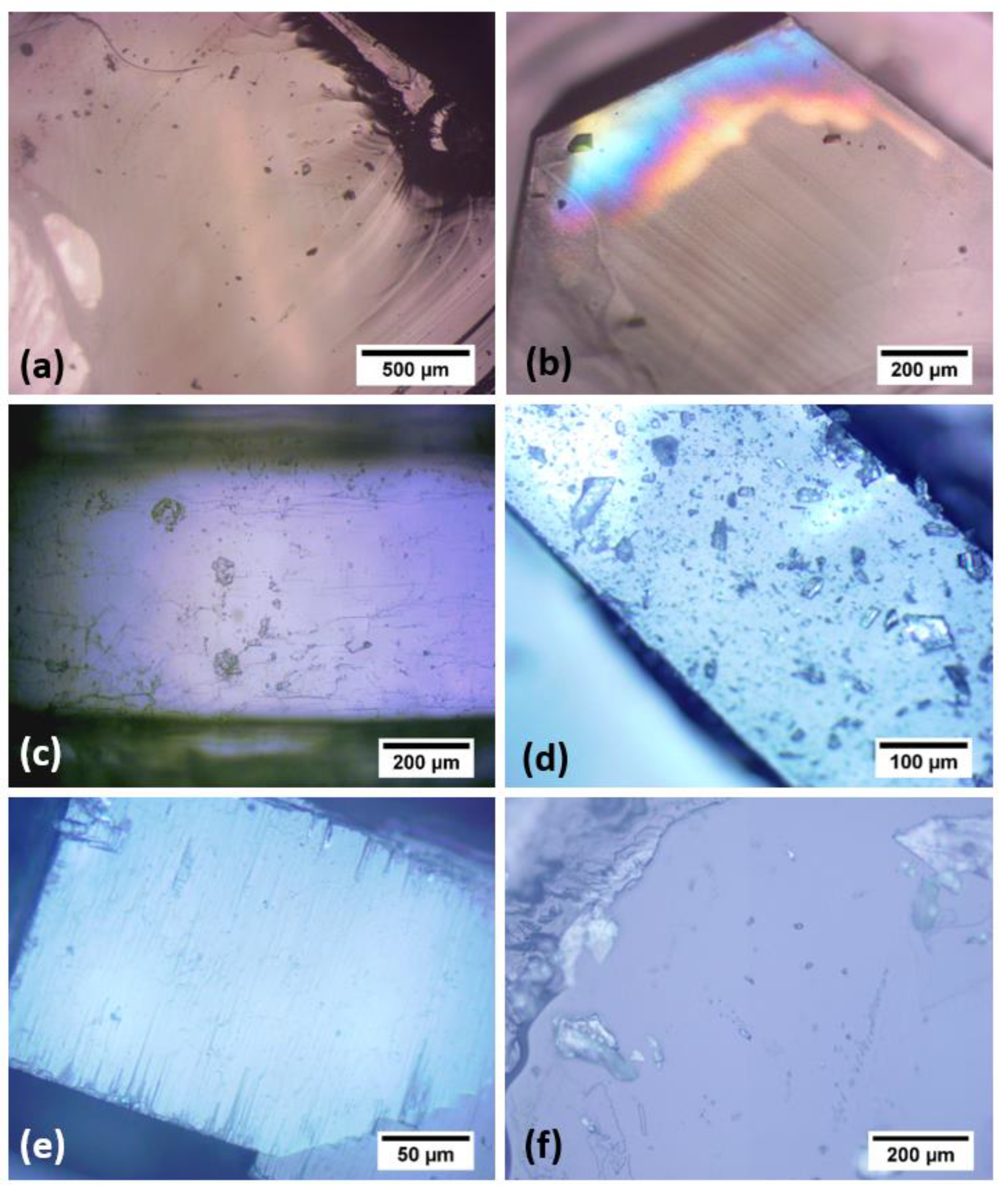
3.1. Material Selection
3.2. Thermal Characterization
3.3. Hardness and Modulus Measurements
3.4. Incipient Plasticity and Fracture Behavior
3.5. Assessment of Experimental Uncertainty due to Crystal Properties and Anisotropy.
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Matyáš, R.; Šelešovský, J.; Musil, T. Sensitivity to friction for primary explosives. J. Hazard. Mater. 2012, 213, 236–241. [Google Scholar] [CrossRef]
- Gibbs, T.R.; Popolato, A. LASL Explosive Property Data; University of California: Berkeley, CA, USA, 1980. [Google Scholar]
- Kubas, G.; Rees, W.; Caguiat, J.; Asch, D.; Fagan, D.; Cortes, P. Identification of peptide sequences that selectively bind to pentaerythritol trinitrate hemisuccinate-a surrogate of PETN, via phage display technology. Pept. Sci. 2017, 108, e22997. [Google Scholar] [CrossRef] [PubMed]
- Konek, C.; Wilkinson, J.; Esenturk, O.; Heilweil, E.; Kemp, M. Terahertz spectroscopy of explosives and simulants: RDX, PETN, sugar, and L-tartaric acid. In Proceedings of the Terahertz Physics, Devices, and Systems III: Advanced Applications in Industry and Defense, Orlando, FL, USA, 30 April 2009; Volume 7311, p. 73110K. [Google Scholar]
- Carter, J.C.; Angel, S.M.; Lawrence-Snyder, M.; Scaffidi, J.; Whipple, R.E.; Reynolds, J.G. Standoff detection of high explosive materials at 50 meters in ambient light conditions using a small Raman instrument. Appl. Spectrosc. 2005, 59, 769–775. [Google Scholar] [CrossRef] [PubMed]
- Yeager, J.D.; Duque, A.L.H.; Shorty, M.; Bowden, P.R.; Stull, J.A. Development of inert density mock materials for HMX. J. Energy Mater. 2018, 36, 253–265. [Google Scholar] [CrossRef]
- Williams, J.H.G.; McCool, A.A. Development of a Mechanically Matched Simulant for AFX-808: AFS-808; Air Force Research Laboratory: Montgomery County, OH, USA, 2014. [Google Scholar]
- Ahmido, T.; Ting, A.; Misra, P. Femtosecond laser-induced breakdown spectroscopy of surface nitrate chemicals. Appl. Opt. 2013, 52, 3048–3057. [Google Scholar] [CrossRef]
- Simpson, R.L.; Pruneda, C.O. Non-Detonable and Non-Explosive Explosive Simulators. U.S. Patent 5,648,636, 15 July 1997. [Google Scholar]
- Dobratz, B.M. Properties of Chemical Explosives and Explosive Simulants; University of California: Berkeley, CA, USA; Lawrence Livermore Lab.: Livermore, CA, USA, 1972. [Google Scholar]
- Liu, C.; Rae, P.J.; Cady, C.M.; Lovato, M.L. Damage & fracture of high-explosive mock subject to cyclic loading. In Mechanics of Time-Dependent Materials and Processes in Conventional and Multifunctional Materials; Springer: Berlin, Germany, 2011; Volume 3, pp. 151–157. [Google Scholar]
- Sheffield, S.A.; Gustavsen, R.L.; Alcon, R.R. Porous HMX initiation studies-sugar as an inert simulant. AIP Conf. Proc. 1998, 429, 575–578. [Google Scholar]
- Taw, M.R.; Yeager, J.D.; Hooks, D.E.; Carvajal, T.M.; Bahr, D.F. The mechanical properties of as-grown noncubic organic molecular crystals assessed by nanoindentation. J. Mater. Res. 2017, 32, 2728–2737. [Google Scholar] [CrossRef]
- Taw, M.R.; Bahr, D.F. The Mechanical Properties of Minimally Processed RDX. Propellants Explos. Pyrotech. 2017, 42, 659–664. [Google Scholar] [CrossRef]
- Burch, A.; Yeager, J.; Bahr, D. Nanoindentation of HMX and Idoxuridine to Determine Mechanical Similarity. Crystals 2017, 7, 335. [Google Scholar] [CrossRef] [Green Version]
- Ramos, K.J.; Hooks, D.E.; Bahr, D.F. Direct observation of plasticity and quantitative hardness measurements in single crystal cyclotrimethylene trinitramine by nanoindentation. Philos. Mag. 2009, 89, 2381–2402. [Google Scholar] [CrossRef]
- Mannepalli, S.; Mangalampalli, K. Indentation Plasticity and Fracture Studies of Organic Crystals. Crystals 2017, 7, 324. [Google Scholar] [CrossRef] [Green Version]
- Zhai, M.; McKenna, G.B. Mechanical properties of pentaerythritol tetranitrate(PETN) single crystals from nano-indentation: Depth dependent response at the nano meter scale. Cryst. Res. Technol. 2016, 51, 414–427. [Google Scholar] [CrossRef]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge structural database. Acta Crystallogr. Sect. B 2016, 72, 171–179. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Matsumura, G.; Haga, N.; Shudo, K. Structure of N, N-bis (2,3,4,5,6-pentafluorophenyl) oxamide. Acta Crystallogr. Sect. C 1992, 48, 558–559. [Google Scholar] [CrossRef]
- Maughan, M.R.; Carvajal, M.T.; Bahr, D.F. Nanomechanical testing technique for millimeter-sized and smaller molecular crystals. Int. J. Pharm. 2015, 486, 324–330. [Google Scholar] [CrossRef]
- Oliver, W.C.; Pharr, G.M. An improved technique for determining harness and elastic modulus using load and displacement sensing indentation experiments. J. Mater. Res. 1992, 7, 1564–1583. [Google Scholar] [CrossRef]
- Morris, D.J.; Myers, S.B.; Cook, R.F. Sharp probes of varying acuity: Instrumented indentation and fracture behavior. J. Mater. Res. 2004, 19, 165–175. [Google Scholar] [CrossRef]
- Burch, A.C.; Yeager, J.D.; Bahr, D.F. Indentation fracture behavior of energetic and inert molecular crystals. J. Mater. Res. 2019, 34, 3954–3963. [Google Scholar] [CrossRef]
- Yeager, J.D.; Woznick, C.S.; Thompson, D.G.; Duque, A.L.; Liu, C.; Burch, A.C.; Bowden, P.R.; Shorty, M.; Bahr, D.F. Development of a new density and mechanical mock for HMX. In Proceedings of the Sixteenth International Detonation Symposium, Cambridge, MD, USA, 16 July 2018; p. 1631. [Google Scholar]
- Shimada, A. Crystal and molecular structure of mesoerythritol. Acta Crystallogr. 1958, 11, 748–749. [Google Scholar] [CrossRef]
- Manner, V.W.; Tappan, B.C.; Scott, B.L.; Preston, D.N.; Brown, G.W. Crystal structure, packing analysis, and structural-sensitivity correlations of erythritol tetranitrate. Cryst. Growth Des. 2014, 14, 6154–6160. [Google Scholar] [CrossRef]
- Cady, H.H.; Larson, A.C. Pentaerythritol tetranitrate II: Its crystal structure and transformation to PETN I; an algorithm for refinement of crystal structures with poor data. Acta Crystallogr. Sect. B 1975, 31, 1864–1869. [Google Scholar] [CrossRef]
- Betz, R.; Gerber, T. 2,4,6-Trifluorobenzoic acid. Acta Crystallogr. Sect. E 2011, 67, o539. [Google Scholar] [CrossRef] [Green Version]
- Shimada, A. Crystal structure and lattice energy of i-erythritol. i. crystal structure of i-erythritol. Bull. Chem. Soc. Jpn. 1959, 32, 325–329. [Google Scholar] [CrossRef] [Green Version]
- Bouwman, E.; Caulton, K.G.; Christou, G.; Folting, K.; Gasser, C.; Hendrickson, D.N.; Huffman, J.C.; Lobkovsky, E.B.; Martin, J.D. Doubly-hydrated hexafluoroacetylacetone as a tetradentate ligand: Synthesis, magnetochemistry, and thermal transformations of a dimanganese (III) complex. Inorg. Chem. 1993, 32, 3463–3470. [Google Scholar] [CrossRef]
- Gdaniec, M. 2,3,4,5,6-Pentafluorobenzamide. Acta Crystallogr. Sect. E Struct. 2003, 59, o1642–o1644. [Google Scholar] [CrossRef]
- Maiti, A.; Gee, R.H. PETN Coarsening–Predictions from Accelerated Aging Data. Propellants Explos. Pyrotech. 2011, 36, 125–130. [Google Scholar] [CrossRef]
- Maiti, A.; Han, Y.; Zaka, F.; Gee, R.H. In-situ Monitoring of Flow-Permeable Surface Area of High Explosive Powder using Small Sample Masses. Propellants Explos. Pyrotech. 2015, 40, 419–425. [Google Scholar] [CrossRef]
- Ramos, K.J.; Bahr, D.F.; Hooks, D.E. Defect and surface asperity dependent yield during contact loading of an organic molecular single crystal. Philos. Mag. 2011, 91, 1276–1285. [Google Scholar] [CrossRef]
- Bahr, D.F.; Kramer, D.E.; Gerberich, W.W. Non-linear deformation mechanisms during nanoindentation. Acta Mater. 1998, 46, 3605–3617. [Google Scholar] [CrossRef]
- Mann, A.B.; Pethica, J.B. The role of atomic size asperities in the mechanical deformation of nanocontacts. Appl. Phys. Lett. 1996, 69, 907–909. [Google Scholar] [CrossRef]
- Monroe, D.C. (U) Historical PETN Pressing Data: 1965–1978; Los Alamos National Lab. (LANL): Los Alamos, NM, USA, 2015. [Google Scholar]
- Ramos, K.J.; Bahr, D.F. Mechanical behavior assessment of sucrose using nanoindentation. J. Mater. Res. 2007, 22, 2037–2045. [Google Scholar] [CrossRef]
- Zhou, X.; Lu, Z.; Zhang, Q.; Chen, D.; Li, H.; Nie, F.; Zhang, C. Mechanical anisotropy of the energetic crystal of 1,1-diamino-2,2-dinitroethylene (FOX-7): A study by nanoindentation experiments and density functional theory calculations. J. Phys. Chem. C 2016, 120, 13434–13442. [Google Scholar] [CrossRef]
- Cook, R.F.; Pharr, G.M. Direct Observation and Analysis of Indentation Cracking in Glasses and Ceramics. J. Am. Ceram. Soc. 1990, 73, 787–817. [Google Scholar] [CrossRef]










| Material | Density (g/cm3) | Crystal Structure |
|---|---|---|
| PETN | 1.778 | Tetragonal [28] |
| 246 TFBA | 1.759 | Monoclinic [29] |
| Erythritol | 1.452 | Tetragonal [30] |
| HFPT | 1.941 | Monoclinic [31] |
| N-BPFPO | 1.932 | Monoclinic [20] |
| PFBA | 1.884 | Monoclinic [32] |
| Material | Melt Temperature (°C) |
|---|---|
| PETN | 141 |
| 246 TFBA | 143 |
| Erythritol | 119 |
| HFPT | 93 |
| N-BPFPO | 235 |
| PFBA | 147 |
| Material | Elastic Modulus (GPa) | Hardness (GPa) |
|---|---|---|
| PETN | 15.2 ± 2.4 | 0.45 ± 0.08 |
| 246 TFBA | 19.2 ± 2.6 | 0.54 ± 0.18 |
| Erythritol | 21.2 ± 2.4 | 1.22 ± 0.13 |
| HFPT | 6.3 ± 0.4 | 0.33 ± 0.07 |
| N-BPFPO | 17.6 ± 0.4 | 0.44 ± 0.07 |
| PFBA | 5.8 ± 1.5 | 0.24 ± 0.03 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burch, A.C.; Wilde, Z.R.; Bahr, D.F.; Yeager, J.D. A Thermal and Nanomechanical Study of Molecular Crystals as Versatile Mocks for Pentaerythritol Tetranitrate. Crystals 2020, 10, 126. https://doi.org/10.3390/cryst10020126
Burch AC, Wilde ZR, Bahr DF, Yeager JD. A Thermal and Nanomechanical Study of Molecular Crystals as Versatile Mocks for Pentaerythritol Tetranitrate. Crystals. 2020; 10(2):126. https://doi.org/10.3390/cryst10020126
Chicago/Turabian StyleBurch, Alexandra C., Zakary R. Wilde, David F. Bahr, and John D. Yeager. 2020. "A Thermal and Nanomechanical Study of Molecular Crystals as Versatile Mocks for Pentaerythritol Tetranitrate" Crystals 10, no. 2: 126. https://doi.org/10.3390/cryst10020126
APA StyleBurch, A. C., Wilde, Z. R., Bahr, D. F., & Yeager, J. D. (2020). A Thermal and Nanomechanical Study of Molecular Crystals as Versatile Mocks for Pentaerythritol Tetranitrate. Crystals, 10(2), 126. https://doi.org/10.3390/cryst10020126





