Crystal Structure, Synthesis and Luminescence Sensing of a Zn(II) Coordination Polymer with 2,5-Dihydroxy-1,4-Terephthalic Acid and 2,2′-Bipyridine as Ligands
Abstract
1. Introduction
2. Experimental
2.1. Materials and Method
2.2. Characterization
2.3. Synthesis of [Zn(Bpy)(DHTA)0.5]n
2.4. Luminescence Sensing Experiments
2.5. X-ray Crystallography
3. Results and Discussions
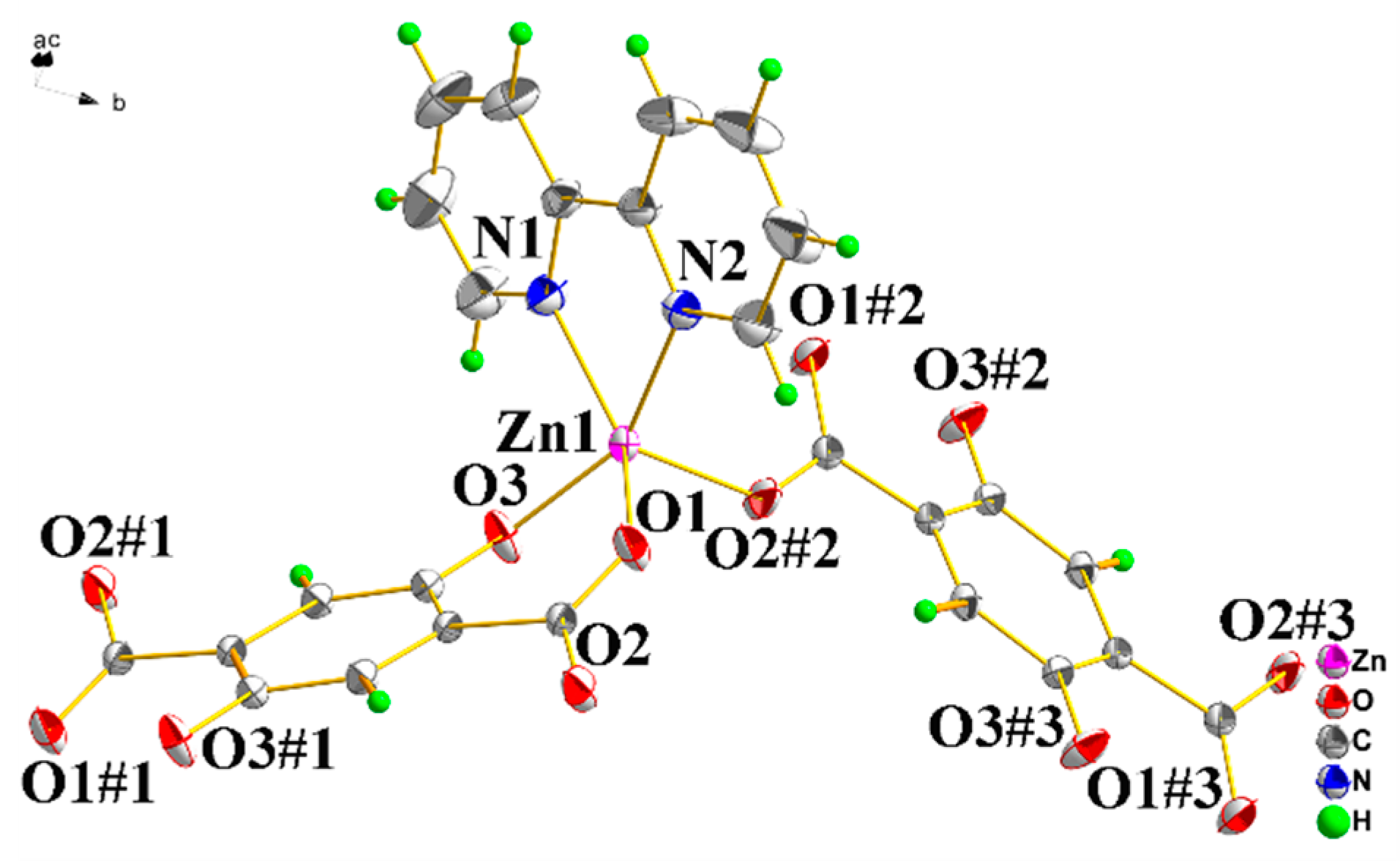
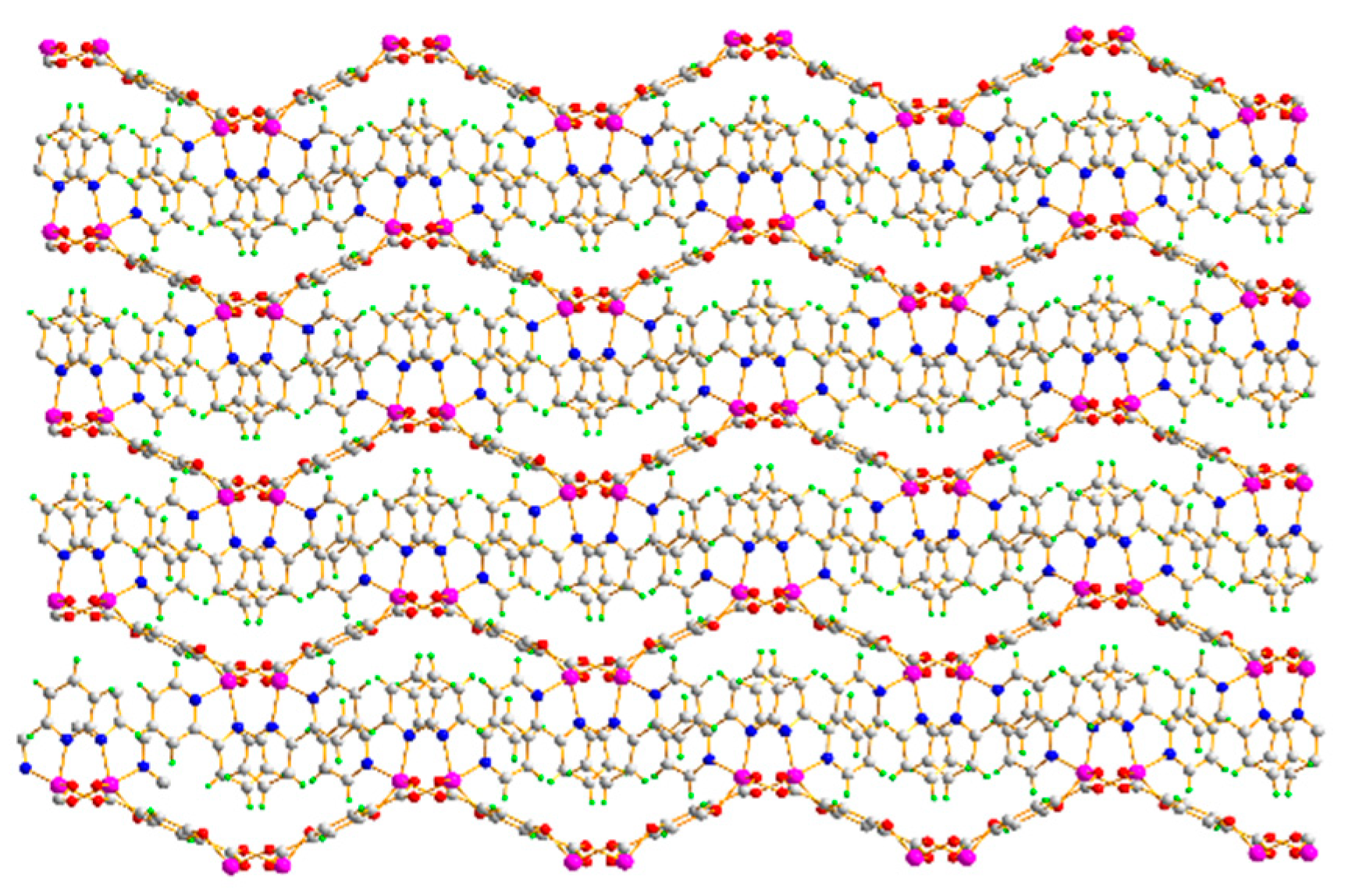
3.1. Structure Description
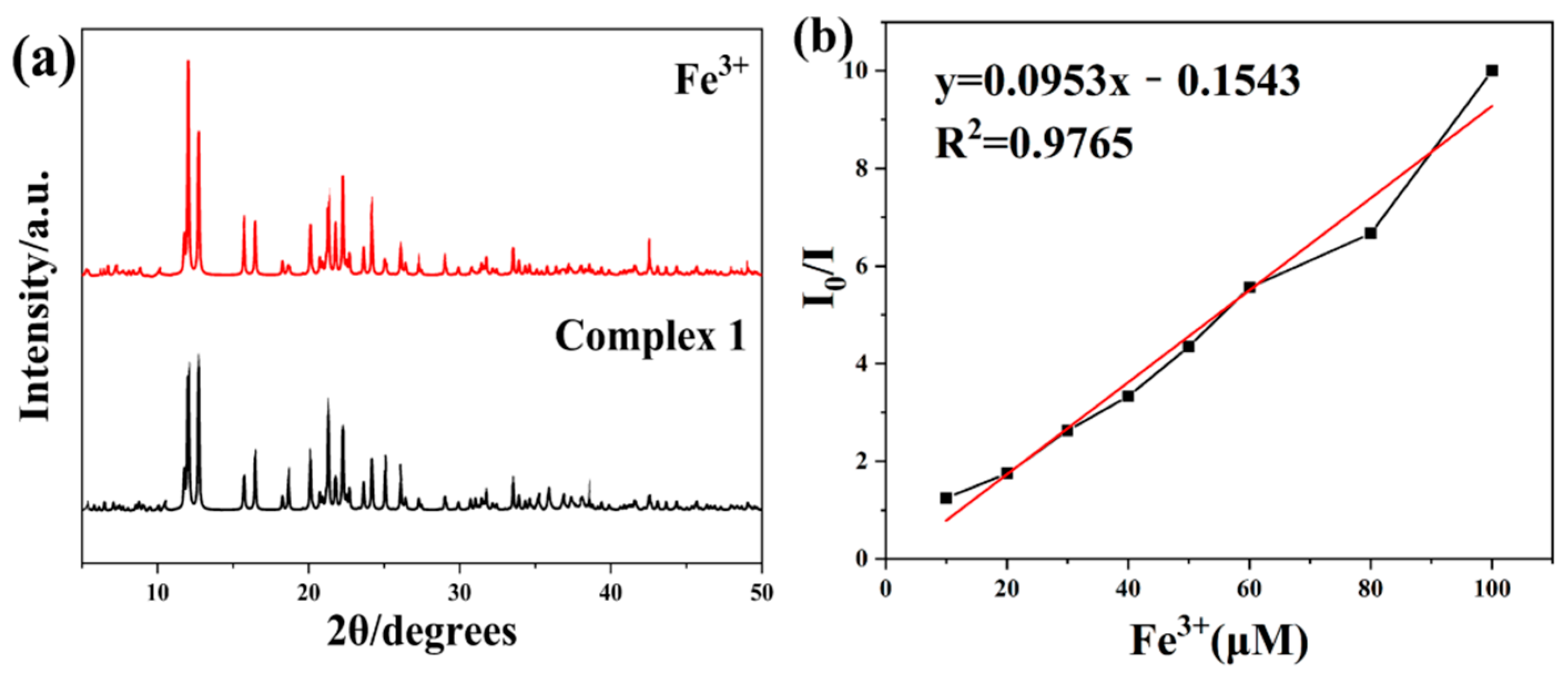
3.2. Powder X-ray Diffraction (PXRD) Analysis
3.3. Stability Properties of the Complex
3.4. Luminescent Property of the Complex with High Sensitivity and Selectivity for Acetone (DMK) and Fe3+
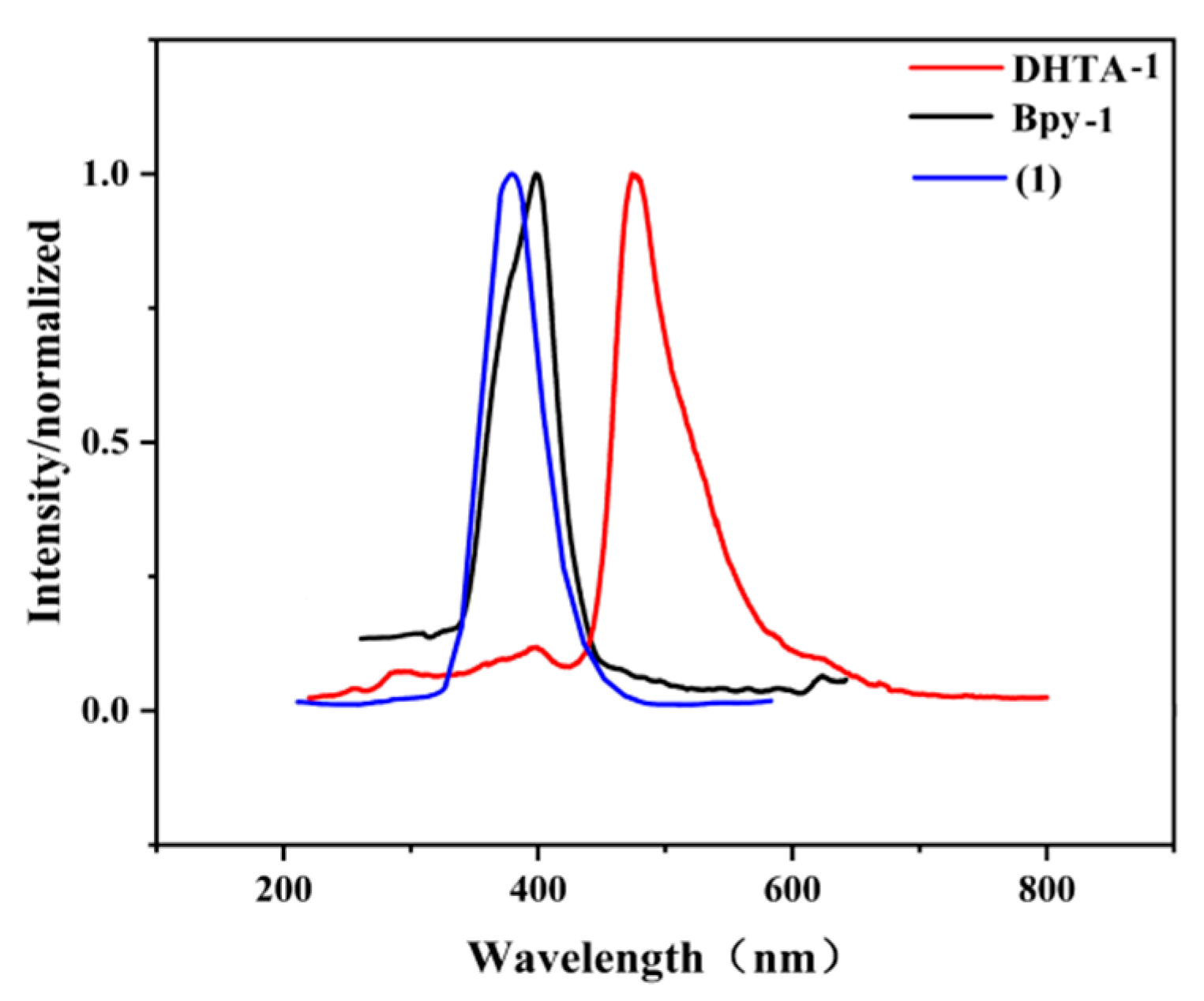
3.4.1. Luminescent Property of the Complex
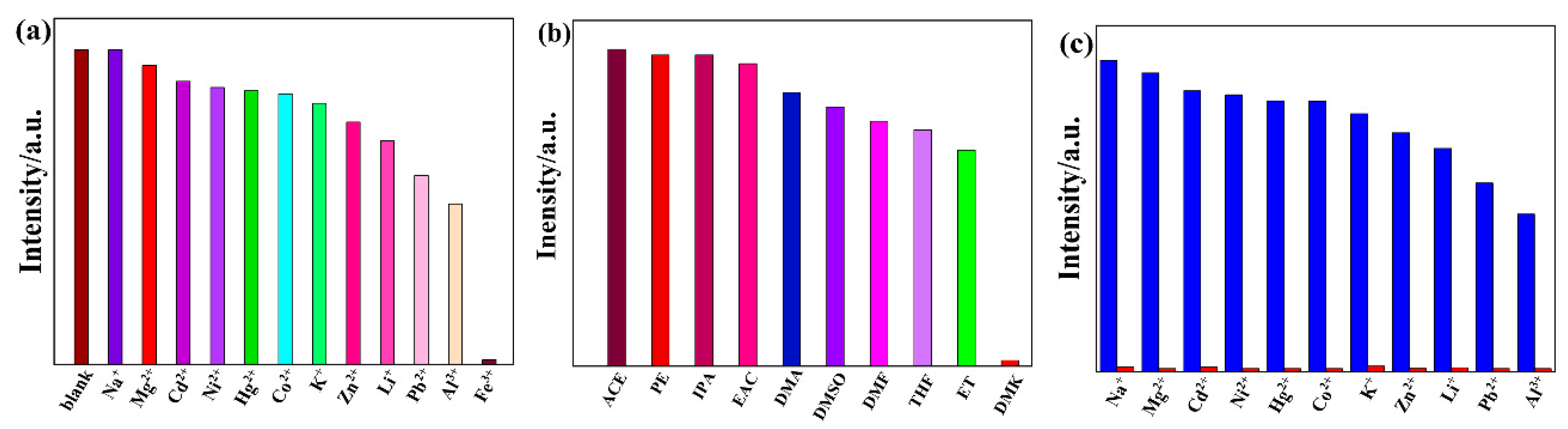
3.4.2. High Sensitivity and Selectivity for Fe3+
3.4.3. High Sensitivity for DMK
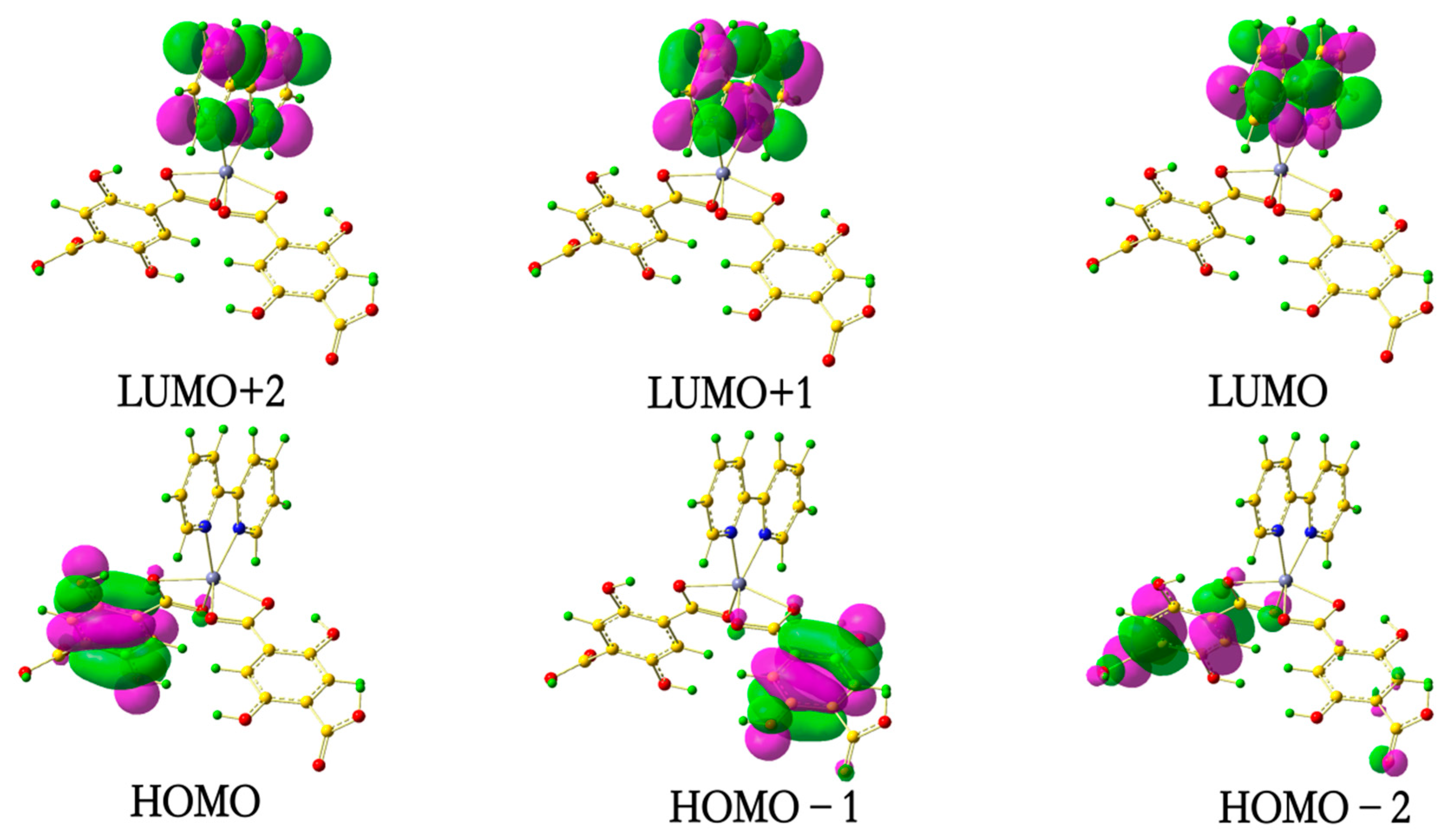
3.5. Theoretical Calculation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fromm, K.M.; Batten, V.S.S.; Neville, S.M.; Turner, D.R. Coordination polymers design, analysis and application. Angew. Chem. 2009, 121, 4986–4987. [Google Scholar] [CrossRef]
- Rowsell, J.L.C.; Yaghi, O.M. Metal–organic frameworks: A new class of porous materials. Microporous Mesoporous Mater. 2004, 73, 3–14. [Google Scholar] [CrossRef]
- Long, J.R.; Yaghi, O.M. The pervasive chemistry of metal–organic frameworks. Chem. Soc. Rev. 2009, 38, 1213–1214. [Google Scholar] [CrossRef]
- Heine, J.; Müller-Buschbaum, K. Engineering metal-based luminescence in coordination polymers and metal–organic frameworks. Chem. Soc. Rev. 2013, 42, 9232–9242. [Google Scholar] [CrossRef]
- Koen, B. Lanthanide-based luminescent hybrid materials. Chem. Rev. 2009, 109, 4283–4374. [Google Scholar]
- Chen, L.; Ye, J.W.; Wang, H.P.; Pan, M.; Yin, S.Y.; Zhang, W.W.; Zhang, L.Y.; Wu, K. Ultrafast water sensing and thermal imaging by a metal-organic framework with switchable luminescence. Nat. Commun. 2017, 8, 15985–15994. [Google Scholar] [CrossRef]
- Scheurle, P.I.; Mähringer, A.; Jakowetz, A.C.; Hosseini, P.; Richter, A.F.; Wittstock, G.; Medina, D.D.; Bein, T. A highly crystalline anthracene-based MOF-74 series featuring electrical conductivity and luminescence. Chem. Sci. 2020, 10, 1039–1043. [Google Scholar] [CrossRef]
- Mark, D.A.; Ronald, J.T.H.; Leanne, A.; Talin, A.A.; Pikarsky, J.; Choudhury, A.; Gall, K.A.; Hesketh, P.J. Stress-induced chemical detection using flexible metal−organic frameworks. J. Am. Chem. Soc. 2008, 130, 14404–14405. [Google Scholar]
- Chen, B.L.; Wang, L.B.; Xiao, Y.; Fronczek, F.R.; Xue, M.; Cui, Y.; Qian, G.D. A luminescent metal-organic framework with lewis basic pyridyl sites for the sensing of metal ions. Angew. Chem. Int. Ed. 2009, 48, 508–511. [Google Scholar] [CrossRef]
- Lan, A.J.; Li, K.H.; Wu, H.H.; Olson, D.H.; Thomas, J.E.; Woosoek, K.C.; Mao, H.; Li, J. A luminescent microporous metal–organic framework for the fast and reversible detection of high explosives. J. Angew. Chem. Int. Ed. 2009, 48, 2370–2374. [Google Scholar] [CrossRef]
- Xie, Z.G.; Ma, L.Q.; DeKrafft, K.E.; Athena, J.; Lin, W.B. Porous phosphorescent coordination polymers for oxygen sensing. J. Am. Chem. Soc. 2010, 132, 922–923. [Google Scholar] [CrossRef] [PubMed]
- White, K.A.; Chengelis, D.A.; Gogick, K.A.; Stehman, J.; Rosi, N.L.; Petoud, S. Near-infrared luminescent lanthanide MOF barcodes. J. Am. Chem. Soc. 2009, 131, 18069–18071. [Google Scholar] [CrossRef]
- Yan, Y.T.; Zhang, W.Y.; Zhang, F.; Cao, F.; Yang, R.F.; Wang, Y.Y.; Hou, L. Four new metal–organic frameworks based on diverse secondary building units: Sensing and magnetic properties. Dalton Trans. 2018, 47, 1682–1692. [Google Scholar] [CrossRef]
- Chen, Y.Z.; Wang, C.M.; Wu, Z.Y.; Xiong, Y.J.; Xu, Q.; Yu, S.H.; Jiang, H.L. From bimetallic metal-organic framework to porous carbon: High surface area and multicomponent active dopants for excellent electrocatalysis. Adv. Mater. 2015, 27, 5010–5016. [Google Scholar] [CrossRef]
- Ni, D.; Chen, Y.X.; Yang, X.W.; Liu, C.C.; Cai, K.F. Microwave-assisted synthesis method for rapid synthesis of tinselenide electrode material for supercapacitors. J. Alloys Compd. 2018, 737, 623–629. [Google Scholar] [CrossRef]
- Rowsell, J.L.; Yaghi, O.M. Strategies for hydrogen storage in metal–organic frameworks. Angew. Chem. Int. Ed. 2005, 44, 4670–4679. [Google Scholar] [CrossRef]
- Kesanli, B.; Cui, Y.; Smith, M.R.; Bittner, E.W.; Bockrath, B.C.; Lin, W.B. Highly interpenetrated metal–organic frameworks for hydrogen storage. Angew. Chem. Int. Ed. 2005, 117, 74–77. [Google Scholar] [CrossRef]
- Dinca, M.; Long, J.R. Hydrogen storage in microporous metal–organic frameworks with exposed metal sites. Angew. Chem. Int. Ed. 2008, 47, 6766–6779. [Google Scholar] [CrossRef]
- Wu, H.H.; Gong, Q.H.; Olson, D.H.; Li, J. Commensurate adsorption of hydrocarbons and alcohols in microporous metal organic frameworks. Chem. Rev. 2012, 112, 836–868. [Google Scholar] [CrossRef]
- Li, J.R.; Kuppler, R.J.; Zhou, H.C. Selective gas adsorption and separation in metal–organic frameworks. Chem. Soc. Rev. 2009, 38, 1477–1504. [Google Scholar] [CrossRef]
- Zhang, M.L.; Zheng, Y.J.; Liu, M.; Ren, Y.X.; Wang, Z.X.; Cao, J.; Wang, J.J. Two zinc(II) coordination polymers based on flexible co-ligands featuring assembly imparted sensing abilities for Cr2O72- and o-NP. RSC Adv. 2019, 9, 21086–21094. [Google Scholar] [CrossRef]
- Zhang, Y.Q.; Vladislav, A.B.; Zheng, T.R.; Yang, C.H.; Qian, L.L.; Li, K.; Li, B.L.; Zhang, B.W.; Blatov, Y.Q.; Zheng, V.A.; et al. A luminescent zinc(II) coordination polymer with unusual (3,4,4)-coordinated self-catenated 3D network for selective detection of nitroaromatics and ferric and chromate ions: A versatile luminescent sensor. Dalton Trans. 2018, 47, 6189–6198. [Google Scholar] [CrossRef] [PubMed]
- Rosales-Vázquez, L.D.; Valdes-García, J.; Bazany-Rodríguez, I.; Germán-Acacio, J.M.; Martínez-Otero, D.; Vilchis-Néstor, A.; Morales-Luckie, R.; Sánchez-Mendieta, V.; Dorazco-González, A. Sensitive photoluminescent chemosensor for cyanide in water based on a zinc coordination polymer bearing ditert-butyl-bipyridine. Dalton Trans. 2019, 48, 12407–12420. [Google Scholar] [CrossRef]
- Kreno, L.E.; Leong, K.; Farha, O.K.; Allendorf, M.; Van Duyne, R.P.; Hupp, J.T. Metal–organic framework materials as chemical sensors. Chem. Rev. 2012, 112, 1105–1125. [Google Scholar] [CrossRef]
- Que, E.L.; Domaille, D.W.; Chang, C.J. Metals in neurobiology: Probing their chemistry and biology with molecular imaging. Chem. Rev. 2008, 108, 1517–1549. [Google Scholar] [CrossRef]
- Chen, W.M.; Meng, X.L.; Zhuang, G.L.; Wang, Z.; Kurmoo, M.; Zhao, Q.Q.; Wang, X.P.; Shan, B.R.; Tung, C.H.; Sun, D. A superior fluorescent sensor for Al3+ and UO22+ based on a Co(II) metal–organic framework with exposed pyrimidyl Lewis base sites. J. Mater. Chem. A 2017, 5, 13079–13085. [Google Scholar] [CrossRef]
- Carter, K.P.; Young, A.M.; Palmer, A.E. Fluorescent sensors for measuring metal ions in living systems. Chem. Rev. 2014, 114, 4564–4601. [Google Scholar] [CrossRef]
- Xu, H.B.; Zhou, S.H.; Xiao, L.L.; Wang, H.H.; Li, S.Z.; Yuan, Q.H. Fabrication of a nitrogen-doped graphene quantum dot from MOF-derived porous carbon and its application for highly selective fluorescence detection of Fe3+. J. Mater. Chem. C 2015, 3, 291–297. [Google Scholar] [CrossRef]
- Tang, S.F.; Li, L.J.; Lv, X.X.; Wang, C.; Zhao, X.B. Tuning the structure of metal phosphonates using uncoordinating methyl group: Syntheses, structures and properties of a series of metal diphosphonates. CrystEngComm 2014, 16, 7043–7052. [Google Scholar] [CrossRef]
- Zhai, F.; Zheng, Q.; Chen, Z.; Ling, Y.; Liu, X.; Weng, L.; Zhou, Y. Crystal transformation synthesis of a highly stable phosphonate MOF for selective adsorption of CO2. CrystEngComm 2013, 15, 2040–2043. [Google Scholar] [CrossRef]
- Xu, H.; Gao, J.K.; Qian, X.F.; Wang, J.P.; He, H.J.; Cui, Y.J.; Yang, Y.; Wang, Z.Y.; Qian, G.D. Metal–organic framework nanosheets for fast-response and highly sensitive luminescent sensing of Fe3+. J. Mater. Chem. A 2016, 16, 4036–4044. [Google Scholar] [CrossRef]
- Lv, R.; Wang, J.Y.; Zhang, Y.P.; Li, H.; Yang, L.Y.; Liao, S.Y.; Gu, W.; Liu, X. An amino-decorated dual-functional metal–organic framework for highly selective sensing of Cr(III) and Cr(VI) ions and detection of nitroaromatic explosives. J. Mater. Chem. A 2016, 4, 15494–15500. [Google Scholar] [CrossRef]
- Zhu, W.Q.; Fan, P.; Li, Y.; Zhu, Y.M.C.; Zhang, J. The Constuction of Double Ligands Zn (II) MOFs and Its Photoluminescence Properties. Imaging Sci. Photochem. 2020, 38, 159–166. [Google Scholar]
- Sun, H.Y.; Li, X.; Wang, Z.R.; Sun, S.Q.; Li, C.B.; Wang, J.J. Synthesis, Crystal Structure and Theoretical Calculations of Two Zn(II) Coordination Polymers Based on 2,5-Dihydroxyterephthalic Acid. J. Clust. Sci. 2018, 29, 1275–1283. [Google Scholar] [CrossRef]
- Altomare, A.; Burla, M.C.; Camalli, M.; Cascarano, G.; Giacovazzo, C.; Guagliardi, A.; Moliterni, G.G.A.; Polidori, G.; Spagna, R. SIR97: A new tool for crystal structure determination and refinement. J. Appl. Crystallogr. 1999, 32, 115–119. [Google Scholar] [CrossRef]
- Guo, F.B.; Zhu, Y.; Xu, G.L.; Zhang, M.M.; Zhang, X.L.; Zhang, J. Tuning structural topologies of five photoluminescent Cd(II) coordination polymers through modifying the substitute group of organic ligand. J. Solid. State Chem. 2013, 199, 42–48. [Google Scholar] [CrossRef]
- Man, Z.M.; Guo, F. Coordination polymers constructed from an asymmetric biphenyl-dicarboxylate and N-donor ligands: Structures and photoluminescent properties. J. Coord. Chem. 2013, 66, 1–8. [Google Scholar] [CrossRef]
- Xiang, J.; Luo, Y.; Zhao, L.L.; Wang, C.H.; Wu, J.S. Ancillary ligands assisted self-assembly of metal organic frameworks: Synthesis, crystal structures and photophysical properties of two Zn(II) complexes containing in-situ formed tetrazole ligands. Inorg. Chem. Commun. 2013, 31, 23–28. [Google Scholar] [CrossRef]
- Wang, X.J.; Liu, Y.H.; Xu, C.Y.; Guo, Q.Q.; Huo, H.W.; Fan, Y.T. Series of Cd(II) metal–organic frameworks based on a flexible tripodal ligand and polycarboxylate acids: Syntheses, structures, and photoluminescent properties. Cryst. Growth Des. 2012, 12, 2435–2444. [Google Scholar] [CrossRef]
- Wang, H.; Han, S.; Wang, J.J.; Dun, L.N.; Zhang, B.S.; Chen, X.; Li, W.F.; Li, C.B. Crystal Structure, Thermal Behavior and Luminescence of a new Manganese(II) coordination polymer constructed with 1, 10-phenanthroline-5, 6-dione and 2, 5-dihydroxyl-1, 4-terephthalic acid. J. Mol. Struct. 2019, 1204, 127466–127479. [Google Scholar] [CrossRef]
- Parmar, B.; Rachuri, Y.; Bisht, K.K.; Laiya, R.; Suresh, E. Mechanochemical and conventional synthesis of Zn(II)/Cd(II) luminescent coordination polymers: Dual sensing probe for selective detection of chromate anions and TNP in aqueous phase. Inorg. Chem. 2017, 56, 2627–2638. [Google Scholar] [CrossRef]
- He, H.M.; Zhang, D.Y.; Guo, F.; Sun, F.X. A versatile microporous Zinc(II) metal–organic framework for selective gas adsorption, cooperative catalysis, and luminescent sensing. Inorg. Chem. 2018, 10, 1021–1030. [Google Scholar] [CrossRef]
- Hu, Z.C.; Deibert, B.J.; Li, J. Luminescent metal–organic frameworks for chemical sensing and explosive detection. Chem. Soc. Rev. 2014, 43, 5815–5840. [Google Scholar] [CrossRef]
- Nagarkar, S.S.; Desai, A.V.; Samanta, P.; Ghosh, S.K. Aqueous phase selective detection of 2,4,6-trinitrophenol using a fluorescent metal–organic framework with a pendant recognition site. Dalton Trans. 2015, 44, 15175–15180. [Google Scholar] [CrossRef]
- Wang, J.; Jiang, M.; Yan, L.; Ren, P.; Meng, J.; Huang, F.; Guo, X.X.; Li, Y.; Wu, P.Y. Multifunctional luminescent Eu(III)-based metal–organic framework for sensing methanol and detection and adsorption of Fe(III) ions in aqueous solution. Inorg. Chem. 2016, 55, 12660–12668. [Google Scholar] [CrossRef]
- Wang, X.; Han, Y.; Han, X.X.; Hou, X.Y.; Wang, J.J.; Fu, F. Highly selective and sensitive detection of Hg2+, Cr2O72-,and nitrobenzene/2,4-dinitrophenol in water via two fluorescent Cd-CPs. New J. Chem. 2018, 42, 19844–19852. [Google Scholar] [CrossRef]
- Pramanik, S.; Zheng, C.; Zhang, X.; Emge, T.J.; Li, J. New microporous metal−organic framework demonstrating unique selectivity for detection of high explosives and aromatic compounds. J. Am. Chem. Soc. 2011, 133, 4153–4155. [Google Scholar] [CrossRef]
- Xu, H.; Liu, F.; Cui, Y.J.; Chen, B.L.; Qian, G.D. A luminescent nanoscale metal–organic framework for sensing of nitroaromatic explosives. Chem. Commun. 2011, 47, 3153–3155. [Google Scholar] [CrossRef]
- Zhang, J.; Zheng, B.; Zhao, T.T.; Li, G.H.; Huo, Q.S.; Liu, Y.L. Topological diversities and luminescent properties of lanthanide metal–organic frameworks based on a tetracarboxylate ligand. Cryst. Growth Des. 2014, 14, 2394–2400. [Google Scholar] [CrossRef]
- Jin, J.C.; Wu, X.R.; Luo, Z.D.; Deng, F.Y.; Liu, J.Q.; Singh, A.; Kumar, A. Luminescent sensing and photocatalytic degradation properties of an uncommon (4,5,5)-connected 3D MOF based on 3,5-di(3′,5′-dicarboxylphenyl)benzoic acid. CrystEngComm 2017, 19, 4368–4377. [Google Scholar] [CrossRef]
- Zhou, J.M.; Shi, W.; Li, H.M.; Li, H.; Cheng, P. Experimental studies and mechanism analysis of high-sensitivity luminescent sensing of pollutional small molecules and ions in Ln4O4 cluster based microporous metal–organic frameworks. J. Phys. Chem. C 2014, 118, 416–426. [Google Scholar] [CrossRef]
- Wu, Y.L.; Yang, G.P.; Zhang, Y.D.; Shi, N.N.; Han, J.; Wang, Y.Y. A new luminescent Cd(II)-MOF as a highly selective chemical probe for Fe3+ in aqueous solution with mixed metal ions. RSC Adv. 2015, 5, 90772–90777. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, H.H.; Yan, B. An Eu3+ post-functionalized nanosized metal–organic framework for cation exchange-based Fe3+-sensing in an aqueous environment. J. Mater. Chem. A 2014, 2, 13691–13697. [Google Scholar] [CrossRef]
- Ju, P.; Zhang, E.S.; Jiang, L.; Zhang, Z.; Hou, X.Y.; Zhang, Y.Q.; Yang, H.; Wang, J.J. A novel microporous Tb-MOF fluorescent sensor for highly selective and sensitive detection of picric acid. RSC Adv. 2018, 8, 21671–21678. [Google Scholar] [CrossRef]
- Hao, J.N.; Yan, B. Ln3+ post-functionalized metal–organic frameworks for color tunable emission and highly sensitive sensing of toxic anions and small molecules. New J. Chem. 2016, 40, 4654–4661. [Google Scholar] [CrossRef]
- Gong, W.J.; Yao, R.; Li, H.X.; Ren, Z.G.; Zhang, J.G.; Lang, J.P. Luminescent cadmium(II) coordination polymers of 1,2,4,5-tetrakis(4-pyridylvinyl)benzene used as efficient multi-responsive sensors for toxic metal ions in water. Dalton Trans. 2017, 46, 16861–16871. [Google Scholar] [CrossRef]
- Li, Y.; Song, H.; Chen, Q.; Liu, K.; Zhao, F.Y.; Ruan, W.J.; Chang, Z. Two coordination polymers with enhanced ligand-centered luminescence and assembly imparted sensing ability for acetone. J. Mater. Chem. A 2014, 2, 9469–9473. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Wu, H.; Dong, X.W.; Liu, H.Y.; Ma, J.F.; Li, S.L.; Yang, J.; Liu, Y.Y.; Su, Z.M. Syntheses, X-ray structures and CVD of titanium(IV) arsine complexes. Dalton Trans. 2008, 39, 5331–5341. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dun, L.; Zhang, B.; Wang, J.; Wang, H.; Chen, X.; Li, C. Crystal Structure, Synthesis and Luminescence Sensing of a Zn(II) Coordination Polymer with 2,5-Dihydroxy-1,4-Terephthalic Acid and 2,2′-Bipyridine as Ligands. Crystals 2020, 10, 1105. https://doi.org/10.3390/cryst10121105
Dun L, Zhang B, Wang J, Wang H, Chen X, Li C. Crystal Structure, Synthesis and Luminescence Sensing of a Zn(II) Coordination Polymer with 2,5-Dihydroxy-1,4-Terephthalic Acid and 2,2′-Bipyridine as Ligands. Crystals. 2020; 10(12):1105. https://doi.org/10.3390/cryst10121105
Chicago/Turabian StyleDun, Linan, Baosheng Zhang, Jiajun Wang, He Wang, Xue Chen, and Chuanbi Li. 2020. "Crystal Structure, Synthesis and Luminescence Sensing of a Zn(II) Coordination Polymer with 2,5-Dihydroxy-1,4-Terephthalic Acid and 2,2′-Bipyridine as Ligands" Crystals 10, no. 12: 1105. https://doi.org/10.3390/cryst10121105
APA StyleDun, L., Zhang, B., Wang, J., Wang, H., Chen, X., & Li, C. (2020). Crystal Structure, Synthesis and Luminescence Sensing of a Zn(II) Coordination Polymer with 2,5-Dihydroxy-1,4-Terephthalic Acid and 2,2′-Bipyridine as Ligands. Crystals, 10(12), 1105. https://doi.org/10.3390/cryst10121105




