Ga-Substituted Cobalt-Chromium Spinels as Ceramic Pigments Produced by Sol–Gel Synthesis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Aqueous Sol–Gel Synthesis
2.3. Preparation of Ceramic Glazes
2.4. Characterization
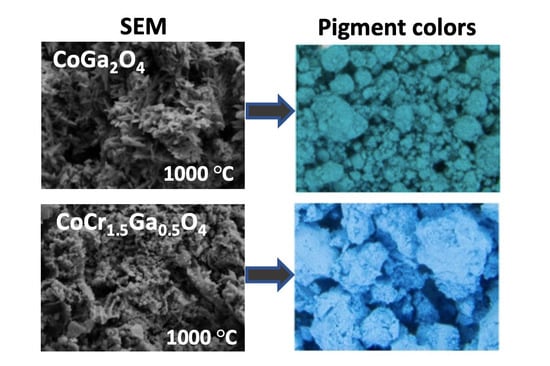
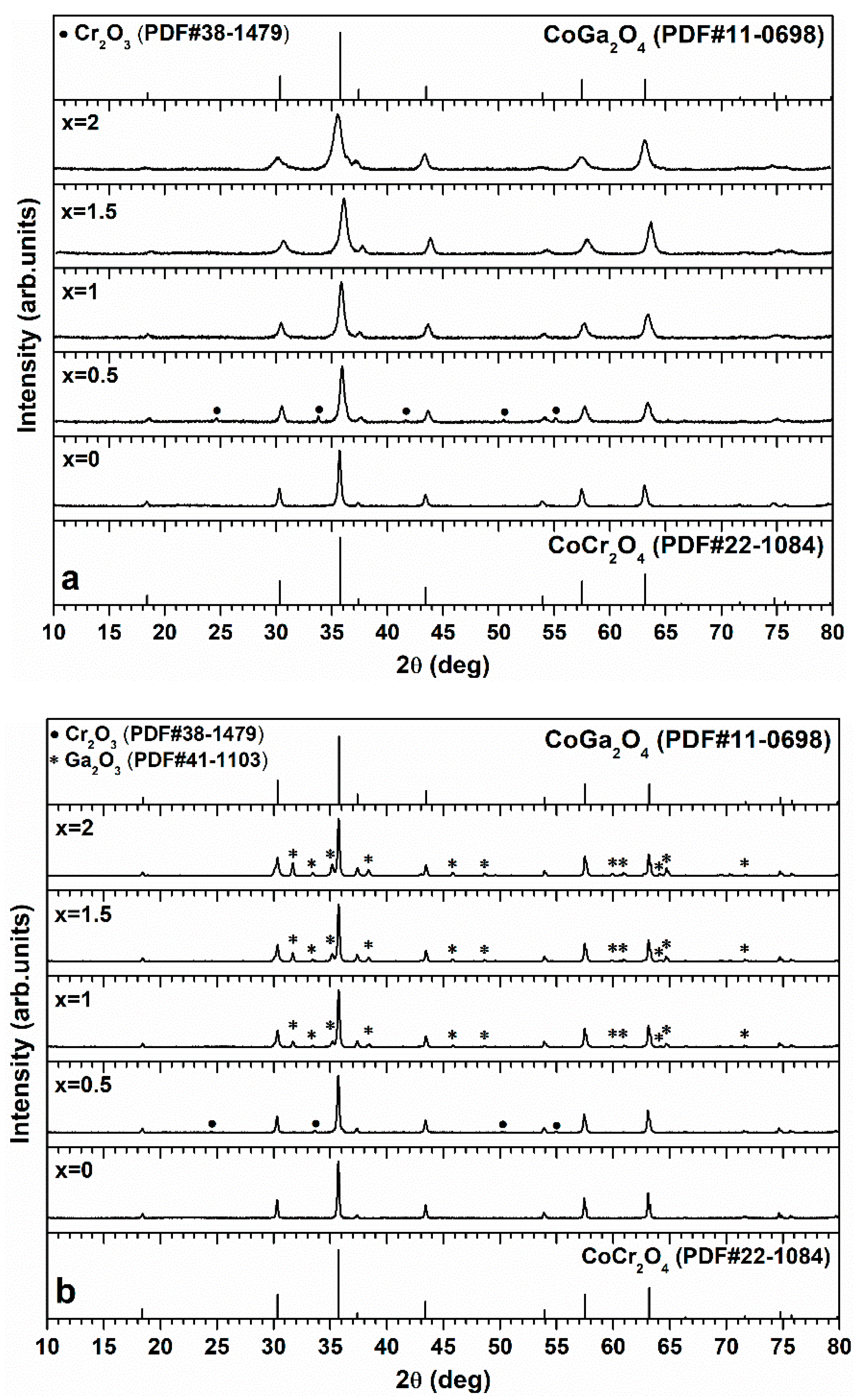
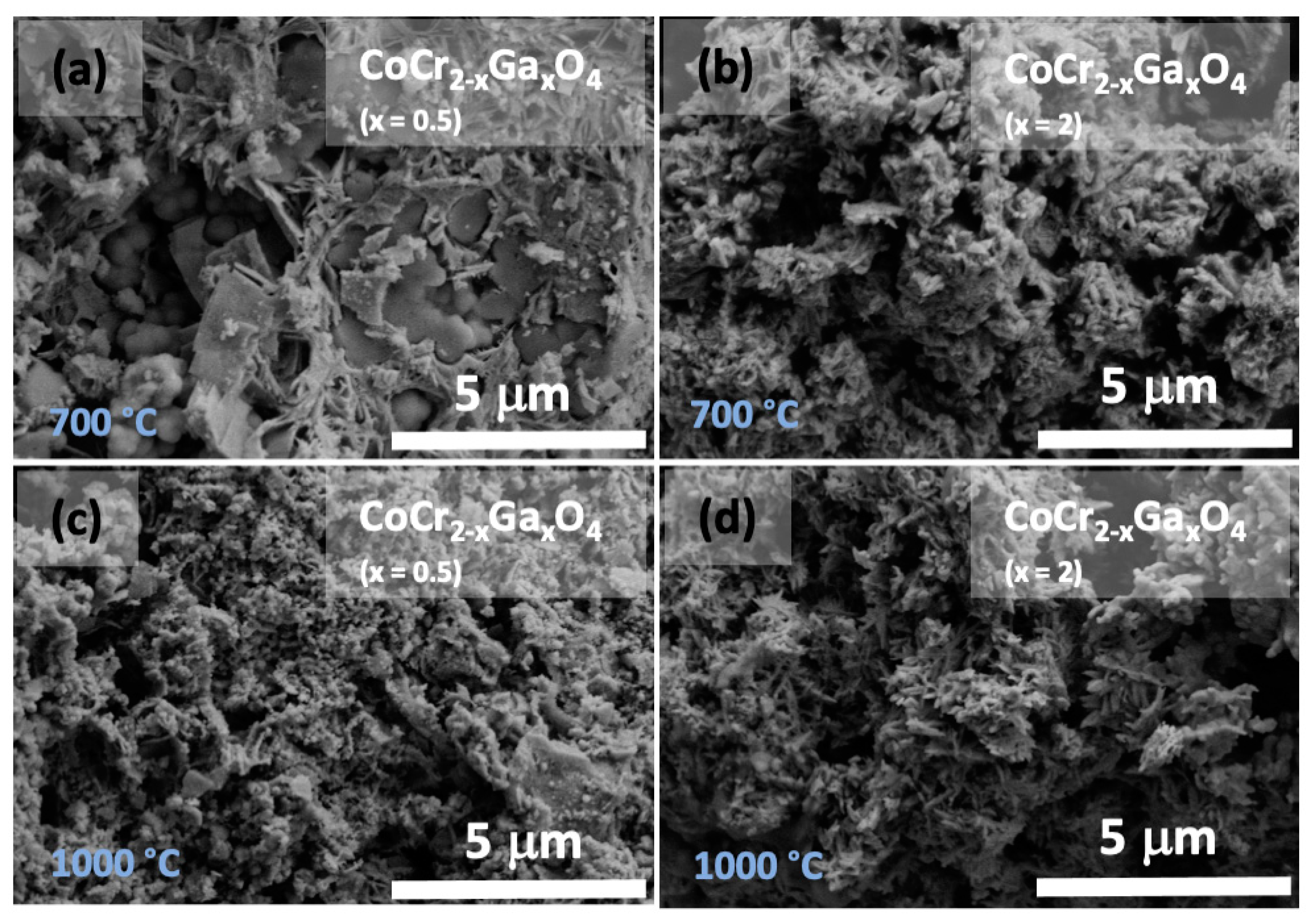
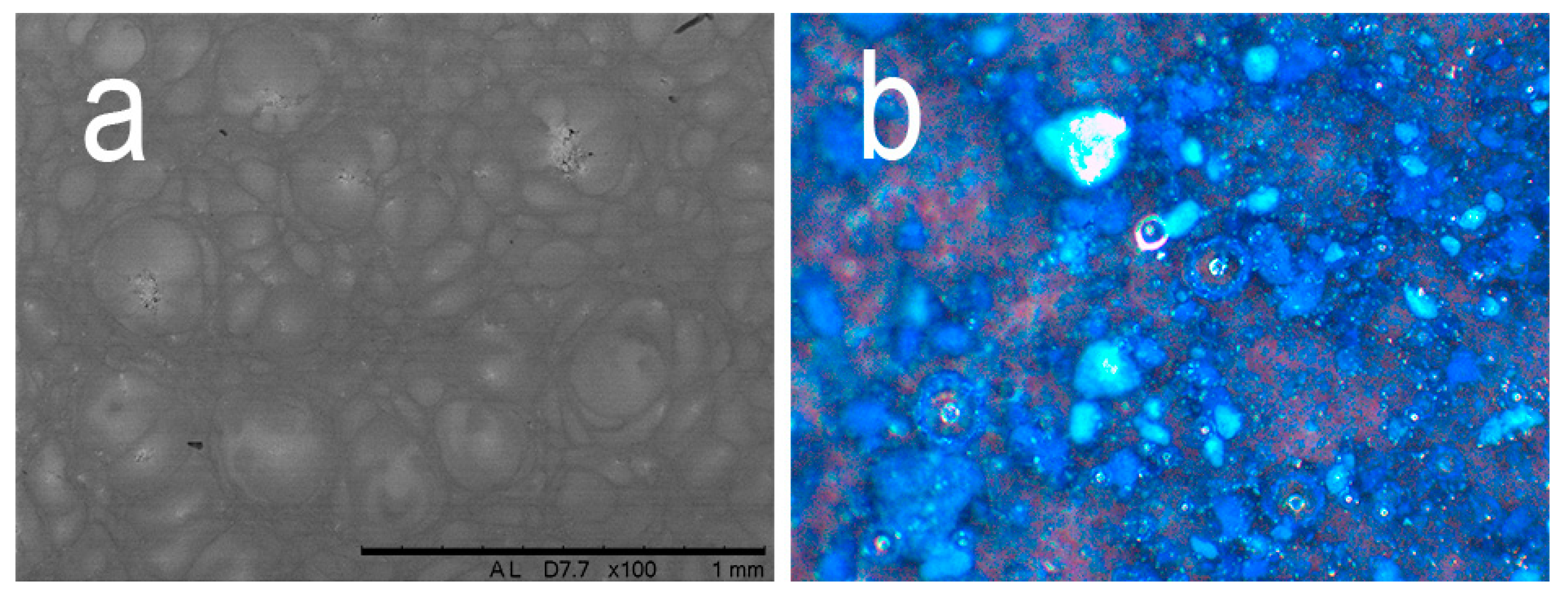
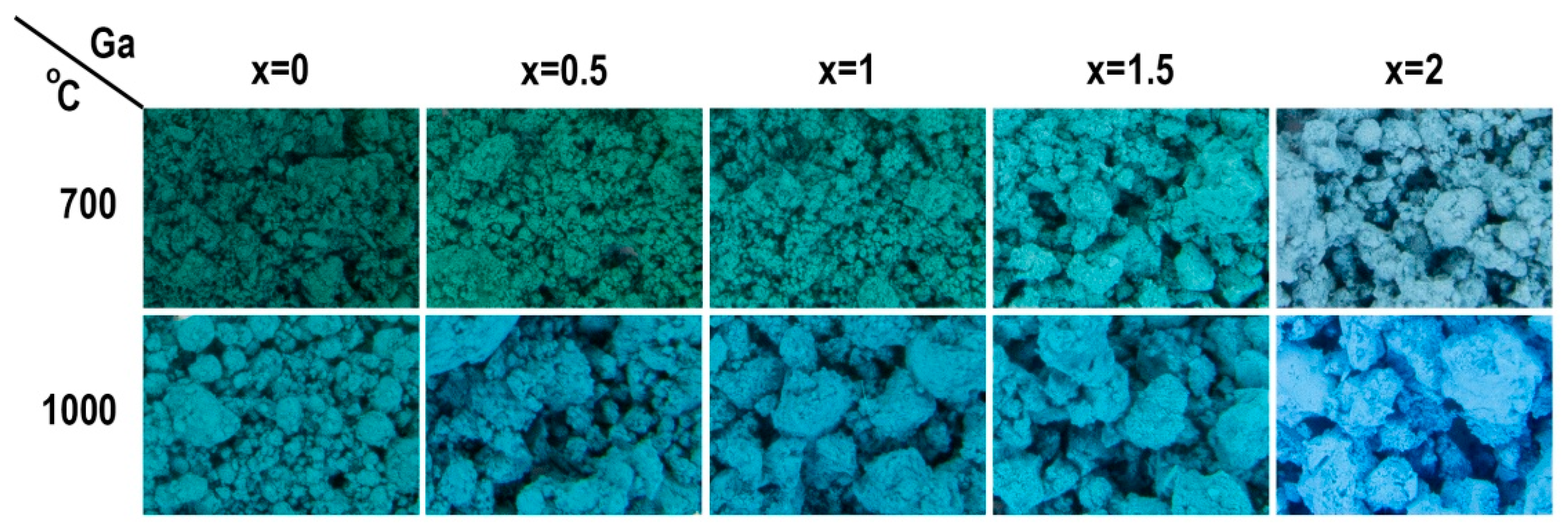
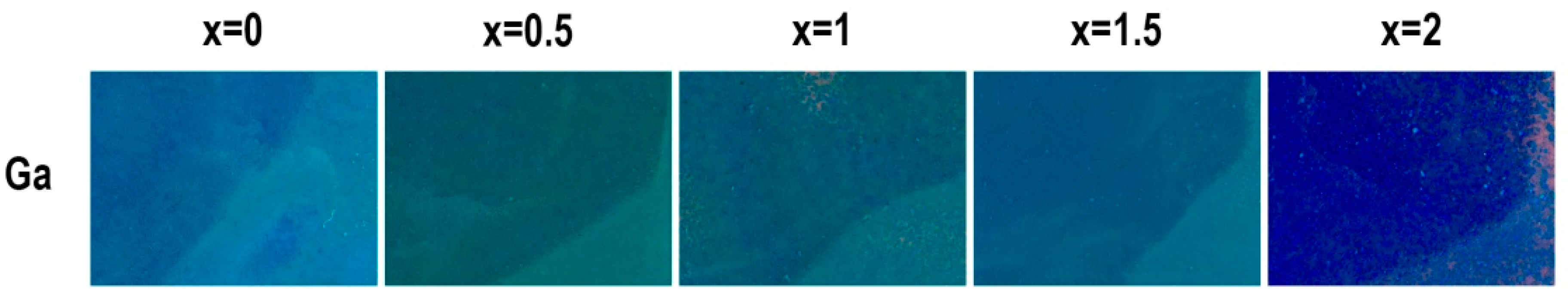
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lorenzi, G.; Baldi, G.; Di Benedetto, F.; Faso, V.; Lattanzi, P.; Romanelli, M. Spectroscopic study of a Ni-bearing gahnite pigment. J. Eur. Ceram. Soc. 2006, 26, 317–321. [Google Scholar] [CrossRef]
- DeSouza, L.; Zamian, J.; Filho, G.N.D.R.; Soledade, L.E.; DosSantos, I.; Souza, A.; Scheller, T.; Angélica, R.S.; Dacosta, C. Blue pigments based on CoxZn1−xAl2O4 spinels synthesized by the polymeric precursor method. Dye. Pigment. 2009, 81, 187–192. [Google Scholar] [CrossRef]
- Cui, H.; Zayat, M.; Levy, D. Sol-Gel Synthesis of Nanoscaled Spinels Using Propylene Oxide as a Gelation Agent. J. Sol-Gel Sci. Technol. 2005, 35, 175–181. [Google Scholar] [CrossRef]
- Jasaitis, D.; Beganskiene, D.; Senvaitiene, J.; Kareiva, A.; Ramanauskas, R.; Juskenas, R.; Selskis, A. Sol-gel synthesis and characterization of cobalt chromium spinel CoCr2O4. Chemija 2011, 22, 125–130. [Google Scholar]
- Hedayati, H.; Alvani, A.A.S.; Sameie, H.; Salimi, R.; Moosakhani, S.; Tabatabaee, F.; Zarandi, A.A. Synthesis and characterization of Co1−xZnxCr2−yAlyO4 as a near-infrared reflective color tunable nano-pigment. Dye. Pigment. 2015, 113, 588–595. [Google Scholar] [CrossRef]
- Hu, D.-S.; Han, A.-J.; Ye, M.; Chen, H.-H.; Zhang, W. 2-xAlxO4 by Low-temperature Combustion Synthesis. J. Inorg. Mater. 2011, 26, 285–289. [Google Scholar] [CrossRef]
- Chamyani, S.; Salehirad, A.; Oroujzadeh, N.; Fateh, D.S. Effect of fuel type on structural and physicochemical properties of solution combustion synthesized CoCr2O4 ceramic pigment nanoparticles. Ceram. Int. 2018, 44, 7754–7760. [Google Scholar] [CrossRef]
- Miranda, E.A.C.; Sepúlveda, A.A.L.; Carvajal, J.F.M.; Gil, S.V.; Baena, O.J.R. Green inorganic pigment production with spinel structureCoCr2O4by solution combustion synthesis. TECCIENCIA 2019, 14, 41–51. [Google Scholar] [CrossRef]
- Lei, S.-L.; Liang, G.-J.; Wang, Y.; Zhou, S.-X.; Zhang, X.; Li, S. Sol-gel combustion synthesis and characterization of CoCr2O4 ceramic powder used as color solar absorber pigment. Optoelectron. Lett. 2020, 16, 365–368. [Google Scholar] [CrossRef]
- Mindru, I.; Gingasu, D.; Marinescu, G.; Patron, L.; Calderon-Moreno, J.M.; Bartha, C.; Andronescu, C.; Crişan, A. Cobalt chromite obtained by thermal decomposition of oxalate coordination compounds. Ceram. Int. 2014, 40, 15249–15258. [Google Scholar] [CrossRef]
- Tanisan, B.; Dondi, M. Cobalt chromite nano pigments synthesis through microwave-assisted polyol route. J. Sol-Gel Sci. Technol. 2017, 83, 590–595. [Google Scholar] [CrossRef]
- Betancur-Granados, N.; Restrepo-Baena, O.J. Flame spray pyrolysis synthesis of ceramic nanopigments CoCr2O4: The effect of key variables. J. Eur. Ceram. Soc. 2017, 37, 5051–5056. [Google Scholar] [CrossRef]
- Grazenaite, E.; Pinkas, J.; Beganskiene, A.; Kareiva, A. Sol–gel and sonochemically derived transition metal (Co, Ni, Cu, and Zn) chromites as pigments: A comparative study. Ceram. Int. 2016, 42, 9402–9412. [Google Scholar] [CrossRef]
- Grazenaite, E.; Jasulaitiene, V.; Ramanauskas, R.; Kareiva, A. Sol–gel synthesis, characterization and application of lanthanide-doped cobalt chromites (CoCr2−xLnxO4; Ln = Tm3+ and Yb3+). J. Eur. Ceram. Soc. 2018, 38, 3361–3368. [Google Scholar] [CrossRef]
- Omata, T.; Ueda, N.; Hikuma, N.; Ueda, K.; Mizoguchi, H.; Hashimoto, T.; Kawazoe, H. New oxide phase with wide band gap and high electroconductivity CdGa2O4spinel. Appl. Phys. Lett. 1993, 62, 499–500. [Google Scholar] [CrossRef]
- Omata, T.; Ueda, N.; Ueda, K.; Kawazoe, H. New ultraviolet--transport electroconductive oxide, ZnGa2O4 spinel. Appl. Phys. Lett. 1994, 64, 1077–1078. [Google Scholar] [CrossRef]
- Mondal, A.; Manam, J. Investigations on spectroscopic properties and temperature dependent photoluminescence of Cr3+ doped MgGa2O4phosphor. Mater. Res. Express 2019, 6, 095081. [Google Scholar] [CrossRef]
- Mondal, A.; Manam, J. Structural and Luminescent Properties of Si4+Co-Doped MgGa2O4:Cr3+Near Infra-Red Long Lasting Phosphor. ECS J. Solid State Sci. Technol. 2017, 6, R88–R95. [Google Scholar] [CrossRef]
- Biswas, S.K.; Sarkar, A.; Pathak, A.; Pramanik, P. Studies on the sensing behaviour of nanocrystalline CuGa2O4 towards hydrogen, liquefied petroleum gas and ammonia. Talanta 2010, 81, 1607–1612. [Google Scholar] [CrossRef]
- Cabello, G.; Lillo-Arroyo, L.; Caro-Díaz, C.; Valenzuela-Melgarejo, F.; Fernández-Pérez, A.; Buono-Core, G.; Chornik, B. Study on the photochemical preparation of nickel gallium oxide spinel doped with Eu(III) ions from carboxylate and β-diketonate complexes and the evaluation of its optical properties. Thin Solid Films 2018, 647, 33–39. [Google Scholar] [CrossRef]
- Galindo, R.; Llusar, M.; Tena, M.A.; Monros, G.; Badenes, J. New pink ceramic pigment based on chromium (IV)-doped lutetium gallium garnet. J. Eur. Ceram. Soc. 2007, 27, 199–205. [Google Scholar] [CrossRef]
- Galindo, R.; Badenes, J.; Llusar, M.; Tena, M.; Ángeles; Monros, G. Synthesis and characterisation of chromium lutetium gallium garnet solid solution. Mater. Res. Bull. 2007, 42, 437–445. [Google Scholar] [CrossRef]
- Monrós, G.; Pinto, H.; Badenes, J.; Llusar, M.; Tena, M.; Ángeles; March, J.A.B. Chromium(IV) Stabilisation in New Ceramic Matrices by Coprecipitation Method: Application as Ceramic Pigments. Z. Anorg. Allg. Chem. 2005, 631, 2131–2135. [Google Scholar] [CrossRef]
- Tamilarasan, S.; Sarma, D.; Reddy, M.L.P.; Natarajan, S.; Gopalakrishnan, J. YGa1−xMnxO3: A novel purple inorganic pigment. RSC Adv. 2013, 3, 3199–3202. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. Sect. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
| CoCr2−xGaxO4 Sample | Crystallite Size, nm | |
|---|---|---|
| 700 °C | 1000 °C | |
| x = 0 | 32.6 ± 0.5 | 54.4 ± 0.7 |
| x = 0.5 | 27.9 ± 0.6 | 51.9 ± 0.5 |
| x = 1.0 | 22.4 ± 0.4 | 49.5 ± 0.5 |
| x = 1.5 | 16.8 ± 0.5 | 48.0 ± 0.6 |
| x = 2.0 | 11.4 ±.0.3 | 46.6 ± 0.7 |
| CoCr2−xGaxO4 Sample | n (Co) | n (Cr) | n (Ga) |
|---|---|---|---|
| x = 0 | 1 | 2.01 | - |
| x = 0.5 | 1 | 1.48 | 0.510 |
| x = 1.0 | 1 | 1.02 | 1.03 |
| x = 1.5 | 1 | 0.491 | 1.52 |
| x = 2.0 | 1 | - | 2.03 |
| Amount of Ga (x) | Pigment | Glaze | ||||
|---|---|---|---|---|---|---|
| L* | a* | b* | L* | a* | b* | |
| 0 | 47.69 | –8.31 | –3.89 | 33.47 | −10.92 | −10.36 |
| 0.5 | 50.25 | −12.39 | −7.56 | 30.86 | −7.59 | −4.41 |
| 1 | 48.48 | −8.49 | −5.60 | 31.53 | −4.01 | −4.16 |
| 1.5 | 52.48 | −11.83 | −8.36 | 30.74 | −4.32 | –6.84 |
| 2 | 54.44 | −9.48 | –14.07 | 27.69 | 3.67 | –9.35 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grazenaite, E.; Garskaite, E.; Stankeviciute, Z.; Raudonyte-Svirbutaviciene, E.; Zarkov, A.; Kareiva, A. Ga-Substituted Cobalt-Chromium Spinels as Ceramic Pigments Produced by Sol–Gel Synthesis. Crystals 2020, 10, 1078. https://doi.org/10.3390/cryst10121078
Grazenaite E, Garskaite E, Stankeviciute Z, Raudonyte-Svirbutaviciene E, Zarkov A, Kareiva A. Ga-Substituted Cobalt-Chromium Spinels as Ceramic Pigments Produced by Sol–Gel Synthesis. Crystals. 2020; 10(12):1078. https://doi.org/10.3390/cryst10121078
Chicago/Turabian StyleGrazenaite, Egle, Edita Garskaite, Zivile Stankeviciute, Eva Raudonyte-Svirbutaviciene, Aleksej Zarkov, and Aivaras Kareiva. 2020. "Ga-Substituted Cobalt-Chromium Spinels as Ceramic Pigments Produced by Sol–Gel Synthesis" Crystals 10, no. 12: 1078. https://doi.org/10.3390/cryst10121078
APA StyleGrazenaite, E., Garskaite, E., Stankeviciute, Z., Raudonyte-Svirbutaviciene, E., Zarkov, A., & Kareiva, A. (2020). Ga-Substituted Cobalt-Chromium Spinels as Ceramic Pigments Produced by Sol–Gel Synthesis. Crystals, 10(12), 1078. https://doi.org/10.3390/cryst10121078