Abstract
The CO2 solubilities (including CO2 Henry’s constants) and viscosities in ionic liquids (ILs)/deep eutectic solvents (DESs)-based hybrid solvents were comprehensively collected and summarized. The literature survey results of CO2 solubility illustrated that the addition of hybrid solvents to ILs/DESs can significantly enhance the CO2 solubility, and some of the ILs-based hybrid solvents are super to DESs-based hybrid solvents. The best hybrid solvents of IL–H2O, IL–organic, IL–amine, DES–H2O, and DES–organic are [DMAPAH][Formate] (2.5:1) + H2O (20 wt %) (4.61 mol/kg, 298 K, 0.1 MPa), [P4444][Pro] + PEG400 (70 wt %) (1.61 mol/kg, 333.15 K, 1.68 MPa), [DMAPAH][Formate] (2.0:1) + MEA (30 wt %) (6.24 mol/kg, 298 K, 0.1 MPa), [TEMA][Cl]-GLY-H2O 1:2:0.11 (0.66 mol/kg, 298 K, 1.74 MPa), and [Ch][Cl]-MEA 1:2 + DBN 1:1 (5.11 mol/kg, 298 K, 0.1 MPa), respectively. All of these best candidates show higher CO2 solubility than their used pure ILs or DESs, evidencing that IL/DES-based hybrid solvents are remarkable for CO2 capture. For the summarized viscosity results, the presence of hybrid solvents in ILs and DESs can decrease their viscosities. The lowest viscosities acquired in this work for IL–H2O, IL–amine, DES–H2O, and DES–organic hybrid solvents are [DEA][Bu] + H2O (98.78 mol%) (0.59 mPa·s, 343.15 K), [BMIM][BF4] + DETA (94.9 mol%) (2.68 mPa·s, 333.15 K), [L-Arg]-GLY 1:6 + H2O (60 wt %) (2.7 mPa·s, 353.15 K), and [MTPP][Br]-LEV-Ac 1:3:0.03 (16.16 mPa·s, 333.15 K) at 0.1 MPa, respectively.
1. Introduction
CO2 emission is an urgent issue due to its main contribution to global warming [1]. It has been evidenced that CO2 capture is a promising route to mitigate CO2 emissions, and in general, cost, energy demand, and environmental impact need to be considered when for selecting the potential CO2 capture technologies [2]. At present, the absorption technology with 30 wt % MEA aqueous solution is the commercialized one. However, this technology with the corresponding solvent has the drawbacks of high energy demand (4.2 GJ/t CO2), high cost ($0.19–1.31/t CO2), low thermal and chemical stability, and high volatility and corrosion [3,4,5,6], which underlines the necessity for developing greener and more efficient solvent for CO2 capture.
Compared with the traditional amine-based solvent, the emerging new absorption solvents of ionic liquids (ILs) and deep eutectic solvents (DESs) have attracted more and more attention due to the merits of recyclability, good solvent stability, low energy demand, and environmentally friendly nature [7]. However, some of the ILs (e.g., [P4442][Cy-Suc], 2567 mPa·s at 303.15 K) [8] and DESs (e.g., [MTPP][Br]-GLY 1:4, 1658 mPa·s at 298.15 K) [9] have high viscosities that influence the rate of absorptions, inhibiting their industry applications. To cope with this disadvantage, hybrid ILs or DESs with water have been strongly recommended [9,10,11,12,13,14]. For example, Zhang et al. investigated the mass transfer feature in [BMIM][NO3] + H2O, evidencing that mass transfer increases with the increase of water content, e.g., the mass transfer of [BMIM][NO3] (95 wt %) + H2O (5 wt %) is 0.55 × 105 m·s−1, while it is 0.64 × 105 m·s−1 for [BMIM][NO3] (90 wt %) + H2O (10 wt %), which may due to the decrease of viscosity that from 35.74 ([BMIM][NO3] (95 wt %) + H2O (5 wt %)) to 12.65 mPa·s ([BMIM][NO3] (90 wt %) + H2O (10 wt %)) [13]. Sarmad et al. studied the viscosity of DES, finding that a small amount of water has a significant effect on DES viscosity [9]. For instance, at 298 K, the viscosity of [TEMA][Cl]-GLY (1:2) is 236.59 mPa·s, while it is 72.75 mPa·s for [TEMA][Cl]-GLY-H2O (1:2:0.055).
Except for adding water to decrease the viscosity, organic solvents (e.g., PEG, TEG, and TG) can substitute water totally or partially to decrease the viscosity or to overcome high energy demand in IL + H2O [15,16,17]. The experimental result indicates that TG can significantly decrease the viscosity of [P66614][4-Triz], especially at low temperatures, i.e., the viscosity of [P66614][4-Triz] is 4640 mPa·s at 278.15 K, while it is 163 mPa·s for [P66614][4-Triz] + TG (58.2 mol%) [18]. Liu et al. found that the addition of a certain amount of PEG200 to [Cho][Gly] + H2O not only decreases the viscosity but also enhances the CO2 solubility, as well as decreases the desorption enthalpy [17].
To further take the benefits of both neoteric and conventional solvents, IL–amine-based and superbase–amine-based DES hybrid absorbents have also been proposed and developed [19,20,21,22,23,24,25]. These hybrid solvents possess certain advantages of low energy demand, low water evaporation, and high CO2 solubility compared to the commercialized MEA aqueous solution [26]. For example, Yang et al. reported that [BMIM][BF4] (40 wt %) + MEA (30 wt %) + H2O (30 wt %) has higher CO2 solubility than MEA (30 wt %) + H2O (70 wt %), and the energy demand reduced by 37.2% with respect to MEA (30 wt %) + H2O (70 wt %) [21]. The CO2 solubilities of four functionalized ILs in MDEA aqueous solution were investigated and compared with MEA + MDEA aqueous solution [27], showing that the CO2 solubility of [N2222][Lys] (15 wt %) + MDEA (15 wt %) + H2O (70 wt %) (0.74 mol/mol) > [N1111][Lys] (15 wt %) + MDEA (15 wt %) + H2O (70 wt %) (0.69 mol/mol) > [N2222][Gly] (15 wt %) + MDEA (15 wt %) + H2O (70 wt %) (0.64 mol/mol) > [N1111][Gly] (15 wt %) + MDEA (15 wt %) + H2O (70 wt %) (0.56 mol/mol) > MEA (15 wt %) + MDEA (15 wt %) + H2O (70 wt %) (0.36 mol/mol). For DES hybrid solvent of [Ch][Cl]-MEA 1:2 + DBN with volume ratio of 1:1, its CO2 solubility improved from 3.29 to 5.11 mol/kg compared with [Ch][Cl]-MEA 1:2 at 298.15 K [25].
To develop the potential IL/DES-based hybrid solvents for CO2 capture, CO2 solubility (in accordance with Henry’s constant for physical absorption) and viscosity are two key properties. Furthermore, the selectively for physical absorption and the CO2 absorption enthalpy for chemical absorption are other concerns in development, while the research is still limited, especially when compared to those for CO2 solubility and viscosity. Several reviews for IL/DES-based hybrid solvents from the aspect of CO2 solubility, Henry’s constant, and viscosity have been published. Babamohammadi et al. summarized the viscosities of IL + H2O and IL + MEA/EG + H2O until 2014 and the CO2 solubilities of ILs (imidazolium- and ammonium-based ILs)-amine hybrid solvents since 2008 [28]. Gao et al. summarized the CO2 solubility of 18 kinds of IL-amine based hybrid solvent until 2015. Huang et al. reviewed the advantages and disadvantages of five kinds of IL–hybrid solvents (i.e., IL–organic, normal IL–amine, normal IL aqueous–amine, functionalized IL–amine, and functionalized IL aqueous–amine) until 2016 for CO2 capture [26], finding that IL–hybrid solvents can significantly reduce the viscosity. Lian et al. introduced the ILs–hybrid processes for CO2 capture and compared the CO2 solubilities for IL–DEA/DMEE/ethanol, indicating that IL–ethanol has the highest solubility of 2.3 mol/mol [29]. Recently, more IL-based hybrid solvents have been developed combined with property measurements, making it necessary to update the latest research progress. Meanwhile, to the best of our knowledge, there is no review article for the DES-based hybrid solvents.
To fulfill this gap and to promote the technology development on CO2 capture in IL/DES-based hybrid solvents, this review summarizes the CO2 solubilities (including Henry’s constants) and viscosities of IL-based hybrid solvents since 2016 and DES-based hybrid solvents since 2014 to avoid the repetition of the published reviews. Finally, the best candidates for IL/DES-based hybrid solvents were obtained and compared with each other.
2. ILs-Based Hybrid Solvents
Regarding the CO2 solubilities for 73 kinds of IL–H2O and 37 kind of IL–organic-based hybrid solvents since 2016, 28 types of IL–amine hybrid solvents since 2018 together with 62 Henry’s constants have been reported. The results were collected and summarized in Table 1 and Table 2. The viscosities for 30 kinds of IL–H2O and 121 IL–organic/organic aqueous solution hybrid solvents since 2016, and 15 kinds of IL–amine/amine aqueous solution hybrid solvents since 2018 have been determined, and these are listed in Table 3. The full names of ILs-based hybrid solvents are displayed in Table S1.
2.1. CO2 Solubility and Henry’s Constant
2.1.1. IL–H2O
The effect of the addition of H2O in [DMAPAH][Formate] (0.5:1, 1.0:1, 2.0:1, 2.5:1) was studied by Vijayaraghavan et al. [30]. Except for the molar ratio of amine to acid of 0.5:1, CO2 solubility initially increased up to a certain water amount of 20 wt % (Figure 1); then, it steadily decreased as the H2O concentration increased. As shown in Figure 1, the best candidate for CO2 capture is [DMAPAH][Formate] (2.5:1) + H2O (20 wt %) (5.69 mol/kg). The CO2 solubility in [TMGH][Im] + H2O (1–25 wt %) was studied by Li et al. [12]. The result indicates that CO2 solubility first increased at a water content range of 1–7 wt %, and then, it decreased when the water content was larger than 7 wt % in [TMGH][Im], resulting in the best absorption capacity of 4.23 mol/kg for [TMGH][Im] + H2O (7 wt %). Huang et al. reported that a [P4442][Suc] structure with basic anion can improve the reaction of CO2 and [P4442][Suc] aqueous solution by forming bicarbonate and conjugate acid [31]. The result indicates that the CO2 solubility of [P4442][Suc] + H2O (3.3 wt %) (1.9 mol/mol) is slightly higher than [P4442][Suc] (1.87 mol/mol); however, the addition of 8.8 and 17.6 wt % of H2O to [P4442][Suc] has a negative effect on their CO2 solubilities compared with [P4442][Suc]. As shown in Table 1, various compositions of [P4444][HCOO] in water were measured at 0.1 MPa in the temperature range of 248.75–324.65 K for CO2 solubility and compared with [N2224][Ac] and [N2222][Ac] [32]. The results indicate that the CO2 solubility of [P4444][HCOO] + H2O first increased from 0.01 to 1 mol/mol at the water contents range of 29–66 mol% and then decreased with the increase of water contents of 70–91 mol%. Temperature also affects the absorption performance of [P4444][HCOO] + H2O. For example, at 273.15 K, the CO2 solubility of [P4444][HCOO] + H2O (<80 mol%) is higher than [N2224][Ac] + H2O and [N2222][Ac] + H2O, while at 298.15 K, it is higher than [N2224][Ac] + H2O at a water content less than 50 mol%, but it is lower at a water content high than 50 mol%. For 323.15 K, the CO2 solubility in [P4444][HCOO] + H2O (<80 mol%) is lower than [N2222][Ac] + H2O. Nathalia et al. [33] evidenced that there are three processes for CO2 capture in [BMMIM][Im] and [BMMIM][Ac] aqueous solutions, i.e., physical, CO2–imidazolium adduct generation, and bicarbonate formation, resulting in a maximum CO2 solubility of 8.15 mol/mol in [BMMIM][Im] + H2O (99.9 mol%). The CO2 solubility in [P4443][Gly] + H2O (59.9, 80.1, 90, 95 wt %) was measured at temperatures ranging from 278.14 to 348.05 K and pressures of 0.1–7.75 MPa [34]. A feature of physical absorption in these hybrid solvents was observed. The best CO2 solubility of 2.44 mol/kg was acquired for [P4443][Gly] + H2O (59.9 wt %) at 298.06 K and 4.6 MPa. Aghaie et al. tested the CO2 solubilities of [HMIM][Tf2N], [HMIM][FAP], and [BMIM][Ac] aqueous solutions [35]. The result indicates that the CO2 solubility reduced by 45% in these three ILs aqueous solutions compared to the solubility of CO2 in pure IL at 298 K and water content of 10 wt %.
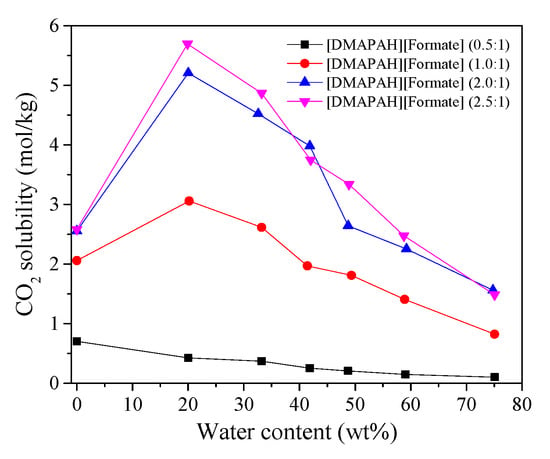
Figure 1.
Effects of water on CO2 solubility in [DMAPAH][Formate]. The values in Figure 1 are cited from Vijayaraghavan et al. [30]. Copyright 2018 Elsevier.
2.1.2. IL–Organic/Organic Aqueous Solution
Huang et al. investigated the CO2 capture capacity in [TETAH][Lys] + ethanol + H2O [36], finding that the CO2 solubility first increases with the increase of volume ratio of ethanol in water from 10:0 to 5:5 v/v, and then, it decreases from 5:5 to 2:8 v/v. Compared with the reported results, the maximum CO2 solubility of 2.45 mol/mol for [TETAH][Lys] + ethanol + H2O (5:5 v/v) is higher than those of [P6444][Lys] [37], [C2NH2MIM][Lys] [38], and [TETAH][Lys] + H2O. Taheri et al. measured the CO2 solubility of [AMIM][Tf2N] + methanol at temperature and pressure ranges of 313.2–353.2 K and 0.98–6.19 MPa, respectively, indicating that the presence of methanol in [AMIM][Tf2N] enhances the CO2 solubility and results in a maximum of 3.89 mol/mol [39].
In order to overcome the drawbacks of high CO2 capture enthalpy and water volatilization in ILs aqueous solution, PEG200 was introduced in [Cho][Gly] + H2O [17]. CO2 solubility was measured in such solvent at 308.15–338.15 K and pressure lower than 0.68 MPa, and the CO2 desorption enthalpy was estimated. Owing to its physical–chemical properties, [Cho][Gly] (30 wt %) + PEG200 (30 wt %) + H2O (40 wt %) has a higher CO2 solubility (0.41–1.23 mol/kg) and regeneration efficiency (95%) compared with [Cho][Gly] (30 wt %) + H2O (70 wt %). Li et al. found that the addition of PEG200 to [Cho][Pro] not only improves the absorption rate but also enhances the desorption efficient, resulting in a maximum CO2 solubility of about 0.6 mol/mol for [Cho][Pro] + PEG200 with the mass ratios of 1:1, 1:2, 1:3, respectively [40]. PEG400 was introduced to [P4444][Gly], [P4444][Ala], and [P4444][Pro] [41], evidencing that [P4444][Pro] + PEG400 (70 wt %) (1.61 mol/kg) > [P4444][Gly] + PEG400 (70 wt %) (1.58 mol/kg) > [P4444][Ala] + PEG400 (70 wt %) (1.57 mol/kg). The effect of three types of PEG (i.e., PEG200, PEG300, and PEG400) and water content on the CO2 solubility in [DETAH][Br] and [DETAH][BF4] at 293.15 K and 0.1 MPa was investigated by Chen et al. [42]. The result evidenced that the CO2 solubility follows the order of [DETAH][Br] + PEG200 (1.18 mol/mol) > [DETAH][Br] + PEG300 (0.87 mol/mol) > [DETAH][BF4] + PEG200 (0.65 mol/mol) > [DETAH][Br] + PEG300 (0.32 mol/mol) at a mass ratio of [DETAH][Br]:PEG = 1:4. Additionally, the CO2 solubility in [DETAH][Br] + PEG200 + H2O (4.7 wt %) (1.18 mol/mol) is higher than that in [DETAH][Br] + PEG200 + H2O (1.3 wt %) (1.05 mol/mol), which may be because water weakens the interaction of the IL cation and anion and enhances the interaction with CO2. Due to the high CO2 solubility of PEO1000 (0.35 mol/mol, 323 K, 4.98 MPa), it is introduced to [N4111][Tf2N]. A maximum CO2 solubility of 1.16 mol/mol was acquired at 323 K, 4.99 MPa for [N4111][Tf2N] + PEO1000 (75 mol%), which is higher than that of pure [N4111][Tf2N] (0.14 mol/mol, 323 K, 5 MPa) [43]. Additionally, a higher amount of PEO1000 in [N4111][Tf2N] corresponds to higher CO2 solubility, which is attributed to the strong interaction between CO2 and PEO. However, Jiang et al. [44] found that increasing the molar fraction of TEG in [BMIM][BF4]/[BMIM][BF4] + H2O results in a decrease of CO2 solubilities, which is on the contrary of the result from Lepre et al. [43]. Moreover, with increasing the [BMIM][BF4] contents in [BMIM][BF4] + TEG mixtures, the Henry’s constant is increased (Table 2) [44]. The Henry’s constant of [BMIM][BF4] + TEG is higher than [BMIM][BF4] but lower than TEG, indicating that CO2 is more soluble in [BMIM][BF4]. The CO2 solubilities of [P66614][3-Triz] + TG (30 mol%) and [P66614][4-Triz] + TG (30 mol%) at 313.15–353.6 K and pressure less than 3 MPa were measured by Fillion et al. [18]. The CO2 solubility of [P66614][4-Triz] + TG (30 mol%) is 2.23 mol/mol at 313.15 K and 3.03 MPa, which is higher than [P66614][3-Triz] + TG (30 mol%) (1.55 mol/mol, 313.15 K, and 2.68 MPa). [TEPAH][2-MI] combined with propan-1-ol (NPA) and EG was used for CO2 capture [45]. The result indicates that the CO2 solubility in [TEPAH][2-MI] + NPA + EG can reach up to 1.72 mol/mol, which was much higher than that of [C3OHmim]Cl + MEA (0.3 mol/mol) [46], AMP + MEA + H2O (0.5 mol/mol) [47], [P66614][Gly] (1.26 mol/mol) [48], and TETA + AMP + ethanol (1.03 mol/mol) [49].
2.1.3. IL–Amine/Amine Aqueous Solution
Three base-rich diamino ILs of [DMAPAH][Formate], [DMEDAH][Formate], and [DMAPAH][Octanoate] were synthesized with different molar ratios of base to acid (0.5:1, 1.0:1, 2.0:1, and 2.5:1, respectively) and hybrid with MEA for CO2 capture, respectively [30]. According to Table 1 and Figure 2, the hybrid solvents of the synthesized ILs with an additional MEA showed enhanced CO2 solubility, which agrees with the studies from Zeng et al. [50] and Meng et al. [51] that applied MDEA and DMEE as the hybrid solvents to [DMAPAH][Ac] and [N1111][Lys], respectively. Among them, [DMAPAH][Formate] (2.0:1) + MEA (30 wt %) with 6.24 mol/kg was identified to be the best one for CO2 capture. The CO2 capture performance in [BMPyrr][DCA] (5 wt %) + DEA (35 wt %) + H2O (60 wt %) and [BMPyrr][DCA] (10 wt %) + DEA (30 wt %) + H2O (60 wt %) were studied by Salleh et al. [52] and compared with DEA (40 wt %) + H2O (60 wt %). The result indicates that the CO2 solubility increases with increasing the [BMPyrr][DCA] amount in the hybrid solvent. However, the CO2 solubilities of these two hybrid solvents are lower than those in DEA (40 wt %) + H2O (60 wt %).
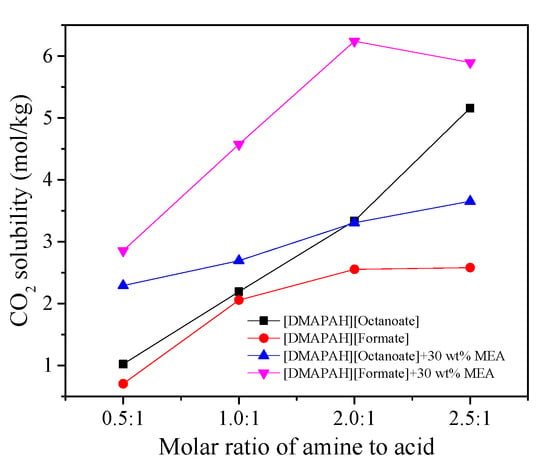
Figure 2.
Effects of MEA on CO2 solubilities in [DMAPAH][Formate] and [DMAPAH][Octanoate] [30]. Copyright 2018 Elsevier.
In conclusion, (1) a certain amount of water in ILs (mainly for chemical-based ILs) can enhance the CO2 solubility, due to the decrease in viscosity and the formation of new products (e.g., carbamate and bicarbonate). However, excess water in ILs corresponds to a low ILs concentration and results in the decrease of CO2 solubility; (2) the IL–organic and IL–organic aqueous solution as absorbents exhibit remarkable CO2 capture performances, including high absorption capacity and low desorption enthalpy. The organic molecular weight, type, and water content in ILs can affect their CO2 capture performance. Based on the summarized result, the organic solvent with low molecular weight together with a certain amount of water is beneficial for capturing CO2; (3) IL–MEA shows better CO2 capture performance than that of IL–MDEA and IL–DMEE; additionally, the IL–amine based hybrid solvent has higher CO2 solubility than that of IL–H2O and IL–organic hybrid solvents. The best for each of them are [DMAPAH][Formate] (2.0:1) + MEA (30 wt %) (6.24 mol/kg, 298 K, 0.1 MPa), [DMAPAH][Formate] (2.5:1) + H2O (20 wt %) (4.61 mol/kg, 298 K, 0.1 MPa), and [P4444][Pro] + PEG400 (70 wt %) (1.61 mol/kg, 333.15 K, 1.68 MPa). Sometimes, the presence of water in IL–organic/amine hybrid solvents improves the CO2 solubility.
2.2. Viscosity
2.2.1. IL–H2O
The viscosities of IL–H2O hybrid solvents are given in Table 3. Figure 3 displays the viscosities of [DEA][Bu] + H2O [14]; the result indicated that the [DEA][Bu] has a strong hygroscopic characteristic. The viscosity of [DEA][Bu] + H2O decreases with the increase of water content and temperature. Yasaka et al. found that the viscosities of [P4444][HCOO] + H2O decreased with water contents from 25 (356 mPa·s) to 91 mol% (14.4 mPa·s), corresponding to an increase of the CO2 solubility between the water conetnt of 25 and 50 mol%, and then, they decrease from 50 to 91 mol%, which is regarded as the typical property of carboxylate ILs [32]. Aghaie et al. measured the viscosities of [HMIM][Tf2N], [HMIM][FAP], and [BMIM][Ac] aqueous solutions at 298–333 K, 2 MPa, and water mass percentages of 0.1, 1, 2, 5, and 10 wt %, respectively [35]. The result indicates that water has a significant effect on [BMIM][Ac] viscosity, e.g., the viscosity of [BMIM][Ac] decreased from 47.64 (pure IL) to 3.77 (10 wt % H2O) mPa·s at 333 K. However, for [HMIM][Tf2N] and [HMIM][FAP], their viscosities only slightly decrease at 0.1–10 wt % water. For example, the viscosity of [HMIM][FAP] at 333 K is 20.72 mPa·s, while it is 20.47 mPa·s for [HMIM][FAP] + H2O (10 wt %). Additionally, increasing the water amount in these three ILs results in the decrease of CO2 solubility.
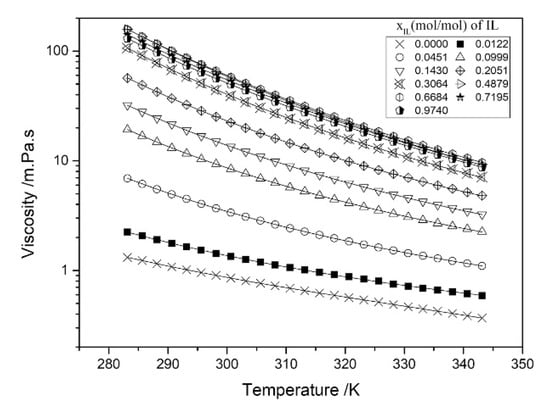
Figure 3.
Viscosity of [DEA][Bu] + H2O, 283.15–343.15 K, 0.1 MPa [14]. Copyright 2018 Elsevier.
2.2.2. IL–Organic/Organic Aqueous Solution
For [TETAH][Lys] + H2O + ethanol, it was observed that its viscosity increased with the decrease amount of ethanol, corresponding to a decreased CO2 solubility [36]. Liu et al. found that the viscosities of [Cho][Gly] (84.3–7.2 mPa·s), PEG200 (31.8–8.8 mPa·s), and [Cho][Gly] + PEG200 (70 wt %) (101.3–28.6 mPa·s) are much higher than that of [Cho][Gly]/H2O + PEG200 (30 wt %) (7.96–3.43 mPa·s) at 308.15–338.15 K [17]. To avoid the decrease of absorption rate by increasing PEG200, less than 30 wt % of PEG200 was recommended to [Cho][Gly] + PEG200 + H2O. The viscosities of [P4444][Gly], [P4444][Ala], and [P4444][Pro] hybrid with PEG400 were measured at 298.15–393.15 K and 0.1 MPa [41]. The result indicates that the viscosities of these amino-acid ILs + PEG400 are about half with respect to the pure amino acid ILs at 298.15 K. Chen et al. reported that the viscosity of the [DETAH][Br] + PEG200 (80 wt %) is 71.7 mPa·s at 293.15 K and 0.1 MPa [42]. Despite the viscosities’ increases with the increasing long-chain polymers, i.e., [N4111][Tf2N] + PEO222 < [N4111][Tf2N] + PEO500 < [N4111][Tf2N] + PEO1000, [N4111][Tf2N] + PEO1000 was proposed for CO2 capture due to the higher CO2 solubility of the pure PEO1000 [43]. The viscosities for 18 kinds of ILs (imidazolium- and phosphonium-based) + TG at different of mole ratio of each of these ILs at 278.15–323.15 K and 0.1 MPa were studied [18], evidencing that the presence of TG can significantly decrease the viscosity, resulting in about 50 mPa·s for these hybrid solvents (Table 3).
2.2.3. IL–Amine
As shown in Table 3, the viscosities of the [N1111][Lys] + DMEE and [BMIM][BF4] + DETA hybrid solvents increased with increasing the content of ILs and decreasing temperature [51,53]. Based on the study of Meng et al. [51], the viscosity of [N1111][Lys] + DMEE significantly decreased when the IL is <60 wt % compared to the pure [N1111][Lys].
From Table 3, it can be found that the viscosities of IL-based hybrid solvents are very sensitive to H2O, organic, and amine solvents. Their viscosities can significantly decrease with the increased amount of H2O, PEG, TEG, TG, DMEE, and DETA; however, it increases with the increased amount of methanol. The lowest viscosity obtained from Table 3 for IL–H2O, IL–organic, and IL–amine based hybrid solvents are [DEA][Bu] + H2O (98.78 mol%) (0.59 mPa·s, 343.15 K), [N1114][Tf2N] + PEO222 (75.06 mol%) (2.57 mPa·s, 353.15 K), and [BMIM][BF4] + DETA (94.9 mol%) (2.68 mPa·s, 353.15 K) at 0.1 MPa, respectively.

Table 1.
CO2 solubility of ionic liquid (IL)-based hybrid solvents.
Table 1.
CO2 solubility of ionic liquid (IL)-based hybrid solvents.
| IL-Based Hybrid Solvents | T/K | P/MPa | CO2 Solubility (mol CO2/kg Absorbent) | CO2 Solubility (mol CO2/mol IL) | Ref. |
|---|---|---|---|---|---|
| IL–H2O | |||||
| [DMAPAH][Formate] (0.5:1, 1.0:1, 2.0:1, 2.5:1) + H2O (20, 33, 42, 49, 59, 75 wt %) | 298.15 | 0.1 | 0.41–0.14, 2.73–0.77, 4.23–1.41, 4.61–1.41 | [30] | |
| [P4442][Suc] + H2O (3.3, 8.8, 17.6 wt %) | 293 | 0.1 | 1.9, 1.24, 0.81 | [31] | |
| [TMGH][Im] + H2O (1, 2, 3, 5, 7, 10, 15, 20, 25 wt %) | 313.15 | 0.1 | 3.39–4.23 | [12] | |
| [P4444][HCOO] + H2O (29, 32, 38, 50, 66, 70, 73, 79, 86, 91 mol%) | 248.75–333.15 | 0.1 | 0.01–1 | [32] | |
| [BMMIM][Im] + H2O (67, 91, 99, 99.9 mol%) | / | 2 | 1.29, 2.30, 0.83, 0.55 | 0, 0, 0.36, 8.15 | [33] |
| [BMMIM][Im] + H2O (99 mol%) | / | 1 | 0.45 | [33] | |
| [BMMIM][Ac] + H2O (67, 99.9 mol%) | / | 2 | 0, 6.95 | [33] | |
| [P4443][Gly] + H2O (59.9, 80.1, 90, 95 wt %) | 278.09–348.10 | 0.103–7.53 | 0.091–2.44 | 0.23–12.13 | [34] |
| [HMIM][Tf2N] + H2O (0.1, 1, 2, 5, 10 wt %) | 298–333 | 2 | 0.30–0.13 | [35] | |
| [HMIM][FAP] + H2O (0.1, 1, 2, 5, 10 wt %) | 298–333 | 2 | 0.48–0.14 | [35] | |
| [BMIM][Ac] + H2O (0.1, 1, 2, 5, 10 wt %) | 298–323 | 2 | 0.46–0.32 | [35] | |
| [Cho][Gly] + H2O (70 wt %) | 308.15–338.15 | 0.0046–0.68 | 0.36–1.24 | 0.21–0.74 | [17] |
| IL–organic/organic aqueous solution | |||||
| [TETAH][Lys] + ethanol + H2O (H2O:ethanol = 8:2, 6:4, 5:5, 4:6, 3:7, 2:8 v:v) | 303 | 0.1 | 2.45–1.53 | [36] | |
| [AMIM][Tf2N] + methanol (20, 50, 80 wt %) | 313.2–353.2 | 0.98–6.19 | 2.15–3.89 | [39] | |
| [Cho][Gly] (30 wt %) + PEG200 (10 wt%) + H2O (60 wt %) | 308.15–338.15 | 0.0054–0.68 | 0.37–1.22 | 0.22–0.72 | [17] |
| [Cho][Gly] (30 wt %) + PEG200 (20 wt%) + H2O (50 wt %) | 308.15–338.15 | 0.0039–0.68 | 0.41–1.21 | 0.24–0.72 | [17] |
| [Cho][Gly] (30 wt %) + PEG200 (30 wt%) + H2O (40 wt %) | 308.15–338.15 | 0.0065–0.68 | 0.41–1.23 | 0.24–0.73 | [17] |
| [Cho][Pro] + PEG200 (50, 67, 75 wt %) | 308.15–353.15 | 0.0041–0.11 | 0.099–0.61 | [40] | |
| [P4444][Gly] + PEG400 (70 wt %) | 333.15–413.15 | 0.088–1.7 | 0.19–1.58 | 0.19–1.23 | [41] |
| [P4444][Ala] + PEG400 (70 wt %) | 333.15–413.15 | 0.093–1.7 | 0.11–1.57 | 0.11–1.26 | [41] |
| [P4444][Pro] + PEG400 (70 wt %) | 333.15–413.15 | 0.096–1.71 | 0.15–1.61 | 0.17–1.41 | [41] |
| [DETAH][Br] + PEG200 (80 wt %) | 293.15 | 0.1 | 1.18 | [42] | |
| [DETAH][Br] + PEG300 (80 wt %) | 293.15 | 0.1 | 0.87 | [42] | |
| [DETAH][Br] + PEG400 (80 wt %) | 293.15 | 0.1 | 0.32 | [42] | |
| [DETAH][BF4] + PEG200 (80 wt %) | 293.15 | 0.1 | 0.65 | [42] | |
| [DETAH][Br] + PEG200 + H2O (1.3, 4.7 wt %) | 293.15 | 0.1 | 1.05, 1.18 | [42] | |
| [N1114][Tf2N] + PEO1000 (10.44, 28.27, 50.22, 75.31 mol%) | 323, 343 | 0.02–0.5 | 0.0057–1.16 | [43] | |
| [BMIM][BF4] + TEG (20, 50, 80 mol%) | 273.15–353.15 | 0.42–3.55 | 0.051–1.72 | [44] | |
| [BMIM][BF4] (56 mol%) + TEG (14 mol%) + H2O (30 mol%) | 293.15–333.15 | 0.38–4.17 | 0.051–0.96 | [44] | |
| [BMIM][BF4] (35 mol%) + TEG (35 mol%) + H2O(30 mol%) | 293.15–333.15 | 0.57–4.37 | 0.079–1.4 | [44] | |
| [BMIM][BF4] (14 mol%) + TEG (56 mol%) + H2O(30 mol%) | 293.15–333.15 | 0.64–4.46 | 0.15–1.84 | [44] | |
| [P66614][3-Triz] + TG (30 mol%) | 313.15–353.6 | 0.037–2.75 | 0.06–1.55 | [18] | |
| [P66614][4-Triz] + TG (30 mol%) | 313.15–353.6 | 0.07–3.03 | 0.075–2.23 | [18] | |
| [TEPAH][2-MI] + NPA + EG | 303.15 | 0.1 | 1.72 | [45] | |
| IL-amine/amine aqueous solution | |||||
| [BMPyrr][DCA] (5 wt %) + DEA (35 wt%) + H2O (60 wt %) | 333.15 | 0.5–0.7 | 0.49–0.71 | [52] | |
| [BMPyrr][DCA] (10 wt %) + DEA (30wt%) + H2O (60 wt %) | 333.15 | 0.5–0.7 | 0.58–0.81 | [52] | |
| [DMAPAH][Formate] (1.0:1, 2.5:1) + MEA | 298.15 | 0.1 | 3.82, 4.52 | [30] | |
| [DMEDAH][Formate] (1.0:1, 2.5:1) + MEA | 298.15 | 0.1 | 3.52, 4.07 | [30] | |
| [DMAPAH][Formate] (0.5:1, 1.0:1, 2.0:1, 2.5:1) + MEA (30 wt %) | 298.15 | 0.1 | 2.85, 4.57, 6.24, 5.89 | [30] | |
| [DMAPAH][Octanoate] (0.5:1, 1.0:1, 2.0:1, 2.5:1) + MEA (30 wt %) | 298.15 | 0.1 | 2.29, 2.69, 3.30, 3.65 | [30] | |
| [DMAPAH][Ac] + MDEA (20, 33, 43, 50, 60, 67, 80 mol%) | 308.15 | 0.0–3.0 | 0–3.13 | [50] | |
| [DMAPAH][Ac] + MDEA (50 mol%) | 298.15–328.15 | 0.0–3.0 | 0–3.21 | [50] | |
| [N1111][Lys] + DMEE (95, 90, 80, 60, 40 wt %) | 303 | 0.1 | 0.28–1.69 | 1.22–0.61 | [51] |
| [N1111][Lys] + DMEE (80 wt %) | 313, 323 | 0.1 | 0.76, 0.72 | [51] | |
The CO2 solubility unit with mole scale for reference [45] is mol CO2/mol absorbent, while in [52] is mol CO2/mol amine.

Table 2.
Henry’s constant of IL-based hybrid solvents.
Table 2.
Henry’s constant of IL-based hybrid solvents.
| IL-Based Hybrid Solvents | T (K) | Henry’s Constant (MPa) | Ref. |
|---|---|---|---|
| IL–H2O | |||
| [P4443][Gly] + H2O (59.9, 80.1, 90%, 95 wt %) | 278.14–348.05 | 2.8–5.05, 1.53–3.3, 0.87–2.81, 0.35–1.03 | [34] |
| [Cho][Gly] + H2O (70 wt %) | 308.15–338.15 | 40.56–58 | [17] |
| IL–organic | |||
| [Cho][Gly] (30 wt %) + PEG200 (10 wt %) + H2O (60 wt %) | 308.15–338.15 | 36.6–52.2 | [17] |
| [Cho][Gly] (30 wt %) + PEG200 (20 wt %) + H2O (50 wt %) | 308.15–338.15 | 33–47.03 | [17] |
| [Cho][Gly] (30 wt %) + PEG200 (30 wt %) + H2O (40 wt %) | 308.15–338.15 | 31.49–46.79 | [17] |
| [BMIM][BF4] + TEG (20, 50, 80 mol%) | 273.15–353.15 | 4.84–20.02, 5.65–22, 6.48–27.63 | [44] |

Table 3.
Viscosity of IL-based hybrid solvents.
Table 3.
Viscosity of IL-based hybrid solvents.
| IL-Based Hybrid Solvents | T/K | P/MPa | Viscosity (mPa·s) | Ref. |
|---|---|---|---|---|
| IL-H2O | ||||
| [DEA][Bu] + H2O (98.78, 95.49, 90.01, 85.7, 79.49, 69.36, 51.21, 33.16, 2.6 mol%) | 283.15–343.15 | 0.1 | 2.24–0.59, 6.91–1.10, 19.26–2.25, 32.03–3.24, 56.72–4.81, 106.48–7.09, 158.58–9.28, 158.29–9.64, 130.18–8.70 | [14] |
| [P4444][HCOO] + H2O (25, 50, 60, 74, 80, 91 mol%) | 298.15 | 0.1 | 356, 146, 97, 48, 35.3, 14.4 | [32] |
| [HMIM][Tf2N] + H2O (0.1, 1, 2, 5, 10 wt %) | 298–333 | 2 | 69.18–18.6, 66.54–18.2, 63.71–17.54, 55.95–15.7, 45.05–13.01 | [35] |
| [HMIM][FAP] + H2O (0.1, 1, 2, 5, 10 wt %) | 298–333 | 2 | 88.09–20.71, 84.54–20.70, 80.76–20.66, 70.4–20.6, 56.01–20.47 | [35] |
| [BMIM][Ac] + H2O (0.1, 1, 2, 5, 10 wt %) | 298–333 | 2 | 389–45.30, 225.86–30.04, 135.5–20.45, 44.08–8.78, 14.34–3.77 | [35] |
| IL–organic/organic aqueous solution | ||||
| [TETAH][Lys] + ethanol + H2O (H2O:ethanol = 8:2, 6:4, 5:5, 4:6, 3:7, 2:8 v:v) | 303 | 0.1 | 2.57, 3.00, 3.50, 3.81, 3.74, 3.51 | [36] |
| [Cho][Gly] + PEG200 (70 wt %) | 308.15–338.15 | 0.1 | 101.3–28.6 | [17] |
| [Cho][Gly]/H2O + PEG200 (30 wt %) | 308.15, 338.15 | 0.1 | 7.96, 3.43 | [17] |
| [P4444][Gly] + PEG400 (70 wt %) | 298.15–393.15 | 0.1 | 180.47–8.96 | [41] |
| [P4444][Ala] + PEG400 (70 wt %) | 298.15–393.15 | 0.1 | 216.64–9.13 | [41] |
| [P4444][Pro] + PEG400 (70 wt %) | 298.15–393.15 | 0.1 | 481–14.1 | [41] |
| [DETAH][Br] + PEG200 (80 mol%) | 293.15 | 0.1 | 71.7 | [42] |
| [N1114][Tf2N] + PEO222 (26.23, 50.07, 75.06 mol%) | 293.15–353.15 | 0.1 | 135.22–2.57 | [43] |
| [N1114][Tf2N] + PEO500 (20.90, 44.22, 70.40 mol%) | 293.15–353.15 | 0.1 | 135.22–7.51 | [43] |
| [N1114][Tf2N] + PEO1000 (25.04, 50.22, 75.31 mol%) | 318.15–353.15 | 0.1 | 51.72–17.22 | [43] |
| [P66614][4-NO2imid] + TG (18.9, 40.4 mol%) | 278.15–323.15 | 0.1 | 1503–57 | [18] |
| [P66614][4,5-CNimid] + TG (20.1, 40.1 mol%) | 278.15–323.15 | 0.1 | 1051–63 | [18] |
| [P66614][Tf2N] + TG (19.1, 36.6, 55.9, 66.4 mol%) | 278.15–323.15 | 0.1 | 589–51 | [18] |
| [P66614][2-CH3,5-NO2imid] + TG (15.1, 39.9 mol%) | 278.15–323.15 | 0.1 | 2535–57 | [18] |
| [P66614][DCA] + TG (9.9, 20.3, 29.9, 50.1, 65.2 mol%) | 278.15–323.15 | 0.1 | 1130–54 | [18] |
| [HMIM][Tf2N] + TG (9.9, 19, 27.6, 41.3, 54.4 mol%) | 278.15–293.15 | 0.1 | 171–50 | [18] |
| [P66614][BrBnIm] + TG (10.2, 13.8, 21.8, 34.6, 47.8, 63.8 mol%) | 278.15–323.15 | 0.1 | 4400–51 | [18] |
| [P66614][Ac] + TG (10.3, 20.7, 29.9, 40, 49.9 mol%) | 278.15–323.15 | 0.1 | 1130–57 | [18] |
| [HMMIM][Tf2N] + TG (10.2, 20.5, 30.6, 39.9, 49.9, 59.9 mol%) | 278.15–323.15 | 0.1 | 370–48 | [18] |
| [P66614][4-Triz] + TG (4.6, 12.6, 19.6, 24.9, 30.6, 39, 48.4, 58.2 mol%) | 278.15–323.15 | 0.1 | 3290–53 | [18] |
| [P66614][3-Triz] + TG (8.3, 12.2, 20.7, 31, 39.8, 50.1, 70 mol%) | 278.15–323.15 | 0.1 | 1180–51 | [18] |
| [P44412][3-Triz] + TG (6.2, 12.3, 15.5, 21.4, 30.5, 39.5, 49.5, 59.6 mol%) | 278.15–323.15 | 0.1 | 1980–57 | [18] |
| [P2228][4-NO2pyra] + TG (5.1, 10.2, 19.9, 30, 40.4, 50.3 mol%) | 278.15–323.15 | 0.1 | 1010–55 | [18] |
| [P2228][4-NO2imid] + TG (10, 20, 30.1, 40.1, 50.1 mol%) | 278.15–323.15 | 0.1 | 700–55 | [18] |
| [P2228][2-CH3,5-NO2imid] + TG (3.6, 6.7, 11.5, 23.3, 30, 39.9, 50, 59.8 mol%) | 278.15–323.15 | 0.1 | 2730–51 | [18] |
| [mm(butene)im][4-NO2pyra] + TG (4.8, 10, 19.9, 29.9, 39.9, 50, 60 mol%) | 278.15–323.15 | 0.1 | 4300–51 | [18] |
| [P2224][2-CH3,5-NO2imid] + TG (4.8, 10.2, 20.1, 29.9, 40.1, 50.1 mol%) | 278.15–323.15 | 0.1 | 1540–57 | [18] |
| [pmmim][4-NO2pyra] + TG (5, 10, 20.1, 30.2, 39.9, 49.9, 60 mol%) | 278.15–323.15 | 0.1 | 5420–59 | [18] |
| [TEPAH][2-MI] + NPA + EG | 303.15 | 0.1 | 3.66 | [45] |
| IL–amine | ||||
| [N1111][Lys] + DMEE (95, 90, 80, 60, 40 wt %) | 303–333 | 0.1 | 12.09–4.88, 20.70–6.98, 30.00–9.30, 80.46–20, 101.86–25.35 | [51] |
| [BMIM][BF4] + DETA (94.9, 80.14, 70.02, 60.05, 50.08, 40.95, 30.26, 19.88, 10.52, 5.04 mol%) | 298.15–333.15 | 0.1 | 6.71–2.68, 12.1–4.89, 17.35–6.69, 23.66–8.72, 31.54–11.19, 40.07–13.88, 52.59–17.57, 67.10–21.30, 82.58–24.18, 91.94–24.54 | [53] |
3. DESs-Based Hybrid Solvents
The CO2 solubility data for 33 kinds of DESs-based hybrid solvents, together with viscosities for six types of DESs-based hybrid solvents since 2016, and Henry’s constants for 21 kinds of DES-based hybrid solvents since 2013 have been reported, as summarized in Table 4 and Table 5. The full names of the studied components of DESs are given in Table S1.

Table 4.
CO2 solubilities and viscosities of deep eutectic solvent (DES)-based hybrid solvents.

Table 5.
Henry’s constant of DES–H2O hybrid solvents.
3.1. CO2 Solubility
3.1.1. DES–H2O
The CO2 solubility of DESs using water as a hybrid solvent was investigated by Sarmad et al. at 298.15 K and pressure up to 2 MPa (Table 4) [9]. From this study, the CO2 solubility of DES-based hybrid solvents can be affected by four factors. (1) The first is pressure: the CO2 solubility increased with the increasing pressure. For instance, the CO2 solubility of [TEMA][Cl]-GLY-H2O 1:2:0.11 increased from 0.025 to 0.66 mol/kg at a pressure range of 0.14 to 1.74 MPa. (2) The second factor is the mole ratio of water: the CO2 solubility of [TEMA][Cl]-GLY-H2O 1:2:0.05 first decreased at pressure range of 0.23–0.85 MPa, and then, it increased from 1.25 to 1.98 MPa compared with [TEMA][Cl]-GLY 1:2. Upon increasing the mole ratio of water, the CO2 solubility of [TEMA][Cl]-GLY-H2O 1:2:0.11 significantly enhanced with respect to [TEMA][Cl]-GLY 1:2 and [TEMA][Cl]-GLY-H2O 1:2:0.05. This result agrees with the DES-based hybrid solvent of [BTMA][Cl]-GLY-H2O 1:2:0.011 and 1:2:0.05. (3) The third factor is the type of hydrogen bond acceptor (HBA)—for example, the CO2 solubility of [TEMA][Cl]-GLY-H2O 1:2:0.05 (1.98 MPa, 0.66 mol/kg) > [BTMA][Cl]-GLY-H2O 1:2:0.05 (2.02 MPa, 0.29 mol/kg).
Harifi-Mood et al. investigated the Henry’s constants for [Ch][Cl]-EG aqueous solution at temperatures of 303.15–323.15 K [54]. As shown in Table 5, the results indicate that the Henry’s constant of CO2 increases with increasing water amount in the absorbent, corresponding to a decrease of CO2 solubility. This result agrees with the measured Henry’s constants of [Ch][Cl]-EG, [Ch][Cl]-GLY, and [Ch][Cl]-MA aqueous solution from 303.15 to 313.15 K by Lin et al. [55].
3.1.2. DES–Organic
The CO2 solubilities in DESs–organic hybrid solvents are given in Table 4. The result indicates that the addition of 0.03 mol of acetic acid in [MTPP][Br]-LEV 1:3 significantly enhanced the CO2 solubility and decreased the viscosity compared with [MTPP][Br]-LEV 1:3 [9].
A superbase can participate in the reaction of DES and CO2, thus increasing the CO2 solubility. Bhawna et al. studied the CO2 solubility by three hybrid superbases of TBD, DBN, and DBU with DESs of [Ch][Cl]-Urea 1:2 and [Ch][Cl]-EG 1:2, respectively [25]. The result indicates that all of these three superbases can enhance the CO2 solubility, and among them, TBD has the highest capacity, followed by DBU and DBN. The further addition of glycerol in these hybrid solvents decreased the CO2 solubility. For the effect of these three superbases on different male ratio of DESs, it is found that [Ch][Cl]-MEA 1:2 + DBN (5.11 mol/kg) > [Ch][Cl]-MEA + TBD 1:4 (3.91 mol/kg) > [Ch][Cl]-MEA 1:2 + DBU (3.54 mol/kg). Additionally, the same phenomenon was observed that the addition of glycerol in these [Ch][Cl]-MEA-based hybrid solvents can decrease the CO2 solubility.
An imidazole (Im)-derived DESs of [BMIM][Cl]-Im was synthesized for CO2 capture by hybrid with DBN [56]. These hybrid solvents show remarkable CO2 capture capacity up to 1.00 mol/mol, following the order of DBN-[BMIM][Cl]-Im 1:1:2 > DBN-[BMIM][Cl]-Im 1:1:1 > DBN-[BMIM][Cl]-Im 1:2:1. The theoretical calculation indicates that DBN plays a key role in the absorption process by forming a strong hydrogen bond with the derived [BMIM+-COO−].
In conclusion, the obtained best DES–H2O hybrid solvent is [TEMA][Cl]-GLY-H2O 1:2:0.11 (0.66 mol/kg, 298 K, 1.74 MPa), while it is [Ch][Cl]-MEA 1:2 + DBN 1:1 (5.11 mol/kg, 298 K, 0.1 MPa) for DES–organic hybrid solvent. These values are lower than the best IL–H2O and IL–organic hybrid solvents, respectively.
3.2. Viscosity
The DESs consisting of glycerol as the hydrogen bond donor (HBD) exhibited high viscosity. Meanwhile, their viscosities increased considerably with an increase in the amount of dissolved CO2. As shown in Table 4, using water as a hybrid solvent in glycerol-based DESs can significantly decrease the viscosity of the DES [9]. For example, the viscosity of [BTMA][Cl]-GLY 1:2 decreased from 1017.67 to 70.76 mPa·s when adding a 0.05 molar ratio of water in [BTMA][Cl]-GLY 1:2 (i.e., [BTMA][Cl]-GLY-H2O 1:2:0.05), but limiting the contribution of H2O to CO2 solubility. Meanwhile, increasing the water content of 0.11 mol in [BTMA][Cl]-GLY 1:2 results in a considerably reduced viscosity, which agrees with the results in the [TEMA][Cl]-GLY-H2O system. Additionally, the addition of 0.11 mol of water to the DES of [BHDE][Cl]-GLY 1:3 decreased the viscosity from 32.76 to 17.11 mPa·s at 298.15 K and 0.23–2.02 MPa, and it increased the CO2 solubility from 0.037 to 0.21 mol/kg. The viscosity of the [L-Arg]-GLY 1:6 as a function of water content from 0 to 60 wt % was measured, which indicates that viscosity of the DES decreased sharply with the increase of water contents, giving an option to lower the viscosity [57].
In a word, adding water and organic solvents in DES can significantly decrease the viscosity.
4. Comparison of CO2 Solubility and Viscosity
The obtained best candidates of IL–H2O, IL–organic, IL–amine, DES–H2O, and DES–organic hybrid solvents were compared with each other and their pure ILs and DESs (Figure 4). As shown in Figure 4, for either the IL-based or DES-based hybrid solvents, their CO2 solubilies are higher than their pure IL/DES under the same condition. For example, the CO2 solubility of [DMAPAH][Formate] (2.5:1) + H2O (20 wt %) is 4.61 mol/kg at 298 K and 0.1 MPa, while it is 2.32 mol/kg for [DMAPAH][Formate] (2.5:1). This result indicates that IL/DES-based hybrid solvents are remarkable ones for CO2 capture. Additionally, the IL-based hybrid solvent shows better CO2 capture performance compared with the DES-based hybrid solvent, as shown in Figure 4. Figure 5 gives the comparison of viscosities for these IL/DES-based hybrid solvents and pure IL and DES at 333.15 K and 0.1 MPa. As shown in Figure 5, the addition of hybrid solvents can significantly decrease the viscosity compared to pure ILs and DESs, which are beneficial to accelerate mass transfer during capturing CO2.
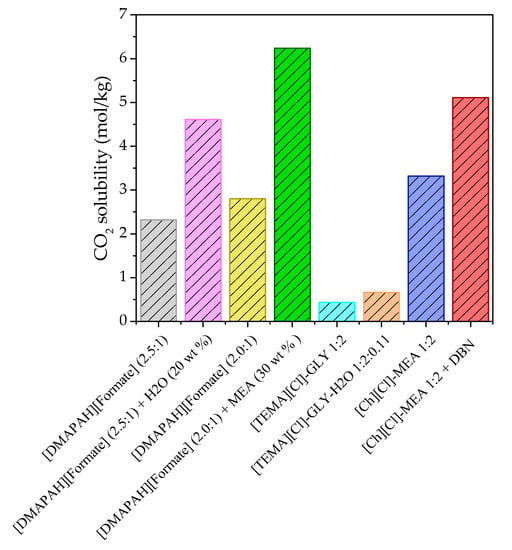
Figure 4.
Comparison of CO2 solubility for IL and IL-based hybrid solvent, as well as DES and DES-based hybrid solvent.
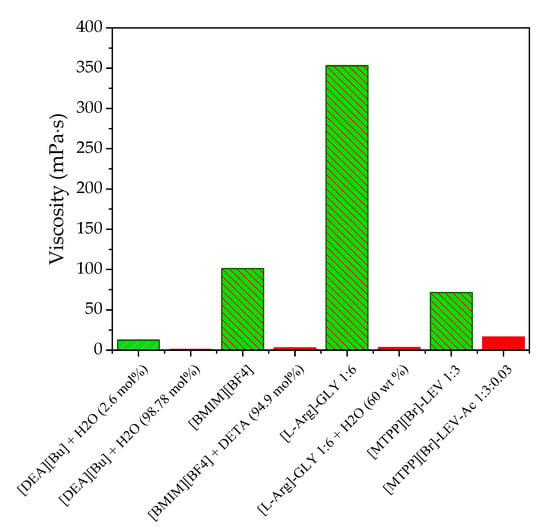
Figure 5.
Comparison of viscosity for IL and IL-based hybrid solvent, as well as DES and DES-based hybrid solvent at 333.15 K and 0.1 MPa.
Viscosity is the key factor for impeding the mass transfer of gas in absorbent [58]. For example, Gómez-Coma et al. investigated the viscosity and mass transfer performance of [EMIM][Ac] + H2O for CO2 capture [59], finding that the viscosity of [EMIM][Ac] + H2O decreased from 150 to 20 mPa·s with the increase of water content from 0–40 wt %. For the mass transfer coefficient, it is first increased from 1.7 × 10−5 to 9.34 × 10−5 m·s−1 with the increasing of water from 0 to 30 wt % in [EMIM][Ac] and then decreased to 6.81 × 10−5 m·s−1 when water content up to 40 wt %. Huang et al. evidenced that the low viscosity of IL–MEA aqueous solution corresponds to a high mass transfer performance [60], i.e., [EMIM][Br] (20 wt %) + MEA (5 wt %) + H2O (75 wt %) (11.57 × 106 mol·m−3·s−1·Pa−1, 1.23 mPa·s) > [BMIM][Br] (20 wt %) + MEA (5 wt %) + H2O (75 wt %) (11.04 × 106 mol·m−3·s−1·Pa−1, 1.3 mPa·s) > [EMIM][Br] (30 wt %) + MEA (5 wt %) + H2O (65 wt %) (9.86 × 106 mol·m−3·s−1·Pa−1, 1.42 mPa·s) > [BMIM][Br] (30 wt %) + MEA (5 wt %) + H2O (65 wt %) (9.67 × 106 mol·m−3·s−1·Pa−1, 1.6 mPa·s). A similar phenomenon can be found in DESs hybrid solvent. Ma et al. indicated that a small amount of water in [BTMA][Cl]-GLY 1:2 not only decreases the viscosity but also improves the CO2 solubility due to the increase of the mass transfer [61], while excess water in [BTMA][Cl]-GLY 1:2 results in a decrease of CO2 solubility, which is in agreement with Li et al. [12].
5. Conclusions
This review summarizes the research work on developing ILs/DESs-based hybrid solvents (i.e., IL–H2O, IL–organic/organic aqueous solution, IL–amine, DES–H2O, and DES–organic) for CO2 capture, including CO2 solubility, Henry’s constant, and viscosity. The results illustrate that the addition of hybrid solvents to ILs and DESs can decrease the viscosity and enhance the CO2 solubility. IL–amine based hybrid solvents are super to IL–H2O and IL–organic/organic aqueous solution, and some of the IL-based hybrid solvents show better performance than that of DES-based hybrid solvents. Additionally, some of the IL/DES hybrid solvents have higher CO2 solubility compared to their pure IL/DES, indicating that the addition of hybrid solvent to IL/DES is possible to develop greener and more efficient absorbents for CO2 capture. To develop the efficient IL/DES hybrid solvents for CO2 capture, the following aspects are suggested for consideration to decrease the viscosity and increase the CO2 solubility: (1) hybrid of functional ILs/DESs that have high CO2 solubilities with a certain amount of water; (2) the addition of organic solvent which has a small molecular weight to the ILs/DESs; and (3) applying amine solvent which has good CO2 capture capacity to ILs and DESs.
Supplementary Materials
The following is available online at https://www.mdpi.com/2073-4352/10/11/978/s1, Table S1: Full names and abbreviations of ILs, components of DESs and hybrid solvents.
Author Contributions
Writing—original draft preparation, Y.L.; investigation, Z.D., writing—review and editing, F.D., conceptualization and supervision, X.J. All authors have read and agreed to the published version of the manuscript.
Funding
This work is financially supported by Carl Tryggers Stiftelse foundation (No. 18:175). X.J. thanks the financial support from the Swedish Energy Agency (No. P47500-1) and K. C. Wang Education Foundation (No. GJTD-2018-04). F.D. thanks the financial support from the National Nature Science Foundation of China (21808223).
Conflicts of Interest
The author declares no conflict of interest.
References
- Zhou, W.; Wang, J.; Chen, P.; Ji, C.; Kang, Q.; Lu, B.; Li, K.; Liu, J.; Ruan, R. Bio-mitigation of carbon dioxide using microalgal systems: Advances and perspectives. Renew. Sustain. Energy Rev. 2017, 76, 1163–1175. [Google Scholar] [CrossRef]
- MacDowell, N.; Florin, N.; Buchard, A.; Hallett, J.; Galindo, A.; Jackson, G.; Adjiman, C.S.; Williams, C.K.; Shah, N.; Fennell, P. An overview of CO2 capture technologies. Energy Environ. Sci. 2010, 3, 1645. [Google Scholar] [CrossRef]
- Oko, E.; Zacchello, B.; Wang, M.H.; Fethi, A. Process analysis and economic evaluation of mixed aqueous ionic liquid and monoethanolamine (MEA) solvent for CO2 capture from a coke oven plant. Greenh. Gases 2018, 8, 686–700. [Google Scholar] [CrossRef]
- Kothandaraman, A.; Nord, L.; Bolland, O.; Herzog, H.J.; McRae, G.J. Comparison of solvents for post-combustion capture of CO2 by chemical absorption. Energy Procedia 2009, 1, 1373–1380. [Google Scholar] [CrossRef]
- Lawal, A.; Wang, M.; Stephenson, P.; Obi, O. Demonstrating full-scale post-combustion CO2 capture for coal-fired power plants through dynamic modelling and simulation. Fuel 2012, 101, 115–128. [Google Scholar] [CrossRef]
- Ramdin, M.; de Loos, T.W.; Vlugt, T.J.H. State-of-the-art of CO2 capture with ionic liquids. Ind. Eng. Chem. Res. 2012, 51, 8149–8177. [Google Scholar] [CrossRef]
- Zeng, S.J.; Zhang, X.P.; Bai, L.; Zhang, X.C.; Wang, H.; Wang, J.J.; Bao, D.; Li, M.D.; Liu, X.Y.; Zhang, S.J. Ionic-liquid-based CO2 capture systems: Structure, interaction and process. Chem. Rev. 2017, 117, 9625–9673. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.J.; Cui, G.K.; Wang, H.Y.; Li, Z.Y.; Wang, J.J. Tuning ionic liquids with imide-based anions for highly efficient CO2 capture through enhanced cooperations. J. CO2 Util. 2018, 28, 299–305. [Google Scholar] [CrossRef]
- Sarmad, S.; Xie, Y.J.; Mikkola, J.-P.; Ji, X.Y. Screening of deep eutectic solvents (DESs) as green CO2 sorbents: From solubility to viscosity. New J. Chem. 2017, 41, 290–301. [Google Scholar] [CrossRef]
- Liu, H.J.; Pan, Y.; Yao, H.; Zhang, Y. Enhancement of carbon dioxide mass transfer rate by (ionic liquid)-in-water emulsion. Adv. Mater. Res. 2014, 881–883, 113–117. [Google Scholar] [CrossRef]
- Chu, C.Y.; Zhang, F.B.; Zhu, C.Y.; Fu, T.T.; Ma, Y.G. Mass transfer characteristics of CO2 absorption into 1-butyl-3-methylimidazolium tetrafluoroborate aqueous solution in microchannel. Int. J. Heat Mass Trans. 2019, 128, 1064–1071. [Google Scholar] [CrossRef]
- Li, F.F.; Bai, Y.G.; Zeng, S.J.; Liang, X.D.; Wang, H.; Huo, F.; Zhang, X.P. Protic ionic liquids with low viscosity for efficient and reversible capture of carbon dioxide. Int. J. Greenh. Gas Control 2019, 90, 102801. [Google Scholar] [CrossRef]
- Zhang, X.; Bao, D.; Huang, Y.; Dong, H.F.; Zhang, X.P.; Zhang, S.J. Gas-liquid mass-transfer properties in CO2 absorption system with ionic liquids. AIChE J. 2014, 60, 2929–2939. [Google Scholar] [CrossRef]
- Alcantara, M.L.; Santos, J.P.; Loreno, M.; Ferreira, P.I.S.; Paredes, M.L.L.; Cardozo-Filho, L.; Silva, A.K.; Lião, L.M.; Pires, C.A.M.; Mattedi, S. Low viscosity protic ionic liquid for CO2/CH4 separation: Thermophysical and high-pressure phase equilibria for diethylammonium butanoate. Fluid Phase Equilibr. 2018, 459, 30–43. [Google Scholar] [CrossRef]
- Li, J.; You, C.J.; Chen, L.F.; Ye, Y.M.; Qi, Z.W.; Sundmacher, K. Dynamics of CO2 Absorption and Desorption Processes in Alkanolamine with Cosolvent Polyethylene Glycol. Ind. Eng. Chem. Res. 2012, 51, 12081–12088. [Google Scholar] [CrossRef]
- Yang, Z.-Z.; He, L.-N.; Zhao, Y.-N.; Li, B.; Yu, B. CO2 capture and activation by superbase/polyethylene glycol and its subsequent conversion. Energy Environ. Sci. 2011, 4, 3971. [Google Scholar] [CrossRef]
- Liu, S.D.; Li, H.; Chen, Y.F.; Yang, Z.H.; Wang, H.L.; Ji, X.Y.; Lu, X.H. Improved CO2 separation performance of aqueous choline-glycine solution by partially replacing water with polyethylene glycol. Fluid Phase Equilibr. 2019, 495, 12–20. [Google Scholar] [CrossRef]
- Fillion, J.J.; Bennett, J.E.; Brennecke, J.F. The viscosity and density of ionic liquid + tetraglyme mixtures and the effect of tetraglyme on CO2 Solubility. J. Chem. Eng. Data 2017, 62, 608–622. [Google Scholar] [CrossRef]
- Sairi, N.A.; Ghani, N.A.; Aroua, M.K.; Yusoff, R.; Alias, Y. Low pressure solubilities of CO2 in guanidinium trifluoromethanesulfonate-MDEA systems. Fluid Phase Equilibr. 2015, 385, 79–91. [Google Scholar] [CrossRef]
- Sairi, N.A.; Yusoff, R.; Alias, Y.; Aroua, M.K. Solubilities of CO2 in aqueous N-methyldiethanolamine and guanidinium trifluoromethanesulfonate ionic liquid systems at elevated pressures. Fluid Phase Equilibr. 2011, 300, 89–94. [Google Scholar] [CrossRef]
- Yang, J.; Yu, X.H.; Yan, J.Y.; Tu, S.-T. CO2 capture using amine solution mixed with ionic liquid. Ind. Eng. Chem. Res. 2014, 53, 2790–2799. [Google Scholar] [CrossRef]
- Xu, F.; Gao, H.S.; Dong, H.F.; Wang, Z.L.; Zhang, X.P.; Ren, B.Z.; Zhang, S.J. Solubility of CO2 in aqueous mixtures of monoethanolamine and dicyanamide-based ionic liquids. Fluid Phase Equilibr. 2014, 365, 80–87. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, F.; Huang, K.; Ma, J.-W.; Wu, Y.-T.; Zhang, Z.-B. Absorption of CO2 in amino acid ionic liquid (AAIL) activated MDEA solutions. Int. J. Greenh. Gas Control 2013, 19, 379–386. [Google Scholar] [CrossRef]
- Baj, S.; Siewniak, A.; Chrobok, A.; Krawczyk, T.; Sobolewski, A. Monoethanolamine and ionic liquid aqueous solutions as effective systems for CO2 capture. J. Chem. Technol. Biotechnol. 2013, 88, 1220–1227. [Google Scholar] [CrossRef]
- Bhawna; Pandey, A.; Pandey, S. Superbase-added choline chloride-based deep eutectic solvents for CO2 capture and sequestration. ChemistrySelect 2017, 2, 11422–11430. [Google Scholar] [CrossRef]
- Huang, K.; Chen, F.-F.; Tao, D.-J.; Dai, S. Ionic liquid–formulated hybrid solvents for CO2 capture. Curr. Opin. Green Sustain. 2017, 5, 67–73. [Google Scholar] [CrossRef]
- Zhang, F.; Fang, C.-G.; Wu, Y.-T.; Wang, Y.-T.; Li, A.-M.; Zhang, Z.-B. Absorption of CO2 in the aqueous solutions of functionalized ionic liquids and MDEA. Chem. Eng. J. 2010, 160, 691–697. [Google Scholar]
- Babamohammadi, S.; Shamiri, A.; Aroua, M.K. A review of CO2 capture by absorption in ionic liquid-based solvents. Rev. Chem. Eng. 2015, 31, 383–412. [Google Scholar] [CrossRef]
- Lian, S.H.; Song, C.F.; Liu, Q.L.; Duan, E.H.; Ren, H.W.; Kitamura, Y. Recent advances in ionic liquids-based hybrid processes for CO2 capture and utilization. J. Environ. Sci. 2021, 99, 281–295. [Google Scholar] [CrossRef]
- Vijayaraghavan, R.; Oncsik, T.; Mitschke, B.; MacFarlane, D.R. Base-rich diamino protic ionic liquid mixtures for enhanced CO2 capture. Sep. Purif. Technol. 2018, 196, 27–31. [Google Scholar] [CrossRef]
- Huang, Y.J.; Cui, G.K.; Zhao, Y.L.; Wang, H.Y.; Li, Z.Y.; Dai, S.; Wang, J.J. Reply to the correspondence on “Preorganization and cooperation for highly efficient and reversible capture of low-concentration CO2 by ionic liquids”. Angew. Chem. 2019, 58, 386–389. [Google Scholar] [CrossRef]
- Yasaka, Y.; Kimura, Y. Effect of temperature and water concentration on CO2 absorption by tetrabutylphosphonium formate ionic liquid. J. Chem. Eng. Data 2016, 61, 837–845. [Google Scholar] [CrossRef]
- Simon, N.M.; Zanatta, M.; dos Santos, F.P.; Corvo, M.C.; Cabrita, E.J.; Dupont, J. Carbon dioxide capture by aqueous ionic liquid solutions. ChemSusChem 2017, 10, 4927–4933. [Google Scholar] [CrossRef]
- Chen, Y.; Guo, K.H.; Huangpu, L. Experiments and modeling of absorption of CO2 by amino-cation and amino-anion dual functionalized ionic liquid with the addition of aqueous medium. J. Chem. Eng. Data 2017, 62, 3732–3743. [Google Scholar] [CrossRef]
- Aghaie, M.; Rezaei, N.; Zendehboudi, S. Assessment of carbon dioxide solubility in ionic liquid/toluene/water systems by extended PR and PC-SAFT EOSs: Carbon capture implication. J. Mol. Liq. 2019, 275, 323–337. [Google Scholar] [CrossRef]
- Huang, Q.S.; Jing, G.H.; Zhou, X.B.; Lv, B.H.; Zhou, Z.M. A novel biphasic solvent of amino-functionalized ionic liquid for CO2 capture: High efficiency and regenerability. J. CO2 Util. 2018, 25, 22–30. [Google Scholar] [CrossRef]
- Qian, Y.H.; Jing, G.H.; Lv, B.H.; Zhou, Z.M. Exploring the general characteristics of amino-acid-functionalized ionic liquids through experimental and quantum chemical calculations. Energy Fuels 2017, 31, 4202–4210. [Google Scholar] [CrossRef]
- Zhou, X.B.; Jing, G.H.; Liu, F.; Lv, B.H.; Zhou, Z.M. Mechanism and kinetics of CO2 absorption into an aqueous solution of a triamino-functionalized ionic liquid. Energy Fuels 2017, 31, 1793–1802. [Google Scholar] [CrossRef]
- Taheri, M.; Dai, C.N.; Lei, Z.G. CO2 capture by methanol, ionic liquid, and their binary mixtures: Experiments, modeling, and process simulation. AIChE J. 2018, 64, 2168–2180. [Google Scholar] [CrossRef]
- Li, X.Y.; Hou, M.Q.; Zhang, Z.F.; Han, B.X.; Yang, G.Y.; Wang, X.L.; Zou, L.Z. Absorption of CO2 by ionic liquid/polyethylene glycol mixture and the thermodynamic parameters. Green Chem. 2008, 10, 879. [Google Scholar] [CrossRef]
- Li, J.; Dai, Z.D.; Usman, M.; Qi, Z.W.; Deng, L.Y. CO2 /H2 separation by amino-acid ionic liquids with polyethylene glycol as co-solvent. Int. J. Greenh. Gas Con. 2016, 45, 207–215. [Google Scholar] [CrossRef]
- Chen, Y.; Hu, H. Carbon dioxide capture by diethylenetriamine hydrobromide in nonaqueous systems and phase-change formation. Energy Fuels 2017, 31, 5363–5375. [Google Scholar] [CrossRef]
- Lepre, L.F.; Pison, L.; Siqueira, L.J.A.; Ando, R.A.; Costa Gomes, M.F. Improvement of carbon dioxide absorption by mixing poly(ethylene glycol) dimethyl ether with ammonium-based ionic liquids. Sep. Purif. Technol. 2018, 196, 10–19. [Google Scholar] [CrossRef]
- Jiang, Y.F.; Taheri, M.; Yu, G.Q.; Zhu, J.Q.; Lei, Z.G. Experiments, modeling, and simulation of CO2 dehydration by ionic liquid, triethylene glycol, and their binary mixtures. Ind. Eng. Chem. Res. 2019, 58, 15588–15597. [Google Scholar] [CrossRef]
- Liu, F.; Shen, Y.; Shen, L.; Sun, C.; Chen, L.; Wang, Q.L.; Li, S.J.; Li, W. Novel amino-functionalized ionic liquid/organic solvent with low viscosity for CO2 capture. Environ. Sci. Technol. 2020, 54, 3520–3529. [Google Scholar] [CrossRef]
- Huang, Q.; Li, Y.; Jin, X.B.; Zhao, D.; Chen, G.Z. Chloride ion enhanced thermal stability of carbon dioxide captured by monoethanolamine in hydroxyl imidazolium based ionic liquids. Energy Environ. Sci. 2011, 4, 2125. [Google Scholar] [CrossRef]
- Fu, D.; Hao, H.M.; Liu, F. Experiment and model for the viscosity of carbonated 2-amino-2-methyl-1-propanol-monoethanolamine and 2-amino-2-methyl-1-propanol-diethanolamine aqueous solution. J. Mol. Liq. 2013, 188, 37–41. [Google Scholar] [CrossRef]
- Goodrich, B.F.; de la Fuente, J.C.; Gurkan, B.E.; Zadigian, D.J.; Price, E.A.; Huang, Y.; Brennecke, J.F. Experimental measurements of amine-functionalized anion-tethered ionic liquids with carbon dioxide. Ind. Eng. Chem. Res. 2011, 50, 111–118. [Google Scholar] [CrossRef]
- Liu, F.; Jing, G.H.; Zhou, X.B.; Lv, B.H.; Zhou, Z.M. Performance and mechanisms of triethylene tetramine (TETA) and 2-Amino-2-methyl-1-propanol (AMP) in aqueous and nonaqueous solutions for CO2 capture. ACS Sustain. Chem. Eng. 2017, 6, 1352–1361. [Google Scholar] [CrossRef]
- Zheng, W.-T.; Huang, K.; Wu, Y.-T.; Hu, X.-B. Protic ionic liquid as excellent shuttle of MDEA for fast capture of CO2. AIChE J. 2018, 64, 209–219. [Google Scholar] [CrossRef]
- Meng, Y.N.; Wang, X.D.; Zhang, F.; Zhang, Z.B.; Wu, Y.T. IL-DMEE Nonwater system for CO2 capture: Absorption performance and mechanism investigations. Energy Fuels 2018, 32, 8587–8593. [Google Scholar] [CrossRef]
- Salleh, R.M.; Jamaludin, S.N. Thermodynamic equilibrium solubility of diethanolamine–N-butyl-1-methylpyrrolidinium dicyanamide [DEABMPYRR DCA] mixtures for carbon dioxide capture. IOP Conf. Ser. Mater. Sci. Eng. 2018, 358, 012010.53. [Google Scholar] [CrossRef]
- Ahmad, W.; Al-Ajmi, A.; Vakili-Nezhaad, G.R. Investigation of physico-chemical properties for the 1-butyl-3-methylimidazolium tetrafluoroborate ([Bmim][BF4])–diethylenetriamine (DETA) system for CO2 capture. J. Solut. Chem. 2019, 48, 578–610. [Google Scholar] [CrossRef]
- Harifi-Mood, A.R.; Mohammadpour, F.; Boczkaj, G. Solvent dependency of carbon dioxide Henry’s constant in aqueous solutions of choline chloride-ethylene glycol based deep eutectic solvent. J. Mol. Liq. 2020, 319, 114173. [Google Scholar] [CrossRef]
- Lin, C.-M.; Leron, R.B.; Caparanga, A.R.; Li, M.-H. Henry’s constant of carbon dioxide-aqueous deep eutectic solvent (choline chloride/ethylene glycol, choline chloride/glycerol, choline chloride/malonic acid) systems. J. Chem. Thermodyn. 2014, 68, 216–220. [Google Scholar] [CrossRef]
- Zhang, N.; Huang, Z.H.; Zhang, H.M.; Ma, J.W.; Jiang, B.; Zhang, L.H. Highly efficient and reversible CO2 capture by task-specific deep eutectic solvents. Ind. Eng. Chem. Res. 2019, 58, 13321–13329. [Google Scholar] [CrossRef]
- Ren, H.W.; Lian, S.H.; Wang, X.; Zhang, Y.; Duan, E.H. Exploiting the hydrophilic role of natural deep eutectic solvents for greening CO2 capture. J. Clean. Prod. 2018, 193, 802–810. [Google Scholar] [CrossRef]
- Siani, G.; Tiecco, M.; Di Profio, P.; Guernelli, S.; Fontana, A.; Ciulla, M.; Canale, V. Physical absorption of CO2 in betaine/carboxylic acid-based natural deep eutectic solvents. J. Mol. Liq. 2020, 315, 113708. [Google Scholar] [CrossRef]
- Gómez-Coma, L.; Garea, A.; Irabien, Á. Hybrid solvent ([emim][Ac]+water) to improve the CO2 capture efficiency in a PVDF hollow fiber contactor. ACS Sustain. Chem. Eng. 2017, 5, 734–743. [Google Scholar] [CrossRef]
- Huang, Z.L.; Deng, Z.Y.; Ma, J.Y.; Qin, Y.H.; Zhang, Y.; Luo, Y.B.; Wu, Z.K. Comparison of mass transfer coefficients and desorption rates of CO2 absorption into aqueous MEA + ionic liquids solution. Chem. Eng. Res. Des. 2017, 117, 66–72. [Google Scholar] [CrossRef]
- Ma, C.Y.; Sarmad, S.; Mikkola, J.-P.; Ji, X.Y. Development of low-cost deep eutectic solvents for CO2 capture. Energy Procedia 2017, 142, 3320–3325. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).