1. Introduction
Titanium has attracted much attention in various fields such as aerospace [
1], marine industry [
2], electronics [
3,
4,
5,
6,
7], and medical industry [
8] due to its high strength, lightness, corrosion-resistance, bio-compatibility, and luxury color. Recently, titanium has been used not only in the industrial fields but also in civil and construction areas. For example, the Guggenheim museum, the best architecture in the twentieth century, is covered with titanium on its exterior part called “metal flower”. The color of titanium was tuned by anodic oxidation by which the structure color of anodized-titanium showed various colors, like a chameleon, concerning changes in the angle of sunshine [
9]. Together with the multifunctionality of the material itself, the aesthetic element gained much significance in modern technology.
To obtain various titanium colors, various thicknesses of titanium oxide layers are required [
10,
11,
12]. The most popular technique for coloring the titanium is the anodization method, which enables titanium surface to form a thin oxide layer with a rough structure [
13]. When the degree of roughness is changed by the applied voltage, the structure color of titanium could be changed. However, these approaches require preparation step, acidic condition, low temperature, and applied voltages. Recently, plasma treatment and laser-induced methods have been developed to form the oxide layer onto the metal surface [
14]. The plasma treatment catalyzes the reaction within a few minutes; however, it requires toxic gases and elevated temperature to react on titanium surfaces. In the laser-induced method, the formation area of the oxide layer depends on the tiny spot size of the layer and requires complicated optical equipment. Therefore, obtaining an environmentally friendly, fast, and straightforward fabrication method remains a challenge.
To endow the multifunctional properties to colored-titanium, various colors and superhydrophobicity are required. The structure or roughness of titanium has to be changed in advance. The hydrophobic silane could then react to the slightly oxidized titanium surface via chemical covalent bonding [
15]. The superhydrophobic surface enhances the “self-cleaning” by contaminants being attracted to repellent for water and its low surface energy [
16].
In this study, we introduced the torching method to fabricate the titanium’s rough surface within 1 min. The torching method for coloring enables the fast and straightforward reaction in less than one minute to form the titanium dioxide, which provides roughness to the surface titanium. A smooth titanium surface was obtained by electrical polishing in order to investigate the surface of the oxide layer with respect to the torching time. The roughness and phase of oxide layers by torching method were studied using SEM, Atomic Force Microscope (AFM), and Raman spectroscopy. Subsequently, the octadecyltriethoxylsilane (ODTS) was covalently bound to the oxide layers to endow the hydrophobic property. For superhydrophobicity, titanium was rubbed by the sandpaper to provide macroscopic roughness and the oxide layer provided microscopic roughness which is formed during torching the titanium. A standard cross-cut tape test was conducted to test the mechanical stability of the oxide layer and ODTS onto the substrate.
2. Materials and Methods
2.1. Materials
Titanium sheet with 300 μm thickness was obtained from Sigma-Aldrich. Deionized water (18.3 MΩ∙cm) was obtained from a reverse osmosis water system (Human Science, Hanam, Korea). The torch was purchased from Daehan science (Sungnam, Korea). Octadecyltriethoxylsilane (ODTS) was purchased from Polypore (Singapore). For polishing the titanium surface, perchloric acid, 1-butanol, and methanol were purchased from Merck (Darmstadt, Germany). The CW-100 and CW-400 sandpaper were obtained from HAWK (Sejong, Korea).
2.2. Fabrication of Coloring Titanium and ODTS Treatment
1.5 × 1.5 cm2 titanium substrates were electrically polished to obtain a smooth surface. The substrate was connected to the cathode while carbon electrode was connected to the anode in a solution of 1:6:9 ratio of perchloric acid, 1-butanol, and methanol under 20 V at −20 °C for 10 min. For macroscopic roughness, the other substrates were rubbed by CW-100 and CW-400 sandpaper, sequentially. Both electropolished and rubbed substrates were reacted less than 1 min by torch. The electropolished and rubbed titanium torched with various torching time were denoted as Ti-pX and Ti-sX, respectively (X = 0, 1, 2, 3, 4, 5, where 0 means no torching while 1, 2, 3, 4 and 5 indicate 5, 10, 15, 20, and 40 s torching). According to the torching time, the temperature of the torch flame was varied from 200 °C to 700 °C. Then, each substrate was immersed in 10 mM ODTS toluene solutions for 3 h.
2.3. Characterization
The morphology and phase of titanium were characterized by field-emission scanning electron microscopy (SEM), atomic force microscopy (AFM), and Raman spectroscopy with 488 nm wavelength. After the treatment by ODTS, the contact angle of each substrate was measured using SmartDrop goniometer (Femtofab, Korea). The mechanical stability of ODTS and titanium nanostructure were tested based on ASTM standard D 3359-02 method.
3. Results and Discussion
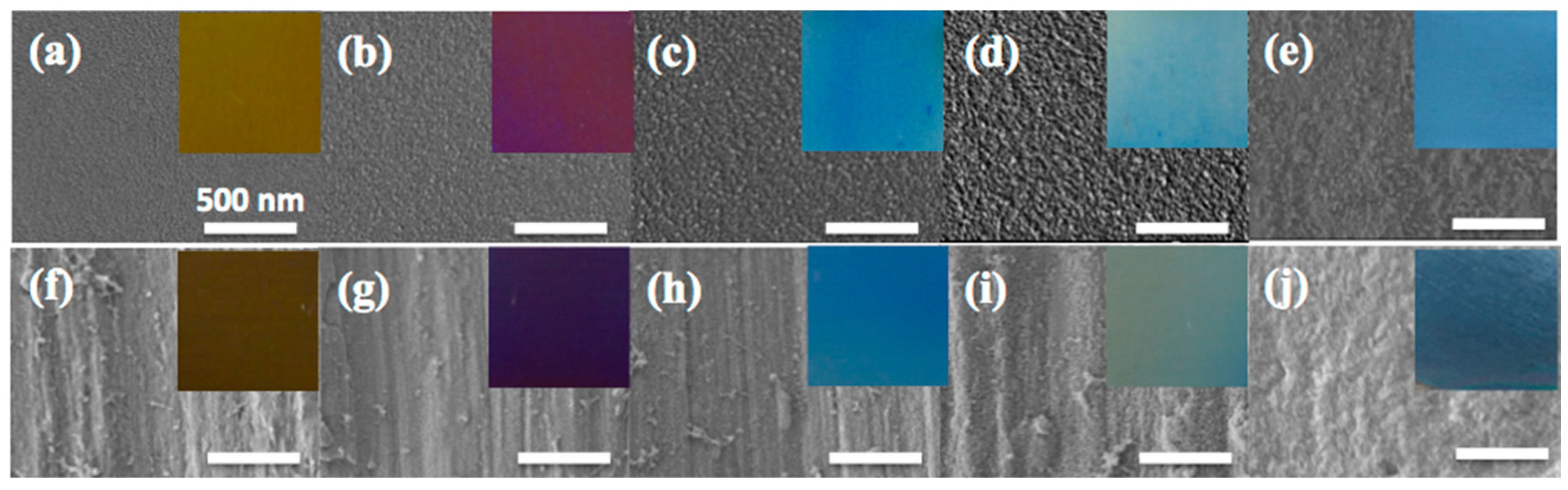
Figure 1 shows the SEM and optical images of torched-titanium substrates with different reaction times. The polished-titanium substrates were denoted as Ti-p1, Ti-p2, Ti-p3, Ti-p4, Ti-p5 while the rubbed titanium substrates were denoted as Ti-s1, Ti-s2, Ti-s3, Ti-s4, Ti-s5. As can be seen from
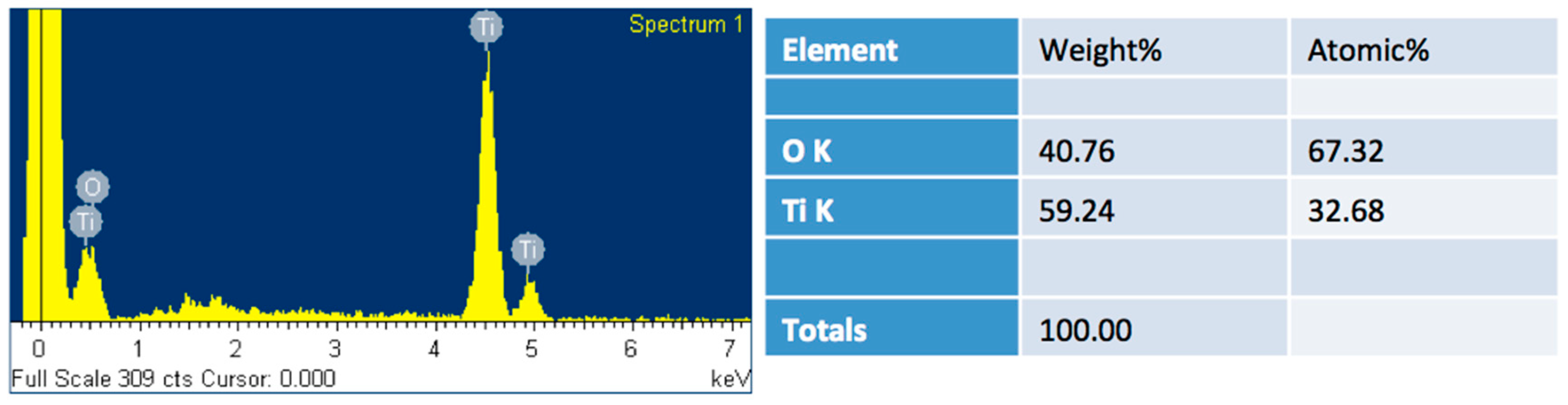
Figure 1a–d, the polished-titanium substrates became rougher after torching the substrate. The substrates’ roughness increased upon increasing the torching time. The roughness will be discussed later in Figure 3. EDS analysis, as shown in the
Appendix A (
Figure A1), revealed that the torched-surface is composed of titanium and oxygen with titanium to oxygen atomic percentage ratio of 1:2. It implies that the titanium surfaces reacted with oxygen and formed titanium dioxide during the reaction. The phase of the oxide layer will be discussed by Raman spectroscopy in
Figure 2. During longer reaction times, the substrate could react with more oxygen at the ambient conditions.
Similarly, the rubbed titanium substrates showed the particles after torching and also the macroscale roughness by sandpaper. From the optical images in the inset of
Figure 1, the color of the torched-titanium substrate was different regarding its morphology and roughness. As the reaction time increased, the colors changed to yellow, wine, blue, sky blue, and dark blue. The surface morphology and roughness are related to the light reflectance, so that the relationship of the roughness of the oxide layer will be discussed in
Figure 3. It is noted that the macroscale roughness (i.e., the inset in the second row of
Figure 1) scattered more light than the polished substrates (i.e., the inset in the first row of
Figure 1) so that the rubbed by sandpaper substrates exhibited darker color than polished substrates [
17].
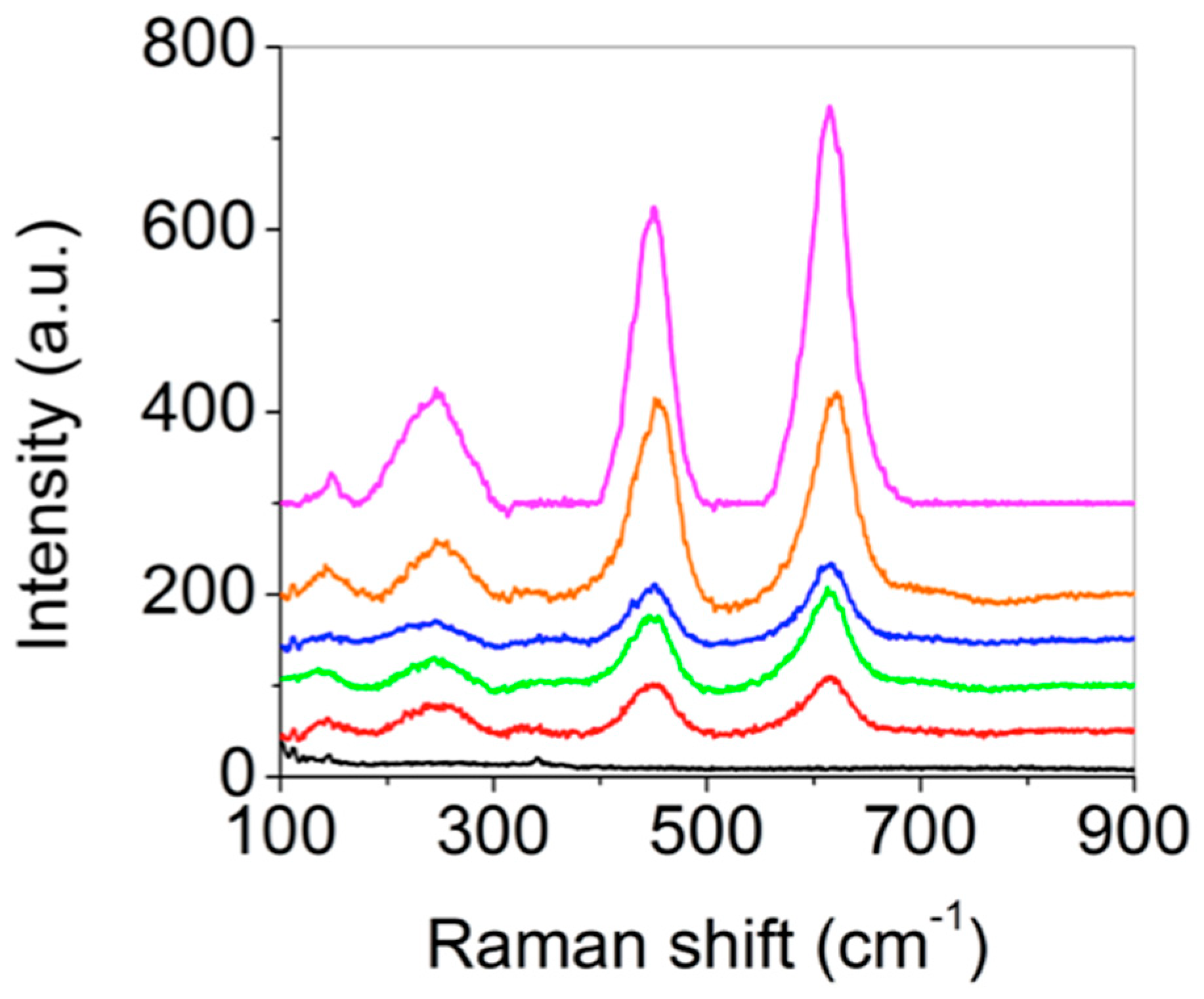
To explore the phase of oxide layer by the torch, Ti-pX substrates were used for Raman spectroscopy instead of Ti-sX due to the reduced light scattering. In
Figure 2, Ti-p0 did not exhibit any peaks while Ti-p1, Ti-p2, Ti-p3, Ti-p4, and Ti-p5 showed four characteristic peaks that were assigned at 146 cm
−1 (
B1g), 443 cm
−1 (
Eg), 609 cm
−1 (
A1g), and 243 cm
−1 (second-order scattering). The phase of titanium oxide layer is assigned to rutile phase, and it is well-matched with existing literature [
18,
19]. It is noted that x-ray diffraction (XRD) measurement could not define the phase of the torched-titanium substrates. XRD peaks showed no difference between the metal titanium and torched-titanium (results are not shown here). It implies that the oxide layers formed by the torching method were mainly amorphous. As torching time increased, the peak intensities also increased. It infers that the size of titanium oxide increased [
18,
20].
To investigate the torching effect on roughness, the polished substrates (Ti-pX substrates) were used instead of measuring the substrates rubbed by sandpaper (Ti-sX substrates).
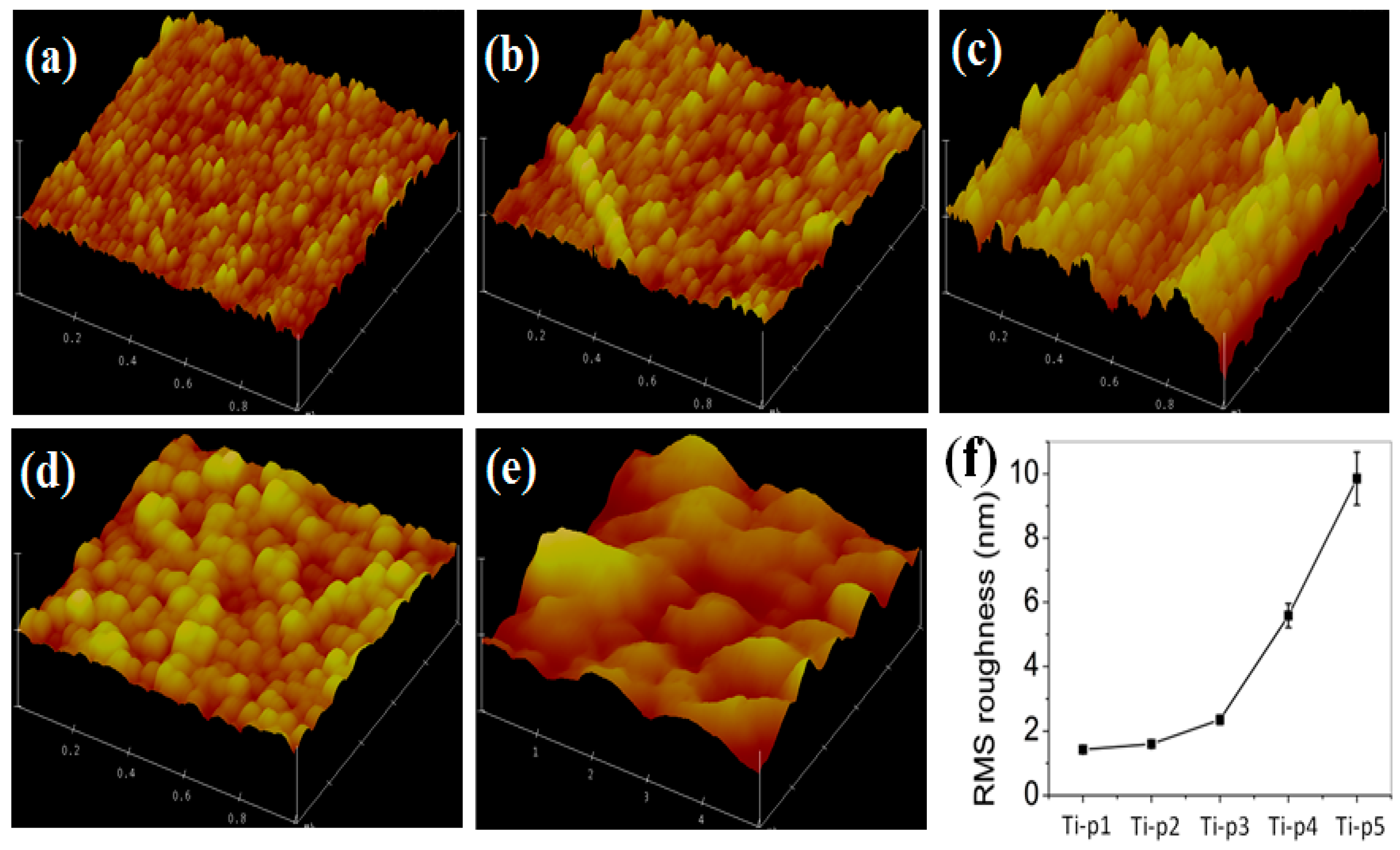
Figure 3 presents the AFM images, RMS roughness, and optical images of the torched-titanium substrates with various reaction times. Similar to that of SEM images shown in
Figure 1, the particle-like morphology appeared in
Figure 3a–e. However, in
Figure 3d, the particles aggregated onto the substrate over 20 s reaction time. This indicates that increasing the torching time leads to increasing the reaction temperature so that the titanium oxide becomes large. The RMS roughness increased from 1.423 ± 0.0845 nm to 9.854 ± 0.825 nm when increasing reaction time due to increasing the particle size, as shown in
Figure 3f. Torched substrates with different roughness showed different colors because the roughened surfaces scattered the incident light more efficiently than the bare surface. Another reason could be attributed to the difference in refractive index (
n) of titanium (
n = 2.6112) and titanium dioxide (
n = 2.6142) [
21].
Both electropolished (Ti-pX) and rubbed (Ti-sX) torched-titanium substrates were functionalized with ODTS to investigate the superhydrophobic property.
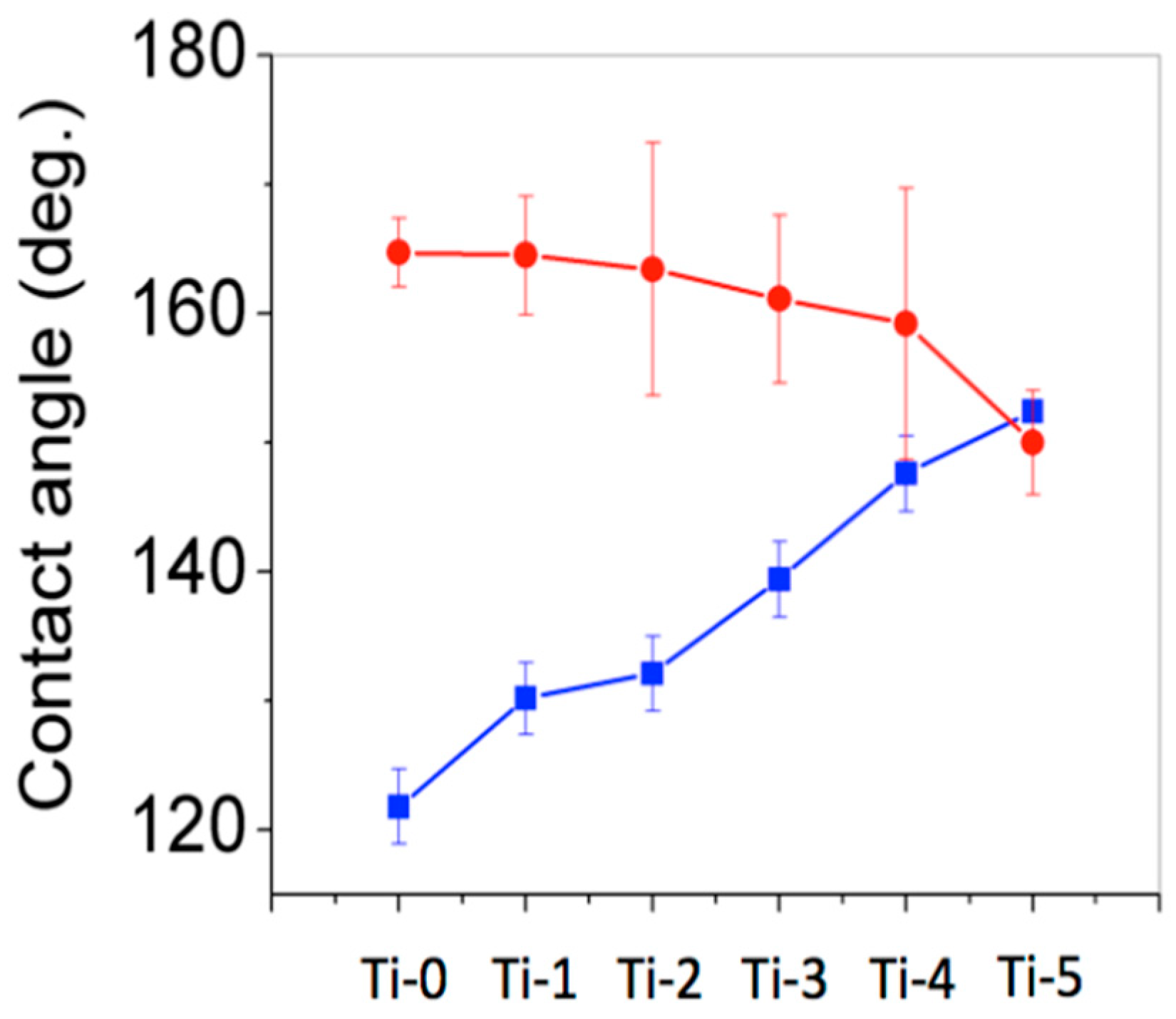
Figure 4 shows that the water contact angle increased as the torching time increased for the electropolished substrates while the water contact angle slightly decreased for rubbed by sandpaper substrates. The standard deviation of Ti-sX substrates was larger than that of Ti-pX. This is because the surfaces of Ti-sX were more irregular than that of Ti-pX. For Ti-pX substrates, the contact angle increased by increasing torching time because the torching led to roughness increase in the microscopic scale. On the other hands, Ti-sX substrates showed superhydrophobic property in an untorched substrate because rubbing by sandpaper provided roughness in both macroscopic and microscopic scales. The slight decrease in contact angle is a result of the formation and aggregation of the oxide layer, forming a relatively smooth surface in the microscopic scale. The results revealed that the sandpaper method offered a fast and simple route to endow the superhydrophobic property compared to the electropolishing method.
The cross-cut tape test was conducted onto the torched-titanium substrate to evaluate the mechanical stability of the torched-titanium substrate. The substrates were scratched in cross-cut using a cutter, and then the adhesion of titanium dioxide layer was tested using adhesion tape.
Figure 5a,b showed the mechanical stability of titanium dioxide layer on macroscopic scale, before and after the adhesion test. After the adhesion test, the optical images revealed that it was stable under pressure stress and maintained its unique color. The titanium dioxide is formed and strongly bound onto the titanium substrate. However, the microscope images have a limitation in the macroscopic scale. To investigate the microscopic mechanical stability test, the mechanical stability test was conducted for the mechanical stability of titanium dioxide layer and ODTS on titanium dioxide layer.
Figure 5c,d showed the water contact angle before and after the adhesion test. As can be observed, the contact angles were over 160
o on the ODTS-coated surface, and it maintained a high contact angle even after the adhesion test because ODTS is bound to the titanium dioxide with covalent bonding. From the cross-cut adhesion test, the titanium dioxide layer was strongly bound to the metal titanium surface. It indicates that the titanium dioxide layer strongly adhered to the surface. This color-tuned and superhydrophobic titanium substrate could be used as self-cleaning multicolor materials.
4. Conclusions
A facile and rapid method for fabricating color-tuned titanium was developed using a torch at less than one minute at room temperature under ambient air conditions. The surface roughness of titanium dioxide on titanium surfaces was controlled via torching time, changing the colors of surfaces. Furthermore, the refractive index of titanium dioxide, which varies with the time of torch heating, changed the color of the surface. The sandpaper method offered macroscopic and microscopic roughness, while the electropolishing method gave rise to a smooth surface. In the sandpaper method with very high roughness, the light scattering increased so that the color turns darker than electropolished titanium. The sandpaper method exhibits more hydrophobic surfaces than the electropolished method due to the high roughness of sandpaper rubbed surfaces. Cross-cut adhesion tape test was performed to evaluate the mechanical and functional group stability of the titanium dioxide layer. Through the test, the ODTS layer-coated torched-titanium substrate maintained its color and contact angle. By exploiting sandpaper and torching method, self-cleaned metal flower surfaces of titanium were rapid and easily fabricated. This technique may be used in various fields such as engineering, medicine, and architectures in which various colors and self-cleaning effects are required.