Abstract
For the first time, an equiatomic refractory high entropy alloy (RHEA) TiNbZrHfTa compact with a single-phase body-centered cubic (BCC) structure was fabricated via a titanium hydride (TiH2) assisted powder metallurgy approach. The constituent pure Ti, Zr, Nb, Hf, and Ta powders were mechanically alloyed (MA) with titanium hydride (TiH2) powder. The resultant MA powder was dehydrogenated at 1073 K for 3.6 ks and subsequently sintered through spark plasma sintering (SPS). Additionally, TiNbZrHfTa counterparts were prepared from pure elements without MA with TiH2. It was observed that the compact prepared from pure powders had a chemically heterogeneous microstructure with hexagonal close packed (HCP) and dual BCC phases. On the other hand, despite containing many constituents, the compact fabricated at 1473 K for 3.6 ks via the hydride approach had a single-phase BCC structure. The Vickers microhardness of the TiNbZrHfTa alloy prepared via the hydride process was Hv 520 (±30). The exceptional microhardness of the alloy is greater than any individual constituent, suggesting the operation of a simple solid-solution-like strengthening mechanism and/or precipitation hardening. In addition, the heat treatments were also carried out to analyze the phase stability of TiNbZrHfTa prepared via the hydride process. The results highlight the substantial changes in the phase as a function of temperature and/or time.
1. Introduction
High entropy alloys (HEAs) are multicomponent alloys composed of equiatomic or near-equiatomic mixtures (between 5 to 35 at.%) of five or more principal elements, wherein the mixing entropy is maximized. It leads to the extended solid solubility and formation of a single or a dual-phase structure. In general, binary/ternary phase diagrams suggest that alloys having multi-elements may develop several phases and intermetallic compounds, resulting in complex and brittle microstructures that may have very limited practical value [1,2]. In contrast, the equiatomic (or near-equiatomic) HEAs exhibit simple body-centered cubic (BCC), face-centered cubic (FCC), hexagonal close packed (HCP), or a mixture of these, crystal structures due to their good mutual solubility [3,4,5,6].
The recently developed equiatomic TiNbZrHfTa Refractory High-Entropy Alloys (RHEA) belong to the BCC structure family of the HEAs. It is a promising alloy with excellent high-temperature properties due to its composition consisting of refractory elements with high melting points from 1940 K (Ti) to 3290 K (Ta) [7,8,9]. Therefore, it is considered as a replacement for heat-resistant Ni-superalloys, which are currently used for aerospace applications [10,11]. A good combination of high strength and elongation and its reduced density of 9.94 g/cm3 makes it an attractive class of structural material [12,13,14]. Additionally, TiNbZrHfTa is a biomaterial due to its biocompatible constituent elements [15].
In general, different fabrication methods, such as arc melting (liquid metallurgy), powder metallurgy (solid-state), electrodeposition method, etc., applied to the same starting compositions produce materials with distinctly different microstructures and properties [16,17,18,19,20]. The powder metallurgy has emerged as an efficient method to produce materials with a variety of compositions, controlled properties, and near-net-shape characteristics. Recently, TiNbZrHfTa RHEA was prepared from pure powders via different powder metallurgy (PM) methods [21,22,23]. However, the phase of the TiNbZrHfTa alloy is hardly controlled in most of the powder metallurgy processes [21,22,23]. These undesirable microstructures generally deteriorated the mechanical properties of alloys, which limits the potential applications of the RHEAs.
Recent studies have highlighted the prominence of the utilization of titanium hydride (TiH2) powder and its mixture with a pure Ti powder, to obtain Ti and their alloys. The ultra-fine or even nanocrystalline powders can be synthesized by mechanical milling of pure elements with brittle TiH2 powder [24,25,26]. Combined with spark plasma sintering (SPS), the nanocrystalline powders can be rapidly consolidated in high vacuum conditions to achieve a chemically homogeneous and fine-grained solid solution of the alloy. Additional advantages of the mixture of Ti and TiH2 powder include full recovery of milled powder after milling i.e., control over agglomeration and cold-welding of powder with milling media, lower initial price, fewer impurity contents, high-density compacts, and relative ease of handling during the milling process [24,25,26,27,28]. Moreover, the sintering of TiH2 powder at high temperatures prevents the surface oxidation of the powder and improves the sintering performance. Based on these advantages of the TiH2 based fabrication approach, the present work aims to analyze the feasibility of producing a single-phase BCC TiNbZrHfTa RHEA from a mixture of pure powders with TiH2 powder.
In the present study, TiNbZrHfTa compacts were prepared from a mechanically alloyed (MA) mixture of elemental and TiH2 powders through a two-step rapid sintering-based powder metallurgy process (hydride approach) [25]. Additionally, TiNbZrHfTa compacts were prepared from elemental powders via the conventional route of mixing and sintering. The influence of the process parameters (i.e., sintering time and temperature) on the microstructure and properties of the as-sintered compacts were studied. In addition, the phase stability of the TiNbZrHfTa compacts, prepared by the hydride method, was analyzed by heat-treatments at different temperatures and/or time.
2. Experimental Procedure
2.1. Starting Material
To prepare equiatomic TiNbZrHfTa RHEA, the elemental powders of Ti, Zr, Nb, Hf, Ta are used, along with TiH2 powder, as starting materials. The information on the starting powder materials is provided in Table 1.

Table 1.
Purity, particle size, and manufacturer of the starting powder materials.
The detailed composition of the elements required to prepare the TiNbZrHfTa RHEA compacts is shown in Table 2. The weighting and mixing of the powders was performed under an Ar protective atmosphere. As per Table 2, an equal atomic percentage was taken for each element, whereas the weight% is different and also shown.

Table 2.
Various compositions of initial powder mixtures.
2.2. Mechanical Alloying (MA)
In the hydride method, the MA powder was prepared from a mixture of elemental powders and TiH2 powder (with the composition shown in Table 2) by planetary ball milling (200 rpm) in an Ar atmosphere for 180 ks using stainless steel balls. The powder to ball ratio was kept at 1:5. MA powder samples were taken for microstructural evaluation to obtain the morphology of the powder particles.
2.3. Sintering of Powders via Spark Plasma Sintering
The elemental powder mixture and MA powder mixture were consolidated by SPS (Dr. Sinter, Sumitomo Coal Mining Co. Ltd., Tokyo, Japan) in high vacuum conditions (~10−3 Pa) using graphite die and punch. In the hydride process, to minimize the amount of hydrogen content in the compacts, the MA powder was dehydrogenated in SPS, without applying any external load, at 1073 K for 7.2 ks under high vacuum conditions. Subsequently, the dehydrogenated MA powders were sintered. The various SPS conditions are provided in Table 3. After SPS, disc-shaped specimens with the dimensions of 15 mm (dia.) and 4 mm (height) were obtained. Some of the sintered compacts of the hydride process were subjected to heat treatments to analyze the thermal stability of the RHEA compacts. Heat treatments were carried out at 1273 K for 86.4 ks, 1473 K for 86.4 ks, and 1623 K for 0.6 ks.

Table 3.
Different dehydrogenation and/or sintering conditions of the spark plasma sintering (SPS) process.
2.4. Microstructural and Phase Analysis
X-ray diffraction was carried out (XRD, Shimadzu Lab-X XRD-6100) with a CuKα (λ = 0.15406 nm) source. The morphology and microstructure were studied by a scanning electron microscope (SEM, JEOL JSM-7200F) equipped with back scattered electron (BSE) and electron back scattered diffraction (EBSD). The EBSD data were analyzed by TSL OIM data collection software (TSL OIM Analysis 8). The chemical characterization was carried out by energy dispersive X-ray spectroscopy (EDS). The microstructure of the as-fabricated sample was characterized by a transmission electron microscope (TEM, JEOL JEM-2100 plus, 200 kV). The thin samples used for the TEM observations were carefully prepared through a focused ion beam (FIB, Hitachi FB-2100).
2.5. Mechanical Properties
Vickers microhardness (SHIMADZU HMV-G21) was used to measure the hardness by indentation under the load of 980.7 mN (Hv 0.1) with a dwelling time of 10 s. All indentations were carried out randomly during the hardness measurement. The distance between two neighboring indentations was kept at least three times the size of the indent and an average of 20 indentations was used as a hardness value for further analysis.
3. Results and Discussion
3.1. Morphology of Initial Powders
The morphologies of the starting powder materials are shown in Figure 1. Pure Ti had a spherical shape whereas TiH2, Nb, Zr, Hf, and Ta powder particles exhibited irregular shaped morphology. All the starting powders contain both coarse and fine-sized particles.

Figure 1.
SEM micrographs of the starting powders.
3.2. Phase and Morphology of MA Powder
Figure 2 shows the XRD patterns of the MA powder, obtained through the hydride process. As shown in Figure 2, only diffraction peaks corresponding to HCP (α) and BCC (β) phases were identified. A maximum intensity peak of the BCC phase, along with a few other small peaks of the BCC phase, are visible in the pattern. Along the BCC phase, comparatively small peaks of α phase are also noticed. Therefore, it is evident from the XRD results that the MA powder has existence of both the phases. However, the higher intensity of the BCC phase shows that it is the dominating phase in MA powder.
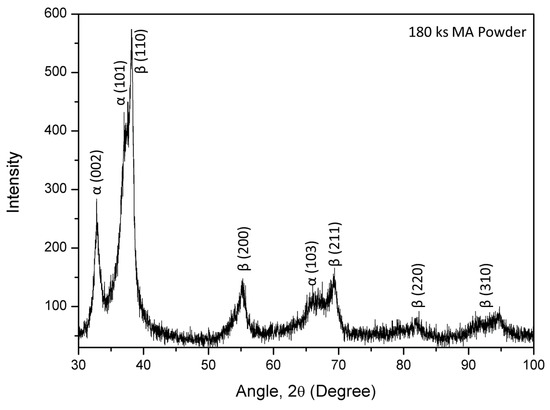
Figure 2.
XRD patterns of the mechanically alloyed powder.
Comparing the diffraction peaks of the MA powder and the individual elements, it can be deduced that the diffraction peaks corresponding to α and β are either associated with HCP and BCC solid solutions, respectively, or they could be broadened and overlapped peaks of initial powders. Therefore, it is complex to identify particular peaks associated with the elements. However, it is interesting to notice that the diffraction peaks are significantly broadened and their intensities are also significantly reduced compared to the initial powders, which can be attributed to the severe plastic deformation and fragmentation of powder particles during milling [29]. Therefore, it is reasonable to mention that when fabricating the HEAs via the powder metallurgy process, it is not essential for the milling process to form a single BCC solid solution phase. It also shows that no significant oxide peaks were observed and confirmed the absence of any significant quantity of oxides. Figure 3 illustrates the morphology of the MA powder. Interestingly, irrespective of the shapes of the initial powder particles, the maximum volume of spherical shape is observed in the MA powder particles. The average particle size of spherical shaped power is approximately 10 μm.
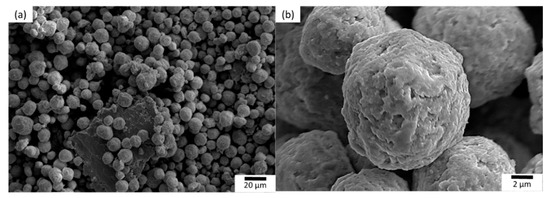
Figure 3.
SEM images of mechanically alloyed powder at; (a) low, and (b) high magnifications.
3.3. Phase and Microstructure of Compacts Prepared from Pure Elemental Powders
Figure 4 shows the XRD patterns of the TiNbZrHfTa RHEA compacts developed from Ti, Nb, Zr, Hf, and Ta pure elemental powders at 1273 and 1473 K. It is clear from Figure 4 that the compacts prepared from pure elemental powder exhibit multiple phases, which were confirmed as one HCP phase (α) and two BCC phases (β1 and β2). However, with an increase in sintering temperature and time, several minor peaks of the β2 phase disappeared. The lattice parameters of both the compacts are shown in Table 4.
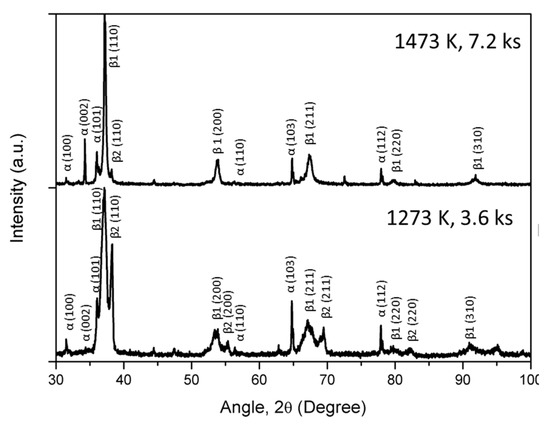
Figure 4.
XRD patterns of the TiNbZrHfTa compact prepared from pure elemental powders.

Table 4.
Lattice parameters of the TiNbZrHfTa compacts prepared from pure elements.
The microstructure (BSE) of the TiNbZrHfTa compact obtained from pure elemental powders is shown in Figure 5. Figure 5a is the representative micrograph of the TiNbZrHfTa compact at low magnification, and Figure 5b is the magnified image of the dark black region frequently observed in the microstructure matrix. From Figure 5a,b, it is apparent that the TiNbZrHfTa compacts are basically composed of four distinct distinguishable areas, i.e., a light grey matrix, a “brighter” white area, a dark black region, and a dark grey region. To analyze the chemical compositions of these distinct regions, chemical composition analysis was carried out using SEM-EDX mapping (Table 5). The bright white region is distinctly enriched in the Ta element. This qualitatively implies that the bright white area may be enriched in elements with abundant electrons. Simultaneously, significant compositional segregation of Zr and Hf in the dark grey matrix is also detected, indicating an incomplete alloying process. A large fraction of the dark black phase was detected as the Zr rich area, which remained undissolved in the microstructure matrix during the sintering process. On the other hand, the matrix region with a light grey appearance is a BCC solid solution, which is composed of an approximately equiatomic composition of the constituent elements. Therefore, it was observed that a chemically heterogeneous microstructure was achieved after the sintering of pure elemental powders, wherein the majority of the elements are either unalloyed or partially alloyed under the given conditions of the temperature and time (as shown in Table 3). Therefore, it can be assumed that due to the large particle sizes of the initial powders, the solid solutions of the pure elements have extremely high melting points, such as Nb: 2741 K, Zr: 2125 K, Hf: 2495 K, and Ta: 3269 K, which requires sintering at a higher temperature for a prolonged time.
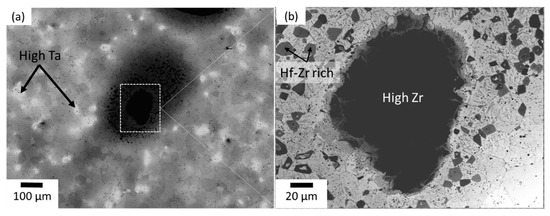
Figure 5.
Back scattered electron (BSE) images of the TiNbZrHfTa alloy prepared from pure elements. (a) Representative micrograph at low magnification, (b) magnified image of the dark black area.

Table 5.
Chemical composition (in wt.%) of the alloy compact prepared from pure elements.
3.4. Phase and Microstructure of Compacts Prepared from the Hydride Process
Figure 6 shows the XRD patterns of the TiNbZrHfTa RHEA compacts prepared from the hydride process involving a mixture of TiH2 powder with constituent elemental powders at different temperatures and time. It is clear from Figure 6 that the compacts prepared at a lower temperature of 1273 K (3.6 ks) exhibit multiple phases, which were confirmed as α (HCP) and β (BCC) phases. However, with an increase in sintering temperature up to 1473 K (3.6 ks), approximately all the peaks of the α phase disappeared and diffraction peaks corresponding to the approximately single-phase β structure were observed. However, interestingly, increasing the sintering time up to 7.2 ks at 1473 K leads to the diffraction peaks of the α phase. Therefore, it can be assumed that with increasing holding time at high temperatures, α precipitation occurs. It is to be noted that the diffraction peaks related to oxides or hydrides were not observed. The lattice parameters of both the compacts are shown in Table 6. The lattice parameters of the RHEA compacts were comparable to those reported in the literature [12,30], which show the feasibility of the hydride process to achieve equiatomic TiNbZrHfTa alloys with a BCC solid solution of constituent elements. It is worth noting that this RHEA system could be successfully achieved by MA followed by the SPS process (hydride process), and it could not be fabricated by the SPS process of pure elements only (without the milling).
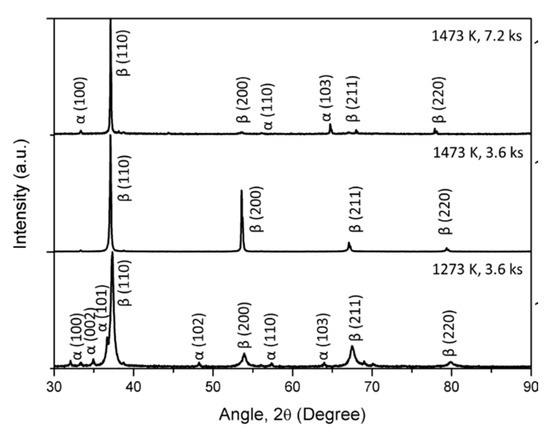
Figure 6.
XRD patterns of the TiNbZrHfTa compacts prepared by the hydride process.

Table 6.
Lattice parameters of the alloy compacts prepared by the hydride process.
Figure 7 illustrates the BSE micrographs, presenting the microstructure of the TiNbZrHfTa compacts prepared by the hydride process. The compact sintered at 1273 K for 3.6 ks has a grain size of about 3 μm and increases to ~500 μm after higher temperature sintering. Therefore, significant grain growth occurs with increasing temperature. It can be observed that at a low temperature of 1273 K (3.6 ks), a chemically heterogeneous microstructure was achieved wherein bright and dark areas can be distinguished from the grey matrix (Figure 7a). To identify the chemical compositions of the matrix and inclusions, a semi-quantitative energy dispersive X-ray spectrometer (EDS) analysis was conducted (Table 7). The spot EDS analysis results indicates that the chemical composition of the matrix of the compact, sintered at 1473 K for 3.6 ks, well matches the nominal composition of the equiatomic TiNbZrHfTa (Table 2). However, at a lower temperature of 1273 K (3.6 ks), compared to the matrix phase, the bright and darker regions of Ta-rich and Hf-rich areas, respectively, were observed. Therefore, the EDS results with the XRD patterns combinedly indicate that the α phase is associated with the dark phase (Hf-Zr rich) whereas the β phase is majorly associated with the matrix with near equiatomic fractions of constituent elements. However, at a higher temperature (1473 K, 3.6 ks), a majorly grey phase can be seen, which can also be confirmed by XRD results showing a single β phase (Figure 6). In addition, visible porosities were observed. It is worth mentioning that the TiNbZrHfTa alloy with a single β phase was obtained by the hydride process (Figure 7b). All the constituent elements were equally distributed, forming the BCC phase. However, minor traces of Ta rich areas were noticed, and these traces were still present even after prolonged sintering at the same temperature (1473 K, 7.2 ks). In addition, in some areas, precipitation was noticed at the grain boundaries (Figure 7c). Figure 8 illustrates the TEM images and selected area diffraction (SAD) pattern of the TiNbZrHfTa compact prepared at 1473 K for 3.6 ks by the hydride method. The SAD pattern of the matrix is centered along with the [012]bcc beam direction, which represents a BCC structure. However, fine particles were seldom noticed in the microstructure matrix, which might indicate that fine Ta rich regions are present in the compact. Therefore, it can be deduced that at a certain sintering condition, i.e., 1473 K for 3.6 ks, a bcc phase was achieved for the hydride-based PM processed TiNbZrHfTa alloy, which starts to dissociate after sintering for a prolonged time.
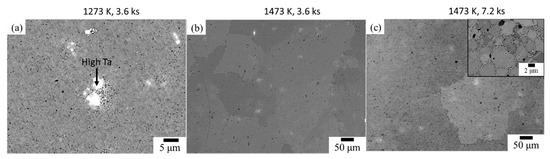
Figure 7.
BSE micrographs of the TiNbZrHfTa refractory high entropy alloy (RHEA) compacts prepared by the hydride process, at; (a) 1273 K, 3.6 ks, (b) 1473 K, 3.6 ks, and (c) 1473 K, 7.2 ks.

Table 7.
Chemical analysis (wt.%) of the alloy compacts prepared by the hydride process.
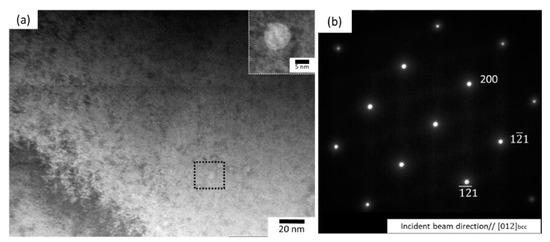
Figure 8.
(a) TEM image (magnified image of the marked fine precipitate is shown in inset), and (b) selected area diffraction (SAD) image (at the matrix) of the TiNbZrHfTa alloy prepared at 1473 K for 3.6 ks.
To achieve a homogeneous microstructure, the BCC stabilized compact of TiNbZrHfTa RHEA, sintered at 1473 K (3.6 ks), was heat-treated in an ultra-high vacuum furnace (UlVAC MILA 5000). The heat treatment was carried out at different temperatures and times, i.e., 1273 K for 86.4 ks (Figure 9a), 1473 K for 86.4 ks (Figure 9b), and 1623 K for 0.6 ks (Figure 9c). Despite the heat treatments at higher temperatures for a prolonged time, element segregation was noted in the matrix. The elemental segregation during heat treatment is consistent with the results obtained during prolonged sintering (Figure 7c) wherein fine precipitates of Hf-Zr rich areas were noticed. The chemical analysis data are provided separately in the supplementary file (Figure S1 (Supplementary Materials)). These results are not consistent with the equiatomic TiNbZrHfTa RHEA fabricated by ingot metallurgy wherein phase decompositions are usually reported during annealing below 1273 K and at a temperature above 1273 K, a single bcc phase is usually observed [12,20,31,32,33]. The chemical composition and presence of fine elements may have a significant influence on the phase stability during heat treatments of the TiNbZrHfTa compact prepared by the powder metallurgy process; however, this is a task for further investigations.
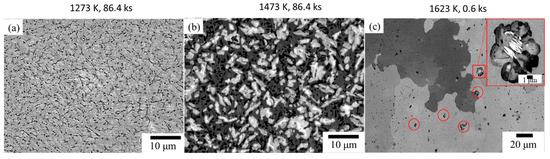
Figure 9.
BSE image of the TiNbZrHfTa RHEA fabricated by the hydride process after heat treatment at; (a) 1273 K for 86.4 ks, (b) 1473 K for 86.4 ks, and (c) 1623 K for 0.6 ks.
The average Vickers microhardness values for the as-fabricated and heat-treated compacts of the TiNbZrHfTa RHEAs are shown in Figure 10. It is clear that the microhardness of the as-fabricated TiNbZrHfTa RHEA gradually increases with the increasing sintering temperature and/or time. Based on the microstructure characterization above, the improved microhardness of the as-sintered compacts is probably attributed to the homogeneous microstructure, which can be evidenced by standard deviation from the average hardness value, e.g., the specimen fabricated at 1473 K for 3.6 ks. However, holding for a prolonged time leads to the nucleation of fine precipitates of the Hf-Zr phase. Therefore, among the as-fabricated alloy compacts, the compact fabricated at 1473 K (7.2 ks) exhibited the highest hardness with a wide standard deviation. However, after annealing, the hardness of the TiNbZrHfTa alloy compacts was increased depending on the heat treatment conditions. In general, it is well-known that the mechanical properties of materials are basically influenced by various strengthening mechanisms, such as precipitate, solid solution, grain boundary, dislocations, etc. Based on the rule of mixtures guidelines [30], the microhardness of the equiatomic TiNbZrHfTa RHEA can be roughly calculated to be Hv 119. However, for the present alloy fabricated by the hydride process, the microhardness does not follow the rule of mixtures. The significantly higher microhardness of the present alloy has likely originated from the combined effect of solid solution strengthening, and the presence of different phases and/or fine precipitates (depending on the fabrication temperature/time). Similar results of higher hardness were also reported in the literature [30,31,34,35,36].
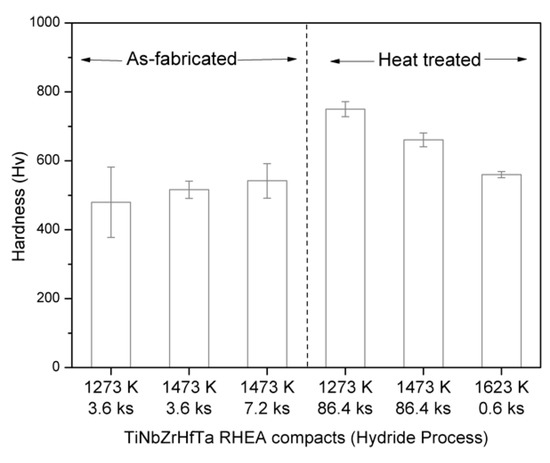
Figure 10.
The Vickers microhardness of the TiNbZrHfTa compact prepared with different sintering and heat treatment conditions.
4. Conclusions
In this study, an equiatomic TiNbZrHfTa refractory high entropy alloy (RHEA) was successfully fabricated with the hydride-based powder metallurgy approach, involving mechanical alloying (MA) of titanium hydride powder with other constituent elemental powders, i.e., Ti, Nb, Zr, Hf, and Ta. Subsequently, the bulk specimens were prepared by SPS under different sintering temperatures from 1273 K to 1473 K and sintering times of 3.6 ks to 7.2 ks. The MA powder shows a single-phase BCC solid solution due to the fine powder particles formed during milling with brittle type titanium hydride powder. After dehydrogenation and sintering, the single BCC solid solution of the TiNbZrHfTa RHEA was achieved without any trace of oxides or hydrides. The significantly high hardness of Hv 520 (±30) proves the promising applications of the alloy. The higher hardness was attributed to the combined effects of the solid solution of the β phase and fine α precipitates.
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-4352/10/11/1020/s1, Figure S1 (The chemical analysis of the TiNbZrHfTa RHEA compact heat treated at 1473 K for 86.4 ks).
Author Contributions
Conceptualization, B.S. and K.A.; methodology, B.S.; software, B.S.; formal analysis, B.S., H.F.; investigation, B.S., K.N., K.K.S.; resources, B.S., K.A.; writing—original draft preparation, B.S.; writing—review and editing, B.S., K.K.S.; supervision, K.A., H.F.; project administration, B.S.; funding acquisition, B.S., H.F., K.A. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Japan Society for the Promotion of Science (JSPS) Grants-in-Aid for Scientific Research (KAKENHI) grant numbers 20K15064, 19H02466, and 18H05455. This support is gratefully appreciated.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ogut, S.; Rabe, K.M. Band gap and stability in the ternary intermetallic compounds NiSnM (M = Ti,Zr,Hf): A first-principles study. Phys. Rev. B 1995, 51, 10443. [Google Scholar] [CrossRef]
- Tsai, M.; Yeh, J.W. High-Entropy Alloys: A Critical Review. Mater. Res. Lett. 2014, 2, 107–123. [Google Scholar] [CrossRef]
- Guo, N.N.; Wang, L.; Luo, L.S.; Li, X.Z.; Su, Y.Q.; Guo, J.J.; Fu, H.Z. Microstructure and mechanical properties of refractory MoNbHfZrTi high-entropy alloy. Mater. Des. 2015, 81, 87–94. [Google Scholar] [CrossRef]
- Otto, F.; Yang, Y.; Bei, H.; George, E.P. Relative effects of enthalpy and entropy on the phase stability of equiatomic high-entropy alloys. Acta Mater. 2013, 61, 2628–2638. [Google Scholar] [CrossRef]
- Santodonato, L.J.; Zhang, Y.; Feygenson, M.; Parish, C.M.; Gao, M.C.; Weber, R.; Neuefeind, J.; Tang, Z.; Liaw, P. Deviation from high-entropy configurations in the atomic distributions of a multi-principal-element alloy. Nat. Commun. 2015, 6, 5964. [Google Scholar] [CrossRef] [PubMed]
- Hemphill, M.A.; Yuan, T.; Wang, G.Y.; Yeh, J.W.; Tsai, C.W.; Chuang, A.; Liaw, P.K. Fatigue behavior of Al0.5CoCrCuFeNi high entropy alloys. Acta Mater. 2012, 60, 5723–5734. [Google Scholar] [CrossRef]
- Maiti, S.; Steurer, W. Structural-disorder and its effect on mechanical properties in single-phase TaNbHfZr high-entropy alloy. Acta Mater. 2016, 106, 87–97. [Google Scholar] [CrossRef]
- Senkov, O.N.; Senkova, S.V.; Woodward, C. Effect of aluminum on the microstructure and properties of two refractory high entropy alloys. Acta Mater. 2014, 68, 214–228. [Google Scholar] [CrossRef]
- Zou, Y.; Ma, H.; Spolenak, R. Ultrastrong ductile and stable high-entropy alloys at small scales. Nat. Commun. 2015, 6, 7748. [Google Scholar] [CrossRef]
- Miracle, D.B.; Senkov, O.N. A critical review of high entropy alloys and related concepts. Acta Mater. 2017, 122, 448–511. [Google Scholar] [CrossRef]
- Čapek, J.; Kyncl, J.; Kolařík, K.; Beránek, L.; Pitrmuc, Z.; Medřický, J.; Pala, Z. Grinding of Inconel 713 superalloy for gas turbines. Manuf. Technol. 2016, 16, 38–45. [Google Scholar] [CrossRef]
- Senkov, O.; Scott, J.; Senkova, S.; Meisenkothen, F.; Miracle, D.; Woodward, C.F. Microstructure and elevated temperature properties of a refractory TaNbHfZrTi alloy. J. Mater. Sci. 2012, 47, 4062–4074. [Google Scholar] [CrossRef]
- Dirras, G.; Lilenstein, L.; Djemia, P.; Laurent-Brocq, M.; Tingaud, D.; Couzinie, J.P.; Perriere, L.; Chauveau, T.; Guillot, I. Elastic and plastic properties of as-cast equimolar TiHfZrTaNb high-entropy alloy. Mater. Sci. Eng. A 2016, 654, 30–38. [Google Scholar] [CrossRef]
- Juan, C.; Tsai, M.; Tsai, C.; Hsu, W.; Lin, C.; Chen, S.; Lin, S.; Yeh, J. Simultaneously increasing the strength and ductility of a refractory high-entropy alloy via grain refining. Mater. Lett. 2016, 184, 200–203. [Google Scholar] [CrossRef]
- Biesiekierski, A.; Wang, J.; Gepreel, M.; Wen, C. A new look at biomedical Ti-based shape memory alloys. Acta Biomater. 2012, 8, 1661–1669. [Google Scholar] [CrossRef]
- Senkov, O.N.; Semiatin, S.L. Microstructure and properties of a refractory high-entropy alloy after cold working. J. Alloys Compd. 2015, 649, 1110–1123. [Google Scholar] [CrossRef]
- Dirras, G.; Couque, H.; Lilensten, L.; Heczel, A.; Tingaud, D.; Couzinie, J.; Perriere, L.; Gubicza, J.; Guillot, I. Mechanical behaviour and microstructure of Ti20Hf20Zr20Ta20Na20 high entropy alloy loaded under quasi-static and dynamic compression conditions. Mater. Charact. 2016, 111, 106–113. [Google Scholar] [CrossRef]
- Juan, C.; Tsai, M.; Tsai, C.; Lin, C.; Wang, W.; Yang, C.; Chen, S.; Lin, S.; Yeh, J. Enhanced mechanical properties of HfMoTaTiZr and HfMoNbTaTiZr refractory high-entropy alloys. Intermetallics 2015, 62, 76–83. [Google Scholar] [CrossRef]
- Chen, S.; Tseng, K.; Tong, Y.; Li, W.; Tsai, C.; Yeh, J.; Liaw, P. Grain growth and Hall-Petch relationship in a refractory HfNbTaZrTi high-entropy alloy. J. Alloys Compd. 2019, 795, 19–26. [Google Scholar] [CrossRef]
- Senkov, O.N.; Pilchak, A.L.; Semiatin, S.L. Effect of Cold Deformation and Annealing on the Microstructure and Tensile Properties of a HfNbTaTiZr Refractory High Entropy Alloy. Metall. Mater. Trans. A 2018, 49, 2876–2892. [Google Scholar] [CrossRef]
- Malek, J.; Zyka, J.; Lukac, F.; Vilemova, M.; Vlasak, T.; Cizek, J.; Melikhova, O.; Machackova, A.; Kim, H.S. The Effect of Processing Route on Properties of HfNbTaTiZr High Entropy Alloy. Metals 2019, 12, 4022. [Google Scholar] [CrossRef] [PubMed]
- Shkodich, N.; Sedegov, A.; Kuskov, K.; Busurin, S.; Scheck, Y.; Vadchenko, S.; Moskovskikh, D. Refractory High-Entropy HfTaTiNbZr-Based Alloys by Combined Use of Ball Milling and Spark Plasma Sintering: Effect of Milling Intensity. Metals 2020, 10, 1268. [Google Scholar] [CrossRef]
- Malek, J.; Zyka, J.; Lukac, F.; Cizek, J.; Kuncicka, L.; Kocich, R. Microstructure and Mechanical Properties of Sintered and Heat-Treated HfNbTaTiZr High Entropy Alloy. Metals 2019, 9, 1324. [Google Scholar] [CrossRef]
- Sharma, B.; Vajpai, S.; Ameyama, K. An Efficient Powder Metallurgy Processing Route to Prepare High-Performance β-Ti–Nb Alloys Using Pure Titanium and Titanium Hydride Powders. Metals 2018, 8, 516. [Google Scholar] [CrossRef]
- Sharma, B.; Vajpai, S.K.; Ameyama, K. Preparation of strong and ductile pure titanium via two-step rapid sintering of TiH2 powder. J. Alloys Compd. 2016, 683, 51–55. [Google Scholar] [CrossRef]
- Sharma, B.; Vajpai, S.; Ameyama, K. Microstructure and properties of beta Ti–Nb alloy prepared by powder metallurgy route using titanium hydride powder. J. Alloys Compd. 2016, 656, 978–986. [Google Scholar] [CrossRef]
- Biasotto, M.; Riceeri, R.; Scuor, N.; Schmid, C.; Sandrucci, M.A.; Dilenarda, R.; Matteazzi, P. Porous titanium obtained by a new powder metallurgy technique: Preliminary results of human osteoblast adhesion on surface polished substrates. J. Appl. Biomater. Biomech. 2003, 1, 172–177. [Google Scholar]
- Mimoto, T.; Nakanishi, N.; Umeda, J.; Kondoh, K. Fabrication of Powder Metallurgy Pure Ti Material by Using Thermal Decomposition of TiH2. J. High-Temp. Soc. 2011, 37, 326–331. [Google Scholar] [CrossRef][Green Version]
- Zhang, D.L. Processing of advanced materials using high-energy mechanical milling. Prog. Mater. Sci. 2004, 49, 537–560. [Google Scholar] [CrossRef]
- Senkov, O.; Scott, J.; Senkova, S.; Miracle, D.; Woodward, C. Microstructure and room temperature properties of a high-entropy TaNbHfZrTi alloy. J. Alloys Compd. 2011, 509, 6043–6048. [Google Scholar] [CrossRef]
- Chen, S.Y.; Tong, Y.; Tseng, K.K.; Yeh, J.W.; Poplawsky, J.D.; Wen, J.G.; Gao, M.C.; Kim, G.; Ren, Y.; Feng, R.; et al. Phase transformations of HfNbTaTiZr high-entropy alloy at intermediate temperatures. Scr. Mater. 2019, 158, 50–56. [Google Scholar] [CrossRef]
- Stepanov, N.D.; Yurchenko, N.; Zherebtsov, S.; Tikhonovsky, M.; Salishchev, G. Aging behavior of the HfNbTaTiZr high entropy alloy. Mater. Lett. 2018, 211, 87–90. [Google Scholar] [CrossRef]
- Schuh, B.; Volker, B.; Todt, J.; Schell, N.; Perriere, L.; Li, J.; Couzinie, J.P.; Hohenwarter, A. Thermodynamic instability of a nanocrystalline, single-phase TiZrNbHfTa alloy and its impact on the mechanical properties. Acta Mater. 2018, 142, 201–212. [Google Scholar] [CrossRef]
- Zherebtsov, S.; Yurchenko, N.; Shaysultanov, D.; Tikhonovsky, M.; Salishchev, G.; Stepanov, N. Microstructure and Mechanical Properties Evolution in HfNbTaTiZr Refractory High-Entropy Alloy During Cold Rolling. Adv. Eng. Mater. 2020, 22, 2000105. [Google Scholar] [CrossRef]
- Wang, S.; Wu, M.; Zhu, G.; Wang, D.; Sun, B. Mechanical instability and tensile properties of TiZrHfNbTa high entropy alloy at cryogenic temperatures. Acta Mater. 2020, 201, 517–527. [Google Scholar] [CrossRef]
- Eleti, R.; Stepanov, N.; Zherebtsov, S. Mechanical behavior and thermal activation analysis of HfNbTaTiZr body-centered cubic high-entropy alloy during tensile deformation at 77 K. Scr. Mater. 2020, 188, 118–123. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).