Molecular and Crystal Structure of 7,7-Dimethyl-2-pyridin-4-yl-6,7-dihydro-1,2,4-triazolo[1,5-a][1,3,5]triazin-5-amine [1]
Abstract
: When crystallized from ethanol, 7,7-dimethyl-2-pyridin-4-yl-6,7-dihydro-1,2,4- triazolo[1,5-a][1,3,5]triazin-5-amine forms crystals which have monoclinic (P21/n) symmetry with unit cell dimensions a = 7.3326(5) Å, b = 19.4897(14) Å, c = 8.6586(6) Å, α = 90°, β = 106.069(2)°, γ = 90°, V = 1189.06(14) Å3, Z = 4. The triazine ring in the molecule has a flattened boat conformation with gem-dimethyl groups as flagpole and bowsprit at the bow. The puckering parameters for the ring are: Q = 0.2996(14) Å, θ = 111.7(3)° and φ = 124.1(3)°. In the crystal, molecules are arranged in the three types of chains generated by the intermolecular NH⋯N hydrogen bonds. The extended chains with the C(11) graph-set motif running along a [010] axis are formed by the amino group hydrogen atom and the pyridine nitrogen atom of another molecule. The C(4)C(6) chains with the R22(8) binary graph-set motif running along a [101] direction are formed by linking the amino group hydrogen atom and the hydrogen atom at the triazine nitrogen atom with the triazole and triazine nitrogen atoms of another molecule, respectively. The centrosymmetric inverted dimers are formed via the C-H⋯π interactions between the methyl group hydrogen and the pyridine ring of the pair molecule.1. Introduction
Compounds with 1,2,4-triazolo[1,5-a]triazine ring system have not been found in nature. However, this heterocyclic system resembles purine which is one of the most important heterocycles in nature. The additional bridge nitrogen atom of 1,2,4-triazolo[1,5-a]triazines makes them 5-azaisosters of purines. The chemistry and biological activity of 1,2,4-triazolo[1,5-a]triazines have been extensively explored and this heterocyclic system has been well recognized as a promising scaffold for the construction of molecules with diverse biological effects [2].
In our search for potential therapeutic agents in this class of compounds we developed a number of effective synthetic procedures for 1,2,4-triazolo[1,5-a]triazines [3-7] with particular interest to the heterocyclizations of 1,2,4-triazol-3(5)-yl guanidines [4-7]. Consequently, a series of structural investigations of 1,2,4-triazolo[1,5-a]triazines using X-ray diffraction analysis [8-12] appeared complementing an earlier work by Gilardi [13].
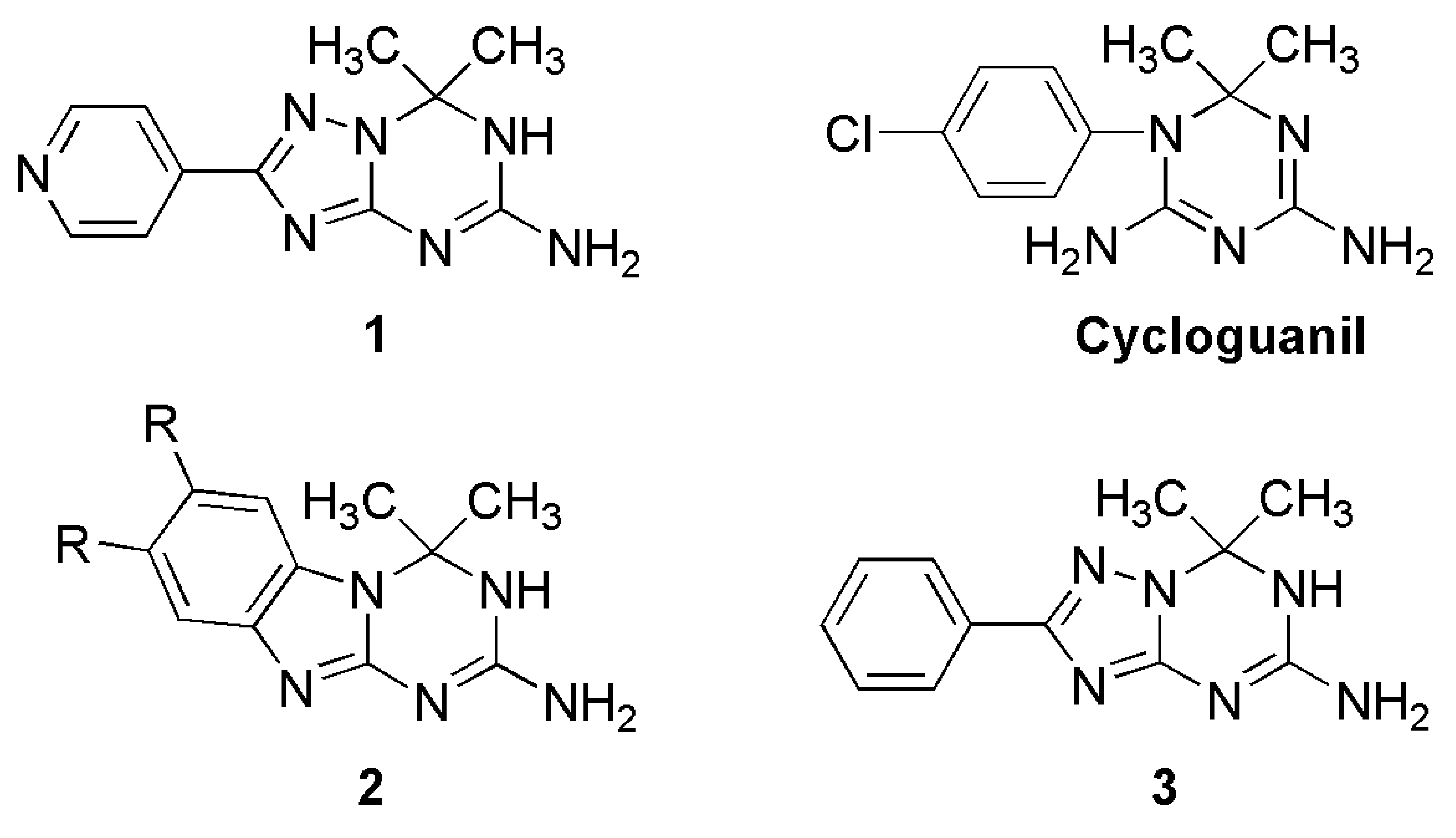
In continuation of our research program on the synthesis and structural analysis of potentially bioactive 1,2,4-triazolo[1,5-a]triazines, we synthesized 7,7-dimethyl-2-pyridin-4-yl-6,7-dihydro-1,2,4- triazolo[1,5-a][1,3,5]triazin-5-amine (1) according to the previously reported general procedure [6,7] and report herein its molecular and crystal structure. The molecule 1 shares gem-dimethyl substituted dihydro-1,3,5-triazin-5-amine structure moiety with the antifolate drug cycloguanil and its fused analogues 2 (Figure 1) also inhibiting dihydrofolate reductase [14-16].
2. Results and Discussion
Crystals suitable for the X-ray diffraction analysis were obtained by recrystallization from ethanol (colorless blocks, m.p. 302 °C). The crystal and instrumental parameters used in the unit cell determination and data collection are summarized in Table 1.
2.1. Molecular Structure
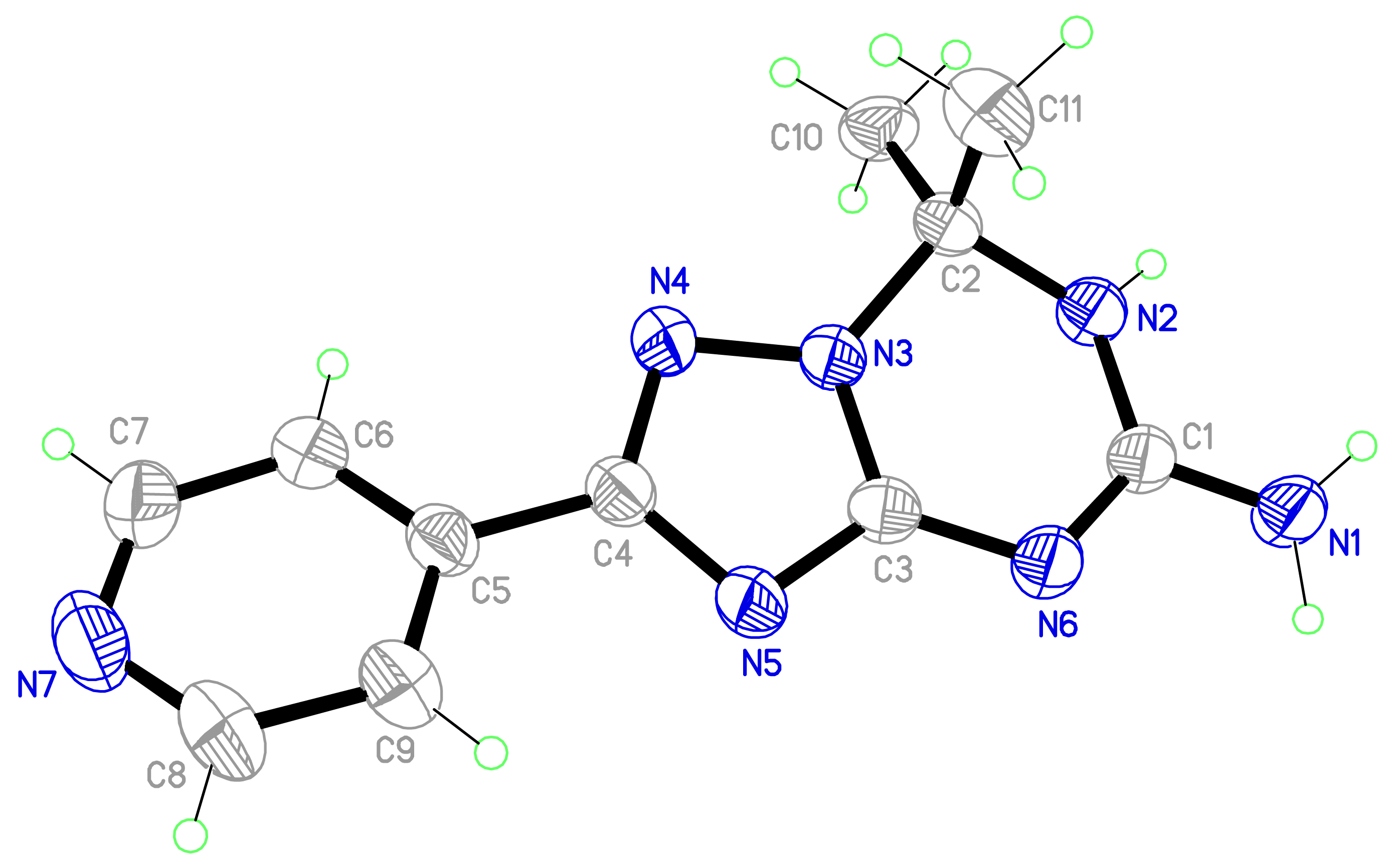
The drawing of the molecule with the atom-labeling scheme (displacement thermal ellipsoids are drawn at the 50% probability level) is shown in Figure 2. The bond lengths and angles comprising the key features of the 6,7-dihydro-1,2,4-triazolo[1,5-a][1,3,5]triazine core are given in Table 2. Bond distances indicate extensive delocalization within the heterocyclic ring system and the amino group. In general, the geometry of the 7,7-dimethyl-6,7-dihydro-1,2,4-triazolo[1,5-a][1,3,5]triazin-5-amine skeleton is similar to that of the earlier reported [11] structure with the 2-phenyl substitution (compound 3, Figure 1).
The 1,2,4-triazolo[1,5-a][1,3,5]triazine core of the molecule is nearly planar with C2 atom deviated to higher extent (viz. 0.2455(14) Å) from the mean plane of the system due to sp3 hybridized nature of the carbon C2 atom. The mean planes of the 1,2,4-triazolo[1,5-a][1,3,5]triazine (C1/N2/C2/N3/N4/C4/N5/C3/N6) and pyridine (C5/C6/C7/N7/C8/C9) rings make a dihedral angle of 21.49(7)°. This is more than twice higher than the corresponding value for 3.
The triazine ring C1/N2/C2/N3/C3/N6 in the molecule adopts a flattened boat conformation with atoms C2 and N6 at the bow and stern. The angle C10-C2-C11 between the flagpole and bowsprit geminal methyl groups is 111.88(13)°. This value is within the range (111.33–112.40) observed for the structurally related molecules [11,17].
The sum of the magnitudes of the six intraring torsion angles (P) around the 1,3,5-triazine ring is 19.51(8)° that indicates a reasonable level of the ring planarity but allow the Cremer-Pople analysis of the ring puckering [18]. The Cremer-Pople system describes six-atom rings using puckering amplitude (Q) and orientation angles (θ and φ). The puckering amplitude (Q), which is a measure of the average displacement of the ring atoms away from a best-fit plane, is equal to 0.2996(14) Å for the triazine ring C1/N2/C2/N3/C3/N6. The angle θ describes where the puckering occurs around the ring and φ is an inversion angle that accounts for the possibility of inverted ring forms. Ideally, boat conformers should have θ = 90° and φ = 0, 60, 120, 180, 240 or 300°. The orientation angles θ and φ characterizing the triazine ring are 111.7(3)° and 124.1(3)°, respectively that confirms the flattened boat conformation of the ring. Similar conformation was observed for structurally related 7,7-dimethyl-2-phenyl-6,7-dihydro-1,2,4-triazolo[1,5-a][1,3,5]triazin-5-amine [11]. However, changing type of the ring fused to the gem-dimethyl substituted amino-1,3,5-triazine [11,17] or position of the fusion [10] appear to affect the triazine ring conformation.
2.2. Crystal Structure
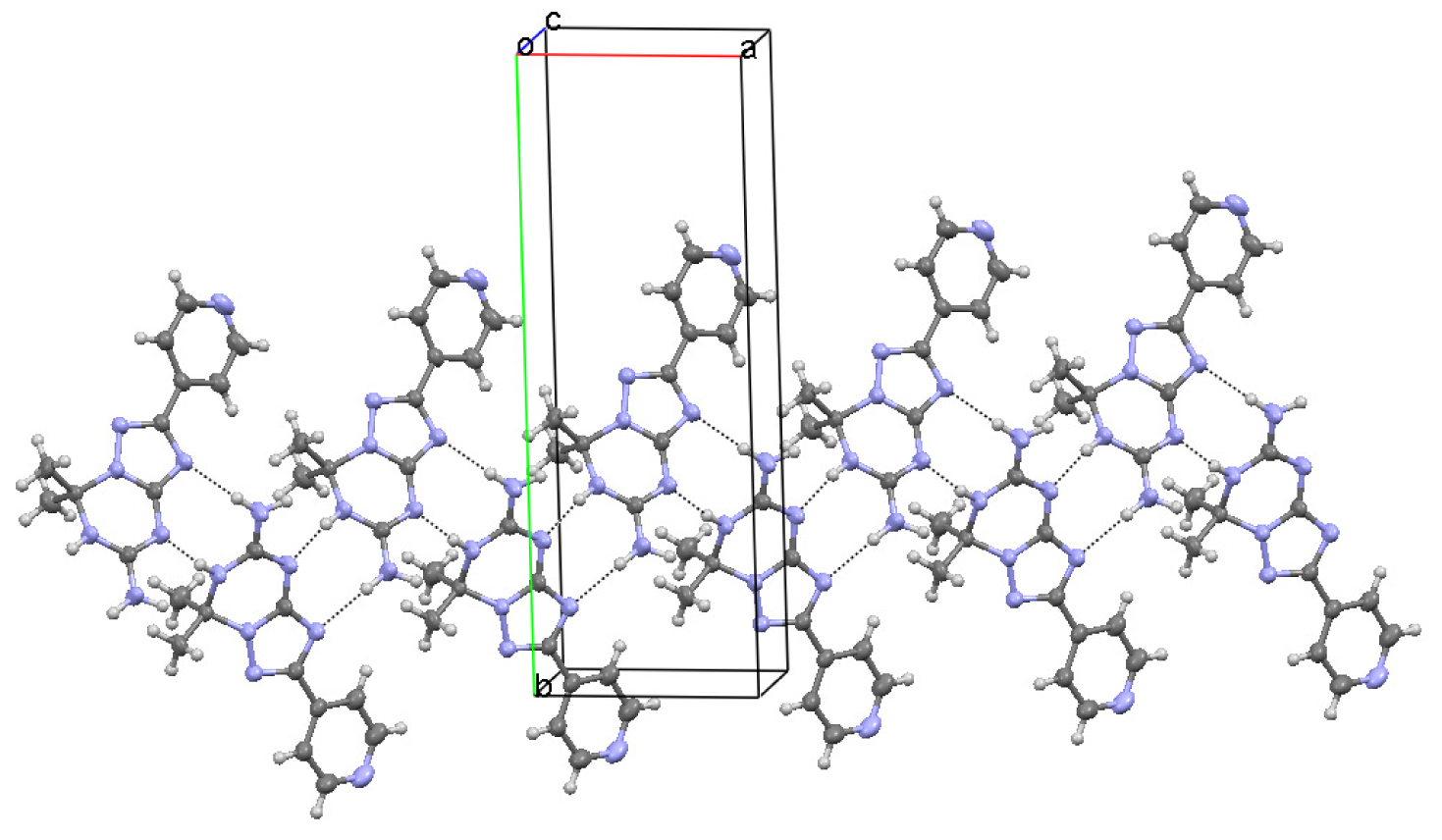
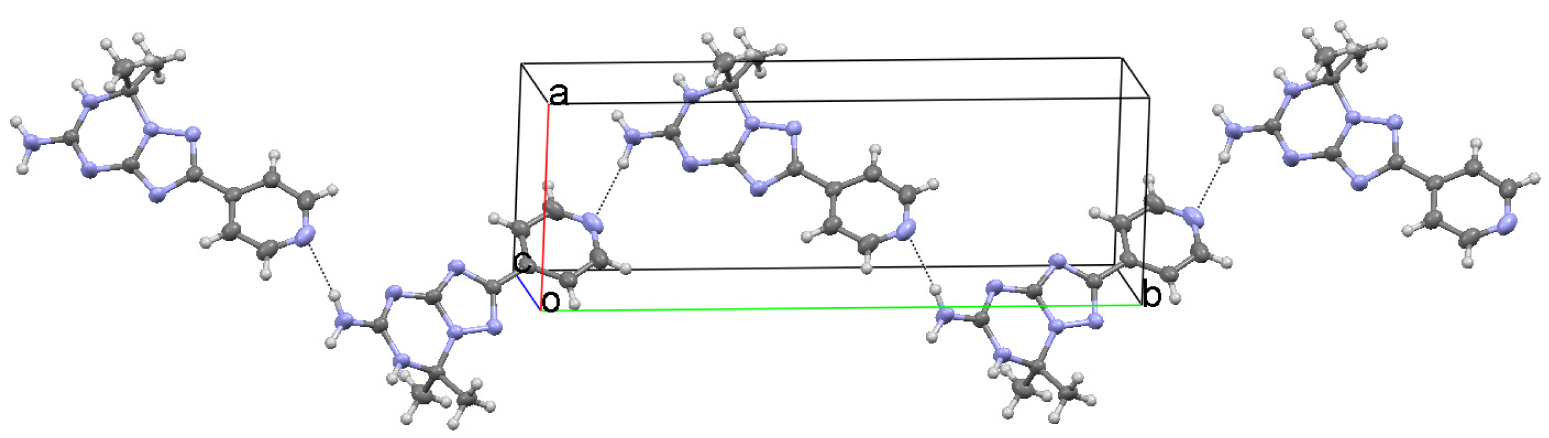
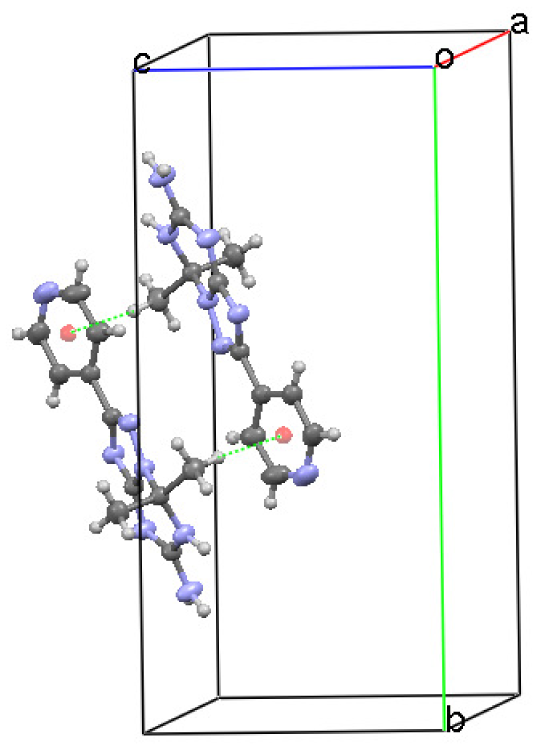
In the crystal of 7,7-dimethyl-2-pyridin-4-yl-6,7-dihydro-1,2,4-triazolo[1,5-a][1,3,5]triazin-5-amine (1), molecules form a hydrogen bond connected network of three types of chains. A hydrogen atom of the primary amino group N1-H1A and the hydrogen atom at the endocyclic N2 atom act as hydrogen donors forming intermolecular N⋯HN hydrogen bonds with the triazole and triazine N5 and N6 atoms, respectively (Table 3). These contacts arrange molecules into the running along a [101] direction C(4)C(6) chains with the R22(8) binary graph-set motif [19] (Figure 3). The C(11) extended chains parallel to a [010] axis consist of the molecules linked via the NH⋯N hydrogen bonds between the second hydrogen atom of the amino group N1-H1B and the N7 atom of the pyridine ring (Table 3, Figure 4).
The C-H⋯π contacts [20] with the classical T shape geometry between the methyl group hydrogen and the pyridine ring (C5/C6/C7/N7/C8/C9) at (−x, −y, 1−z) combine two molecules into a centrosymmetric inverted dimer (Table 2, Figure 5).
3. Experimental Section
The X-ray diffraction data for the compound were measured at room temperature on a Bruker SMART APEX CCD diffractometer using Mo Kα (λ = 0.71073 Å) radiation. The empirical absorption corrections were applied by the multi-scan method using SADABS [21]. Intensity data were collected in the ω-φ scan mode using SMART [22] and were reduced using SAINT [22]. The structure was solved by direct methods using SHELXS97 [23] and difference Fourier synthesis using SHELXL97 [23]. The positions and anisotropic displacement parameters of all non-hydrogen atoms were included in the full-matrix least-square refinement using SHELXL97 [23] and the procedures were carried out for a few cycles until convergence was reached. A total of 8323 reflections were collected, resulting in 2736 (Rint = 0.037) independent reflections of which the number of reflections satisfying I > 2σ(I) criteria was 2282. All the N-bound hydrogen atoms were located in a difference map and refined freely. The hydrogen atoms attached to the carbon atoms were placed in calculated positions (0.94 Å for Caryl-H and 0.97 Å for the methyl groups) and included in the final cycles of refinement using a riding model, with Uiso(H) = 1.2Ueq(Caryl) and Uiso(H) = 1.5Ueq(Cmethyl). A rotating group model was used for the methyl groups. The R factor for observed data finally converged to R1 = 0.0476 with wR2 = 0.1188 in the compound. The maximum and minimum values of residual electron density were 0.268 and −0.213 eÅ−3. The geometry calculations were performed using PLATON [24]. SHELXTL [23] and MERCURY [25] were used for the diagram generation.
4. Conclusions
The molecular structure of 7,7-dimethyl-2-pyridin-4-yl-6,7-dihydro-1,2,4-triazolo[1,5-a][1,3,5] triazin-5-amine has been determined and compared with structurally similar molecules. The classical NH⋯N hydrogen bonds and C-H⋯π interactions stabilizing packing of the molecules in the crystal has been described.
| Crystal data | Refinement | ||
|---|---|---|---|
| Empirical formula | C11H13N7 | Refinement method | Full-matrix least-squares on F2 |
| Formula weight | 243.28 | ||
| Temperature | 295(2) K | Data/restraints/parameters | 2736/0/177 |
| Wavelength, λ | 0.71073 Å | ||
| Crystal system | monoclinic | Goodness-of-fit on F2 | 1.057 |
| Space group | P21/n | Final R indices | R1 = 0.0476, |
| Unit cell dimensions | a = 7.3326(5) Å | [I > 2σ (I)] | wR2 = 0.1188 |
| b = 19.4897(14) Å | R indices (all data) | R1 = 0.0582, | |
| c = 8.6586(6) Å | wR2 = 0.1255 | ||
| α = 90° | |||
| β = 106.069(2)° | Largest diff. peak and hole | 0.268 and −0.213 eÅ−3 | |
| γ = 90° | |||
| Volume | 1189.06(14) Å3 | ||
| Z | 4 | ||
| Density (calculated) | 1.359 mg/m3 | ||
| Absorption coefficient | 0.091 mm−1 | ||
| F(000) | 512 | ||
| Crystal size | 0.70 × 0.40 × 0.20 mm | ||
| Bond Lengths, Å | |||
| N1-C1 | 1.3308(19) | N4-C4 | 1.3236(18) |
| N2-C1 | 1.3459(17) | N5-C3 | 1.3270(18) |
| N2-C2 | 1.4607(18) | N5-C4 | 1.3588(18) |
| N3-C3 | 1.3447(17) | N6-C1 | 1.3249(17) |
| N3-N4 | 1.3669(16) | N6-C3 | 1.3544(18) |
| N3-C2 | 1.4659(16) | ||
| Bond Angles, ° | |||
| C1-N2-C2 | 123.57(12) | N6-C1-N2 | 123.45(13) |
| C3-N3-N4 | 110.73(11) | N1-C1-N2 | 118.64(13) |
| C3-N3-C2 | 122.48(12) | N2-C2-N3 | 103.42(11) |
| N4-N3-C2 | 125.76(11) | N5-C3-N3 | 109.47(12) |
| C4-N4-N3 | 101.20(11) | N5-C3-N6 | 126.38(13) |
| C3-N5-C4 | 102.89(11) | N3-C3-N6 | 124.13(12) |
| C1-N6-C3 | 113.86(12) | N4-C4-N5 | 115.70(12) |
| N6-C1-N1 | 117.87(13) | ||
| D-H⋯A | D-H | H⋯A | D⋯A | D-H⋯A |
|---|---|---|---|---|
| N1-H1A⋯N5i | 0.88(2) | 2.06(2) | 2.9444(18) | 173.6(17) |
| N1-H1B⋯N7ii | 0.89(2) | 2.21(2) | 3.094(2) | 170.3(16) |
| N2-H2⋯N6i | 0.841(18) | 2.128(18) | 2.9648(17) | 172.9(16) |
| C10-H10C⋯Cgiii | 0.96 | 2.60 | 3.5557(17) | 173 |
Cg is the centroid of the pyridine ring (C5/C6/C7/N7/C8/C9); Symmetry codes:(i)x + 1/2, −y + 1/2, z + 1/2;(ii)− x − 1/2, y + 1/2, −z + 1/2;(iii)− x, −y, 1−z.
Acknowledgments
This work was supported by the School of Pharmacy, Curtin University of Technology, and the National Medical Research Council, Singapore (NMRC/NIG/0019/2008).
References and Notes
- Part 20 in the series “Fused heterocyclic systems with s-triazine ring”, for part 19 see:Dolzhenko, A.V.; Tan, G.K.; Dolzhenko, A.V.; Koh, L.L.; Chui, W.K. 7-(4-Fluorobenzylamino)-2-phenyl-1, 2,4-triazolo[1,5-a][1,3,5]triazin-5-amine methanol disolvate. Acta Crystallogr. 2011, E67, o1183–o1184. [Google Scholar]
- Dolzhenko, A.V.; Dolzhenko, A.V.; Chui, W.K. 1,2,4-Triazolo[1,5-a][1,3,5]triazines (5-azapurines): synthesis and biological activity. Heterocycles 2006, 68, 1723–1753. [Google Scholar]
- Dolzhenko, A.V.; Dolzhenko, A.V.; Chui, W.K. Synthesis of 5,7-diamino[1,2,4]triazolo[1,2-a][1,3,5]triazines via annulation of 1,3,5-triazine ring onto 3(5)-amino-1,2,4-triazoles. Heterocycles 2007, 71, 429–436. [Google Scholar]
- Dolzhenko, A.V.; Tan, B.J.; Dolzhenko, A.V.; Chiu, G.N.C.; Chui, W.K. Synthesis and biological activity of fluorinated 7-aryl-2-pyridyl-6,7-dihydro[1,2,4]triazolo[1,5-a][1,3,5]triazin-5-amines. J. Fluorine Chem. 2008, 129, 429–434. [Google Scholar]
- Dolzhenko, A.V.; Pastorin, G.; Dolzhenko, A.V.; Chui, W.K. A convenient method for the synthesis of 7-amino-substituted 1,2,4-triazolo[1,5-a][1,3,5]triazin-5-amines. Tetrahedron Lett. 2008, 49, 7180–7183. [Google Scholar]
- Dolzhenko, A.V.; Dolzhenko, A.V.; Chui, W.K. Practical synthesis of regioisomeric 5(7)-amino-6,7(4,5)-dihydro[1,2,4]triazolo[1,5-a][1,3,5]triazines. Tetrahedron 2007, 63, 12888–12895. [Google Scholar]
- Dolzhenko, A.V.; Syropyatov, B.Ya. Method of obtaining condensed gem-dimethyl substituted amino-1,3,5-triazines. Russian Patent 2330854, 10 August 2008. [Google Scholar]
- Dolzhenko, A.V.; Tan, G.K.; Dolzhenko, A.V.; Koh, L.L.; Chui, W.K. 2-Phenyl-7-(4-pyridylmethylamino)-1,2,4-triazolo[1,5-a][1,3,5]triazin-5(4H)-one. Acta Crystallogr. 2011, E67, o85–o86. [Google Scholar]
- Dolzhenko, A.V.; Tan, G.K.; Koh, L.L.; Woo, S.F.; Chui, W.K. 7-Dimethylamino-2-phenyl-1,2,4-triazolo[1,5-a][1,3,5]triazin-5-amine methanol solvate. Acta Crystallogr. 2008, E64, o2021. [Google Scholar]
- Dolzhenko, A.V.; Tan, G.K.; Koh, L.L.; Dolzhenko, A.V.; Chui, W.K. 5,5-Dimethyl-2-phenyl-4,5-dihydro[1,2,4]triazolo[1,5-a][1,3,5]triazin-7-amine. Acta Crystallogr. 2007, E63, o2797. [Google Scholar]
- Dolzhenko, A.V.; Tan, G.K.; Koh, L.L.; Dolzhenko, A.V.; Chui, W.K. 7,7-Dimethyl-2-phenyl-6,7-dihydro[1,2,4]triazolo[1,5-a][1,3,5]triazin-5-amine. Acta Crystallogr. 2007, E63, o2796. [Google Scholar]
- Khankischpur, M.; Hansen, F.K.; Geffken, D. Convenient synthesis of 5-substituted 2-amino[1,2,4]triazolo[1,5-a][1,3,5]triazin-7(6H)-ones from N-triazolide imidates and 1,2,4-triazole-3,5-diamine. Synthesis 2010, 1645–1648. [Google Scholar]
- Gilardi, R.D. Crystal structure of a compound with an uncommon asapurine ring system. 5,7-Bis(dimethylamino)-2-(methylthio)-s-triazolo[1,5-a]-s-triazine. Acta Crystallogr. 1973, B29, 2089–2095. [Google Scholar]
- Dolzhenko, A.V.; Chui, W.K. Synthesis and biological activity of 1,3,5-triazino[1,2-a]benzimidazol-2-amines. Pharm. Chem. J. 2007, 41, 470–473. [Google Scholar]
- Dolzhenko, A.V.; Chui, W.K. Synthesis of 2-amino-s-triazino[1,2-a]benzimidazoles as potential antifolates from 2-guanidino- and 2-guanidino-5-methylbenzimidazoles. J. Heterocycl. Chem. 2006, 43, 95–100. [Google Scholar]
- Toyoda, T.; Brobey, R.K.B.; Sano, G.; Horii, T.; Tomioka, N.; Itai, A. Lead discovery of inhibitors of the dihydrofolate reductase domain of Plasmodium falciparum dihydrofolate reductase-thymidylate synthase. Biochem. Biophys. Res. Commun. 1997, 235, 515–519. [Google Scholar]
- Dolzhenko, A.V.; Tan, G.K.; Dolzhenko, A.V.; Koh, L.L.; Chui, W.K. 4,4-Dimethyl-3,4-dihydropyrido[2′,3′:3,4]pyrazolo[1,5-a][1,3,5]triazin-2-amine ethanol monosolvate. Acta Crystallogr. 2011, E67, o83–o84. [Google Scholar]
- Cremer, D.; Pople, J.A. General definition of ring puckering coordinates. J. Am. Chem. Soc. 1975, 97, 1354–1358. [Google Scholar]
- Bernstein, J.; Davis, R.E.; Shimoni, L.; Chang, N.-L. Patterns in hydrogen bonding: functionality and graph set analysis in crystals. Angew. Chem. Int. Ed. 1995, 34, 1555–1573. [Google Scholar]
- Nishio, M.; Umezawa, Y.; Honda, K.; Tsuboyama, S.; Suezawa, H. CH/π hydrogen bonds in organic and organometallic chemistry. CrystEngComm 2009, 11, 1757–1788. [Google Scholar]
- Sheldrick, G.M. SADABS; University of Göttingen: Göttingen, Germany, 2001. [Google Scholar]
- Bruker. SMART and SAINT; Bruker AXS GmbH: Karlsruhe, Germany, 2001. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. 2008, A64, 112–122. [Google Scholar]
- Spek, A.L. Single-crystal structure validation with the program PLATON. J. Appl. Crystallogr. 2003, 36, 7–13. [Google Scholar]
- Macrae, C.F.; Bruno, I.J.; Chisholm, J.A.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Rodriguez- Monge, L.; Taylor, R.; van de Streek, J.; Wood, P.A. Mercury CSD 2.0 - new features for the visualization and investigation of crystal structures. J. Appl. Crystallogr. 2008, 41, 466–470. [Google Scholar]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Dolzhenko, A.V.; Tan, G.K.; Koh, L.L.; Dolzhenko, A.V.; Chui, W.K. Molecular and Crystal Structure of 7,7-Dimethyl-2-pyridin-4-yl-6,7-dihydro-1,2,4-triazolo[1,5-a][1,3,5]triazin-5-amine [1]. Crystals 2011, 1, 136-144. https://doi.org/10.3390/cryst1030136
Dolzhenko AV, Tan GK, Koh LL, Dolzhenko AV, Chui WK. Molecular and Crystal Structure of 7,7-Dimethyl-2-pyridin-4-yl-6,7-dihydro-1,2,4-triazolo[1,5-a][1,3,5]triazin-5-amine [1]. Crystals. 2011; 1(3):136-144. https://doi.org/10.3390/cryst1030136
Chicago/Turabian StyleDolzhenko, Anton V., Geok Kheng Tan, Lip Lin Koh, Anna V. Dolzhenko, and Wai Keung Chui. 2011. "Molecular and Crystal Structure of 7,7-Dimethyl-2-pyridin-4-yl-6,7-dihydro-1,2,4-triazolo[1,5-a][1,3,5]triazin-5-amine [1]" Crystals 1, no. 3: 136-144. https://doi.org/10.3390/cryst1030136
APA StyleDolzhenko, A. V., Tan, G. K., Koh, L. L., Dolzhenko, A. V., & Chui, W. K. (2011). Molecular and Crystal Structure of 7,7-Dimethyl-2-pyridin-4-yl-6,7-dihydro-1,2,4-triazolo[1,5-a][1,3,5]triazin-5-amine [1]. Crystals, 1(3), 136-144. https://doi.org/10.3390/cryst1030136




