Microwave-Assisted One-Step Conversion of Wood Wastes into Levulinic Acid
Abstract
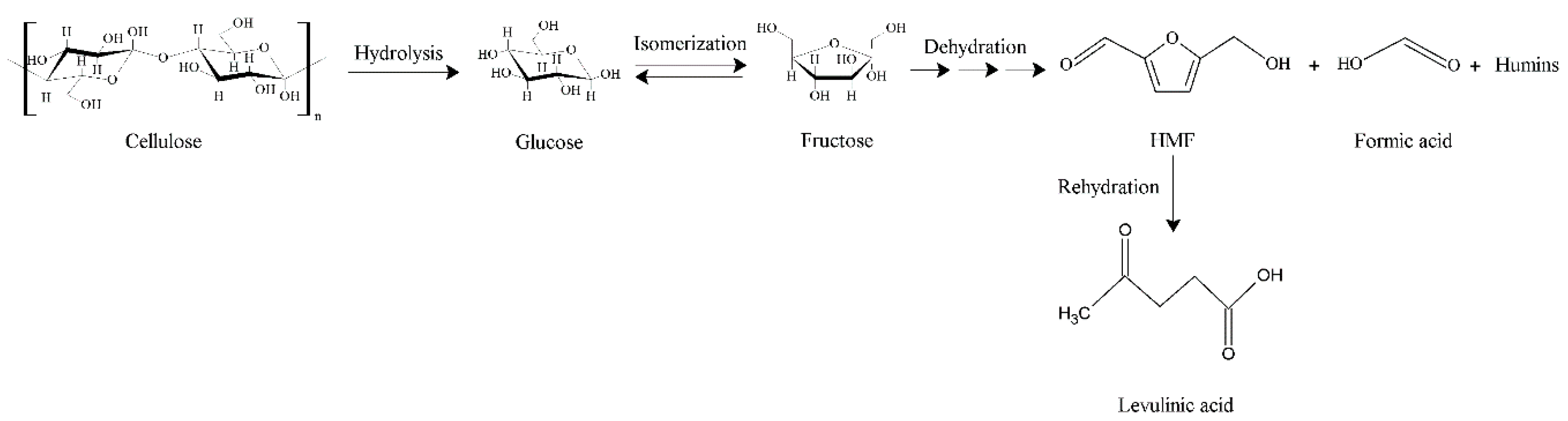
1. Introduction
2. Results and Discussion
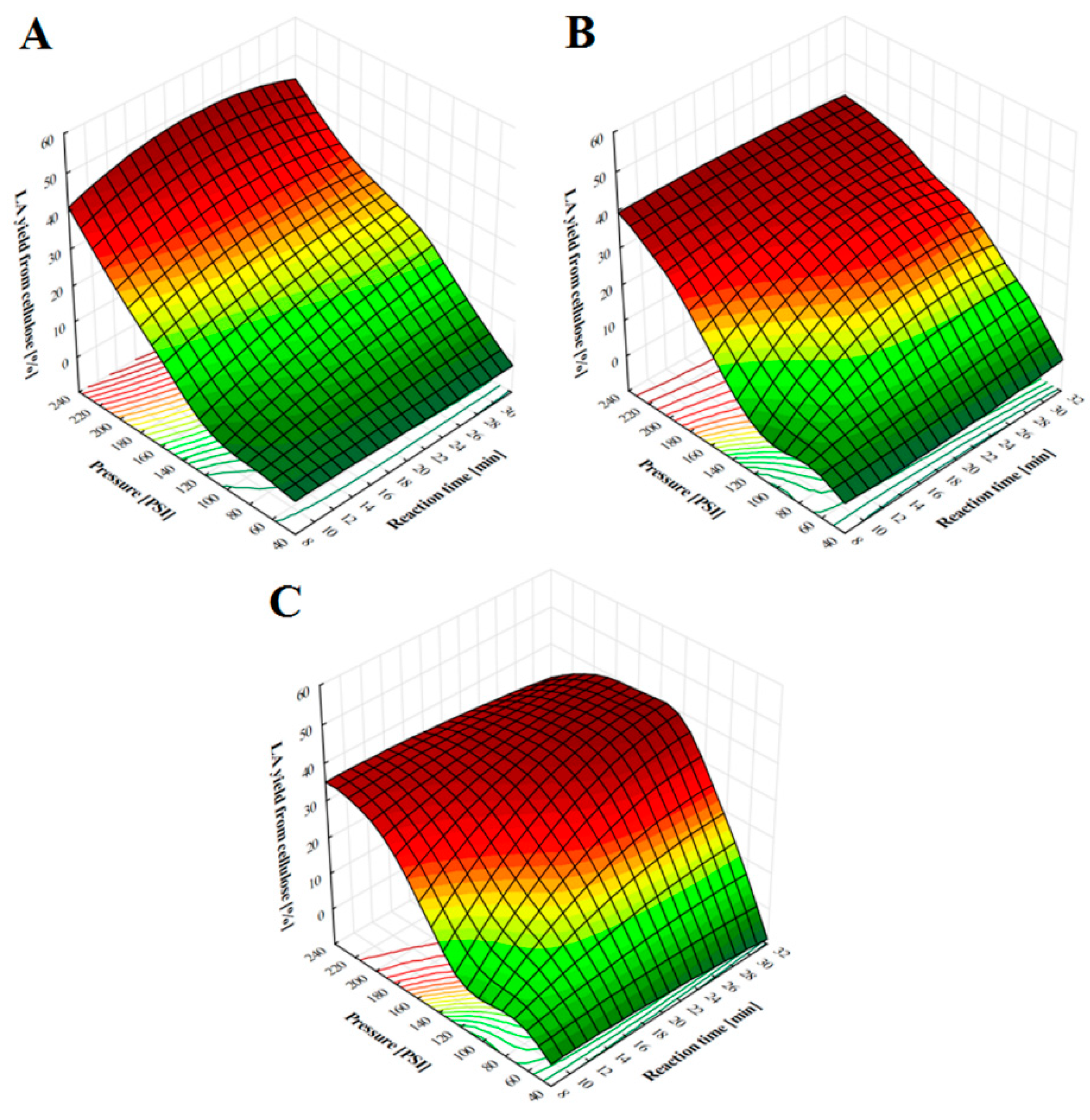
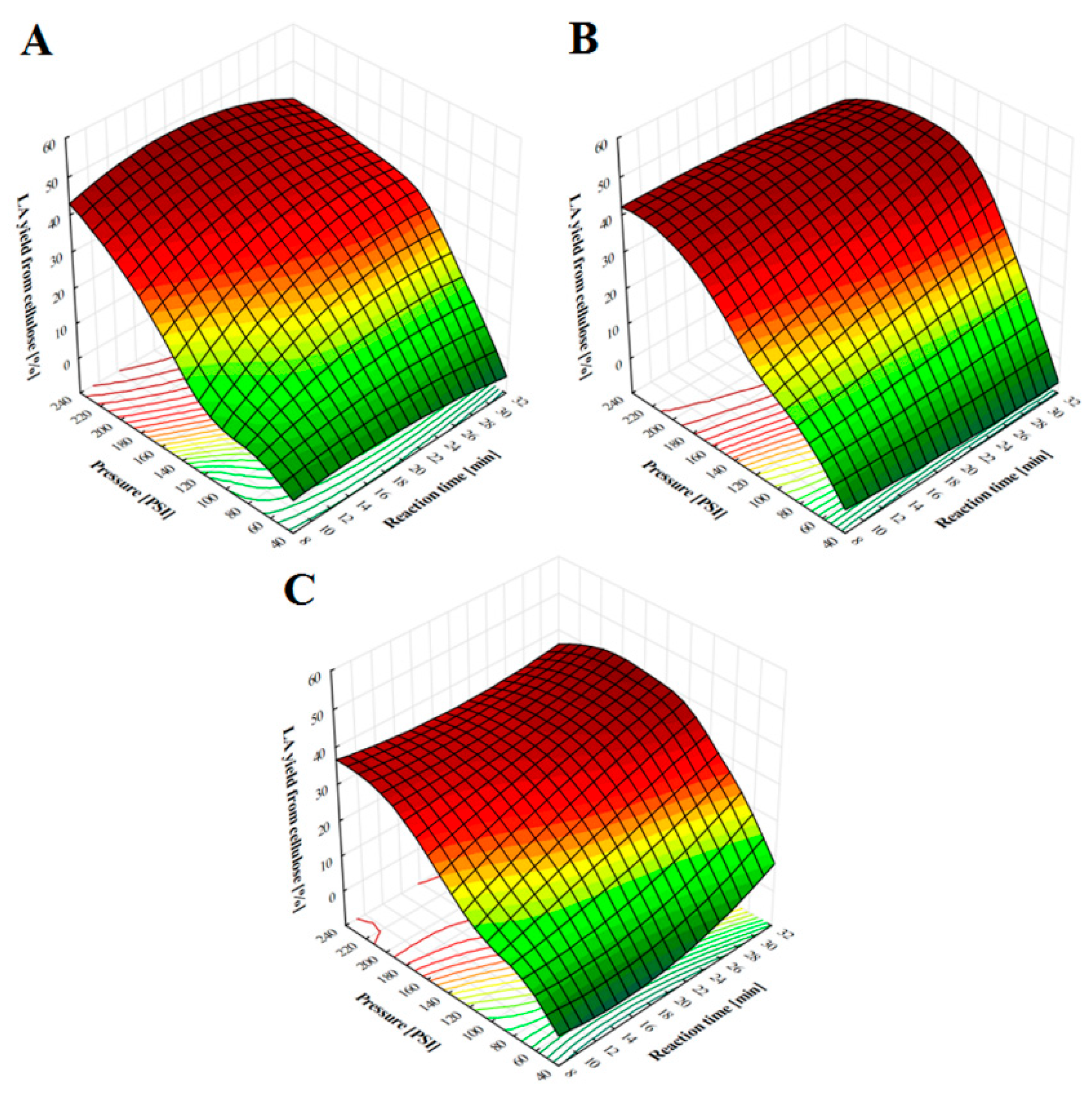
2.1. LA Production from Pine Chips at Different Pressure Values
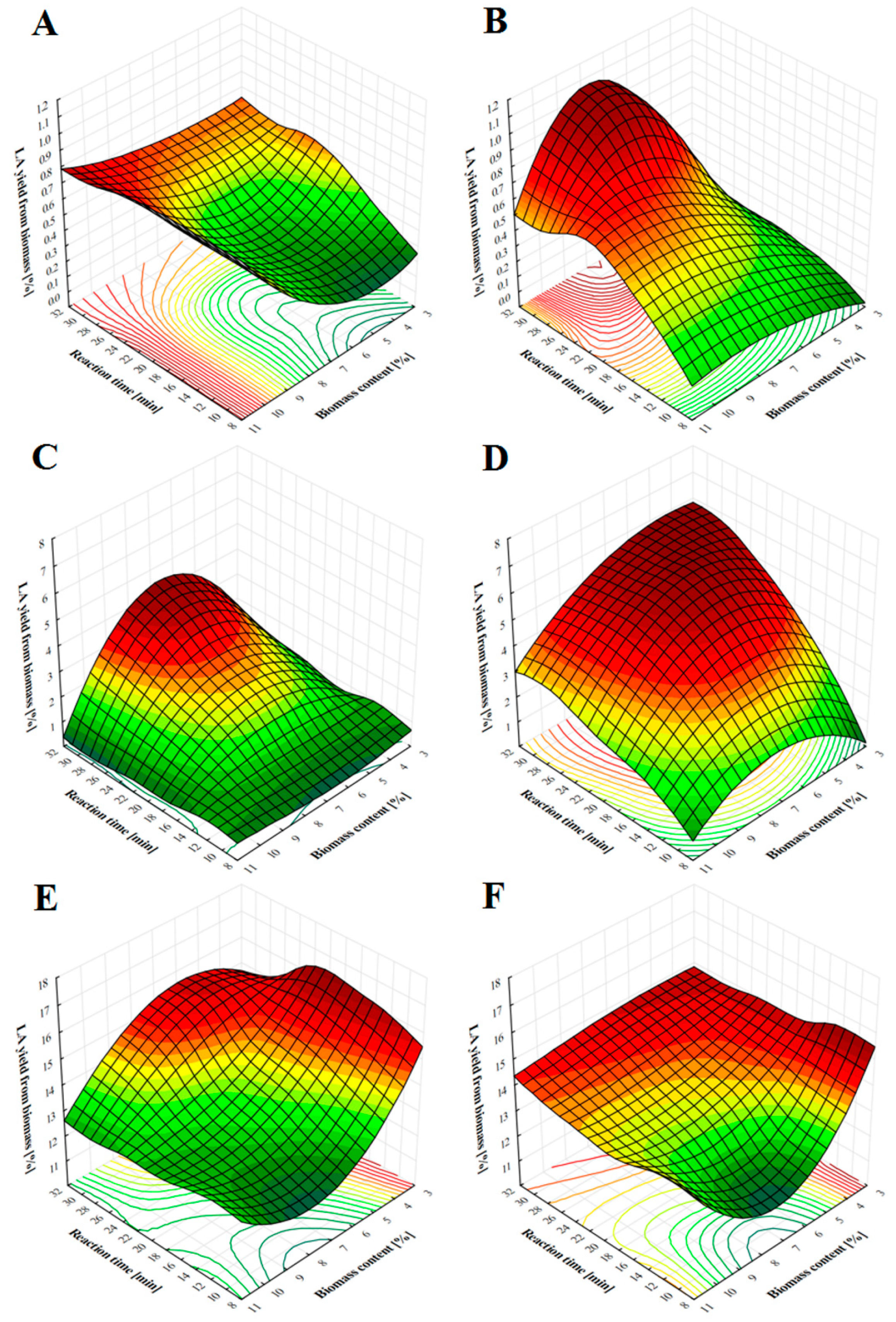
2.2. LA Production from Soft- and Hardwood Chips Using Nitric, Phosphoric and Sulfuric Acid under Various Process Conditions
3. Materials and Methods
3.1. Raw Material
3.2. Chemical Reagents
3.3. Production of Levulinic Acid Using Microwave Radiation
3.4. Analytical Methods
3.4.1. Analysis of Wood Waste Composition
3.4.2. HPLC Analysis
3.5. Computational Methods
3.6. Statistics
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Compliance with Ethics Requirements
References
- Sharma, A.; Pareek, V.; Zhang, D. Biomass pyrolysis—A review of modelling, process parameters and catalytic studies. Renew. Sustain. Energy Rev. 2015, 50, 1081–1096. [Google Scholar] [CrossRef]
- Kang, S.; Yu, J. Hydrophobic organic compounds from hydrothermal liquefaction of bacterial biomass. Biomass Bioenergy 2015, 74, 92–95. [Google Scholar] [CrossRef]
- Ruiz, J.A.; Juárez, M.C.; Morales, M.P.; Muñoz, P.; Mendívil, M.A. Biomass gasification for electricity generation: Review of current technology barriers. Renew. Sustain. Energy Rev. 2013, 18, 174–183. [Google Scholar] [CrossRef]
- Kang, S.; Li, X.; Fan, J.; Chang, J. Solid fuel production by hydrothermal carbonization of black liquor. Bioresour. Technol. 2012, 110, 715–718. [Google Scholar] [CrossRef] [PubMed]
- Balat, M. Production of bioethanol from lignocellulosic materials via the biochemical pathway: A review. Energy Convers. Manag. 2011, 52, 858–875. [Google Scholar] [CrossRef]
- Chianese, S.; Loipersböck, J.; Malits, M.; Rauch, R.; Hofbauer, H.; Molino, A.; Musmarra, D. Hydrogen from the high temperature water gas shift reaction with an industrial Fe/Cr catalyst using biomass gasification tar rich synthesis gas. Fuel Process. Technol. 2015, 132, 39–48. [Google Scholar] [CrossRef]
- Lang, C.; Sécordel, X.; Kiennemann, A.; Courson, C. Water gas shift catalysts for hydrogen production from biomass steam gasification. Fuel Process. Technol. 2017, 156, 246–252. [Google Scholar] [CrossRef]
- Ma, H.; Liu, W.W.; Chen, X.; Wu, Y.J.; Yu, Z.L. Enhanced enzymatic saccharification of rice straw by microwave pretreatment. Bioresour. Technol. 2009, 100, 1279–1284. [Google Scholar] [CrossRef]
- Liu, Q.; Li, W.; Ma, Q.; An, S.; Li, M.; Jameel, H.; Chang, H.M. Pretreatment of corn stover for sugar production using a two-stage dilute acid followed by wet-milling pretreatment process. Bioresour. Technol. 2016, 211, 435–442. [Google Scholar] [CrossRef]
- Sun, Y.; Cheng, J.J. Dilute acid pretreatment of rye straw and bermudagrass for ethanol production. Bioresour. Technol. 2005, 96, 1599–1606. [Google Scholar] [CrossRef]
- Ye, S.; Cheng, J. Hydrolysis of lignocellulosic materials for ethanol production: A review. Bioresour. Technol. 2002, 83, 1–11. [Google Scholar]
- Jørgensen, H.; Kristensen, J.B.; Felby, C. Enzymatic conversion of lignocellulose into fermentable sugars: Challenges and opportunities. Biofuels Bioprod. Biorefining 2007, 1, 119–134. [Google Scholar] [CrossRef]
- Werpy, T.A.; Holladay, J.E.; White, J.F. Top Value Added Chemicals from Biomass: Results of Screening for Potential Candidates from Sugars and Synthesis Gas; Pacific Northwest National Laboratory: Richland, WA, USA, 2004. [Google Scholar]
- Fang, Q.; Hanna, M.A. Experimental studies for levulinic production from whole kernel grain sorghum. Bioresour. Technol. 2002, 81, 187–192. [Google Scholar] [CrossRef]
- Kang, S.; Fu, J.; Zhang, G. From lignocellulosic biomass to levulinic acid: A review on acid-catalyzed hydrolysis. Renew. Sustain. Energy Rev. 2018, 94, 340–362. [Google Scholar] [CrossRef]
- Morone, A.; Apte, M.; Pandey, R.A. Levulinic acid production from renewable waste resources: Bottlenecks, potential remedies, advancements and applications. Renew. Sustain. Energy Rev. 2015, 51, 548–565. [Google Scholar] [CrossRef]
- Chang, C.; Cen, P.; Ma, X. Levulinic acid production from wheat straw. Bioresour. Technol. 2007, 98, 1448–1453. [Google Scholar] [CrossRef]
- Sweygers, N.; Dewil, R.; Appels, L. Production of levulinic acid and furfural by microwave-assisted hydrolysis from model compounds: Effect of temperature, acid concentration and reaction time. Waste Biomass Valorization 2018, 9, 343–355. [Google Scholar] [CrossRef]
- Shrotri, A.; Kobayashi, H.; Fukuoka, A. Cellulose depolymerization over heterogeneous catalysts. Acc. Chem. Res. 2018, 51, 761–768. [Google Scholar] [CrossRef]
- Yoon, S.Y.; Han, S.H.; Shin, S.J. The effect of hemicelluloses and lignin on acid hydrolysis of cellulose. Energy 2014, 77, 19–24. [Google Scholar] [CrossRef]
- Galletti, A.M.R.; Antonetti, C.; Luise, V.D.; Licursi, D.; Nassi, D. Levulinic acid production from waste biomass. BioResources 2012, 7, 1824–1835. [Google Scholar]
- Shen, J.; Wyman, C.E. Hydrochloric acid-catalyzed levulinic acid formation from cellulose: Data and kinetic model to maximize yields. AIChE J. 2012, 58, 236–246. [Google Scholar] [CrossRef]
- Jeong, H.; Jang, S.K.; Hong, C.Y.; Kim, S.H.; Lee, S.; Lee, S.M.; Choi, J.W.; Choi, I.G. Levulinic acid production by two-step acid-catalyzed treatment of Quercus mongolica using dilute sulfuric acid. Bioresour. Technol. 2017, 225, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Bundhoo, Z.M.A. Microwave-assisted conversion of biomass and waste materials to biofuels. Renew. Sustain. Energy Rev. 2018, 82, 1149–1177. [Google Scholar] [CrossRef]
- Yemiş, O.; Mazza, G. Optimization of furfural and 5-hydroxymethylfurfural production from wheat straw by a microwave-assisted process. Bioresour. Technol. 2012, 109, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Mӧller, M.; Harnisch, F.; Schrӧder, U. Microwave-assisted hydrothermal degradation of fructose and glucose in subcritical water. Biomass Bioenergy 2012, 39, 389–398. [Google Scholar] [CrossRef]
- Budarin, V.L.; Clark, J.H.; Lanigan, B.A.; Shuttleworth, P.; Macquarrie, D.J. Microwave assisted decomposition of cellulose: A new thermochemical route. Bioresour. Technol. 2010, 101, 3776–3779. [Google Scholar] [CrossRef]
- Monteil-Rivera, F.; Huang, G.H.; Paquet, L.; Deschamps, S.; Beaulieu, C.; Hawari, J. Microwave-assisted extraction of lignin from triticale straw: Optimization and microwave effects. Bioresour. Technol. 2012, 104, 775–782. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Dai, L.; Fan, L.; Shan, S.; Liu, Y.; Ruan, R. Review of microwave-assisted lignin conversion for renewable fuels and chemicals. J. Anal. Appl. Pyrolysis 2016, 119, 104–113. [Google Scholar]
- Gong, G.; Liu, D.; Huang, Y. Microwave-assisted organic acid pretreatment for enzymatic hydrolysis of rice straw. Biosyst. Eng. 2010, 107, 67–73. [Google Scholar] [CrossRef]
- Zhu, S.; Wu, Y.; Yu, Z.; Wang, C.; Yu, F.; Jin, S.; Ding, Y.; Chi, R.; Liao, J.; Zhang, Y. Comparison of three microwave/chemical pretreatment processes for enzymatic hydrolysis of rice straw. Biosyst. Eng. 2006, 93, 279–283. [Google Scholar] [CrossRef]
- Zhu, S.; Wu, Y.; Yu, Z.; Zhang, X.; Wang, C.; Yu, F.; Jin, S. Production of ethanol from microwave-assisted alkali pretreated wheat straw. Process Biochem. 2006, 41, 869–873. [Google Scholar] [CrossRef]
- Appels, L.; Houtmeyers, S.; Degrève, J.; Van Impe, J.; Dewil, R. Influence of microwave pre-treatment on sludge solubilization and pilot scale semi-continuous anaerobic digestion. Bioresour. Technol. 2013, 128, 598–603. [Google Scholar] [CrossRef] [PubMed]
- Ebenezer, A.V.; Kaliappan, S.; Adish Kumar, S.; Yeom, I.T.; Banu, J.R. Influence of deflocculation on microwave disintegration and anaerobic biodegradability of waste activated sludge. Bioresour. Technol. 2015, 185, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Asomaning, J.; Haupt, S.; Chae, M.; Bressler, D.C. Recent developments in microwave-assisted thermal conversion of biomass for fuels and chemicals. Renew. Sustain. Energy Rev. 2018, 92, 642–657. [Google Scholar] [CrossRef]
- Markovića, M.; Markov, S.; Grujić, O.; Mojović, L.; Kocić-Tanackov, S.; Vukašinović, M.; Pejina, J. Microwave as a pre-treatment of triticale for bioethanol fermentation and utilization of the stillage for lactic acid fermentation. Biochem. Eng. J. 2014, 85, 132–138. [Google Scholar] [CrossRef]
- Moodley, P.; Kana, E.B.G. Development of a steam or microwave-assisted sequential salt-alkali pretreatment for lignocellulosic waste: Effect on delignification and enzymatic hydrolysis. Energy Convers. Manag. 2017, 148, 801–808. [Google Scholar] [CrossRef]
- Lacerda, V.S.; López-Sotelo, J.B.; Correa-Guimarães, A.; Hernández-Navarro, S.; Sánchez-Bascones, M.; Navas-Gracia, L.M.; Martín-Ramos, P.; Pérez-Lebeña, E.; Martín-Gil, J. A kinetic study on microwave-assisted conversion of cellulose and lignocellulosic waste into hydroxymethylfurfural/furfural. Bioresour. Technol. 2015, 180, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, V.; Mushrif, S.H.; Ho, C.; Anderko, A.; Nikolakis, V.; Marinkovic, N.S.; Frenkel, A.I.; Sandler, S.I.; Vlachos, D.G. Insights into the interplay of Lewis and Brønsted acid catalysts in glucose and fructose conversion to 5-(hydroxymethyl) furfural and levulinic acid in aqueous media. J. Am. Chem. Soc. 2013, 135, 3997–4006. [Google Scholar] [CrossRef]
- Girisuta, B.; Danon, B.; Manurung, R.; Janssen, L.P.B.M.; Heeres, H.J. Experimental and kinetic modelling studies on the acid-catalysed hydrolysis of the water hyacinth plant to levulinic acid. Bioresour. Technol. 2008, 99, 8367–8375. [Google Scholar] [CrossRef]
- Cao, L.; Yu, I.K.M.; Cho, D.-W.; Wang, D.; Tsang, D.C.W.; Zhang, S.; Ding, S.; Wang, L.; Ok, Y.S. Microwave-assisted low-temperature hydrothermal treatment of red seaweed (Gracilaria lemaneiformis) for production of levulinic acid and algae hydrochar. Bioresour. Technol. 2018, 273, 251–258. [Google Scholar] [CrossRef]
- Hu, X.; Song, Y.; Wu, L.; Gholizadeh, M.; Li, C.Z. One-pot synthesis of levulinic acid/ester from C5 carbohydrates in a methanol medium. ACS Sustain. Chem. Eng. 2013, 1, 1593–1599. [Google Scholar] [CrossRef]
- Mukherjee, A.; Dumont, M.-J.; Raghavan, V. Sustainable production of hydroxymethylfurfural and levulinic acid: Challenges and opportunities. Biomass Bioenergy 2015, 72, 143–183. [Google Scholar] [CrossRef]
- Fachri, B.A.; Abdilla, R.; Bovenkamp, H.; Rasrendra, C.; Heeres, H.J. Experimental and kinetic modeling studies on the sulphuric acid catalyzed conversion of D-Fructose to 5-Hydroxymethylfurfural and levulinic acid in water. ACS Sustain. Chem. Eng. 2015, 3, 3024–3034. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhao, Z.K. Microwave-assisted conversion of lignocellulosic biomass into furans in ionic liquid. Bioresour. Technol. 2010, 101, 1111–1114. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Zhou, Y.; Liu, L. Selective conversion of cellulose to levulinic acid via microwave-assisted synthesis in ionic liquids. Bioresour. Technol. 2013, 129, 616–619. [Google Scholar] [CrossRef]
- Maiti, S.; Gallastegui, G.; Suresh, G.; Pachapur, V.L.; Brar, S.K.; Bihan, Y.L.; Drogui, P.; Buelna, G.; Verma, M.; Galvez-Cloutier, R. Microwave-assisted one-pot conversion of agro-industrial wastes into levulinic acid: An alternate approach. Bioresour. Technol. 2018, 265, 471–479. [Google Scholar] [CrossRef]
- Liang, C.; Hu, Y.; Wang, Y.; Wu, L.; Zhang, W. Production of levulinic acid from corn cob residue in a fed-batch acid hydrolysis process. Process Biochem. 2018, 73, 124–131. [Google Scholar] [CrossRef]
- Ruiz, R.; Date, T.E. Determination of Carbohydrates in Biomass by High Performance Liquid Chromatography; Laboratory Analytical Procedure No. 002; National Renewable Research Laboratory: Golden, CO, USA; Citeseer: Forest Grove, OR, USA, 1996. [Google Scholar]
- Mikulski, D.; Kłosowski, G.; Menka, A.; Koim-Puchowska, B. Microwave-assisted pretreatment of maize distillery stillage with the use of dilute sulfuric acid in the production of cellulosic ethanol. Bioresour. Technol. 2019, 278, 318–328. [Google Scholar] [CrossRef]
- Rana, V.; Rana, D.; Ahring, B.K. Process modeling of enzymatic hydrolysis of wet-exploded corn stover. Bioenergy Res. 2014, 7, 450–459. [Google Scholar] [CrossRef]
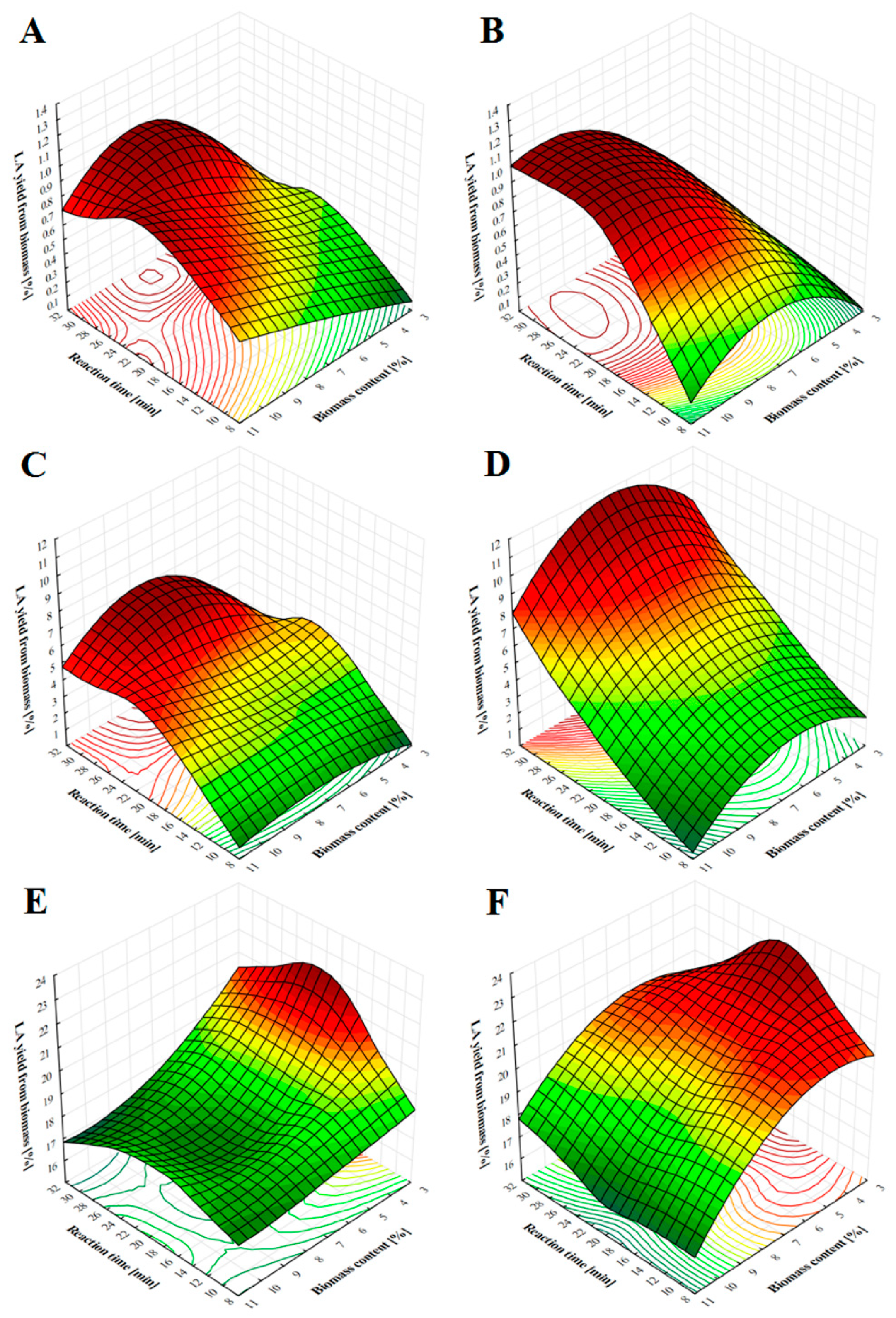
| Reaction Time (min) | Biomass Content (%) | Concentration of Levulinic Acid (g/L) | Production of Levulinic Acid (mg/g of DW) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1% H2SO4 | 2% H2SO4 | 1% H3PO4 | 2% H3PO4 | 1% HNO3 | 2% HNO3 | 1% H2SO4 | 2% H2SO4 | 1% H3PO4 | 2% H3PO4 | 1% HNO3 | 2% HNO3 | ||
| 10 | 10.0 | 4.37 ± 0.09 | 4.49 ± 0.20 | 0.42 ± 0.10 | 0.48 ± 0.30 | 0.15 ± 0.01 | 0.10 ± 0.03 | 175.1 ± 3.4 | 179.5 ± 7.8 | 16.8 ± 4.0 | 19.1 ± 11.8 | 5.9 ± 0.2 | 4.1 ± 1.4 |
| 10 | 5.0 | 4.62 ± 0.10 | 5.34 ± 0.22 | 0.39 ± 0.11 | 0.87 ± 0.19 | 0.07 ± 0.01 | 0.09 ± 0.02 | 185.1 ± 3.8 | 213.5 ± 8.6 | 15.6 ± 4.4 | 34.7 ± 7.4 | 2.8 ± 0.4 | 3.6 ± 0.8 |
| 10 | 3.3 | 4.71 ± 0.05 | 5.28 ± 0.04 | 0.23 ± 0.02 | 0.59 ± 0.06 | 0.04 ± 0.04 | 0.03 ± 0.03 | 188.4 ± 2.0 | 211.1 ± 1.4 | 9.2 ± 0.8 | 23.6 ± 2.4 | 1.6 ± 1.6 | 1.1 ± 1.0 |
| 20 | 10.0 | 4.43 ± 0.22 | 4.34 ± 0.21 | 1.15 ± 0.53 | 1.10 ± 0.29 | 0.22 ± 0.01 | 0.25 ± 0.01 | 177.2 ± 8.8 | 173.5 ± 8.2 | 46.0 ± 21.2 | 44.0 ± 11.6 | 8.7 ± 0.2 | 10.0 ± 0.4 |
| 20 | 5.0 | 4.77 ± 0.12 | 5.41 ± 0.52 | 0.84 ± 0.19 | 1.48 ± 0.21 | 0.13 ± 0.01 | 0.14 ± 0.02 | 190.8 ± 4.8 | 216.5 ± 20.7 | 33.47 ± 7.4 | 59.1 ± 8.2 | 5.1 ± 0.2 | 5.5 ± 0.6 |
| 20 | 3.3 | 5.41 ± 0.07 | 5.79 ± 0.40 | 0.97 ± 0.34 | 1.26 ± 0.06 | 0.11 ± 0.04 | 0.05 ± 0.02 | 216.4 ± 2.8 | 231.5 ± 16.0 | 38.8 ± 13.6 | 50.4 ± 2.4 | 4.4 ± 1.6 | 1.9 ± 0.6 |
| 30 | 10.0 | 4.28 ± 0.22 | 4.67 ± 0.04 | 1.44 ± 0.43 | 2.10 ± 0.14 | 0.22 ± 0.02 | 0.26 ± 0.01 | 171.3 ± 8.8 | 186.7 ± 1.4 | 57.6 ± 17.2 | 84.0 ± 5.6 | 8.7 ± 0.6 | 10.5 ± 0.2 |
| 30 | 5.0 | 4.77 ± 0.10 | 5.27 ± 0.14 | 1.42 ± 0.33 | 2.54 ± 0.27 | 0.19 ± 0.01 | 0.16 ± 0.05 | 190.7 ± 3.8 | 210.8 ± 5.6 | 56.7 ± 13.0 | 101.6 ± 10.8 | 7.6 ± 0.4 | 6.3 ± 1.8 |
| 30 | 3.3 | 5.10 ± 0.28 | 5.18 ± 0.44 | 0.77 ± 0.22 | 2.10 ± 0.15 | 0.09 ± 0.01 | 0.06 ± 0.02 | 203.9 ± 11.2 | 207.1 ± 17.4 | 30.8 ± 8.8 | 84.0 ± 6.0 | 3.5 ± 0.2 | 2.4 ± 0.8 |
| Reaction Time (min) | Biomass Content (%) | Yield of Levulinic Acid Production (% of Theoretical) | |||||
|---|---|---|---|---|---|---|---|
| 1% H2SO4 | 2% H2SO4 | 1% H3PO4 | 2% H3PO4 | 1% HNO3 | 2% HNO3 | ||
| 10 | 10.0 | 48.9 ± 1.0 | 50.2 ± 2.2 | 4.7 ± 1.1 | 5.3 ± 3.3 | 1.6 ± 0.1 | 1.2 ± 0.4 |
| 10 | 5.0 | 51.7 ± 1.1 | 59.7 ± 2.4 | 4.4 ± 1.2 | 9.7 ± 2.1 | 0.8 ± 0.1 | 1.0 ± 0.2 |
| 10 | 3.3 | 52.7 ± 0.6 | 59.0 ± 0.4 | 2.6 ± 0.2 | 6.6 ± 0.7 | 0.5 ± 0.5 | 0.3 ± 0.3 |
| 20 | 10.0 | 49.5 ± 2.5 | 48.5 ± 2.3 | 12.9 ± 5.9 | 12.3 ± 3.2 | 2.4 ± 0.1 | 2.8 ± 0.1 |
| 20 | 5.0 | 53.3 ± 1.3 | 60.5 ± 5.8 | 9.4 ± 2.1 | 16.5 ± 2.3 | 1.4 ± 0.1 | 1.5 ± 0.2 |
| 20 | 3.3 | 60.5 ± 0.8 | 64.7 ± 4.5 | 10.9 ± 3.8 | 14.1 ± 0.7 | 1.2 ± 0.5 | 0.5 ± 0.2 |
| 30 | 10.0 | 47.9 ± 2.5 | 52.2 ± 0.4 | 16.1 ± 4.8 | 23.5 ± 1.6 | 2.4 ± 0.2 | 2.9 ± 0.1 |
| 30 | 5.0 | 53.3 ± 1.1 | 58.9 ± 1.6 | 15.8 ± 3.6 | 28.4 ± 3.0 | 2.1 ± 0.1 | 1.8 ± 0.5 |
| 30 | 3.3 | 57.0 ± 3.1 | 57.9 ± 4.9 | 8.6 ± 2.5 | 23.5 ± 1.7 | 1.0 ± 0.1 | 0.7 ± 0.2 |
| Reaction Time (min) | Biomass Content (%) | Concentration of Levulinic Acid (g/L) | Production of Levulinic Acid (mg/g of DW) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1% H2SO4 | 2% H2SO4 | 1% H3PO4 | 2% H3PO4 | 1% HNO3 | 2% HNO3 | 1% H2SO4 | 2% H2SO4 | 1% H3PO4 | 2% H3PO4 | 1% HNO3 | 2% HNO3 | ||
| 10 | 10.0 | 3.13 ± 0.15 | 3.24 ± 0.14 | 0.19 ± 0.08 | 0.49 ± 0.19 | 0.17 ± 0.05 | 0.06 ± 0.06 | 125.1 ± 5.8 | 129.6 ± 5.6 | 7.6 ± 3.2 | 19.6 ± 7.6 | 6.8 ± 2.0 | 2.4 ± 2.4 |
| 10 | 5.0 | 3.34 ± 0.25 | 3.20 ± 0.18 | 0.15 ± 0.05 | 0.58 ± 0.24 | 0.06 ± 0.06 | 0.03 ± 0.03 | 133.6 ± 10.0 | 127.9 ± 7.0 | 5.9 ± 1.8 | 23.2 ± 9.6 | 2.3 ± 2.2 | 1.1 ± 1.0 |
| 10 | 3.3 | 3.83 ± 0.30 | 3.76 ± 0.20 | 0.20 ± 0.10 | 0.32 ± 0.04 | 0.07 ± 0.07 | 0.00 ± 0.00 | 153.1 ± 11.8 | 150.4 ± 8.0 | 8.0 ± 4.0 | 12.7 ± 1.4 | 2.8 ± 2.8 | 0.0 ± 0.0 |
| 20 | 10.0 | 3.17 ± 0.22 | 3.35 ± 0.14 | 0.34 ± 0.13 | 0.87 ± 0.30 | 0.17 ± 0.01 | 0.16 ± 0.07 | 126.8 ± 8.8 | 133.9 ± 5.4 | 13.6 ± 5.2 | 34.7 ± 11.8 | 6.8 ± 0.4 | 6.4 ± 2.8 |
| 20 | 5.0 | 3.64 ± 0.11 | 3.52 ± 0.19 | 0.55 ± 0.28 | 1.33 ± 0.55 | 0.09 ± 0.04 | 0.09 ± 0.06 | 145.5 ± 4.2 | 140.8 ± 7.6 | 22.0 ± 11.2 | 53.2 ± 22.0 | 3.6 ± 1.6 | 3.5 ± 2.2 |
| 20 | 3.3 | 4.04 ± 0.10 | 3.70 ± 0.18 | 0.27 ± 0.01 | 1.12 ± 0.31 | 0.14 ± 0.07 | 0.04 ± 0.04 | 161.5 ± 3.8 | 148.0 ± 7.2 | 10.8 ± 0.4 | 44.7 ± 12.2 | 5.5 ± 2.6 | 1.5 ± 1.4 |
| 30 | 10.0 | 3.36 ± 0.03 | 3.57 ± 0.21 | 0.46 ± 0.10 | 0.99 ± 0.01 | 0.18 ± 0.07 | 0.19 ± 0.08 | 134.4 ± 1.2 | 142.7 ± 8.2 | 18.3 ± 3.8 | 39.5 ± 0.25 | 7.2 ± 2.8 | 7.6 ± 3.2 |
| 30 | 5.0 | 3.89 ± 0.23 | 3.70 ± 0.09 | 0.92 ± 0.45 | 1.47 ± 0.29 | 0.15 ± 0.04 | 0.17 ± 0.06 | 155.6 ± 9.2 | 147.9 ± 3.4 | 36.8 ± 18.0 | 58.7 ± 11.4 | 6.0 ± 1.6 | 6.7 ± 2.2 |
| 30 | 3.3 | 3.74 ± 0.16 | 3.70 ± 0.34 | 0.47 ± 0.05 | 1.47 ± 0.38 | 0.15 ± 0.07 | 0.00 ± 0.00 | 149.7 ± 6.2 | 148.0 ± 13.6 | 18.7 ± 1.8 | 58.7 ± 15.0 | 6.0 ± 2.8 | 0.0 ± 0.0 |
| Reaction Time (min) | Biomass Content (%) | Yield of Levulinic Acid Production (% of Theoretical) | |||||
|---|---|---|---|---|---|---|---|
| 1% H2SO4 | 2% H2SO4 | 1% H3PO4 | 2% H3PO4 | 1% HNO3 | 2% HNO3 | ||
| 10 | 10.0 | 33.6 ± 1.6 | 34.8 ± 1.5 | 2.0 ± 0.9 | 5.3 ± 2.0 | 1.8 ± 0.5 | 0.7 ± 0.6 |
| 10 | 5.0 | 35.9 ± 2.7 | 34.4 ± 1.9 | 1.58 ± 0.5 | 6.2 ± 2.6 | 0.6 ± 0.6 | 0.3 ± 0.3 |
| 10 | 3.3 | 41.1 ± 3.2 | 40.4 ± 2.2 | 2.2 ± 1.1 | 3.4 ± 0.4 | 0.8 ± 0.8 | 0.0 ± 0.0 |
| 20 | 10.0 | 34.1 ± 2.4 | 36.0 ± 1.5 | 3.7 ± 1.4 | 9.3 ± 3.2 | 1.8 ± 0.1 | 1.7 ± 0.8 |
| 20 | 5.0 | 39.1 ± 1.1 | 37.8 ± 2.0 | 5.9 ± 3.0 | 14.3 ± 5.9 | 1.0 ± 0.4 | 0.9 ± 0.6 |
| 20 | 3.3 | 43.4 ± 1.0 | 39.8 ± 1.9 | 2.9 ± 0.1 | 12.0 ± 3.3 | 1.5 ± 0.7 | 0.4 ± 0.4 |
| 30 | 10.0 | 36.1 ± 0.3 | 38.3 ± 2.2 | 4.9 ± 1.0 | 10.6 ± 0.1 | 1.9 ± 0.8 | 2.0 ± 0.9 |
| 30 | 5.0 | 41.8 ± 2.5 | 39.7 ± 0.9 | 9.9 ± 4.8 | 15.8 ± 3.1 | 1.6 ± 0.4 | 1.8 ± 0.6 |
| 30 | 3.3 | 40.2 ± 1.7 | 39.8 ± 3.7 | 5.0 ± 0.5 | 15.8 ± 4.0 | 1.6 ± 0.8 | 0.0 ± 0.0 |
| Wood Waste | Dry weight Content (%) | Cellulose Content (% DW) | Hemicellulose Content (% DW) | Lignin Content (% DW) |
|---|---|---|---|---|
| Pine chips | 93.07a ± 0.12 | 49.93a ± 0.87 | 14.33a ± 2.49 | 26.68a ± 0.27 |
| Sweet cherry chips | 93.97a ± 0.09 | 52.07a ± 0.76 | 21.10b ± 0.62 | 12.44b ± 0.23 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kłosowski, G.; Mikulski, D.; Menka, A. Microwave-Assisted One-Step Conversion of Wood Wastes into Levulinic Acid. Catalysts 2019, 9, 753. https://doi.org/10.3390/catal9090753
Kłosowski G, Mikulski D, Menka A. Microwave-Assisted One-Step Conversion of Wood Wastes into Levulinic Acid. Catalysts. 2019; 9(9):753. https://doi.org/10.3390/catal9090753
Chicago/Turabian StyleKłosowski, Grzegorz, Dawid Mikulski, and Aleksandra Menka. 2019. "Microwave-Assisted One-Step Conversion of Wood Wastes into Levulinic Acid" Catalysts 9, no. 9: 753. https://doi.org/10.3390/catal9090753
APA StyleKłosowski, G., Mikulski, D., & Menka, A. (2019). Microwave-Assisted One-Step Conversion of Wood Wastes into Levulinic Acid. Catalysts, 9(9), 753. https://doi.org/10.3390/catal9090753








